Abstract
Investigation of 3 families with bleeding symptoms demonstrated a defect in the collagen-binding activity of von Willebrand factor (VWF) in association with a normal VWF multimeric pattern. Genetic analysis showed affected persons to be heterozygous for mutations in the A3 domain of VWF: S1731T, W1745C, and S1783A. One person showed compound heterozygosity for W1745C and R760H. W1745C and S1783A have not been reported previously. The mutations were reproduced by site-directed mutagenesis and mutant VWF expressed in HEK293T cells. Collagen-binding activity measured by immunosorbent assay varied according to collagen type: W1745C and S1783A were associated with a pronounced binding defect to both type I and type III collagen, whereas the principal abnormality in S1731T patients was a reduction in binding to type I collagen only. The multimer pattern and distribution of mutant proteins were indistinguishable from wild-type recombinant VWF, confirming that the defect in collagen binding resulted from the loss of affinity at the binding site and not impairment of high-molecular-weight multimer formation. Our findings demonstrate that mutations causing an abnormality in the binding of VWF to collagen may contribute to clinically significant bleeding symptoms. We propose that isolated collagen-binding defects are classified as a distinct subtype of von Willebrand disease.
Introduction
von Willebrand factor (VWF) is a large multimeric glycoprotein that has 2 important roles in hemostasis. These are stabilizing factor VIII (FVIII), by acting as its carrier protein in the circulation, and attaching activated platelets to the subendothelium via binding to the GpIb receptor on platelets and to collagen in the subendothelial matrix. Enhancement of the platelet-subendothelium interaction is vital at sites of vascular injury during conditions of high shear stress. VWF binds to collagen via 2 sites: the A3 domain (residues 1683-1874) contains the main site for fibrillar collagen types I and III found within perivascular connective tissue,1,2 and a second site in the A1 domain (residues 1260-1471) binds nonfibrillar collagen type VI within the subendothelial matrix.3,4 However, the A1 domain can also bind collagen types I and III, and the relative importance of the 2 domains has been extensively but inconclusively investigated.5,6 In all cases, it appears that affinity of VWF for collagen is heavily dependent on the presence of VWF multimers of high or ultrahigh molecular weight.7
Type 2 von Willebrand disease (VWD) is characterized by a qualitative defect in VWF and is diagnosed by demonstration of a discrepancy between circulating plasma levels of VWF and its functional activity. In addition to measuring GpIbα-dependent function using ristocetin cofactor activity (VWF:RCo), it has been recommended that collagen-binding activity (VWF:CB) is analyzed in the subclassification of type 2 VWD.8 A single defect in VWF collagen binding in association with normal multimeric structure has previously been reported.9
In this report, we describe the phenotypic and genotypic characterization of 3 families with bleeding symptoms in whom the main laboratory abnormality was a reduction in VWF:CB. Analysis of the VWF coding sequence identified 2 novel mutations, W1745C and S1783A, and the previously described mutation S1731T, all within the A3 domain. The mutations have been characterized and shown to be responsible for the phenotype by expression studies. These studies illustrate the physiologic importance of the A3 collagen-binding site and the structural constraints on its function.
Methods
Phenotypic analysis
Bleeding scores were calculated retrospectively from the patient notes using the condensed MCMDM-1VWD questionnaire.10 Venous whole blood was taken into 0.106 M sodium citrate (Sarstedt Monovette 9NC/3-mL tubes, 9:1 vol/vol). Platelet-poor plasma was prepared by double centrifugation at 2000g for 12 minutes at 4°C and stored in aliquots at −45°C until further testing. FVIII:C was analyzed by one-stage clotting assay. VWF antigen (VWF:Ag) was measured by enzyme-linked immunosorbent assay (ELISA),11 and VWF:RCo was measured using lyophilized platelets as previously described.12 VWF:CB was performed by coating Covalink ELISA plates (Nunc) with 2.7 μg/mL placental type III human collagen (Sigma-Aldrich or Southern Biotechnology) or 10 μg/mL placental type I human collagen (Sigma-Aldrich) diluted in 0.14M NaCl, 0.006M Na2HPO4, 0.002M NaH2PO4, and 0.03M KCL (pH 7.3). Plates were blocked with 1.5% bovine serum albumin in phosphate-buffered saline (PBS). Platelet-poor plasma and standard pooled normal plasma were diluted in PBS containing 3% polyethylene glycol. Collagen-bound VWF was detected with horseradish peroxidase (HRP)–conjugated rabbit anti–human VWF antibody (DakoPatts). PFA-100 (Dade-Behring) closure times were determined according to the manufacturer's instructions. Platelet-rich plasma was prepared by centrifugation at 130g for 15 minutes. Platelet function was assessed by optical aggregometry. Ristocetin-induced platelet aggregation (RIPA) was determined by adding ristocetin at concentrations of 0.5, 1.0, 1.25, and 1.5 mg/mL to platelet-rich plasma and monitoring light transmission in an aggregometer (Payton Associates). VWF multimer composition was assessed by sodium dodecyl sulfate agarose gel electrophoresis followed by Western blotting and detection with rabbit anti–human VWF antibody (DakoPatts). The gel was then visualized using a Vectastain ABC-AP kit and a BCIP/NBT Alkaline Phosphate Substrate Kit (Vector Laboratories).
Genetic analysis
Genomic DNA was extracted from peripheral blood lymphocytes using the QIAamp DNA Mini prep kit (QIAGEN) according to the manufacturer's instructions. Fifty-one primer sets were used to amplify the essential regions of VWF (52 exons, flanking intronic regions, 5′ promoter region, and the 3′ untranslated region). Primers were designed to optimally amplify sequences of interest at an annealing temperature of either 57°C or 59°C (sequences and polymerase chain reaction conditions available on request) using the standard polymerase chain reaction methods. Amplicons were purified using microCLEAN (Web Scientific) before cycle sequencing using the Big-Dye Terminator, Version 3.1 Cycle Sequencing Kit (Applied Biosystems). Sequencing reaction products were purified using an in-house ethanol precipitation method and resuspended in formamide (Applied Biosystems) before direct sequencing on an Applied Biosystems AB3130xl DNA sequencer. Mutation detection was performed using the Mutation Surveyor Version 3.2 software package (BioGene Ltd). The numbering and description of mutations are according to the current International Society on Thrombosis and Haemostasis Scientific and Standardization Committee (ISTH SSC) guidelines.13
Molecular modeling
Expression of recombinant VWF
Generation of the expression vector pcDNA3.1 FL-VWF encoding for full-length VWF has been previously described.15 A silent mutation has been previously introduced to mutate the KpnI restriction site located at basepairs 298 to 303, leaving a unique KpnI restriction site located in the VWF A2 domain. The VWF KpnI-AgeI fragment was cloned into pcDNA3.1 and used to generate the mutants, S1731T, W1745C, W1745A, S1783A, R760H, and H1786A using the QuickChange XL site-directed mutagenesis kit (Stratagene). H1786A is a mutation that has not been described in patients but has been shown to abolish collagen binding in expression studies.14 Therefore, this mutant was included as a control. Clones were digested with KpnI and AgeI and subcloned into pcDNA3.1 FL-VWF. All mutations and clones were verified by DNA sequencing.
VWF was expressed in HEK293T cells using 10mM PEI as a transfection reagent as previously described.14 At 3 days after transfection, media were collected and cells were lysed with lysis buffer (50mM Tris-HCl, 150mM NaCl, 1mM ethylenediaminetetraacetic acid, 1% Triton X-100, pH 7.5). For the generation of collagen-binding isotherms, media were collected and concentrated 10-fold using 100-kDa cutoff spin filters (Amicon) and dialyzed into 20mM Tris-HCl, pH 7.4. Concentration of VWF samples did not alter multimeric structure, which was determined by ELISA and gel electrophoresis as previously described.15
Collagen-binding activity of expressed proteins
The collagen-binding activity of VWF was measured by ELISA. Briefly, MaxiSorp plates (Nunc) were coated with either human type III or human type I collagen derived from placenta (Sigma-Aldrich) at a final concentration of 5 μg/mL in carbonate buffer at room temperature. Wells were then blocked with 2.5% bovine serum albumin in PBS. After washing with PBS containing 0.1% Tween (PBS-T), serial dilutions of recombinant VWF samples and reference plasma diluted with PBS-T (1:10-1:320) were added to the wells and incubated for 2 hours at room temperature. To determine binding kinetics, increasing concentrations of VWF were applied to the wells for 1 hour at room temperature. Wells were then washed with PBS-T and incubated with HRP-conjugated rabbit anti–human VWF antibody (DakoPatts) diluted 1/1000 in PBS-T for 45 minutes at room temperature. Bound antibody was detected with Color Fast O–phenylenediamine substrate (Sigma-Aldrich) and the absorbance recorded at 492 nm. Binding curves were generated using GraphPad Prism fitting the data to the one-site binding equation.
Results
Patient phenotype and genotype
Phenotypic and genetic results are shown in Figure 1. All patients had normal platelet counts, platelet aggregometry, RIPA, prothrombin time, activated partial thromboplastin time, and thrombin time.
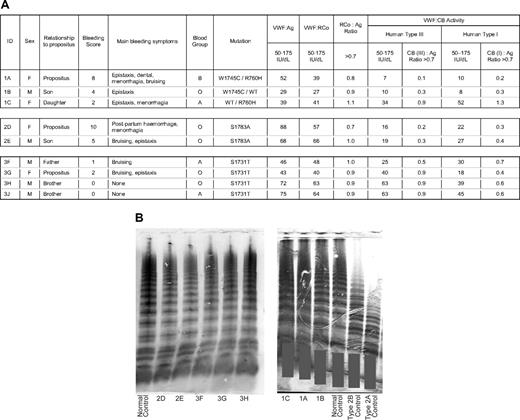
Phenotypic data for patients and family members. (A) Table showing laboratory results. Reference values are given for each assay. Ratios of antigen to activity are shown alongside activity columns. VWF indicates von Willebrand factor; Ag, antigen; RCo, ristocetin cofactor activity; CB, collagen-binding activity; and WT, wild type. Bleeding scores were calculated using the condensed MCMDM-1VWD questionnaire. (B) Gels showing VWF multimer pattern from plasma samples. Gray bars are placed to maintain patient confidentiality. Patient ID or type of control is shown at the bottom of each lane. A sample from 3J was not available for electrophoresis.
Phenotypic data for patients and family members. (A) Table showing laboratory results. Reference values are given for each assay. Ratios of antigen to activity are shown alongside activity columns. VWF indicates von Willebrand factor; Ag, antigen; RCo, ristocetin cofactor activity; CB, collagen-binding activity; and WT, wild type. Bleeding scores were calculated using the condensed MCMDM-1VWD questionnaire. (B) Gels showing VWF multimer pattern from plasma samples. Gray bars are placed to maintain patient confidentiality. Patient ID or type of control is shown at the bottom of each lane. A sample from 3J was not available for electrophoresis.
In family 1, the proposita (1A) was an elderly woman with lifelong bleeding episodes, including epistaxis, ecchymosis, menorrhagia, and prolonged bleeding after dental extractions, requiring multiple medical interventions. Her 2 offspring, 1B (son) and 1C (daughter), reported minor bleeding symptoms that had not required treatment; 1A demonstrated compound heterozygosity for 2 mutations in the VWF gene. The first is a novel mutation in exon 30:c.5235 G → T resulting in Trp1745Cys and the second is c.2279 G → A resulting in Arg760His. 1B was found to be heterozygous for Trp1745Cys only and 1C was heterozygous for Arg760His only, confirming that these mutations are in trans in 1A. The coexistence of both mutations appears to produce a more significant bleeding disorder as the bleeding score in 1A (8) was higher than that in either of her children (4 and 2).
The position of Trp1745 in the A3 domain is shown in Figure 2. Both persons with this mutation (1A and 1B) had markedly reduced ratios of VWF collagen-binding activity to VWF antigen (CB:Ag) with type III collagen (0.1 and 0.3, respectively) and type I collagen (0.2 and 0.3, respectively). The ratio of VWF:RCo to VWF:Ag (RCo:Ag) was near unity in both persons, and VWF multimer analysis (Figure 1B) and RIPA were normal. Closure times in the PFA-100 were mildly prolonged with the ADP cartridge (1A = 173 seconds, 1B = 189 seconds, NR < 112 seconds). With the epinephrine cartridge, the closure time was prolonged in 1A only (1A = 271 seconds, 1B = 90 seconds, NR < 179 seconds).
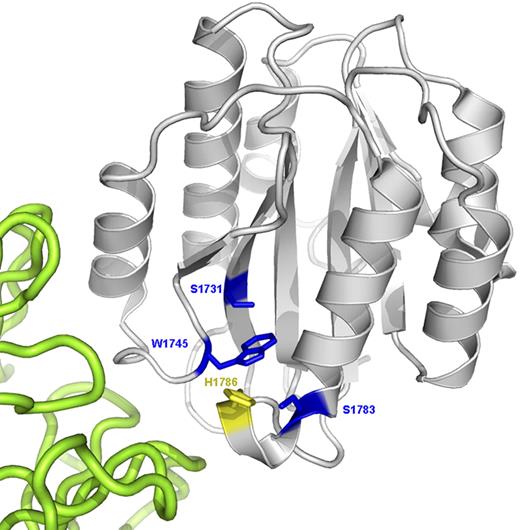
Ribbon diagram of the VWF A3 domain (gray) complexed with the RU5 antibody (green) that inhibits collagen binding (PDB Id: 1FE8). The putative collagen-binding surface is toward the lower left of the A3 domain in this view. The 3 naturally mutated residues are shown as blue sticks. H1786 is shown as a yellow stick.
Ribbon diagram of the VWF A3 domain (gray) complexed with the RU5 antibody (green) that inhibits collagen binding (PDB Id: 1FE8). The putative collagen-binding surface is toward the lower left of the A3 domain in this view. The 3 naturally mutated residues are shown as blue sticks. H1786 is shown as a yellow stick.
In contrast, 1C demonstrated a concordant reduction in VWF:Ag, VWF:RCo, and VWF:CB (to both collagen types) with normal multimer pattern. Thus, Trp1745Cys is associated with a type 2 VWD phenotype with a specific defect in collagen binding, whereas Arg760His in isolation produces a type 1 VWD phenotype with no additional effect on collagen binding. Administration of 0.3 μg/kg 1-desamino-8D-arginine vasopressin (DDAVP) to 1A resulted in correction of both VWF:Ag and VWF:CB into the normal range, but with a persistently discrepant CB:Ag ratio (samples taken 90 minutes after DDAVP administration showed VWF:Ag 162 IU/dL, VWF:RCo 140 IU/dL, VWF:CB 66 IU/dL, CB:Ag ratio = 0.4).
The proposita (2D) in family 2 was investigated after a postpartum hemorrhage requiring blood transfusion. She gave a longstanding history of menorrhagia, and her calculated bleeding score was 10, the highest of all the persons in this study. Her son 2E had mild bruising and epistaxis. Both were shown to be heterozygous for a novel mutation in exon 31:c.5347 T → G resulting in Ser1783Ala (Figure 2). VWF:Ag, VWF:RCo, and multimer analysis were all normal. VWF:CB was significantly reduced with both types I and III collagen, resulting in CB:Ag ratios of 0.2 to 0.4. Closure times in the PFA-100 were normal in 2D with both cassettes (Coll/Epi, 111 seconds; Coll/ADP, 98 seconds). The discrepant reduction in VWF:CB persisted after DDAVP administration to 2D despite normalization of both VWF:Ag and VWF:CB (samples taken 90 minutes after DDAVP administration showed VWF:Ag 191 IU/dL, VWF:CB 67 IU/dL, CB:Ag ratio = 0.35).
In family 3, the female proposita (3G) presented in childhood with epistaxis and easy bruising. Her father (3F), who reported mild bruising symptoms, and both asymptomatic younger male siblings (3H and 3J) were also investigated. All family members were found to be heterozygous for a previously described mutation in exon 30:c.5191 T → A, resulting in Ser1731Thr.9 VWF:Ag and VWF:RCo were borderline low in 3F and 3G but normal in the asymptomatic brothers, 3H and 3J. Multimer analysis was normal in all family members tested (Figure 1). VWF:CB binding to type I collagen was reduced in all family members (CB/Ag ratio 0.4-0.7), whereas only one (3G) showed a reduction in CB:Ag ratio with type III collagen.
Expression of recombinant VWF mutants
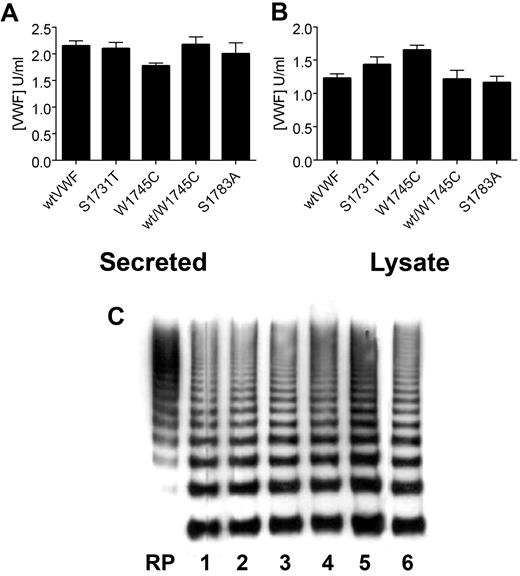
The recombinant proteins wt-rVWF, W1745C-rVWF, W1745A-rVWF, S1783A-rVWF, S1731T-rVWF, R760H-rVWF, and H1786A-rVWF were all expressed in HEK293T cells. Cotransfections of W1745C-rVWF with wt-rVWF and R760H-rVWF were also expressed to mimic the heterozygous or compound heterozygous states. VWF ELISA demonstrated that R760H-rVWF was expressed at a significantly lower level than wt-rVWF. All the other recombinant proteins were expressed and secreted at similar levels to wt-rVWF (∼ 2 μg/mL) with a similar amount of VWF retained within the cell (∼ 1.5 μg/mL; Figure 3). Electrophoretic analysis demonstrated that all the mutant proteins had a similar multimer distribution to that of wt-rVWF (Figure 3C).
Expression of recombinant VWF mutants. Recombinant wt and mutant VWF were transiently expressed in HEK293T cells, and VWF ELISA was used to determine the concentration of secreted VWF (A) and VWF retained within the cell lysate (B). Error bars represent mean ± SD of 3 separate experiments each performed in duplicate. (C) The multimer composition of recombinant VWF was analyzed in 1.3% agarose gels and visualized with HRP-conjugated anti-VWF polyclonal antibodies. RP indicates reference plasma. Lane 1 indicates wt-rVWF; lane 2, S1731T; lane 3, W1745C; lane 4, W1745A; lane 5, wt/W1745C; lane 6, S1783A. All mutants are expressed at similar levels to wild-type rVWF, with similar supernatant to lysate ratios and normal multimer distribution.
Expression of recombinant VWF mutants. Recombinant wt and mutant VWF were transiently expressed in HEK293T cells, and VWF ELISA was used to determine the concentration of secreted VWF (A) and VWF retained within the cell lysate (B). Error bars represent mean ± SD of 3 separate experiments each performed in duplicate. (C) The multimer composition of recombinant VWF was analyzed in 1.3% agarose gels and visualized with HRP-conjugated anti-VWF polyclonal antibodies. RP indicates reference plasma. Lane 1 indicates wt-rVWF; lane 2, S1731T; lane 3, W1745C; lane 4, W1745A; lane 5, wt/W1745C; lane 6, S1783A. All mutants are expressed at similar levels to wild-type rVWF, with similar supernatant to lysate ratios and normal multimer distribution.
Collagen-binding function of recombinant VWF mutants
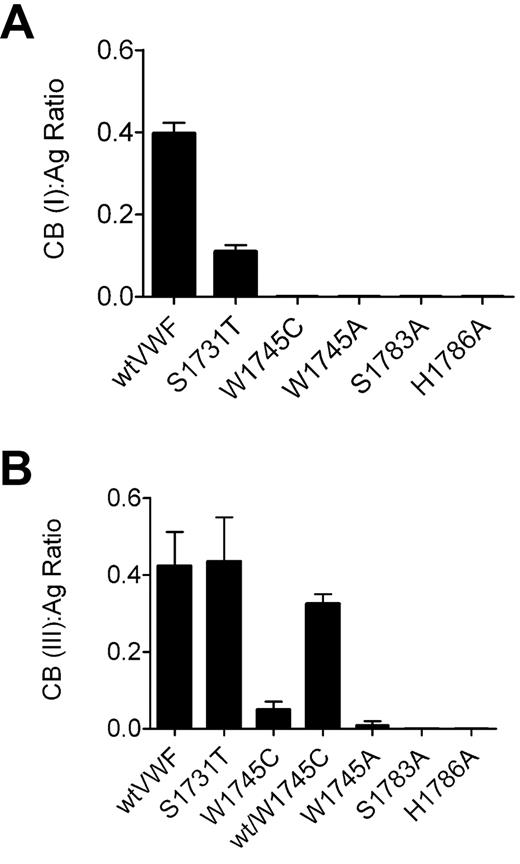
In the diagnosis of type 2M VWD, a CB:Ag ratio more than 0.7 is taken to reflect normal collagen-binding function.16 The CB:Ag ratio for wt-rVWF binding to collagen type III is below this at 0.4 (Figure 3B). This is most probably the result of the difference in multimer distribution observed between plasma-derived and recombinant VWF (Figure 3C). However, all recombinant VWF proteins had multimeric patterns that were indistinguishable from wt-rVWF, and we therefore attribute differences in collagen binding to differences in affinity at the collagen-binding site between the molecules. H1786A-rVWF and S1783A-rVWF did not bind to type III collagen (Figures 4B, 5B). W1745C-rVWF and W1745A-rVWF both produced CB:Ag ratios of less than 0.05, confirming that it is the loss of the large side chain of tryptophan, rather than the availability of an additional cysteine residue for disulphide bond formation, that affects collagen-binding (Figure 4B). The defect in collagen binding was partly corrected when W1745C-rVWF was cotransfected with wt-rVWF. S1731T-rVWF had a CB:Ag ratio with type III collagen that was similar to wt-rVWF (0.43; Figure 2A).
CB:Ag ratio of recombinant VWF mutants. Collagen-binding assays to (A) type I collagen and (B) type III collagen were performed in parallel with VWF:Ag ELISA. Error bars represent mean ± SD of 3 separate experiments performed in duplicate. Wild-type recombinant VWF and H1786A (a mutation previously shown to abolish collagen binding) are shown as controls. Only S1731T shows significant binding, which is greater with collagen type III. Coexpression of wild-type VWF with W1745C significantly improved binding to type III collagen.
CB:Ag ratio of recombinant VWF mutants. Collagen-binding assays to (A) type I collagen and (B) type III collagen were performed in parallel with VWF:Ag ELISA. Error bars represent mean ± SD of 3 separate experiments performed in duplicate. Wild-type recombinant VWF and H1786A (a mutation previously shown to abolish collagen binding) are shown as controls. Only S1731T shows significant binding, which is greater with collagen type III. Coexpression of wild-type VWF with W1745C significantly improved binding to type III collagen.
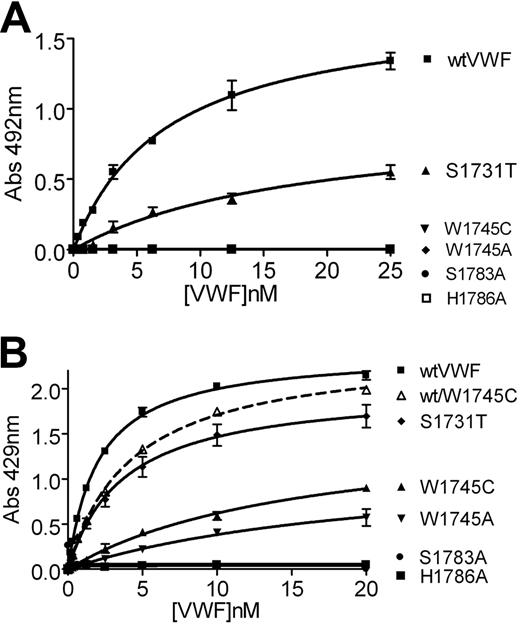
Binding isotherms of the collagen-VWF interactions. Microtiter plates were coated with either type I (A) or type III (B) human collagen at a final concentration of 5 μg/mL and incubated with decreasing concentrations of wild-type or mutant recombinant VWF. As previously reported, H1786A shows no binding to either collagen type and only S1731T binds significantly to type 1 collagen.
Binding isotherms of the collagen-VWF interactions. Microtiter plates were coated with either type I (A) or type III (B) human collagen at a final concentration of 5 μg/mL and incubated with decreasing concentrations of wild-type or mutant recombinant VWF. As previously reported, H1786A shows no binding to either collagen type and only S1731T binds significantly to type 1 collagen.
As with type III collagen, the CB:Ag ratio for wt-rVWF with type I collagen (0.39) was below the normal plasma range. W1745C-rVWF, S1783A-rVWF, S1731T-rVWF, and H1786A-rVWF did not bind to type I collagen, and so a ratio could not be determined. S1731T-rVWF had a reduced CB:Ag ratio of 0.12. This is consistent with the findings of Ribba et al9 and demonstrates that mutation of S1731 affects binding to collagen type I but not type III.
R760H-rVWF showed similar CB:Ag ratio to wt-rVWF with both collagen types. Coexpression of W1745C-rVWF with R760H-rVWF showed the same results as for W1745C-rVWF/wt-rVWF (data not shown).
To further investigate the binding of VWF to collagen, binding isotherms were generated to calculate the KD,app for the VWF-collagen interactions. As previously determined, wt-rVWF bound type III collagen with high affinity (KD,app 2.0 ± 0.1 nM; Figure 5B). In accordance with previous studies and the results obtained for the CB:Ag ratio, the control H1786A-rVWF mutant and the S1783A-rVWF mutants did not bind to collagen type III. S1731T-rVWF bound type III collagen with similar affinity to wt-rVWF, KD,app 3.7 plus or minus 0.5 nM (Figure 5B). W1745C-rVWF and W1745A-rVWF both bound type III collagen with reduced affinity, KD,app 16 plus or minus 2.6 nM and 21.3 plus or minus 6.3 nM, respectively (Figure 5B).
No binding of H1786A-rVWF, S1783A-rVWF, W1745C-rVWF, or W1745A-rVWF was observed to type I collagen in keeping with the CB:Ag ratio results. S1731T-rVWF bound type I collagen with reduced affinity, KD,app 27.1 plus or minus 5.2 nM compared with 7.3 plus or minus 0.8 nM for wt-rVWF (Figure 4B), confirming that the S1731T mutation only affects binding to collagen type I.
Discussion
We have reported 3 unrelated families with bleeding symptoms in which the principal detectable abnormality was defective collagen binding, not attributable to loss of HMW multimers. Genetic analysis showed that affected persons had mutations in the A3 domain of VWF, which contains the main binding site for fibrillar collagen. Mapping of the mutants onto the crystal structure of the A3 domain demonstrated their close proximity to the putative collagen-binding site. Experiments with recombinant mutant proteins confirmed that the defects in collagen binding were the result of reduced affinity at the binding site rather than abnormalities of multimer formation.
Two novel A3 domain mutations have been described in this report in families with clinically significant bleeding: W1745C and S1783A. Both mutations result in reduced VWF affinity toward collagen types I and III. Previous studies have shown that S1783 is essential for collagen type III binding, but a mutation at this residue has not previously been reported in a patient.17,18 In our study, the W1745C kindred includes a member with milder bleeding symptoms than the affected members and who did not carry the W1745C mutation. This person did, however, have a mild concordant reduction in VWF levels and functional activity (including collagen binding). The variation in VWF levels in this family results partly from the difference in ABO blood group and also from the presence of the R760H mutation. Expression studies showed that, although R760H results in decreased VWF production, it has no effect on collagen binding or multimer distribution. Therefore, we conclude that its presence does not alter the interpretation of the effects of W1745C and confirmed this by coexpression studies. The effect of R760H is consistent with the position of this mutation in the VWF propeptide, a part of the protein that directs posttranslational modification and secretion. R760H has previously been reported in the VWF database (http://www.VWF.group.shef.ac.uk/index.html) but without description of the phenotype. R760C is also reported in association with the type 2N subtype, indicating that mutation at this residue can affect the factor VIII binding function of VWF that is contained in the adjacent D′ domain. The mechanism underlying the abnormalities seen with R760C has been analyzed in previous expression studies.19 It appears that R760 is part of the recognition motif for furin-dependent cleavage of the propeptide, which is impaired by this substitution. However, the presence of His at P4 in the furin cleavage site has been reported to result in satisfactory processing.20 There was no evidence that recombinant R760H-VWF retained the propeptide. This suggests that the effect of mutation at R760 is more complex than previously thought and requires further analysis that is beyond the scope of this study. The mixture of phenotypes in one pedigree emphasizes the difficulty in attributing phenotypic changes to mutations in VWF on a highly heterogeneous background. Nonetheless, in family 1, the most significant bleeding symptoms (BS = 8) were present in member 1A who had reduced collagen binding associated with normal VWF:Ag and a normal RCo:Ag ratio.
In family 2, both members carry the S1783A mutant, both are blood group O, and both have normal levels of VWF:Ag and VWF:RCo but reduced collagen binding with a normal multimer pattern. As both have increased bleeding scores, this indicates that a clinically significant phenotype can be associated with a pure defect in collagen binding that is not caused by loss of high-molecular-weight multimers.
The third mutation (S1731T) has previously been reported in 2 persons with moderate bleeding symptoms9 similar to those reported here. In our study, there was variation in the clinical and laboratory phenotypes between family members, all of whom had S1731T. Two of the offspring did not report bleeding symptoms, including after tonsilloadenoidectomy, indicating that this mutation may be clinically silent in some cases. The 2 symptomatic persons in this family had lower levels of VWF:Ag, VWF:RCo, and VWF:CB than their 2 asymptomatic relatives, indicating that the phenotype probably has a complex composition. It is not possible to distinguish the contributions of VWF level and reduced collagen-binding activity to the symptoms, but the milder phenotype is in keeping with the milder defect seen with S1731T-rVWF in the collagen-binding studies compared with the other recombinant mutants.
The clinical importance of these mutations can be assessed by comparison of the bleeding scores. With the bleeding questionnaire used, a positive score is considered to be 4 or more. All the persons heterozygous for W1745C or S1783A had scores of 4 or more with the highest values of 10 for 2D with S1783A and 8 for 1A with W1745C/R760H. In contrast, the scores among those heterozygous for S1731T were 0 to 2. Although the numbers involved are too small for statistical analysis, there does appear to be a correlation between bleeding score and laboratory phenotype, with the lowest ratios for collagen binding (CB:Ag 0.1 and 0.2 in 1A and 0.2 and 0.3 in 2D, Figure 1A) found in the persons with the greater bleeding symptoms. Similarly, the near normality of the laboratory phenotype in some of the affected persons in family 3 correlates with the normality of the bleeding score.
In the original report, the S1731T mutation was associated with reduced binding to type I collagen (CB:Ag ratio = 0.12).9 Binding to type III collagen was not assessed, but the results reported here show that it is similar to wild-type rVWF (CB:Ag ratio = 0.45). This finding implies that mutations affecting binding to both types of fibrillar collagen may be clinically more significant than those affecting binding to type I collagen only. It may also indicate more stringent structural requirements for binding type I compared with type III collagen. This may be of importance when considering the type of collagen that should be used in binding assays.
The A3 domain is considered to be the major binding site for types I and III collagen. However, a recent study demonstrated that A3-deficient VWF mutants could bind normally to collagen via the A1 domain under conditions of flow.5 Subsequently, it has been shown that mutations in the A3 domain abolishing collagen binding did not impair the ability of VWF to correct the bleeding time in VWF−/− mice, although they did result in delayed thrombus formation after FeCl3 injury.21 Similarly, antibody-mediated inhibition of the interaction between VWF and collagen abolished thrombus formation in a baboon model of mechanical arterial injury.22 Our results indicate that isolated defects in the A3 domain are associated with abnormal hemostatic function in vivo in the presence of a normal A1 domain.
Identification of these mutations in patients with VWD can give useful insights into the structural requirements for the interaction between VWF and collagen. Figure 2 shows the positions of the 3 mutations identified in the families on a crystal structure of the VWF A3 domain complexed with an antibody that prevents the binding of collagen. This model indicates that the A3 domain is a rectangular hexahedron with the stabilizing C1686-C1872 disulphide bond on one surface. The putative collagen-binding surface is located on an adjacent side of the domain formed by 3 loops.14,18,23 It appears that the residues at the surface of the binding site form a shallow groove that can accommodate the triple helix of collagen microfibrils.
W1745 is in loop β3α2 in the center of the RU5 antibody-binding surface. The replacement of the large hydrophobic residue W1745 by a much smaller cysteine residue probably influences domain structure by affecting local packing and topology. The similar defect in collagen binding seen with W1745A indicates that it is not the result of the provision of an alternative position for aberrant disulphide bond formation. Further consideration of the crystal structure of the VWF A3 domain showed that W1745 may participate in a hydrophobic interaction with Y1780, and we note that a previous study demonstrated that the mutation Y1780A also significantly reduced collagen binding.18 To test this hypothesis, we expressed rVWF with the mutation W1745F; and as predicted, this did not significantly affect collagen binding (data not shown), supporting the hypothesis that W1745 and Y1780 participate in an internal aromatic interaction that helps to maintain the structural configuration of the A3 domain. The effect of W1745 on collagen binding was assessed by Vanhoorelbeke et al with a separate monoclonal antibody (82D6A3) that also binds to the A3 domain and inhibits collagen binding.24 In their study, it did not appear that W1745C formed part of the epitope recognized by the antibody but interacted with residues in that epitope via its side chain. Thus, it appears that mutation at W1745 might affect collagen binding by both direct and indirect mechanisms.
S1783 is in loop α3β4, which also contains H1786. Mutation of the residues in these loops to alanine (S1783A and H1786A) has previously shown them to be critical residues that abolish collagen binding.17 H1786 is expected to make direct contact with collagen but S1783 is not (Figure 2),18 so its effect is probably via perturbation of the loop as a whole. As serine and alanine differ only in the replacement of a hydroxyl group by a hydrogen in the side chain, this demonstrates that even minor changes in conformation of the loop cannot be easily tolerated. S1731 is part of a β-sheet that runs through the center of the domain and is not solvent exposed. This residue probably does not make direct contact with collagen, and any effects on collagen binding are probably via a secondary effect on the surface of the A3 domain. Secondary effects of this nature might well be more subtle and less deleterious than changes in residues directly involved in ligand binding, particularly as serine and the residue to which it is mutated in this case, threonine, have similar side chains.
The defects observed in this study would have been missed had collagen-binding function not been analyzed. Without the collagen-binding assay, these patients would have been misclassified with type 1 VWD (subjects 1A and 1B) or the diagnosis of VWD might have been missed altogether (subjects 2D and 2E). In our laboratory, the PFA-100 is not used in the diagnosis of VWD as previous studies have demonstrated its limitations as a screening tool.25,26 It may be used to monitor the response to therapy in selected patients as was seen in this study. The requirement for collagen in the PFA-100 cassettes suggests that this could be a sensitive test for defects of collagen binding. However, the normal closure times obtained in subjects 1B and 2D indicate that this is not the case. Thus, correct identification of these mutations requires the use of a collagen-binding assay in the initial diagnostic assessment of all patients with possible VWD. Furthermore, the preferential binding to specific collagen types shown with some of the defects raises the question as to what type(s) of collagen should be used in the laboratory measurement of collagen binding. Previous studies have shown that laboratories use a wide variety of different collagen types in the VWF:CB assay and that this has a significant effect on the result.27 Collagen-binding assays are useful in the detection of HMW multimers, and it has been suggested that this is best achieved using a mixture of type I (95%) and type III (5%) collagen.28 Although the use of a single type or source of collagen may be sufficient for detecting abnormalities in multimer distribution, this approach may miss clinically important defects affecting affinity at the collagen-binding site. The issues of which collagen type is the most important in the clinical setting and whether mixing collagen types in a single assay is appropriate for detecting loss of affinity at the collagen-binding site remain unclear. Further evaluation of naturally occurring mutations with correlation to clinical phenotype is therefore required.
Collagen-binding assays are sometimes used as an alternative to multimer analysis because they are particularly sensitive to the absence of high-molecular-weight multimers. In this study, multimer pattern was normal both in plasma samples and in expressed proteins. ELISA studies with expressed proteins showed that the defective collagen binding was caused by reduced affinity to collagen. An explanation for this observation was provided by modeling of the mutants in the crystal structure of the A3 domain, which showed that they were involved in the putative collagen-binding site. These findings demonstrate that abnormalities in collagen binding require further investigation with multimer and genetic analysis.
In the recently updated ISTH SSC VWD classification, isolated collagen-binding defects are included within the type 2M subgroup.29 The impairment of the VWF-dependent adhesion of platelets to the endothelium in type 2M VWD occurs in the presence of a full range of VWF multimers. This is a key characteristic of the 2M subtype, which is also a feature of the mutants described here. However, there are 3 important differences with the 2M group. First, type 2M mutations are clustered in the A1 domain (residues 1260-1471) of VWF, whereas these mutations affecting collagen binding are found in A3. Second, 2M is characterized by impaired binding to platelet GpIbα as shown by a reduced ratio of RCo:Ag. All 8 of the persons in this study with collagen-binding mutations had normal RCo:Ag ratios with no evidence of a qualitative defect in GpIb binding. Finally, one of the aims of the revised ISTH recommendations was to correlate VWD phenotype with clinical observations. An important feature of the 2M phenotype for clinical practice is that it tends to be associated with a poor functional response to DDAVP. The 2 patients in this study who received DDAVP demonstrated good functional responses. This included a rise in VWF:CB, which was the result of an overall increase in the amount of circulating VWF. The VWF secreted in response to DDAVP continued to show a qualitative defect in collagen binding as shown by a persistence of the abnormal CB:Ag ratio. Thus, there are important genotypic, phenotypic, and therapeutic differences between the mutations described here and those previously included in the type 2M subgroup. The logical conclusion of these findings is to recommend that the classification of VWD is altered to acknowledge these differences. However, before doing so, it is important to consider whether these mutations have a significant impact in clinical practice. It is clear from the increased bleeding scores in 2 of the pedigrees that defects at the collagen-binding site are associated with clinically significant bleeding symptoms. Therefore, we propose that these defects are classified as a distinct subtype, which may be designated type 2CB and characterized by defective collagen binding in the presence of normal multimer formation and normal GpIb binding. Recognition of this subtype will require the incorporation of collagen-binding assays in the first-line diagnostic assessment of patients with suspected VWD.
In conclusion, this study demonstrates that mutations in the A3 domain of VWF causing reduction in collagen binding, independent of multimer composition, may give rise to clinically significant bleeding symptoms. The severity of the defect varies according to the specific mutation and is probably modified by other factors. This type of VWD is currently included in the 2M subtype but has important differences compared with other mutations in this group. The use of VWF:RCo as the only functional assay will not detect this abnormality. It is therefore probable that defects at the collagen-binding site are underdiagnosed, and we propose that these defects are classified as a distinct subtype of VWD.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We gratefully acknowledge the contribution of Dr V. Andrews, Medway Maritime Hospital, Gillingham, United Kingdom, the referring physician for one of the families.
This work was supported by the National Institute for Health Research Biomedical Research Centre Funding Scheme.
Authorship
Contribution: A.F.R., T.A.J.M., G.M., S.G., and M.S. obtained laboratory data; K.G., C.M.M., and S.A.B. obtained clinical data; and K.G., A.F.R., M.A.L., C.M.M., and T.A.J.M. wrote the report. All authors were involved in analyses and interpretation of the results; read, commented on, and approved the final version of the manuscript; and had full access to the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Keith Gomez, Katharine Dormandy Haemophilia Centre and Thrombosis Unit, The Royal Free and University College Medical School, Royal Free Campus, Rowland Hill St, Hampstead, London NW3 2PF, United Kingdom; e-mail: kgomez@medsch.ucl.ac.uk.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal