Abstract
HIV-1 has developed several strategies to evade natural killer (NK)–cell antiviral functions. One of these mechanisms is the HIV-1–induced expansion of highly dysfunctional NK-cell subsets. Here, we analyze a large cohort of HIV-1–infected patients in early or chronic phases of infection, both cross-sectionally and longitudinally. We demonstrate that a striking decrease in the surface expression of sialic acid–binding immunoglobulin-like lectin 7 (Siglec-7) represents the earliest marker of the aberrant NK-cell dysregulation, which precedes the down-modulation of CD56 mostly occurring in patients with chronic HIV-1 viremia. The combined detection of Siglec-7 and CD56 allows the identification of 2 new pathologic NK-cell subsets expanded preferentially in early (Siglec-7−/CD56+) or chronic (Siglec-7−/CD56−) stages of HIV-1 infection. Remarkably, these phenotypic abnormalities were directly associated with progressive and distinct impairments of NK-cell functions. The aforementioned NK-cell aberrancies could be observed only in the presence of high levels of viral replication and not in patients with low or undetectable HIV-1 viremia, such as long-term nonprogressors or patients having undergone antiretroviral therapy. High frequencies of Siglec-7−/CD56+ and Siglec-7−/CD56− pathologic NK cells reflect the immune and clinical status of HIV-1 infection and can also track the effectiveness of therapy.
Introduction
Natural killer (NK) cells were first identified in the middle 1970s1,2 on the basis of their capacity to rapidly kill, without previous sensitization, tumor cells lacking major histocompatibility complex class I (MHC-I) molecules (missing-self hypothesis).3,4 Our understanding of these important effectors of innate immune responses has certainly advanced over the past 30 years, and today NK cells are no longer considered only for their cytolytic activity against cancer cells. Among the several other NK-cell immune functions, there are protection against viral and parasitic infections, secretion of important immune-modulatory cytokines, and regulation of the maternofetal tolerance. Moreover, NK cells have been shown to function as regulatory cells by interacting with other cellular components of the immune system, such as dendritic cells (DCs), to link innate with adaptive immunity and ensure optimal antigen-specific immune responses.5-10
NK-cell activation and target cell recognition represent the final step of a complex process that is based on both activating and inhibitory signals that are simultaneously delivered through several NK-cell receptors after the engagement of their putative ligands. Inhibitory NK-cell receptors (iNKRs) belong either to the superfamily of immunoglobulin or to the c-type lectin receptors and recognize mainly MHC-I molecules. Appropriate interactions between iNKRs and MHC-I turn NK cells “off” and ensure tolerance to self. Diminution or absence of surface levels of MHC-I molecules after viral infection or tumor transformation results in a reduced engagement of iNKRs, thereby allowing NK cells to eliminate a missing-self cellular target.11,12 The acquisition of NK-cell cytotoxicity during evolution has been achieved through a highly sophisticated process that educates NK cells to ensure self-recognition. Activating NK-cell receptors and coreceptors detect the presence of specific ligands on stressed, infected, or tumor-transformed cells, and this binding leads to the elimination of the targeted cells. Therefore, the dynamic balance of antagonistic pathways on interaction with neighboring cells regulates NK-cell immune functions.13-15
HIV-1 has been shown to severely alter the phenotype and function of NK cells.16,17 In particular, high levels of chronic viral replication in HIV-1–infected patients lead to an aberrant NK cell–surface expression of several inhibitory and activating NK-cell receptors and to high frequencies of a pathologic CD56−/CD16+ (CD56−) NK-cell population that is rarely represented in uninfected donors.18-20 These phenotypic abnormalities have been shown to severely affect NK-cell cytolytic function against tumor cell targets and against autologous CD4+ HIV-1–infected cells, to impair the NK-cell production of antiviral cytokines, and to disrupt NK-cell interactions with autologous DCs.21-25 Although NK cells are not directly infected by HIV-1, it has been shown in cross-sectional studies that the chronic suppression of viral replication to undetectable level in patients undergoing antiretroviral therapy (ART) restores NK-cell phenotype and functions.22,26 The kinetics of NK-cell aberrancies in phenotype and function associated with HIV-1 viremia or with suppression of viral replication are largely unclear, and longitudinal analyses on large cohorts of HIV-1–infected persons at different stages of infection are required to answer these questions. Furthermore, a cellular marker highly sensitive to viral replication that can be associated with NK-cell abnormalities starting from the initial stages of HIV-1 infection has yet to be identified.
In this study, we analyzed the effect of HIV-1 viremia on the repertoire of NK-cell receptors from 144 infected patients either naive for treatment or whose ART had been discontinued at the time of the study. This cohort included 21 early, 96 chronic, and 27 long-term nonprogressor (LTNP) HIV-1–infected patients. Moreover, 33 chronic viremic patients who underwent ART were followed longitudinally for 24 months. By the analysis of a large panel of activating and inhibitory NK-cell receptors and coreceptors, we could detect an altered expression of sialic acid–binding immunoglobulin-like lectin 7 (Siglec-7). This molecule, also termed p75/AIRM1, was originally identified as an inhibitory receptor constitutively expressed on all NK-cell subsets.27,28 We show here that the sharp decrease of Siglec-7 represents the earliest marker of the aberrant dysregulation of NK cells preceding the down-modulation of CD56. The combined use of Siglec-7 and CD56 allows the identification of 2 new pathologic and dysfunctional NK-cell subsets correlating with different stages of HIV-1 infection.
Methods
Study subjects
Three cohorts of early viremic, chronic viremic, and LTNP HIV-1–infected patients were studied (Table 1). Early infection was defined on the basis of the last seronegative test for HIV-1–specific antibodies (Abs) measured with the enzyme-linked immunosorbent assay technique: 21 patients with high levels of viral replication, naive for ART, without any opportunistic infection or malignancy and with a recently reported history of seroconversion (median, 2 ± 1.9 months) for HIV-1–specific Abs were enrolled in this cohort. The chronic viremic cohort was composed of 96 patients with a prolonged high HIV-1 viremia (≥ 24 months), history of opportunistic diseases, either naive for ART or whose therapeutic regimen had been discontinued. Of these 96 subjects with chronic infection, 33 were successfully treated with ART and followed longitudinally for 24 months. ART included at least one protease inhibitor, one non-nucleoside reverse-transcriptase inhibitor, and/or 2 nucleoside reverse-transcriptase inhibitors. For the 27 LTNPs enrolled in this study, clinic criteria included clinically healthy status, negative history for opportunistic diseases, stable T-cell counts, and very low or undetectable HIV-1 viral load, naive for ART.29
Immunologic and virologic profiles of early, chronic, and LTNP HIV-1–infected patients and of healthy donors
| Cohort . | Time of infection, mo (y) . | Viral load,* copies RNA/mL . | CD4+ T cells/μL . | CD4+/CD8+ ratio . | Antiretroviral therapy . |
|---|---|---|---|---|---|
| Healthy donors (n = 70) | NA | NA | 1315 ± 216 | 1.21 ± 0.8 | NA |
| Early HIV-1 infection (n = 21) | 2 ± 1.9† (0.16 ± 0.16)† | 45 570 ± 99 453 | 815 ± 316 | 0.54 ± 0.25 | Naive |
| Chronic viremic HIV-1 infection (n = 96) | 67.9% ± 63.8‡ (5.7 ± 5.3)‡ | 59 381 ± 83 390 | 380 ± 207 | 0.4 ± 0.28 | Off therapy (≥ 24 mos) |
| Chronic LTNPs (n = 27) | 170.4 ± 63.6‡ (14.2 ± 5.3)‡ | < 50-660 (range) | 912 ± 321 | 1.2 ± 0.1 | Naive |
| Cohort . | Time of infection, mo (y) . | Viral load,* copies RNA/mL . | CD4+ T cells/μL . | CD4+/CD8+ ratio . | Antiretroviral therapy . |
|---|---|---|---|---|---|
| Healthy donors (n = 70) | NA | NA | 1315 ± 216 | 1.21 ± 0.8 | NA |
| Early HIV-1 infection (n = 21) | 2 ± 1.9† (0.16 ± 0.16)† | 45 570 ± 99 453 | 815 ± 316 | 0.54 ± 0.25 | Naive |
| Chronic viremic HIV-1 infection (n = 96) | 67.9% ± 63.8‡ (5.7 ± 5.3)‡ | 59 381 ± 83 390 | 380 ± 207 | 0.4 ± 0.28 | Off therapy (≥ 24 mos) |
| Chronic LTNPs (n = 27) | 170.4 ± 63.6‡ (14.2 ± 5.3)‡ | < 50-660 (range) | 912 ± 321 | 1.2 ± 0.1 | Naive |
Data are median ± SD.
NA indicates not applicable.
Plasma viremia was analyzed by an ultrasensitive bDNA assay (Chiron) with a lower limit detection of 50 copies per milliliter of plasma.
Range of time between the last seronegative screening for anti-HIV-1–specific mAbs and the first positivity to the test.
Since the time of first reported diagnosis.
Peripheral blood mononuclear cells (PBMCs) were obtained by leukapheresis performed in accordance with the clinical protocol approved by the Institutional Review Board (IRB) of the National Institute of Allergy and Infectious Diseases. Each patient signed a consent form that was approved by the above IRBs, in accordance with the Declaration of Helsinki. As negative controls, cells from 70 healthy donors seronegative for HIV-1 were obtained by apheresis generously provided by the Transfusion Medicine Department of the Mark O. Hatfield Clinical Research Center of the National Institutes of Health as a part of IRB-approved clinical studies.
Isolation of PBMCs and flow cytometry
PBMCs were obtained from leukapheresis packs by Ficoll-Hypaque density gradient centrifugation (LSM, MP Biomedicals).30
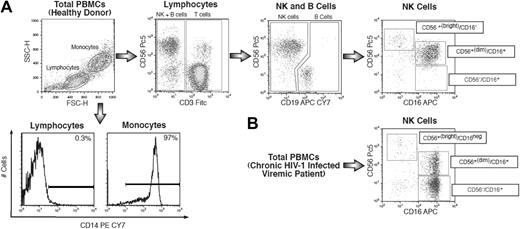
For 6-color flow cytofluorimetric analysis (FACS Canto II, BD Biosciences), fresh PBMCs were stained with directly conjugated monoclonal antibodies (mAbs) labeled with different fluorochromes. Within the lymphocyte gate, NK cells were defined as CD14−, CD3−, and CD19− cells. The expression of Siglec-7 was then analyzed on all NK-cell subsets, which includes CD56 bright/CD16− and CD56dim/CD16+ cell subsets normally represented in healthy donors5 and pathologic CD56−/CD16+ NK cells expanded preferentially in chronic viremic HIV-infected patients22 (Figure 1). To identify NK cells, the following antihuman mAbs were used in this study: fluorescein isothiocyanate–labeled CD3 and PC5-labeled CD56 (Beckman Coulter), allophycocyanin/CY7-labeled CD19 and phycoerythrin/Cy7-labeled CD14 (BD Biosciences PharMingen), and allophycocyanin-labeled CD16 (Miltenyi Biotec). The staining of a large panel of NK-cell surface markers was performed using the following phycoerythrin-labeled mAbs: Siglec-7 (CDw328), NKp46 (CD335), NKp30 (CD337), NKp44 (CD336), NKG2D (CD314), NKG2A (CD159a), CD94, NKp80, 2B4 (CD244), KIR2DL2/DL3/DS2 (CD158b1/b2/j), KIR2DL1/DS1 (CD158 a/h), KIR3DL1/DS1 (CD158e1/e2) and LIR1/ILT2 (Beckman Coulter), and NKG2C, NTB-A, and DNAM-1 (CD226; R&D Systems), and LAIR-1 (BD Biosciences PharMingen). The data were analyzed using FlowJo software (TreeStar).
Functional assays and statistical analyses are presented in supplemental data (available on the Blood website; see the Supplemental Materials link at the top of the online article).
Results
High levels of chronic HIV-1 viremia are required for the expansion of CD56− NK-cell subset
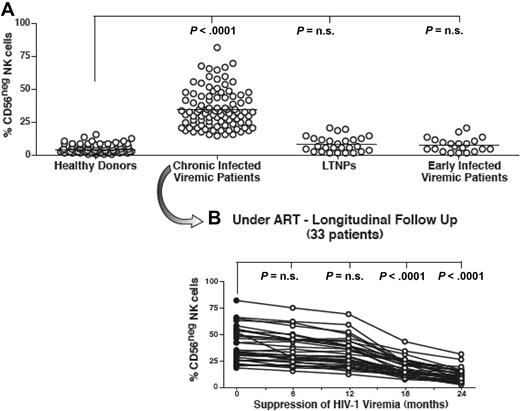
It has been previously reported that the pathologic CD56− NK-cell subset is highly expanded in chronic viremic HIV-1–infected patients and is rarely represented in healthy donors. The suppression of HIV-1 viremia for 24 months or longer in patients having undergone ART in cross-sectional studies restored the CD56 expression on NK cells to levels similar to those of cells from uninfected persons.22 Considering that CD56− NK cells severely interfere with several innate and adaptive immune responses against HIV-1,16 it is important to understand the kinetic of CD56 distribution during the course of HIV-1 infection.
In the present study, CD14−/CD3−/CD19− NK lymphocytes were identified by multicolor flow cytometric analyses of freshly purified PBMCs (Figure 1). The fact that these CD14−/CD3−/CD19− cells were indeed NK cells was also confirmed by their positive expression of other specific NK-cell markers, such as NKp46 and NKp30 (data not shown). As shown in Figure 2A, we did not observe any abnormal expansion of CD56− NK cells in LTNPs, thus confirming that high HIV-1 viral load is required for the expansion of this subset. In addition, we found that, regardless of the very high levels of ongoing viral replication occurring in initial phases of HIV-1 infection, the surface levels of CD56 on NK cells from early HIV-1–infected patients were similar to that of healthy donors. These data confirmed that only chronic insults given by HIV-1 viremia led to the appearance at high frequencies of CD56− NK cells. Indeed, within our cohort of early HIV-1–infected persons, only those patients who did not fully suppress HIV-1 viral load after ART (5 of 21) had a remarkable expansion of CD56− NK cells after 1 year of chronic viral replication (data not shown), similar to data recently published by another report.18
Strategy of NK-cell gating within total freshly purified PBMCs. (A) Within the lymphocyte gate of freshly purified PBMCs, NK-cell subsets were separated from CD14+ (monocytes), CD3+ (T lymphocytes), and CD19+ (B lymphocytes), and then characterized for CD56 and CD16 surface expression. (B) Representative flow cytometric example of expansion of CD56−/CD16+ NK-cell (lower right) subset from a chronic viremic HIV-1–infected patient.
Strategy of NK-cell gating within total freshly purified PBMCs. (A) Within the lymphocyte gate of freshly purified PBMCs, NK-cell subsets were separated from CD14+ (monocytes), CD3+ (T lymphocytes), and CD19+ (B lymphocytes), and then characterized for CD56 and CD16 surface expression. (B) Representative flow cytometric example of expansion of CD56−/CD16+ NK-cell (lower right) subset from a chronic viremic HIV-1–infected patient.
Frequency and kinetics of CD56− NK cells. (A) Summary graphs of statistical dot plots with medians (horizontal black bars) showing the percentages of CD56−/CD16+ (CD56−) NK-cell subsets in healthy donors, chronic viremic, LTNP, and early viremic-infected patients. (B) Summary graph of statistical dot plots showing the kinetic of CD56− NK-cell distribution in 33 chronic viremic HIV-1–infected patients having undergone ART (time 0 or baseline; ●) and followed longitudinally every 6 months for 2 years (times 6, 12, 18, and 24; ○).
Frequency and kinetics of CD56− NK cells. (A) Summary graphs of statistical dot plots with medians (horizontal black bars) showing the percentages of CD56−/CD16+ (CD56−) NK-cell subsets in healthy donors, chronic viremic, LTNP, and early viremic-infected patients. (B) Summary graph of statistical dot plots showing the kinetic of CD56− NK-cell distribution in 33 chronic viremic HIV-1–infected patients having undergone ART (time 0 or baseline; ●) and followed longitudinally every 6 months for 2 years (times 6, 12, 18, and 24; ○).
In line with the slow progressive HIV-1–induced emergence of CD56− NK cells, the suppression of viral load lead to a slow progressive restoration of CD56 on the NK-cell surface. Our longitudinal analyses showed that a minimum of 18 months of undetectable viremia was necessary to observe a statistically significant decrease of CD56− NK-cell subsets in chronic HIV-1–infected viremic patients undergoing ART (P < .001; Figure 2B). Taken together, these data demonstrate that the HIV-1–induced down-modulation of CD56 on NK cells, as well as its recovery after ART, requires high levels of chronic viral replication or prolonged suppression of HIV-1 viremia, respectively. Although the presence of chronic high levels of HIV-1 replication was found to be necessary to detect a significant expansion of CD56− NK cells, we did not observe a direct correlation between the degree of plasma viremia or CD4+ T-cell count and the frequency of this aberrant population (data not shown).
Siglec-7 down-modulation is an early event associated with high levels of HIV-1 replication
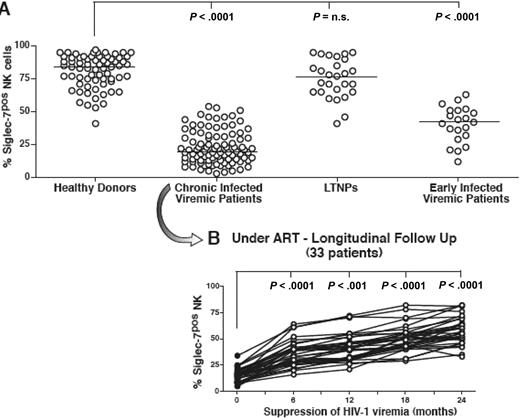
To identify a cellular marker highly sensitive to viral replication that could be associated with NK-cell abnormalities starting from the initial stages of HIV-1 infection, we screened a large panel of surface NK-cell markers. Differently from CD56, we observed that the distribution of Siglec-7 on the NK-cell surface is pathologically altered starting from early phases of HIV-1 infection. Indeed, expression of Siglec-7 was significantly decreased on NK cells from HIV-1–infected patients caught in initial phases of infection compared with that of healthy donors (P < .001). Moreover, the frequency of Siglec-7+ NK cells became even lower in patients with chronic HIV-1 viremia compared with early-infected persons (P < .001). The fact that the decreased frequency of Siglec-7+ NK cells was associated with high levels of HIV-1 replication was also confirmed by the observation that surface levels of Siglec-7 on NK cells from LTNPs were similar to that of uninfected persons (Figure 3A). Again, as observed for the HIV-1–induced down-modulation of CD56, there was not a direct correlation between the degree of HIV-1 plasma viremia or CD4+ T-cell count and the frequency of Siglec-7− NK-cell subset (data not shown).
Frequency and kinetics of Siglec-7+ NK cells. (A) Summary graphs of statistical dot plots with medians (horizontal black bars) showing the percentages of Siglec-7+ NK-cell subsets in healthy donors, chronic viremic, LTNP, and early viremic-infected patients. (B) Summary graph of statistical dot plots showing the kinetics of Siglec-7+ NK-cell distribution in 33 chronic viremic HIV-1–infected patients having undergone ART (time 0 or baseline; ●) and followed longitudinally every 6 months for 2 years (times 6, 12, 18, and 24; ○).
Frequency and kinetics of Siglec-7+ NK cells. (A) Summary graphs of statistical dot plots with medians (horizontal black bars) showing the percentages of Siglec-7+ NK-cell subsets in healthy donors, chronic viremic, LTNP, and early viremic-infected patients. (B) Summary graph of statistical dot plots showing the kinetics of Siglec-7+ NK-cell distribution in 33 chronic viremic HIV-1–infected patients having undergone ART (time 0 or baseline; ●) and followed longitudinally every 6 months for 2 years (times 6, 12, 18, and 24; ○).
In line with the rapid decrement of Siglec-7 expression on NK cells in the presence of high levels of HIV-1 viremia, less than 6 months of successful viral suppression after ART were sufficient to restore Siglec-7 surface levels on NK cells from both early (data not shown) and chronic viremic HIV-1–infected viremic patients (Figure 3B). We could not detect a similar kinetic of rapid modulation coupled with HIV-1 viremia or ART for any other NK-cell receptor, such as natural cytotoxicity receptors or NKG2A or killer-immunoglobulin-like receptors (KIRs), whose NK-cell expression was found pathologically altered in chronic viremic HIV-1–infected patients (data not shown).16
Siglec-7 is also constitutively expressed on monocytes and on a small subset of CD8+ T cells.28 Interestingly, we could not detect any significant change of Siglec-7 expression on these cellular compartments (data not shown), indicating that this HIV-1–associated phenotypic abnormality was restricted to NK cells.
The phenotypic distribution of CD56 and Siglec-7 on NK cells identifies different stages of HIV-1 infection
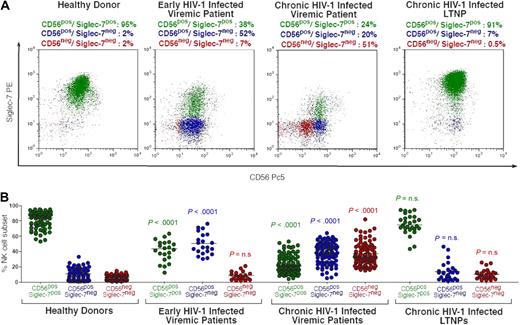
Our cross-sectional and longitudinal phenotypic analyses demonstrate not only that HIV-1 viremia can induce a progressive reduction of both CD56 and Siglec-7 on NK cells, but also that the expression of these 2 NK-cell markers follows different kinetics during the course of the disease. The fact that HIV-1 replication and suppression differently affect the surface expression of Siglec-7 and CD56 represents a unique tool for identifying distinct NK-cell subsets expanded at specific stages of infection. In line with previously reported data,27 the majority of fresh peripheral blood NK cells from healthy donors are characterized by a Siglec-7+/CD56+ phenotype (Figure 4). The decreased expression of Siglec-7, and not of CD56, on NK cells during the early stages of HIV-1 infection resulted in a significant expansion of a novel Siglec-7−/CD56+ NK-cell population that is rarely observed in healthy donors (P < .001). After the subsequent down-modulation of CD56 occurring mostly on NK cells from chronic viremic HIV-1–infected patients, we could detect the emergence of another pathologic Siglec-7−/CD56− subset. This last NK-cell population is almost undetectable in early-infected viremic patients (P < .001) and in uninfected persons (P < .001). As shown in Figures 2 and 3, the very low or undetectable levels of viral replication in LTNPs did not significantly affect the expression of Siglec-7 and CD56 on NK cells. Indeed, the distribution of peripheral blood NK-cell subsets in LTNPs was comparable with that observed in healthy uninfected persons. These data demonstrate that the peculiar NK-cell expression of Siglec-7 and CD56 identifies different aberrant NK-cell phenotypes correlating with different stages of HIV-1 infection. The different kinetics of Siglec-7 and CD56 recovery on NK-cell surface in the presence of a successful viral suppression in patients undergoing ART resulted in a progressive switch from Siglec-7−/CD56− to Siglec-7+/CD56− NK cells within the first 18 months of therapy. A complete restoration of Siglec-7+/CD56+ NK-cell phenotype could be observed only after 24 months of ART (data not shown).
Identification of pathologic NK-cell subset in HIV-1 infection by the distribution on the cell surface of CD56 and Siglec-7. (A) Flow cytometric dot plot graphs showing the distribution of fresh Siglec-7+/CD56+ (green), Siglec-7−/CD56+ (blue), and Siglec-7−/CD56− (red) NK-cell subsets from a representative healthy donor (first column), early viremic (second column), chronic viremic (third column), and LTNP (fourth column) HIV-1–infected patient. (B) Statistical summary graphs of dot plot with medians (horizontal black bars) and P values showing the frequencies of fresh Siglec-7+/CD56+ (green), Siglec-7−/CD56+ (blue), and Siglec-7−/CD56− NK-cell subsets from healthy donors compared with those of HIV-1–infected persons at different stages of disease.
Identification of pathologic NK-cell subset in HIV-1 infection by the distribution on the cell surface of CD56 and Siglec-7. (A) Flow cytometric dot plot graphs showing the distribution of fresh Siglec-7+/CD56+ (green), Siglec-7−/CD56+ (blue), and Siglec-7−/CD56− (red) NK-cell subsets from a representative healthy donor (first column), early viremic (second column), chronic viremic (third column), and LTNP (fourth column) HIV-1–infected patient. (B) Statistical summary graphs of dot plot with medians (horizontal black bars) and P values showing the frequencies of fresh Siglec-7+/CD56+ (green), Siglec-7−/CD56+ (blue), and Siglec-7−/CD56− NK-cell subsets from healthy donors compared with those of HIV-1–infected persons at different stages of disease.
Finally, we did not observe any significant differences in the amount of total NK cells between uninfected and HIV-1–infected persons when we analyzed the frequencies of the entire NK-cell population, which includes normal Siglec-7+/CD56+ and pathologic Siglec-7−/CD56+ and Siglec-7−/CD56− cells (Table 2).
Frequency and absolute numbers of circulating CD56+/Siglec-7+, CD56−/Siglec-7+, and CD56−/Siglec-7− NK-cell subsets from HIV-1–infected patients at different stages of disease compared with those from healthy donors
| Cohort . | NK cells . | |||
|---|---|---|---|---|
| CD56+/Siglec-7+ . | CD56+/Siglec-7− . | CD56−/Siglec-7− . | Total . | |
| Healthy donors (n = 70) | 11.1 ± 4.3 (190 ± 64) | 1.1 ± 1.3 (20 ± 15) | 0.8 ± 0.6 (14 ± 20) | 13.3 ± 6.1 (223 ± 91) |
| Early HIV-1 infection (n = 21) | 6.5 ± 3.3 (108 ± 71, ↓)* | 7.5 ± 3.6 (125 ± 62, ↑)* | 1.4 ± 0.9 (23 ± 19, ∼)† | 15.6 ± 12.3 (258 ± 146, ∼)† |
| Chronic viremic HIV-1 infection (n = 96) | 3 ± 2.1 (49 ± 25, ↓)* | 5.7 ± 3.1 (98 ± 32, ↑)* | 5.1 ± 2.1 (82 ± 38, ↑)* | 14.1 ± 5.6 (231 ± 73, ∼)† |
| Chronic LTNPs (n = 27) | 10.9 ± 4.8 (185 ± 46, ∼)† | 1.74 ± 1.5 (32 ± 25, ∼)† | 1.3 ± 1.1 (25 ± 16, ∼)† | 14.3 ± 6.1 (245 ± 85, ∼)† |
| Cohort . | NK cells . | |||
|---|---|---|---|---|
| CD56+/Siglec-7+ . | CD56+/Siglec-7− . | CD56−/Siglec-7− . | Total . | |
| Healthy donors (n = 70) | 11.1 ± 4.3 (190 ± 64) | 1.1 ± 1.3 (20 ± 15) | 0.8 ± 0.6 (14 ± 20) | 13.3 ± 6.1 (223 ± 91) |
| Early HIV-1 infection (n = 21) | 6.5 ± 3.3 (108 ± 71, ↓)* | 7.5 ± 3.6 (125 ± 62, ↑)* | 1.4 ± 0.9 (23 ± 19, ∼)† | 15.6 ± 12.3 (258 ± 146, ∼)† |
| Chronic viremic HIV-1 infection (n = 96) | 3 ± 2.1 (49 ± 25, ↓)* | 5.7 ± 3.1 (98 ± 32, ↑)* | 5.1 ± 2.1 (82 ± 38, ↑)* | 14.1 ± 5.6 (231 ± 73, ∼)† |
| Chronic LTNPs (n = 27) | 10.9 ± 4.8 (185 ± 46, ∼)† | 1.74 ± 1.5 (32 ± 25, ∼)† | 1.3 ± 1.1 (25 ± 16, ∼)† | 14.3 ± 6.1 (245 ± 85, ∼)† |
Results include the percentages of CD3−/CD19−/CD14− NK-cell subsets within the lymphocyte population, followed by the absolute numbers of cells/μL (values in parentheses), presented as median ± SD. See also Figure 1 for gating strategy.
↑ indicates increased; ↓, decreased; and ∼, stable.
P < .001.
P = not significant.
These data indicate that high levels of HIV-1 replication induce a pathologic redistribution of NK-cell subsets rather than a reduction in the absolute numbers of circulating of NK cells.31
The decreased expression of Siglec-7 and CD56 is associated with impaired functions of NK-cell subsets in HIV-1–infected patients
We next analyzed whether the different NK-cell subsets identified on the basis of the combined use of Siglec-7 and CD56 marker were also characterized by differences in terms of NK-cell functions at different stages of HIV-1 infection. In these experiments, PBMCs from healthy donors and HIV-1–infected persons were activated in vitro with recombinant interleukin-2 (rIL-2) for 24 hours. As shown in Figures 4,Figure 5–6, this short preactivation with rIL-2 did not induce substantial changes in the distribution of Siglec-7+/CD56+, Siglec-7−/CD56+, and Siglec-7−/CD56− NK-cell subsets within the lymphocyte gate of PBMCs from both uninfected and HIV-1–infected persons. In this regard, we previously reported that, on stimulation in vitro with rIL-2, the expression of CD56 was up-regulated on NK cells from chronic HIV-1–infected viremic patients.19 However, we demonstrated that more than 3 days of rIL-2 stimulation was necessary to observe a significant up-modulation of CD56, and the complete recovery of its expression on NK cells required at least 3 weeks of rIL-2 stimulation. In contrast, long-term in vitro rIL-2 stimulation of NK cells from HIV-1–infected donors was unable to restore the normal distribution of the activating and inhibiting NK receptors (ie, natural cytotoxicity receptors, KIRs, and NKG2A) whose surface levels were found to be significantly altered by HIV-1 viremia.19 Similarly, stimulation with rIL-2 did not restore the expression of Siglec-7 on NK cells even after 3 weeks of cellular activation (data not shown).
CD107a expression on rIL-2–activated NK-cell subsets on stimulation with K562. (A) Flow cytometric profiles of CD107a expression on Siglec-7+/CD56+ (green), Siglec-7−/CD56+ (blue), and Siglec-7−/CD56− (red) rIL-2–activated NK-cell subsets from a representative example for a healthy donor (top line), an early (middle line), and a chronic (bottom line) HIV-1–infected patient. (B) The fraction of CD107+ NK-cell subsets is presented as a mean of 5 independent experiments (with P values and bars indicating SDs) performed with cells from 5 unrelated healthy donors, 5 unrelated HIV-1 early-infected persons, and LTNPs.
CD107a expression on rIL-2–activated NK-cell subsets on stimulation with K562. (A) Flow cytometric profiles of CD107a expression on Siglec-7+/CD56+ (green), Siglec-7−/CD56+ (blue), and Siglec-7−/CD56− (red) rIL-2–activated NK-cell subsets from a representative example for a healthy donor (top line), an early (middle line), and a chronic (bottom line) HIV-1–infected patient. (B) The fraction of CD107+ NK-cell subsets is presented as a mean of 5 independent experiments (with P values and bars indicating SDs) performed with cells from 5 unrelated healthy donors, 5 unrelated HIV-1 early-infected persons, and LTNPs.
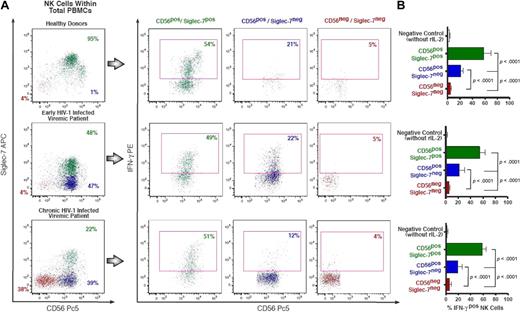
Intracellular IFN-γ production by rIL-2–activated NK-cell subsets. (A) Flow cytometric profiles of intracellular amounts of IFN-γ in Siglec-7+/CD56+ (green), Siglec-7−/CD56+ (blue), and Siglec-7−/CD56− (red) NK-cell subsets from a representative example for a healthy donor (top line), an early (middle line), and a chronic (bottom line) HIV-1–infected patient. (B) The fraction of IFN-γ+ NK-cell subsets is presented as a mean of 5 independent experiments (with P values and bars indicating SDs) performed with cells from 5 unrelated healthy donors, 5 unrelated HIV-1 early-infected persons, and LTNPs.
Intracellular IFN-γ production by rIL-2–activated NK-cell subsets. (A) Flow cytometric profiles of intracellular amounts of IFN-γ in Siglec-7+/CD56+ (green), Siglec-7−/CD56+ (blue), and Siglec-7−/CD56− (red) NK-cell subsets from a representative example for a healthy donor (top line), an early (middle line), and a chronic (bottom line) HIV-1–infected patient. (B) The fraction of IFN-γ+ NK-cell subsets is presented as a mean of 5 independent experiments (with P values and bars indicating SDs) performed with cells from 5 unrelated healthy donors, 5 unrelated HIV-1 early-infected persons, and LTNPs.
Using the CD107a degranulation assay,32 we compared the different ability of the 3 rIL-2–activated Siglec-7+/CD56+, Siglec-7−/CD56+, and Siglec-7−/CD56− NK-cell subsets to degranulate on stimulation with K562. As shown in Figure 5, Siglec-7+/CD56+ cells, which represents the largest NK-cell fraction in healthy donors (median, 84% ± 11%) and in LTNPs (median, 74% ± 13.05%), always expressed very high levels of CD107a. In contrast, the percentage of Siglec-7−/CD56− cells expressing CD107a was very low. This result is in line with our previous studies showing the extremely low cytotoxicity of the pathologic CD56− NK-cell population highly expanded in chronic viremic HIV-1–infected patients.19,22 Although the degranulation levels of Siglec-7−/CD56+ cells, the subset preferentially expanded in early HIV-1 infection, was significantly reduced compared with that of normal Siglec-7+/CD56+ NK cells (P < .001), the amount of Siglec-7−/CD56+ cells expressing CD107a was always significantly higher compared with that of anergic Siglec-7−/CD56− NK cells (P < .001).
We also assessed the ability of these 3 different NK-cell subsets to produce interferon-γ (IFN-γ; Figure 6). To trigger the maximal cytokine production, NK cells were cultured with rIL-2 for 24 hours and then seeded for 4 hours in 96-well plates coated with an anti-CD16 mAb. In line with data obtained with the CD107a degranulation assay, normal Siglec-7+/CD56+ NK cells showed high production of IFN-γ. On the contrary, pathologic Siglec-7−/CD56− cells produced very low or almost undetectable levels of IFN-γ. Even though the ability of Siglec-7−/CD56+ NK cells to secrete IFN-γ was markedly decreased compared with that of normal Siglec-7+/CD56+ NK cells (P < .001), they were still able to produce significantly higher amounts of this cytokine compared with the markedly anergic Siglec-7−/CD56− (P < .001).
These functional data demonstrate that the early down-modulation of Siglec-7 on NK cells during the course of HIV-1 infection identifies a novel pathologic Siglec-7−/CD56+ NK-cell subset displaying an initial impairment of both cytolytic activity and cytokine production.
Discussion
In the present study, we demonstrate that surface expression of Siglec-7 represents a novel and sensitive marker for identifying and tracking NK-cell phenotypic changes and impaired functions during different stages of HIV-1 infection. We show that the early decreased expression of Siglec-7 on NK cells in HIV-1–infected persons precedes the down-regulation of CD56, which mostly occurs in patients in chronic stages of infection and with high levels of ongoing viral replication. The different kinetic of Siglec-7 versus CD56 down-modulation resulted in a reduction of normal Siglec-7+/CD56+ NK cells and allowed the detection of 2 new pathologic Siglec-7−/CD56+ and Siglec-7−/CD56− NK-cell subsets. Siglec-7−/CD56+ NK cells were preferentially expanded in early stages of infection, whereas the Siglec-7−/CD56− phenotype was detectable only in NK cells from chronic viremic HIV-1–infected patients. Remarkably, these phenotypic abnormalities of NK cells were directly associated with progressive and distinct impairments of NK-cell cytolytic activities and cytokine production. The maximal loss of NK-cell function was reached with the expansion of Siglec-7−/CD56− subset in chronic viremic HIV-1–infected patients. The very low or undetectable levels of viral replication in LTNPs did not modify the physiologic surface distribution of Siglec-7 or CD56 on NK cells, thus indicating that these pathologic NK-cell phenotypes cannot be observed in the absence of high HIV-1 viremia. Finally, the possibility of following for 24 months 33 chronic viremic HIV-1–infected patients who successfully suppressed HIV-1 replication to undetectable viremia after ART allowed us to exactly identify the kinetic of NK-cell restoration of Siglec-7 and CD56 expression.
Several studies had shown over the past decade that high levels of chronic HIV-1 replication induce several phenotypic changes in NK cells that progressively reduce several important functions.16,17,33,34 Among the several NK-cell phenotypic aberrancies associated with HIV-1 infection, the decreased expression of CD56 was the first to be identified.20,35 Our cross-sectional and longitudinal data confirm that chronic and high levels of viral replication are needed for a pathologic expansion of CD56− NK cells. Indeed, this aberrant NK-cell population could be detected at very low frequencies in both early viremic HIV-1–infected patients and LTNPs. We also demonstrate here that the suppression of HIV-1 replication to undetectable levels in patients having undergone ART does not result in an immediate recovery of CD56 expression on NK cells, but at least 18 months of treatment is required to observe a significant reduction of CD56− subsets and higher frequencies of normal CD56+ NK cells.
The decreased expression of Siglec-7 on NK cells from early viremic HIV-1–infected persons provides the first evidence of an altered NK-cell phenotype in the early stages of innate immune responses after the infection. We also show here that a relatively short period of HIV-1 suppression to undetectable levels (≤ 6 months) in patients having undergone ART is sufficient to significantly restore Siglec-7 expression on NK cells from infected persons. The different kinetics of modulation of Siglec-7 and CD56 associated with high levels of HIV-1 viremia results in the appearance of 2 different NK-cell populations: (1) Siglec-7−/CD56+ NK-cell subset, which is mostly expanded in early infected patients; and (2) Siglec-7−/CD56− NK-cell subset, which is commonly detected in chronic viremic persons. We demonstrate that these 2 distinct phenotypes are also associated with a different degree of reduced NK-cell functions. In line with the previously reported anergy of the CD56−/CD16− NK-cell subset,19,22 Siglec-7−/CD56− NK cells were highly defective in terms of IFN-γ production and expressed very low levels of CD107a in response to K562 stimulation. Although still significantly impaired if compared with normal Siglec-7+/CD56+ NK cells, Siglec-7−/CD56+ cell subset showed a markedly higher expression of CD107a and production of IFN-γ compared with anergic Siglec-7−/CD56− population. Taken together, these data indicate that sequential loss of Siglec-7 and CD56 expression observed on NK cells from early and chronic HIV-1–infected patients is paralleled by a sequential impairment of NK-cell functions: the initial down-regulation of Siglec-7 is accompanied by mild defects in NK-cell degranulation and cytokine production, whereas the subsequent appearance of Siglec-7−/CD56− phenotype is associated with highly defective NK-cell functions. We have no experimental evidence in regard to possible interactions between CD56 and Siglec-7. Nevertheless, the markedly different degree of anergy between Siglec-7−/CD56+ and Siglec-7−/CD56− NK-cell subsets, the unrelated functions of CD56 and Siglec-7, and their different kinetics of expression during the course of HIV-1 disease suggest that the shift of these 2 markers on NK cells is coincidental.
The remarkable decreased expression of Siglec-7 and CD56 on NK cells did not directly correlate with the degree of HIV-1 plasma viremia. This indicates that the presence of a detectable early or chronic viral replication is sufficient for inducing a remarkable reduction of Siglec-7 (early) and CD56 (chronic) on NK cells, regardless of the quantitative levels of circulating HIV-1 copies. The lack of statistical significance between the kinetics of CD56 or Siglec-7 expression on NK cells and CD4+ T-cell count in patients having undergone ART also suggests that the mechanism that HIV-1 uses to alter NK-cell phenotype is different from the one that induces the loss of CD4+ T cells. Moreover, we further confirm here that, starting from the early phase of disease, HIV-1 replication induces a sequential dysregulation of NK-cell subset distribution without depleting the absolute numbers and frequency of circulating NK cells.16,18 Therefore, the defective functions of NK cells in viremic HIV-1–infected patients are not subsequent to a loss of circulating cells but result from the viral-induced expansion of highly pathologic and anergic NK-cell subsets rarely represented in healthy subjects.
Siglec-7 belongs to a family of 14 sialic-acid-binding immunoglobulin-like lectins and is constitutively expressed on all NK cells and monocytes.28,36 It was first cloned in 1999 from NK cells, and its gene, located on human chromosome 19, encodes for a trans-membrane sialoadhesin characterized by 3 immunoglobulin-like extracellular domains (one NH 2-terminal V-type and 2 C2-type) and a classic immunoreceptor tyrosine-based inhibitory motif in the cytoplasmic portion. Indeed, on cross-linking with specific mAbs, Siglec-7 was shown to inhibit NK-cell cytotoxicity.27 Siglec-7, as well as many other members of its family, is masked at cellular surface because of cis interactions with abundantly expressed low-affinity sialic acid ligands on cell surface. After exposure of cells to sialidase or in some cases after cellular activation, which cleaves the cis interacting low-affinity ligands, Siglec-7 becomes unmasked and is free to interact in trans with highly glycosylated ligands. Even when Siglecs are masked by cis interactions, trans interactions might occur during encounters with other cells or pathogens expressing higher affinity ligands competing with cis interactions.28 Siglec-7 has an unusual binding preference for α2,8-linked disialic acids, such as those displayed by ganglioside GD3. In line with the previously reported inhibitory function of this sialoadhesin,27 the NK-cell–mediated killing of GD3 synthase-transfected P815 cells was remarkably reduced after sialidase treatment of NK cells.37 These findings suggest that the binding of Siglec-7 with sialic acids might have important implication in modulating NK-cell cytolytic activity. Until recently, Siglecs have been mostly studied in the context of adhesion and cellular signaling, but there is growing evidence suggesting their role in endocytosis.38 This is of particular relevance for cells of innate immune system where phagocytic clearance of apoptotic cells and pathogens is critical. In this context, it has been recently shown that Siglec-7 on NK cells is able to recognize sialylated glycans expressed on Campylobacter jejuni lipo-oligosaccharides,39 but neither the signaling pathway involved in this recognition nor the potential NK-cell endocytosis of this pathogen was investigated. HIV-1 envelope is a highly glycosylated protein.40 The fact that Siglec-7 is rapidly decreased on NK cells in the presence of high levels of virus suggests that interactions between Siglec-7 and HIV-1 might occur on NK cells, as it has been recently demonstrated for Siglec-1 on monocytes.41 Further studies are needed to validate this hypothesis and disclose the mechanism(s) underlying the reduction of this sialoadhesin on NK cells in the presence of high levels of HIV-1 replication. The identification of a sialoadhesin as a novel NK-cell marker highly sensitive to HIV-1 viremia opens new scientific perspectives on the potential role of Siglec-7 as a regulator of NK-cell activities during the course of HIV-1 replication.
Although the expression of the CD56 molecule on CD56− NK cells freshly purified from HIV-1–infected viremic patients can be fully recovered after rIL-2 activation in vitro,19 stimulation of Siglec-7− NK cells with rIL-2 did not significantly change or modulate the expression of this sialic acid-binding lectins even after 3 weeks of cell culture (data not shown). The absence of an in vitro modulation in response to rIL-2 stimulation and in the absence of HIV-1 replication suggests that another mechanism, different from HIV-1–driven cell surface regulation, is involved in the decreased expression of Siglec-7. In this regard, similar to the HIV-1 envelope binding to CD209 (DC sign) on DCs,42 the supposed interaction between HIV-1 envelope and Siglec-7 could trigger an endocytic process, possibly explaining the decreased Siglec-7 expression on NK cells starting from the initial phases of HIV-1 infection. The direct contact between HIV-1 envelope and this sugar-binding lectin could also trigger the inhibitory Siglec-7 pathway in NK cells, thus affecting their cytolytic potential. To reproduce in vitro these pathologic NK-cell phenotypes and functions, we exposed NK cells to HIV-1 primary strains or we cocultured NK cells with autologous HIV-1–infected DCs. These experimental approaches did not reproduce the aberrant distribution of CD56 and Siglec-7 on NK cells. Therefore, only a longitudinal follow-up in vivo of bromodeoxyuridine-labeled CD56− and Siglec-7− NK cells in humans or (if possible) in a simian model can definitively prove whether or not a cell turnover is the mean for these NK-cell phenotype shifts. Finally, a potential redistribution of circulating NK-cell population toward peripheral tissues or secondary lymphoid organs in response to HIV-1–induced inflammation starting from the early phases of infection might be taken into account as a possible mechanisms underlying the NK-cell subset redistribution. Additional studies will be needed to further validate these hypotheses.
The aim of the present work was to define exactly the kinetics of the host NK-cell responses in different stages of HIV-1 infection to understand whether the expansion of aberrant cell subsets were responsible for impairments in NK-cell functions starting from the early phases of disease. Here, we characterize 2 new pathologic NK-cell subsets in HIV-1–infected patients on the basis of the combined use of 2 NK-cell surface markers: Siglec-7 and CD56. We identified 2 aberrant NK-cell subsets specifically expanded in early (Siglec-7−/CD56+) or chronic (Siglec-7−/CD56−) stages of the infection and characterized by a different degree of functional impairments. The identification of these 2 viral-induced dysfunctional NK cells further confirms that the expansion of aberrant populations is an important mechanism by which HIV-1 impairs NK-cell antiviral functions, thus affecting the quality of host immune responses. This peculiar distribution and function of NK-cell subsets at different stages of HIV-1 infection can also help physicians better define the clinical stages of the disease and track the effectiveness of treatment.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This paper is dedicated to the memory of Lorenzo Trucco.
The authors thank the patients for their generosity and participation in this study, Dr Anthony S. Fauci for his support and helpful discussion, Mark Connors and Steve Migueles for providing cells from the cohort of LTNPs, and Gregg Roby for patient recruitment.
This work was supported by the intramural research program of National Institute of Allergy and Infectious Diseases, National Institutes of Health, and by intramural program of Istituto Clinico Humanitas.
National Institutes of Health
Authorship
Contribution: E.B., M.F., S.V., L.B., and D.M. performed research and analyzed data; K.L.H. analyzed data; E.M. purified and titrated mAbs; E.B., A.M., and D.M. wrote the manuscript; and D.M. conceived and planned this study.
Conflict-of-interest disclosure: A.M. is founder and shareholder of Innate-Pharma (Marseille, France). The remaining authors declare no competing financial interests.
Correspondence: Domenico Mavilio, Laboratory of Clinical and Experimental Immunology, Istituti di Ricovero e Cura a Carattere Scientifico, Istituto Clinico Humanitas, Via Alessandro Manzoni, 113, Rozzano, Milano, Italy; e-mail: domenico.mavilio@humanitas.it.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal