Abstract
Although the influence of microenvironmental “niche” on the function of a variety of stem cells is undisputed, the details of hematopoietic stem cell/niche interactions at the cellular and molecular level have sparked a continuous debate. We studied the microanatomic partitioning of transplanted normal and α4 integrin-deficient Lin−kit+ cells in trabecular and compact bone before and after irradiation and present robust quantitative data on both. We found that (1) the microanatomic distribution of normal highly enriched progenitor cells is random in nonirradiated recipients based on area distribution analyses, (2) in contrast, in irradiated hosts normal cells distribute preferentially near the endosteum, (3) the overall cell seeding efficiency was higher in trabecular versus compact bone both before and after irradiation, and (4) α4 integrin-deficient cells not only lodge with reduced overall efficiency confirming previous data, but fail to preferentially partition themselves into endosteal regions in irradiated hosts, as normal cells do. A similar phenotype was observed with cells rendered Gi-protein signaling incompetent by pertussis toxin treatment, supporting an active stromal-derived factor 1 (SDF-1) gradient near endosteum after irradiation.
Introduction
The ability of hematopoietic stem cells (HSCs) to exercise their function is vested in their interactive relationships with their neighbor cells and matrix in the place of their residence. This molecularly interactive unit is referred to as “stem cell niche” and its influence on stem cell fate decisions has enjoyed an upsurgence of attention lately. Although HSCs on their developmental journey recruit new anatomic sites for their development and are thereby interacting with different “niches,” it is only the bone marrow (BM) niche in adults that has received the most research attention. Genetic mouse models have been instrumental in uncovering critical cellular components of the niche or critical molecular pathways important in supporting niche function.1-5 However, the precise anatomic localization or the precise cellular elements of the niche in BM have sparked a continuous debate. Newer data aimed to address or redress the challenges have not led to resolution of divergent views. For example, whether quiescent stem cells reside on the endosteum6 or near sinusoids and mostly in central areas7 is not clear. Likewise, whether the coalescence or constellation of few critical cellular components versus 1 or 2 is required to constitute the niche is currently debated. Thus, endosteum cells8,9 or sinusoidal cells,7 or CXCR4-abundant reticular cells,10 or CD146+ osteoprogenitor or sinusoidal progenitor cells11 all have been considered as pivotal cellular contributors to niche formation. Whether these coalesce in specific domains of BM or whether cellular contact between HSCs and these cells is necessary has also not been resolved. Stem cells could respond to gradients of factors generated by the niche or only through their cellular contact with niche cells, but some of the molecular pathways described as responsible for contact (Tie2/Ang-1,6 N-cadherin3 ) have been convincingly challenged recently.10-12
Major technical difficulties in these studies stem from the very small numeric recoveries after transplantation of few highly purified HSCs used as donor cells. Because of the aforementioned limitations (few total sections, few total cells evaluated), we made an effort to provide rigorous quantitative data with normal cells. In addition, we are presenting novel data with α4-deficient cells in many comparative experiments. We believe that our data in aggregate complement and extend current knowledge in the field.
Methods
Animals
B6x129 or C57Bl/6 wild-type mice were used as donors or recipients for most experiments. In addition, mice lacking membrane-bound kit-ligand (Sl/Sld; Jackson Laboratories) served as recipients for some experiments. Tie2+creα4Δ/Δ mice (deleted for α4 integrin in endothelial and hematopoietic lineages, referred to as α4 integrin-deficient throughout) and Tie2+cre−α4f/f littermate controls were used as donors for some experiments as indicated; these mice were previously described.13 Animals were housed at the University of Washington Comparative Medicine Specific Pathogen-Free vivarium, with autoclaved chow and water ad libitum. All procedures were done in agreement with protocols approved by the University of Washington Institutional Animal Care and Use Committee (IACUC).
Transplantation
Recipient mice were conditioned by lethal irradiation with a single dose of 1150 cGy or not conditioned, as indicated. Donor cells (7-30 × 106) were transplanted by injection into the lateral tail vein within 4 hours of irradiation. Mice were painlessly killed 20 hours after transplantation, and femora, tibiae, and sterna were collected. In certain experiments animals were evaluated 8 days after transplantation.
Cells
To generate donor cell populations, cohorts of isogeneic donor mice were painlessly killed using approved protocols, and BM cells were harvested from long bones. Cells were pooled and then either enriched for c-kit–expressing cells or depleted for lineage marker–expressing cells using immunomagnetic isolation with commercially available reagents (biotinylated anti–c-kit antibody [2B8], biotinylated lineage-depletion antibody cocktails, anti–biotin-immunomagnetic beads [Miltenyi Biotec]) following the manufacturer's protocols. Prior to transplantation, purity of the resulting cell populations was routinely assessed; c-kit enrichment regularly resulted in cell populations of approximately 90% purity, and lineage depletion resulted in 75% to 80% lineage-negative cell populations with a c-kit frequency exceeding 75%. In certain experiments Lin− cells were further purified for kit+ cells by fluorescence-activated cell sorting (FACS). α4 integrin expression on wild-type or α4 integrin–deficient donor cell populations were likewise routinely analyzed. For this purpose, flow cytometry, using fluorescence-conjugated antibodies (BD Biosciences), was performed according to standard protocols on the FACSCalibur (BD Immunocytometry Systems). Prior to transplantation, cells were loaded with the cell-permeable dyes carboxyfluorescein succinimidyl ester (CFSE) (green; Molecular Probes) or, for competitive homing experiments, with seminaphtharhodafluor SNARF®-1 (SNARF, red; Molecular Probes). A labeling efficiency of essentially 100% was regularly achieved. Label crossover experiments confirmed equal homing efficiency of green (carboxyfluorescein succinimidyl ester [CFSE]) or red (SNARF) labeled cells.
Homing experiments
For homing studies the proportions of colony-forming unit cell (CFU-C) populations recovered from femurs, peripheral blood, and spleen of irradiated recipients given α4+/+ and α4−/− cells were assessed 24 hours after transplantation relative to the inoculum (CFU-C) transplanted. In addition to cells recovered from flushing femurs in these experiments, cells recovered from fragmented femur segments following collagenase 1 treatment (3 mg/mL collagenase type I [Sigma-Aldrich]) were also evaluated for CFU-C recovery at 24 hours (27 × 106 BM donor cells) and 8 days after transplantation (3 × 106 BM donor cells).
Migration assay
Transwell migration of kit+/α4+/+ or kit+/α4−/− BM cells was performed as previously described.14 Briefly, to engage the α4 integrin, immunomagnetically purified kit+ cells were first incubated in medium containing 10% fetal calf serum, 10% wehi conditioned medium from Walter and Eliza Hall Institute–3 cells, and stem cell factor (100 ng/mL) for 2 hours, then transferred to RetroNectin-coated Transwells in the presence or absence of stromal-derived factor 1α (SDF-1α; 100 ng/mL; Peprotech) in the bottom chamber. Spontaneous or SDF-1α–directed migration was assessed after 4 hours by quantitating the number of migrated cells recovered from the lower chamber as a proportion of the input cells.
Preparation of bone sections, image acquisition, and analysis
Recipient bones were prefixed in 4% paraformaldehyde. Bone sections representing compact bone (entire femur shafts) or trabecular bone (proximal and distal ends of 2 femurs [epiphyses] or sternal bones) were cut into 3 to 5 segments, embedded without decalcification in OCT (Tissue-Tek; Sakura Finetechnical Co), and frozen in acetone/dry ice. Embedded bony fragments were cut into 4- to 5-μm sections (transverse for compact bone, longitudinal for trabecular bone) using a cryostat microtome (CM1850; Leica) equipped with a tungsten carbide knife (HM16 cm/d; Leica) and a CryoJane tape-transfer system (Instrumedics). Sections were mounted with DAPI (4,6 diamidino-2-phenylindole; blue nuclear stain) mounting medium (Vectashield; Vectorlabs). Images were acquired at room temperature using a Leica DMLB fluorescence microscope (with appropriate filters) outfitted with a Leica 10×/0.30 PH1 HC-PL FLUOTAR objective and a Spot RT Slider camera using SPOT Advanced software (Version 4.6; Diagnostic Instruments Inc). For cell counting an unbiased observer counted green (or red) cells relative to their spatial distribution within coded sections using a Nikon Eclipse E800 fluorescence microscope. Three zones were defined within the BM cavity for compact bone: endosteal (≤ 3 cell diameters from endosteal surface: zone 1), subendosteal (4-14 cell diameters from endosteal surface: zone 2), or central (> 14 cell diameters from endosteal surface: zone 3), and 2 zones were defined for trabecular bone: endosteal (≤ 3 cell diameters from endosteal surface) or nonendosteal (> 3 cell diameters from endosteal surface). Sixty to 150 sections were analyzed from either compact or trabecular bone (femur shafts or epiphyses/sternum). Results are expressed as either relative frequency of homed cells in each of the zones among cells homed in all zones (microanatomic homing distribution), or as absolute numbers of cells from all sections counted (Table 1), or of cells recovered per section, normalized to the number of transplanted cells (Figure 3 and Table 3).
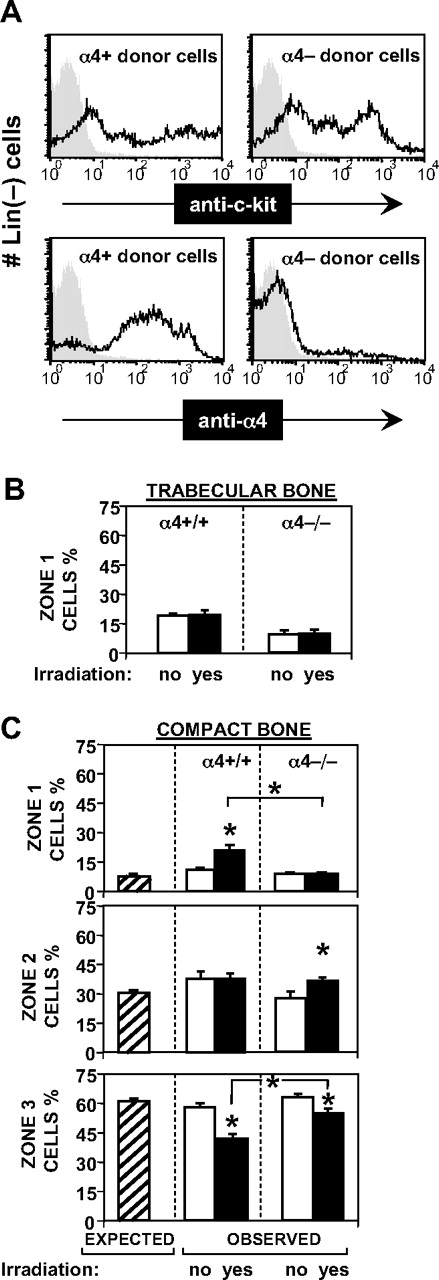
Quantitative microanatomic distribution analysis of wild-type or α4 integrin-deficient Lin−c-kit+ donor cells in nonirradiated or irradiated compact or trabecular bone
| . | No. of experiments . | No. of sections . | No. of cells . | Zone 1, % cells . | Zone 2, % cells . | Zone 3, % cells . |
|---|---|---|---|---|---|---|
| Compact bone (femur diaphyses) | ||||||
| α4+/+ | ||||||
| nonirradiated | 3 | 261 | 12 379 | 10.19 ± 0.96* | 35.60 ± 1.51 | 54.2 ± 0.55† |
| irradiated | 6 | 595 | 7825 | 20.58 ± 2.71* | 38.37 ± 1.88 | 41.04 ± 1.88† |
| α4−/− | ||||||
| nonirradiated | 4 | 489 | 10 042 | 8.69 ± 1.04 | 27.82 ± 1.8‡ | 63.49 ± 2.40‡ |
| irradiated | 5 | 465 | 4305 | 8.98 ± 1.44 | 36.44 ± 2.0‡ | 54.58 ± 2.35‡ |
| Trabecular bone (epiphyses, sternum) | ||||||
| α4+/+ | ||||||
| nonirradiated | 3 | 312 | 61 630 | 17.97 ± 1.85§ | 82.03 ± 1.85 | |
| irradiated | 7 | 638 | 45 869 | 18.54 ± 1.18‖ | 81.46 ± 1.18 | |
| α4−/− | ||||||
| nonirradiated | 3 | 325 | 11 141 | 9.44% ± 1.8§ | 90.56 ± 1.87 | |
| irradiated | 4 | 348 | 8049 | 10.66 ± 2.09‖ | 89.34 ± 2.09 |
| . | No. of experiments . | No. of sections . | No. of cells . | Zone 1, % cells . | Zone 2, % cells . | Zone 3, % cells . |
|---|---|---|---|---|---|---|
| Compact bone (femur diaphyses) | ||||||
| α4+/+ | ||||||
| nonirradiated | 3 | 261 | 12 379 | 10.19 ± 0.96* | 35.60 ± 1.51 | 54.2 ± 0.55† |
| irradiated | 6 | 595 | 7825 | 20.58 ± 2.71* | 38.37 ± 1.88 | 41.04 ± 1.88† |
| α4−/− | ||||||
| nonirradiated | 4 | 489 | 10 042 | 8.69 ± 1.04 | 27.82 ± 1.8‡ | 63.49 ± 2.40‡ |
| irradiated | 5 | 465 | 4305 | 8.98 ± 1.44 | 36.44 ± 2.0‡ | 54.58 ± 2.35‡ |
| Trabecular bone (epiphyses, sternum) | ||||||
| α4+/+ | ||||||
| nonirradiated | 3 | 312 | 61 630 | 17.97 ± 1.85§ | 82.03 ± 1.85 | |
| irradiated | 7 | 638 | 45 869 | 18.54 ± 1.18‖ | 81.46 ± 1.18 | |
| α4−/− | ||||||
| nonirradiated | 3 | 325 | 11 141 | 9.44% ± 1.8§ | 90.56 ± 1.87 | |
| irradiated | 4 | 348 | 8049 | 10.66 ± 2.09‖ | 89.34 ± 2.09 |
Summary of all data from nonirradiated or irradiated recipients of transplanted normal (α4+/+) or α4 integrin-deficient HSC populations: between 60 and 150 sections were analyzed in each experiment. Donor cells given were from 7 to 30 × 106 per recipient. Indicated are, for each of the modalities, the number of experiments, the cumulative number of sections and total cells analyzed, and the relative distribution of homed cells to distinct zones within BM (percentage, mean ± SEM). Statistically significant differences between nonirradiation versus irradiation conditioning in compact bone and between α4+/+ and α4−/− in trabecular bone are indicated by boldface. A relative preference for endosteal seeding in irradiated marrow in compact bone was observed for wild-type HSCs, whereas α4 integrin-deficient cells distributed randomly, as in nonirradiated hosts. Microscopic images illustrating the different microanatomic distribution of transplanted wild-type cells in hosts with/without irradiation are shown in Figure 2G and H.
P = .005;
P = .001;
P = .02;
P = .03;
P = .006.
Immunohistochemical staining
For analysis of CD31 expression, frozen sections were blocked with 10% goat serum followed by subsequent staining with anti-CD31 (MEC13.3; BD Biosciences) or isotype control and Alexa Fluor 594 goat anti–rat immunoglobulin G (Molecular Probes). Slides were mounted and images acquired as described in “Preparation of bone sections, image acquisition, and analysis.”
Statistical analysis
Homing efficiency and relative distribution to the different zones for wild-type versus α4 integrin-deficient cells, or nonirradiated versus irradiated hosts, were compared using Student t test. Mean plus or minus standard error of the mean (SEM) was calculated using Student t tests in Excel Software (Microsoft). A P value less than .05 was considered statistically significant.
Results
Cell recovery in trabecular versus compact bone before and after irradiation
As indicated in “Preparation of bone sections, image acquisition, and analysis,” for compact bone (femoral diaphysis) we chose to use transverse sections of the entire femur shaft because the distances from the center of the bone to the endosteum are maintained through a large number of such consecutive sections. To assure the presence of an adequate number of donor cells in each section, we infused several millions (7 to 30) of CFSE-labeled Lin−Kit+ cells and made our evaluation in an unbiased fashion using a rigid set of criteria (see Figure 1Aii for anatomic zones) chosen to allow comparisons with previously published data. Furthermore, using the same pool of donor cells, observations were made in both irradiated and nonirradiated recipients. To represent trabecular and nontrabecular bones we also evaluated sections from femur epiphyses and sternal bones, adapting a modified set of criteria (2 zones instead of 3, Figure 1Bii). Several experiments were performed and 60 to 150 sections were evaluated from 2 femurs or from proximal and distal epiphyses from each recipient (Table 1). Thus, the entire femur shafts (representing compact bone) or entire epiphyses or sternal bones (representing trabecular bone) were sectioned. Cellular images of nonirradiated and irradiated bone sections are shown in Figure 2A through F. Donor cells seeded in irradiated and nonirradiated tibiae are shown in Figure 2G through H. Total cells recovered per section (from either compact or trabecular bone) were enumerated and normalized to the number of CFSE-labeled donor cells transplanted (Figure 3).
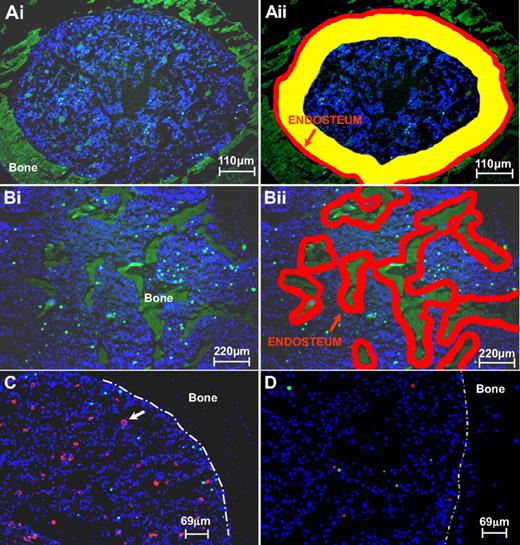
Definition of endosteal, subendosteal, and central zones in BM and demonstration of cell imaging. (Ai-ii) Definition of endosteal, subendosteal, and central zones in BM sections of femur diaphysis representing compact bone. Regions in each transverse bone section were defined as endosteal (≤ 3 cell diameters from endosteal surface; zone 1, depicted in red), subendosteal (4-14 cell diameters from endosteal surface; zone 2, depicted in yellow), or central (> 14 cell diameters from endosteal surface; zone 3). The average relative surface area of each of the 3 regions was calculated, using image analysis software, as 8% ± 0.63%, 31% ± 1.31%, and 61% ± 1.85%, for zones 1, 2, and 3, respectively. (Bi-ii) For trabecular bone sections, 2 zones were evaluated: endosteal in red and the rest. (C) Depiction of homed donor Lin−kit+ cells to irradiated BM. Transplanted cells are labeled with CFSE (green); red-stained cells (anti–CD31-PE) are vascular cells and megakaryocytes (white arrow). (D) Competitive homing of α4+/+ (SNARF-1, red) and α4 integrin–deficient (CFSE, green) labeled hematopoietic Lin−kit+ cells in irradiated BM. The 2 populations were given at a ratio 0.7 (red):1 (green) (see Table 2 for details). The white interrupted line in panels C and D represents the endosteal border.
Definition of endosteal, subendosteal, and central zones in BM and demonstration of cell imaging. (Ai-ii) Definition of endosteal, subendosteal, and central zones in BM sections of femur diaphysis representing compact bone. Regions in each transverse bone section were defined as endosteal (≤ 3 cell diameters from endosteal surface; zone 1, depicted in red), subendosteal (4-14 cell diameters from endosteal surface; zone 2, depicted in yellow), or central (> 14 cell diameters from endosteal surface; zone 3). The average relative surface area of each of the 3 regions was calculated, using image analysis software, as 8% ± 0.63%, 31% ± 1.31%, and 61% ± 1.85%, for zones 1, 2, and 3, respectively. (Bi-ii) For trabecular bone sections, 2 zones were evaluated: endosteal in red and the rest. (C) Depiction of homed donor Lin−kit+ cells to irradiated BM. Transplanted cells are labeled with CFSE (green); red-stained cells (anti–CD31-PE) are vascular cells and megakaryocytes (white arrow). (D) Competitive homing of α4+/+ (SNARF-1, red) and α4 integrin–deficient (CFSE, green) labeled hematopoietic Lin−kit+ cells in irradiated BM. The 2 populations were given at a ratio 0.7 (red):1 (green) (see Table 2 for details). The white interrupted line in panels C and D represents the endosteal border.
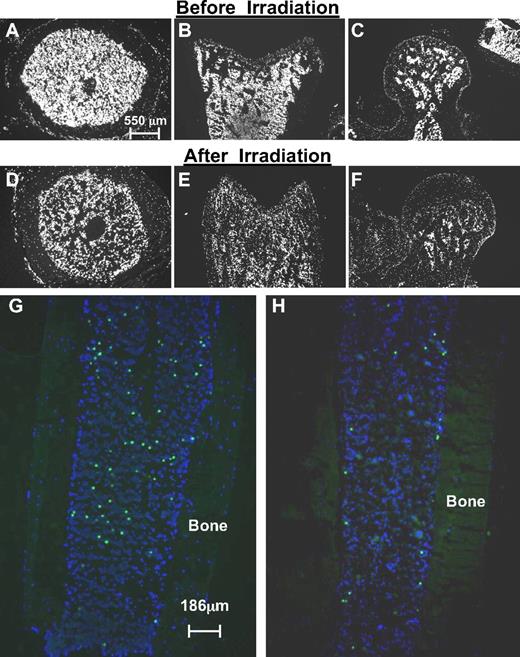
Analysis of transplanted hematopoietic cells in nonirradiated and irradiated bone marrow. (A-F) Cross-sections of nonirradiated (A-C) and irradiated (D-F) bones illustrate the markedly reduced cell density after irradiation (DAPI). Shown are transverse sections of femur shafts (A,D) and longitudinal sections of distal femoral epiphyses (B,E) or femoral heads (C,F). (G-H) Differential distribution of transplanted hematopoietic stem/progenitor cells in nonirradiated (G) and irradiated (H) hosts. Shown here are longitudinal sections of tibiae not used for counting from recipients of similar numbers of donor cells. The efficiency of homing in nonirradiated hosts is higher than in irradiated hosts, but the distribution follows a random pattern, whereas in irradiated hosts, transplanted cells preferentially home to endosteal regions within compact bone. Corresponding quantitative data, generated in transverse sections of femurs, are shown in Table 1.
Analysis of transplanted hematopoietic cells in nonirradiated and irradiated bone marrow. (A-F) Cross-sections of nonirradiated (A-C) and irradiated (D-F) bones illustrate the markedly reduced cell density after irradiation (DAPI). Shown are transverse sections of femur shafts (A,D) and longitudinal sections of distal femoral epiphyses (B,E) or femoral heads (C,F). (G-H) Differential distribution of transplanted hematopoietic stem/progenitor cells in nonirradiated (G) and irradiated (H) hosts. Shown here are longitudinal sections of tibiae not used for counting from recipients of similar numbers of donor cells. The efficiency of homing in nonirradiated hosts is higher than in irradiated hosts, but the distribution follows a random pattern, whereas in irradiated hosts, transplanted cells preferentially home to endosteal regions within compact bone. Corresponding quantitative data, generated in transverse sections of femurs, are shown in Table 1.
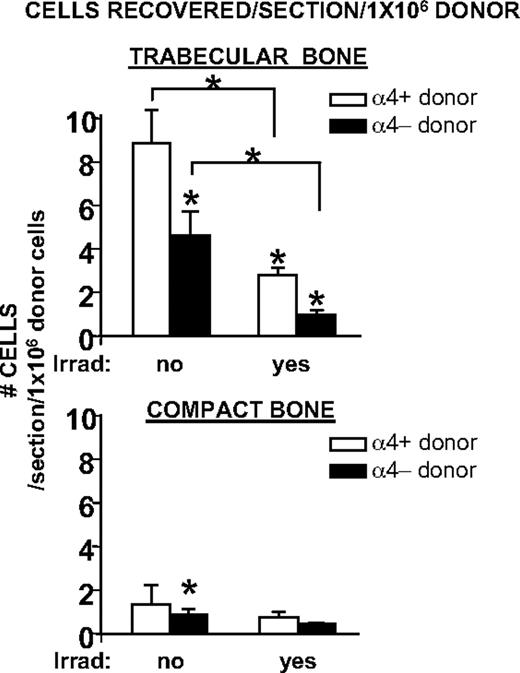
Retention efficiency of transplanted progenitor-enriched cells in nonirradiated and irradiated trabecular or compact bone. Irradiation was associated with a markedly reduced efficiency of BM seeding. This is depicted as number of recovered donor cells/bone section, normalized to 1 × 106 donor cells. Seeding efficiency in trabecular bone was approximately 6-fold higher than in compact bone. α4 integrin-deficient progenitor-enriched cells showed an overall reduced seeding, confirming all prior data on impaired homing of α4 integrin-deficient cells. All columns and cross bars shown represent mean values ± SEM; *P < .05.
Retention efficiency of transplanted progenitor-enriched cells in nonirradiated and irradiated trabecular or compact bone. Irradiation was associated with a markedly reduced efficiency of BM seeding. This is depicted as number of recovered donor cells/bone section, normalized to 1 × 106 donor cells. Seeding efficiency in trabecular bone was approximately 6-fold higher than in compact bone. α4 integrin-deficient progenitor-enriched cells showed an overall reduced seeding, confirming all prior data on impaired homing of α4 integrin-deficient cells. All columns and cross bars shown represent mean values ± SEM; *P < .05.
Two striking differences are immediately apparent: the recovery of intravenously transplanted cells was significantly higher in trabecular bone areas (femoral epiphyses, sterna) than in femoral shafts. Nearly 5-fold differences were seen between the former versus the latter bones in nonirradiated recipients and about 4-fold in irradiated recipients. Overall these data suggest that trabecular bone is more conducive to the entrapment of intravenously infused cells.
Equally important is the conclusion that the total recovery of cells (per section/1 × 106 cells) is much higher in nonirradiated than in irradiated recipients. This difference was more pronounced in compact bone than in trabecular bone. Although the reasons for these quantitative differences before and after irradiation are not immediately apparent, the anatomic changes in cellular density after irradiation may be of relevance. After irradiation there are prominent differences in cellular density in both compact and trabecular bone. In addition to the general decrease in cellular density regularly seen after irradiation (Figure 2), small acellular areas, especially near the center, are not infrequent after irradiation. Large acellular areas were separately evaluated through imaging analysis and found to account for 9% plus or minus 1% of the total space in each section (n = 20). It is thus possible that cellular density, modified by irradiation, influences at least partially and indirectly cellular retention after transplantation. Furthermore, irradiation-induced changes, that is, increase in SDF-1 elaboration, in extramedullary organs are also possible, as these could additionally siphon transplanted cells off into nonhematopoietic tissues thus reducing the number of cells available for BM uptake.
Microanatomic distribution of transplanted normal cells in nonirradiated versus irradiated compact or trabecular bone
In addition to cellular recoveries, in each section we evaluated in detail the microanatomic partitioning of CFSE-labeled Lin−kit+ cells into the 3 designated zones in compact bone (Figure 1Aii) or the 2 zones (endosteal and rest) in trabecular bone (Figure 1Bii). Femur shafts were divided into 3 to 5 segments and from each, 24 to 32 serial sections were prepared and evaluated. Given the thickness of these sections a few cells may have been counted in more than one section, so the error would affect data equally before and after irradiation. Furthermore, in our counting we avoided the recording of small fluorescent events without a nucleus (DAPI stained). Qualitative distribution of donor cells is seen in Figures 1 and 2C. A solitary rather than a clustered pattern is observed and cumulative data from all experiments are presented in Table 1 and Figure 4. In nonirradiated recipients, a consistent proportion of 10.19% plus or minus 0.96% in endosteal zone (< 3 cells from endosteum), of 35.6% plus or minus 1.51% in subendosteal zone, and of 54.21% plus or minus 0.55% in central zone in compact bone is seen. To test whether these values deviated from expected random distribution according to the anatomic area represented by each zone, we evaluated in many sections (by image analysis) the relative anatomic surface area occupied by each zone. The values from each zone (zone 1: 8.0% ± 0.6%; zone 2: 31.0% ± 1.3%; zone 3: 61.0% ± 2.0%) are not considerably different from the proportional cell distribution mentioned 7 to 9 lines above and evaluated independently in each section. This would suggest that, in nonirradiated recipients, the distribution of incoming cells (Lin−kit+) is close to random. In contrast to the near-random distribution seen in nonirradiated hosts, transplanted cells in irradiated hosts showed a preferential endosteal lodgment (Table 1, Figure 2, and supplemental Figure 3, available on the Blood website; see the Supplemental Materials link at the top of the online article). At the same time there was a significant reduction of cells recovered from the central area. Thus it would appear that a shift in population distribution occurring after irradiation (increase near endosteum vs decrease in central zone) should yield a relative change in endosteum (zone 1) or subendosteum (zone 2) or both. To what extent preferential seeding to endosteal zones was due to other than the relative changes mentioned, that is, initial random distribution with subsequent migration (chemotactic?) to endosteal zones and/or enhanced retention in this region because of better preservation of endothelial cells after irradiation,15 was not clear from the data with normal cells and was subsequently addressed using donor cells with deficient adhesion and/or migration properties. In trabecular bone, no significant distribution differences were recorded after irradiation even though the overall cell recovery was also reduced after irradiation as in compact bone (Figures 2–3). However, there are some inherent limitations in interpreting the data with 2-dimensional sections of trabecular bone. Depending on cell size, a few cells scored in zone 2 in one section may be scored to zone 1 in a consecutive section. Although this may alter the frequency of cells with “endosteal” placement, our conclusions with trabecular bone data, that is, differences before and after irradiation or total cell recovery compared with compact bone, would not be altered.
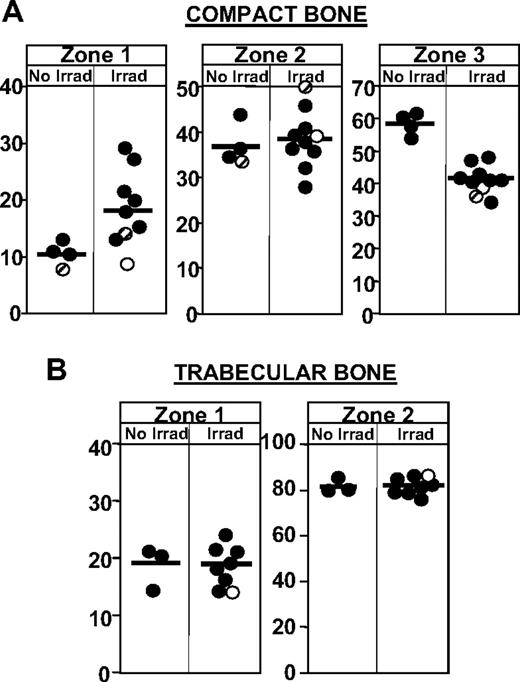
Microanatomic distribution of hematopoietic stem/progenitor cells in endosteal, subendosteal, and central regions within the bone marrow cavity. (A) The relative distribution of transplanted Lin−c-kit+ cells to endosteal (< 3 cells from endosteum; zone 1), subendosteal (4-14 cells from endosteum; zone 2), and central (> 14 cells from endosteum; zone 3) in irradiated and nonirradiated hosts in compact bone is depicted. Each circle depicts the average values from all sections from one experiment (60-150 sections for compact bones and a similar number from trabecular bones per experiment). In compact bones (α4+/+ recipients: ●, and Sl/Sld recipients:  ), the relative frequency of cells seeding close to the endosteal surface is increased in irradiated hosts, at the expense of cell homing to central regions. This relative preference for seeding to endosteal regions in irradiated hosts was not observed when donor cells were treated with the inhibitor of Gi protein signals, pertussis toxin (○). (B) In trabecular bone, irradiation did not significantly affect the relative distribution of transplanted cells to endosteal versus nonendosteal regions.
), the relative frequency of cells seeding close to the endosteal surface is increased in irradiated hosts, at the expense of cell homing to central regions. This relative preference for seeding to endosteal regions in irradiated hosts was not observed when donor cells were treated with the inhibitor of Gi protein signals, pertussis toxin (○). (B) In trabecular bone, irradiation did not significantly affect the relative distribution of transplanted cells to endosteal versus nonendosteal regions.
Microanatomic distribution of hematopoietic stem/progenitor cells in endosteal, subendosteal, and central regions within the bone marrow cavity. (A) The relative distribution of transplanted Lin−c-kit+ cells to endosteal (< 3 cells from endosteum; zone 1), subendosteal (4-14 cells from endosteum; zone 2), and central (> 14 cells from endosteum; zone 3) in irradiated and nonirradiated hosts in compact bone is depicted. Each circle depicts the average values from all sections from one experiment (60-150 sections for compact bones and a similar number from trabecular bones per experiment). In compact bones (α4+/+ recipients: ●, and Sl/Sld recipients:  ), the relative frequency of cells seeding close to the endosteal surface is increased in irradiated hosts, at the expense of cell homing to central regions. This relative preference for seeding to endosteal regions in irradiated hosts was not observed when donor cells were treated with the inhibitor of Gi protein signals, pertussis toxin (○). (B) In trabecular bone, irradiation did not significantly affect the relative distribution of transplanted cells to endosteal versus nonendosteal regions.
), the relative frequency of cells seeding close to the endosteal surface is increased in irradiated hosts, at the expense of cell homing to central regions. This relative preference for seeding to endosteal regions in irradiated hosts was not observed when donor cells were treated with the inhibitor of Gi protein signals, pertussis toxin (○). (B) In trabecular bone, irradiation did not significantly affect the relative distribution of transplanted cells to endosteal versus nonendosteal regions.
Recovery and microanatomic localization of α4 integrin-deficient cells in trabecular versus compact bone before and after irradiation
Having established a template of microanatomic distribution and recovery with normal cells, we then explored the behavior of α4-deficient cells in all parameters studied with normal cells. Our previous studies, confirmed by a number of investigators, documented reduced homing using α4-deficient donor cells, but the ability of these cells to partition to different anatomic zones within BM was not previously tested. Beyond the comparison of α4+/+ cells (α4f/f/cre−) with α4−/− cells we also conducted successful experiments in which both types of donor cells, labeled with different fluorochromes, were given to the same recipients, that is, a “competitive” biodistribution assay (Figure 1D). Quantitative data from these experiments are shown in Table 2 and the variability in cell recovery per section for each zone is shown in Table 3.
Competitive biodistribution of α4+/+ and α4−/− cells
| . | Total sections . | Cells counted . | Zone 1, % . | Zone 2, % . | Zone 3, % . |
|---|---|---|---|---|---|
| Irradiated recipients | |||||
| 11.5 × 106 green α4+/+ cells + 11.8 × 106 red α4−/− cells | |||||
| CB | 100 | 906 green α4+/+ cells277 red α4−/− cells | 15.55 ± 2.78 10.12 ± 1.12 | 49.03 ± 5.71 46.42 ± 6.2 | 35.55 ± 8.07 43.47 ± 6.14 |
| Tr B | 118 | 4509 green α4+/+ cells 910 red α4+/+ cells | 21.29 ± 3.19 12.53 ± 2.2 | 78.71 ± 3.19 87.47 ± 2.2 | |
| 8.5 × 106 red α4+/+ cells + 11.5 × 106 green α4−/− cells | |||||
| CB | 102 | 556 red α4+/+ cells 676 green α4−/− cells | 13.44 ± 4.011 12.78 ± 2.78 | 43.2 ± 7.89 37.14 ± 5.57 | 43.36 ± 11.81 50.08 ± 8.35 |
| Tr B | 99 | 1839 red α4+/+ cells 1942 green α4−/− cells | 19.1 ± 4.06 16.58 ± 1.68 | 80.9 ± 4.06 85.43 ± 2.47 | |
| Nonirradiated recipients | |||||
| 4.25 × 106 green α4+/+ cells + 8.5 × 106 red α4−/− cells | |||||
| CB | 140 | 26 green α4+/+ cells 1212 red α4−/− cells | 14.0 ± 9.0 8.7 ± 1.5 | 38.5 ± 10.0 29.3 ± 1.6 | 47.5 ± 9.0 61.9 ± 3.0 |
| Tr B | 120 | 72 green α4+/+ cells 2935 red α4−/− cells | 12.0 ± 5.5 11.2 ± 1.2 | 87.9 ± 5.5 88.7 ± 1.2 | |
| . | Total sections . | Cells counted . | Zone 1, % . | Zone 2, % . | Zone 3, % . |
|---|---|---|---|---|---|
| Irradiated recipients | |||||
| 11.5 × 106 green α4+/+ cells + 11.8 × 106 red α4−/− cells | |||||
| CB | 100 | 906 green α4+/+ cells277 red α4−/− cells | 15.55 ± 2.78 10.12 ± 1.12 | 49.03 ± 5.71 46.42 ± 6.2 | 35.55 ± 8.07 43.47 ± 6.14 |
| Tr B | 118 | 4509 green α4+/+ cells 910 red α4+/+ cells | 21.29 ± 3.19 12.53 ± 2.2 | 78.71 ± 3.19 87.47 ± 2.2 | |
| 8.5 × 106 red α4+/+ cells + 11.5 × 106 green α4−/− cells | |||||
| CB | 102 | 556 red α4+/+ cells 676 green α4−/− cells | 13.44 ± 4.011 12.78 ± 2.78 | 43.2 ± 7.89 37.14 ± 5.57 | 43.36 ± 11.81 50.08 ± 8.35 |
| Tr B | 99 | 1839 red α4+/+ cells 1942 green α4−/− cells | 19.1 ± 4.06 16.58 ± 1.68 | 80.9 ± 4.06 85.43 ± 2.47 | |
| Nonirradiated recipients | |||||
| 4.25 × 106 green α4+/+ cells + 8.5 × 106 red α4−/− cells | |||||
| CB | 140 | 26 green α4+/+ cells 1212 red α4−/− cells | 14.0 ± 9.0 8.7 ± 1.5 | 38.5 ± 10.0 29.3 ± 1.6 | 47.5 ± 9.0 61.9 ± 3.0 |
| Tr B | 120 | 72 green α4+/+ cells 2935 red α4−/− cells | 12.0 ± 5.5 11.2 ± 1.2 | 87.9 ± 5.5 88.7 ± 1.2 | |
Cumulative data from irradiated and nonirradiated recipients of both α4+/+ and α4−/− cells labeled with different fluorochromes. Note that the data from these competitive distribution experiments are in line with data shown in Table 1 regarding differences between α4+/+ and α4−/− cells in both cell recoveries and microanatomic distribution after irradiation in compact bone. (The numbers for α4+/+ cells in “Nonirradiated recipients” are not included in Table 1).
CB indicates compact bone; Tr B, trabecular bone.
Boldface indicates significant differences between α4+/+ and α4−/− cells.
Variability in cell recovery/section 11.5 × 106 green α4+/+ cells + 11.8 × 106 red α4−/− cells
| Cells counted . | Zone 1, % . | Zone 2, % . | Zone 3, % . |
|---|---|---|---|
| A*1-25† | |||
| Green α4+/+ cells | 1.68 ± 0.3 | 4.48 ± 0.6 | 1.16 ± 0.22 |
| Red α4−/− cells | 0.24 ± 0.09 | 1.20 ± 0.19 | 0.56 ± 0.16 |
| B 1-25 | |||
| Green α4+/+ cells | 0.92 ± 0.2 | 3.8 ± 0.34 | 2.44 ± 0.21 |
| Red α4−/− cells | 0.2 ± 0.08 | 1.52 ± 0.32 | 1.16 ± 0.19 |
| C 1-25 | |||
| Green α4+/+ cells | 1.44 ± 0.22 | 4.84 ± 0.5 | 7.8 ± 0.64 |
| Red α4−/− cells | 0.48 ± 0.12 | 1.36 ± 0.27 | 2.40 ± 0.26 |
| D 1-25 | |||
| Green α4+/+ cells | 1.24 ± 0.24 | 3.64 ± 0.39 | 2.84 ± 0.36 |
| Red α4−/− cells | 0.2 ± 0.08 | 0.8 ± 0.13 | 0.96 ± 0.21 |
| Cells counted . | Zone 1, % . | Zone 2, % . | Zone 3, % . |
|---|---|---|---|
| A*1-25† | |||
| Green α4+/+ cells | 1.68 ± 0.3 | 4.48 ± 0.6 | 1.16 ± 0.22 |
| Red α4−/− cells | 0.24 ± 0.09 | 1.20 ± 0.19 | 0.56 ± 0.16 |
| B 1-25 | |||
| Green α4+/+ cells | 0.92 ± 0.2 | 3.8 ± 0.34 | 2.44 ± 0.21 |
| Red α4−/− cells | 0.2 ± 0.08 | 1.52 ± 0.32 | 1.16 ± 0.19 |
| C 1-25 | |||
| Green α4+/+ cells | 1.44 ± 0.22 | 4.84 ± 0.5 | 7.8 ± 0.64 |
| Red α4−/− cells | 0.48 ± 0.12 | 1.36 ± 0.27 | 2.40 ± 0.26 |
| D 1-25 | |||
| Green α4+/+ cells | 1.24 ± 0.24 | 3.64 ± 0.39 | 2.84 ± 0.36 |
| Red α4−/− cells | 0.2 ± 0.08 | 0.8 ± 0.13 | 0.96 ± 0.21 |
Detailed data on cell recovery per section from one of the experiments described in Table 2's “Irradiated recipients.”
Boldface indicates significant differences between α4+/+ and α4−/− cells.
A,B,C,D are different segments of femur diaphysis and
serial sections (1 to 25).
As depicted in Figure 3 showing cell recoveries for all experiments with α4−/− cells, a significant decrease in cell recovery with α4−/− cells was found compared with normal cells, both in compact and trabecular bone, either before or after irradiation. This difference is consistent with all previous data on homing with these deficient cells using standard homing assays (Scott et al13 and references therein) and reassuring that our current evaluation reflects these changes faithfully. Furthermore, insightful data were obtained in terms of microanatomic distribution of α4−/− cells (Table 1 and Figure 5). In nonirradiated recipients the distribution of α4−/− cells in the 3 anatomic zones was essentially the same as that of α4+/+ cells, and both types of cells had a distribution not significantly deviating from random, estimated by image analysis (P = .63 for zone 1, P = .13 for zone 2, and P = .42 for zone 3 for α4−/− cells). These data would suggest that α4-deficient cells are capable of a random interstitial transmigration within BM. However, in irradiated hosts, a number of differences are seen with these cells: (1) The preferential distribution to the endosteal region, which was observed with α4+/+ donor cells, was not seen when α4−/− cells were transplanted. Thus, values of 8.69% plus or minus 1.04% before irradiation and 8.98% plus or minus 1.44% after irradiation were observed in endosteal zone 1, and both of these values were very similar to the surface area estimated, suggesting a random distribution. (2) In zone 2, more cells were recovered after irradiation (27.82% ± 1.82% vs 36.44% ± 2.02%), whereas and similarly to normal cells, significantly fewer cells were seen in zone 3 (central zone) after irradiation (63.49% ± 2.4% vs 54.58% ± 2.35%). These changes are likely consequent to alterations in cell density seen after irradiation, although one could interpret the increase in zone 2 as a sluggish response of α4−/− cells to stimuli from endosteum. The microanatomic distribution with α4−/− cells gives added credence to our chosen narrow definition of endosteal placement, compared with a more broad one (ie, 12-cell distance from endosteum) used previously.16 In trabecular bone, as in normal cells, no changes were recorded after irradiation.
Microanatomic distribution of transplanted α4+/+ and α−/− cells. (A) Flow cytometric analysis of donor cell populations. Lineage-depleted (using antibodies, magnetic beads, and column) cells were labeled with anti–c-kit (top 2 panels) and with anti-α4 Ab (bottom panels). Kit positivity was 82.8% in α4+ donor cells and 77.3% in α4−/− donor cells. Shaded areas represent isotype control. α4 integrin was expressed on > 90% of normal donor cells and virtually absent on α4-ablated donor cells. (B) Relative distribution of transplanted cells from normal or α4 integrin-deficient donors to nonirradiated or irradiated normal hosts to endosteal regions of trabecular bone. The relative seeding to endosteal regions of trabecular bone was not affected by irradiation, and did not differ significantly between normal and α4 integrin-deficient donor cells. (□, normal donor cells; ■, α4−/− donor cells; mean ± standard error of the mean; see Table 1 for values.) (C) Relative microanatomic distribution of transplanted cells from normal or α4 integrin–deficient donors to nonirradiated or irradiated normal hosts to endosteal, subendosteal, and central regions of compact bone. ▨ in each graph represent the relative surface area estimated by image analysis of each of the 3 zones (Figure 1A). Relative distribution frequencies equivalent to these values would thus indicate random distribution of donor cells throughout the marrow mass. Such random distribution was observed in nonirradiated hosts (□), irrespective of donor cell type, and in irradiated recipients (■) given α4−/− cells. In contrast, normal cells transplanted into irradiated hosts distributed preferentially to the endosteal region (top panel). Asterisks indicate statistically significant differences between normal and α4−/− cells and between no-irradiation (□) and irradiation (■) conditioning.
Microanatomic distribution of transplanted α4+/+ and α−/− cells. (A) Flow cytometric analysis of donor cell populations. Lineage-depleted (using antibodies, magnetic beads, and column) cells were labeled with anti–c-kit (top 2 panels) and with anti-α4 Ab (bottom panels). Kit positivity was 82.8% in α4+ donor cells and 77.3% in α4−/− donor cells. Shaded areas represent isotype control. α4 integrin was expressed on > 90% of normal donor cells and virtually absent on α4-ablated donor cells. (B) Relative distribution of transplanted cells from normal or α4 integrin-deficient donors to nonirradiated or irradiated normal hosts to endosteal regions of trabecular bone. The relative seeding to endosteal regions of trabecular bone was not affected by irradiation, and did not differ significantly between normal and α4 integrin-deficient donor cells. (□, normal donor cells; ■, α4−/− donor cells; mean ± standard error of the mean; see Table 1 for values.) (C) Relative microanatomic distribution of transplanted cells from normal or α4 integrin–deficient donors to nonirradiated or irradiated normal hosts to endosteal, subendosteal, and central regions of compact bone. ▨ in each graph represent the relative surface area estimated by image analysis of each of the 3 zones (Figure 1A). Relative distribution frequencies equivalent to these values would thus indicate random distribution of donor cells throughout the marrow mass. Such random distribution was observed in nonirradiated hosts (□), irrespective of donor cell type, and in irradiated recipients (■) given α4−/− cells. In contrast, normal cells transplanted into irradiated hosts distributed preferentially to the endosteal region (top panel). Asterisks indicate statistically significant differences between normal and α4−/− cells and between no-irradiation (□) and irradiation (■) conditioning.
To test whether the findings with α4-deficient cells can be reproduced in an independent manner, we also did the following: In standard homing assays using α4+/+ and α4−/− cells we recovered not only cells flushed from femur shafts 24 hours later, but also cells from cleaned and fragmented bones treated with collagenase to collect cells from endosteal areas. We cultured each sample and compared the proportional recovery of injected CFU-Cs from flushed femurs and from cells recovered from endosteal areas. The same studies were also repeated 8 days after transplantation. The data (supplemental Figure 1) show again impaired total recovery of α4-deficient cells compared with controls, not only from flushed femurs as expected, but also from endosteal areas, both at 24 hours and 8 days after transplantation. These data reaffirm the compromised retention of α4-deficient cells in endosteal areas.
Microanatomic localization of pertussis toxin–treated donor cells or of normal cells in Sl/Sld recipients before and after irradiation
As described in the previous section, α4-deficient cells do not preferentially accumulate at the endosteal surfaces (zone 1) after irradiation. One of the possibilities responsible for this outcome is their failure to migrate to a putative SDF-1 gradient existing near endosteum.17 A prerequisite for this hypothesis is that donor cells treated with pertussis toxin (PTX) ex vivo would behave similarly. Gi protein signaling inhibition by pertussis toxin induces mobilization similar to that seen in α4-deficient cells18 and when used in combination (ie, pertussis toxin treatment of α4-deficient cells) the homing is synergistically impaired.19 When PTX-treated donor cells (Lin−kit+) were given to irradiated recipients, we found that in femur diaphysis sections representing compact bone (no. of sections = 120), 9.5% plus or minus 2.3% of cells were in the endosteal area (zone 1), 38.5% plus or minus 6.3% were in subendosteum (zone 2); and 48.04% plus or minus 7.3% were in the central area (zone 3; Figure 4). Thus the endosteal distribution of PTX-treated donor cells in irradiated recipients was significantly different from the concurrently studied non–PTX-treated control cells given to another recipient (supplemental Table 1A), and was similar to α4-deficient cells, unlike all other normal cells (Figure 4).
In addition to the cross-talk between α4 integrins and SDF-1 signaling, cooperative signaling was previously demonstrated between α4 integrin and kit/kit ligand (KL).20-22 To study the behavior of normal cells into transplanted hosts lacking membrane bound KL, that is, Sl/Sld mice, we assessed their microanatomic distribution in nonirradiated or irradiated Sl/Sld recipients. We found that in nonirradiated recipients (90 sections, compact bone) the distribution across the 3 zones was 8.01% plus or minus 1.3% (zone 1), 34.29% plus or minus 2.9% (zone 2), and 57.71% plus or minus 4.2% (zone 3), whereas in irradiated recipients (106 sections, compact bone) it was 14.2% plus or minus 2.5% (zone 1), 51.2% plus or minus 2.4% (zone 2), and 34.55% plus or minus 4.76% (zone 3) (Figure 4 and supplemental Table 1B). A modest increase was seen in zone 1 in irradiated S/Sld hosts compared with cells given to normal irradiated recipients but this was at the lowest levels seen in normal recipients (Figure 4). Thus, these data taken together suggest that the α4 integrin, the Gi protein–dependent, and to a lesser degree the kit/KL-dependent signaling pathways play a role in microanatomic partitioning of cells near the endosteum.
Discussion
Hematopoietic stem cell localization at or near the endosteum was presented more than 30 years ago by Lord et al23 but refuted by Maloney et al.24 A number of recent studies have resurrected the endosteal issue of the niche16 and many subsequent enthusiastic supporters provided static or in vivo color images for its support.6,8,9,25-27 However, immunofluorescence images were not usually supplanted with adequate quantitative data and neither the criteria of distance from endosteum (from 2 cells25 to 12 cells16 ) nor the donor cells used were the same (Lin−, LSK CD150+/48−, Flt3+, etc). Nevertheless, despite these inconsistencies, reported studies indicate that there are modest differences, if any, in anatomic sublocalization between populations highly enriched in repopulating stem cells versus progenitor cells. Both the latter16,25 and the highly enriched cells26 were found to be preferentially localized near endosteum, albeit with different criteria defining distance from endosteum.
To provide large-scale quantitative data we decided to use populations of cells and evaluation criteria that allowed comparisons with previously presented data. The differences we observed with Lin−/kit+ cells in irradiated versus nonirradiated recipients are qualitatively similar to those of more purified cells recently reported by Li et al3 and Lo Celso et al,26 although anatomic differences encountered in irradiated BM and potentially quantitatively influencing the results were not considered in these studies. Furthermore, the random distribution (Figure 5) documented in nonirradiated recipients is reminiscent to the one found with highly enriched stem cells (CD150+/48−) described by Kiel et al7 in steady-state hematopoiesis. Such a distribution is also consistent with the random spread across BM of CXCL12-abundant reticular cells supporting stem cells.10 A preferential endosteal localization of highly purified HSCs compared with progenitor enriched cells was reported by Lo Celso et al26 in irradiated recipients, although the differences were modest and, on the average, less than 1 cell diameter. Similar population distinctions were not made, however, in nonirradiated recipients in which the data presented (8 cells from 3 recipients) show no endosteal placement for highly purified LSK CD48−Flk2− cells. (They were all 30 to 50 μm away from the endosteum.) In contrast, in another recent study, 11% (5 cells) of transplanted Lin−Sca+kit+ cells were in direct contact with endosteum (and 95% were within 20 μm from endosteum), whereas only 4% (3.9 cells) of Lin−Sca−kit+ cells did contact endosteum.28 These data led the authors to suggest “distinct” niches for Lin−Sca+kit+ versus Lin−Sca−kit+ cells, although endosteal placement for differentiated cells (macrophages and dendritic cells) was also documented. Thus, a diversity in the microdistribution of HSCs versus hematopoietic progenitor cells has been presented depending on the approach used. Furthermore, it is worth noting that the previously reported homing data (assessed as cells recovered from flushed bones in recipients up to 24 hours later) did not differ between highly purified cells29 (Hoechstlo and Rhodaminelo) and progenitor-enriched cells.30 Thus the weight of the evidence would point to at least no sharp differences in anatomic partitioning between transplanted stem cell–enriched versus progenitor-enriched donor cells, although their responses to niche stimuli may be quite different because of their intrinsic repertoire of factors.
Using the same protocols, contrasting data were obtained with α4-deficient cells. A significant overall decrease in α4−/− cell recoveries was observed (Figure 3, Table 2) consistent with previous data on reduced homing (Scott et al13 and references therein). However, unlike normal cells, preferential endosteal seeding following irradiation was not observed. The significance of these data is 2-fold. First, it was unclear with normal cells to what extent the increased endosteal frequency in irradiated hosts was the result of a preferential migratory shift of transplanted cells toward the endosteal region, or whether it was indirect and consequent to cellular shifts in other areas after irradiation. Clearly the failure of α4−/− cells to preferentially locate to the endosteal region, even in the presence of changes in other areas, favors the former. Second, the inability of α4−/− cells to preferentially seed to the endosteal region may be due to their impaired responsiveness to putative chemoattractants emanated by the radiation-damaged endosteal regions, or to the absence of α4-dependent retention signals in endosteal sinusoids, as previously shown for calvarial sinusoids,31 or in BM sinusoids for immature B cells.32 An impaired ability of α4−/− lymphocytes14 to migrate in vitro toward an SDF-1–dependent chemotactic gradient was previously documented and impairment was also seen with kit+α4−/− cells (supplemental Figure 2). Although these in vitro data are consistent with our in vivo findings, it is acknowledged that these in vitro static data cannot be extrapolated to the in vivo functional behavior of cells, especially when integrins can alter their function quickly, depending on their environmental context. Because there is a cross-talk between α4 and G-protein–coupled receptor–dependent signaling, it is theoretically expected that other cells with impaired responses to an SDF-1 gradient, that is, PTX-treated cells, may behave similarly to α4-deficient cells in vivo. Following this reasoning we used PTX-treated cells in our studies and found that these cells behave similarly to α4-deficient cells after irradiation, that is, their endosteal placement was at levels similar to their random preirradiation distribution. Although these data provide support for the presence of an active SDF-1 gradient near endosteum after irradiation as previously suggested,33 they do not necessarily explain the α4 data, nor do they exclude scattered increases in SDF-1 throughout bone marrow. Furthermore, our data strictly concern α4−/− progenitor enriched cells, and whether highly purified stem cell populations would display different functional behavior remains to be seen. In this context, the recent findings of Köhler et al28 suggesting an inverse correlation between expression of CD49d and distance from endosteum in Lin−Sca+kit+ cells versus Lin−Sca−kit+ cells. However, a positive correlation was found for young versus aged Lin−Sca+kit+. These determinations were recorded in cells ex vivo and may not account for functional differences or for ligand-induced modulation of functional integrin when the cells encounter the in vivo environment.
What are the implications of these findings in terms of short- and/or long-term hematopoietic reconstitution? It was previously argued that an impaired endosteal portioning of incoming cells within nonirradiated BM could account for a decrease in transplantation outcomes observed in irradiated recipients, especially when traditional homing assays did not show any significant deficit.16,25,28,34 It is difficult to reconcile these data with our data, as well as those of Kiel et al,7 Sugiyama et al,10 and Xie et al,27 all of which suggest random distribution of either highly enriched or progenitor-enriched cells in a nonirradiated environment. Moreover, our data further suggest that the capacity for endosteal placement after irradiation, together with reduced homing, as occurs with α4 integrin–deficient cells, may be of consequence for their proliferative expansion short term13 and/or their self-renewal in serial transplantations.35 A partial impairment in endosteal placement of normal cells given to recipients lacking membrane-bound KL (Sl/Sld mice) was previously published in nonirradiated recipients.36 We found that after irradiation the endosteal migration of normal donor cells to the Sl/Sld recipients was moderately impaired in these mice (Figures 4,6). However, short-term engraftment was drastically reduced,18,36 implying that other cues from the microenvironment may have a dominant influence on cell proliferation that follows homing. These data suggest that even with endosteal placement (Figure 6) the response to membrane-bound KL is dominant for the regenerative process in these recipients.
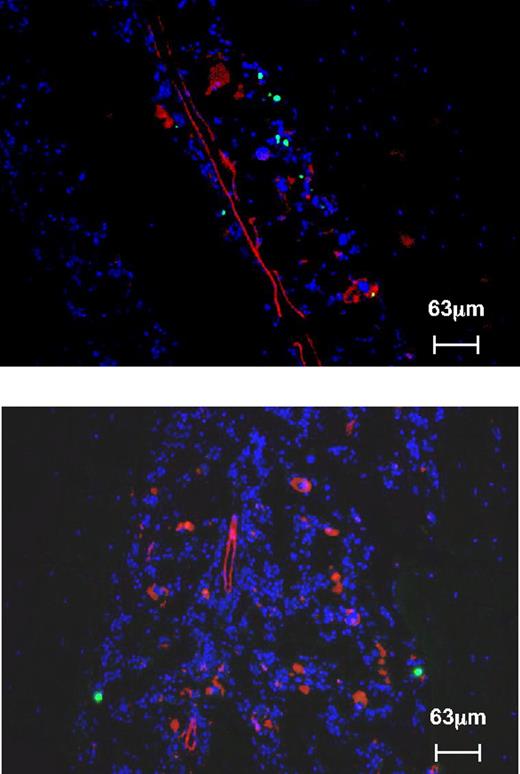
Microanatomic distribution of normal donor cells in irradiated Sl/Sld recipients. Transplanted HSCs display a qualitatively similar preferential lodging to endosteal regions in irradiated Sl/Sld recipients (blue: DAPI, green: CFSE-labeled wild-type HSCs, red: anti-CD31, top panel: tibia, bottom panel: femur), as normal cells do.
Microanatomic distribution of normal donor cells in irradiated Sl/Sld recipients. Transplanted HSCs display a qualitatively similar preferential lodging to endosteal regions in irradiated Sl/Sld recipients (blue: DAPI, green: CFSE-labeled wild-type HSCs, red: anti-CD31, top panel: tibia, bottom panel: femur), as normal cells do.
It has long been observed that early regeneration of hematopoiesis following irradiation23,24,37 begins at or near the endosteum. This challenges the prevailing view of the importance of endosteum in preservation of quiescence6,38 and/or the need for disengagement or dislodgment of HSCs from the endosteal niche toward the central vascular niche for their proliferation.26,39,40 Thus one could propose that the endosteal niche environment, although fostering HSC quiescence under steady-state conditions, shifts the balance toward proliferation/differentiation in response to irradiation or chemotherapy. This constitutes a dynamic adaptation of the niche to meet stress demands by producing factors that both attract and favor proliferative expansion of cells located there. In line with this conclusion are data recently reported suggesting a reciprocal functional interaction between hematopoietic cells and niche cells for hematopoietic regeneration.33,41 Such an interaction is greatly favored by their preferential positioning (in quantitative terms) in trabecular bone.
In summary, our data brought into light several considerations to be taken into account when donor cell microanatomic distribution is compared in irradiated versus nonirradiated hosts. Although it could be argued that our data with Lin−kit+ cells may not represent stringently enriched populations of HSCs, our conclusions relevant to their intramarrow microanatomic partitioning are not dissimilar to those of highly purified cells.7,27,41 Furthermore, if trabecular bone is where the action is, then the issue of preferential endosteal placement may be mostly semantic, as in these areas, cells are never far away from bone or blood vessels.26 Although α4 integrin was frequently included in previous models and reviews of endosteal niche, there was no direct testing of its role previously. Our present experiments show that these cells do not display the expected distribution to endosteal regions in irradiated hosts. In agreement with our in vitro observations, these data suggest that α4 integrin is a critical component for cell migration toward factors elaborated by irradiated endosteal cells, likely including SDF-1. Similar data were encountered with PTX-treated donor cells, presumably due to similar mechanisms. It is important to note that it is only in the postirradiation BM environment that impaired patterns of microanatomic distribution were uncovered with mutant cells and that these may impact on a durable hematopoietic reconstitution. Our data do not resolve the issue of whether cellular contact between HSCs and endosteal cells (perivascular, endothelial, or endosteal osteoblasts) is required42-45 for formation of “stem cell synapse” and whether α4 integrin and its putative ligands at endosteum (ie, osteopontin or vascular cell adhesion molecule 1) are involved in this process. Further experiments with mutant donor cells or mutant microenvironment using other more detailed in vivo imaging approaches will shed light on these issues.
The online version of the article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Betty Nakamoto for assistance in the preparation of the paper and figures, Gregory V. Priestley for his expertise in mouse experiments, Stephen Padilla for excellent care of our mice, and Gabi Spohn for the migration experiments.
This work was supported by National Institutes of Health (NIH) grants HL058734 and HL46557 (T.P.).
National Institutes of Health
Authorship
Contribution: Y.J. was involved in all technical aspects of the experiments described; H.B. performed experiments and cowrote the paper; T.U. and K.C. performed experiments; and T.P. designed and evaluated the experiments and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Thalia Papayannopoulou, University of Washington, Medicine/Hematology, Box 357710, Seattle, WA 98195; email: thalp@uw.edu.