Abstract
Molecular monitoring in chronic myeloid leukemia (CML) is a powerful tool to document treatment responses and predict relapse. Nonetheless, the proliferation of clinical trials and “guidelines” using the molecular endpoints of CML has outpaced practice norms, commercial laboratory application, and reimbursement practices, leaving some anxiety (if not confusion and despair) about molecular monitoring in the day-to-day treatment of CML. This article will try to address these issues by describing how I monitor CML, which, in summary, is with interest and without panic.
Introduction
The study and treatment of chronic myeloid leukemia (CML) have led to several pivotal advances in translational medicine. CML was the first disease where a single chromosomal abnormality, the Philadelphia chromosome (Ph),1 was demonstrated as fundamental to the etiology of the disease. CML was one of the first and best success stories for allogeneic transplantation,2 and it was in this context that molecular monitoring of so-called “minimal residual disease” by sensitive reverse-transcribed polymerase chain reaction (RT-PCR) techniques was found to be predictive of future relapse.3-5 Next came the advent of targeted tyrosine kinase inhibitor (TKI) therapy, which has quickly replaced transplantation as front-line therapy for chronic-phase disease. Given the power of molecular monitoring in the transplantation setting, molecular monitoring was used in the TKI trials as a measure of disease response, and such monitoring is now advocated for the routine clinical care of CML.
However, there is some controversy and confusion about the merits and use of molecular monitoring: what test, how often, and what to do with the results? To put these questions into perspective, let us first summarize what we know about CML, imatinib (IM), other TKI therapy, and monitoring. A glossary of response criteria is shown in Table 1, and the different types of monitoring assays are listed in Table 2.
Methods to detect minimal residual disease in CML
| Method . | Target . | Sensitivity, percentage . | Advantages . | Disadvantages . |
|---|---|---|---|---|
| Morphology | Cellular morphology | 5 | Standard | Poor sensitivity |
| Cytogenetics | Chromosome structure | 1-5 | Widely available | Low sensitivity, bone marrow only |
| FISH | Specific genetic marker(s) | 0.1-5 | Fast (1-2 days) | Does not look for other clonal events |
| QPCR | RNA sequence | 0.001-0.01 | Very sensitive | Poor standardization, laboratory-intensive |
| Method . | Target . | Sensitivity, percentage . | Advantages . | Disadvantages . |
|---|---|---|---|---|
| Morphology | Cellular morphology | 5 | Standard | Poor sensitivity |
| Cytogenetics | Chromosome structure | 1-5 | Widely available | Low sensitivity, bone marrow only |
| FISH | Specific genetic marker(s) | 0.1-5 | Fast (1-2 days) | Does not look for other clonal events |
| QPCR | RNA sequence | 0.001-0.01 | Very sensitive | Poor standardization, laboratory-intensive |
Response criteria in CML
| Level of response . | Definition . |
|---|---|
| Complete hematologic response | Normal complete blood count and differential |
| Minor cytogenetic response | 35%-90% Ph+ metaphases |
| Partial cytogenetic response | 1%-34% Ph+ metaphases |
| Complete cytogenetic response | 0% Ph+ metaphases |
| Major molecular response | ≥ 3-log reduction of BCR-ABL mRNA |
| Complete molecular remission | Negativity by QPCR |
| Level of response . | Definition . |
|---|---|
| Complete hematologic response | Normal complete blood count and differential |
| Minor cytogenetic response | 35%-90% Ph+ metaphases |
| Partial cytogenetic response | 1%-34% Ph+ metaphases |
| Complete cytogenetic response | 0% Ph+ metaphases |
| Major molecular response | ≥ 3-log reduction of BCR-ABL mRNA |
| Complete molecular remission | Negativity by QPCR |
Note that all cytogenetic response categories require the analysis of at least 20 metaphases.
1. TKI therapy is remarkably effective
More than 90% of patients in chronic phase will obtain a hematologic remission; the majority of patients (∼ 80%) who are treated in chronic phase achieve a complete cytogenetic remission (CCyR), and those that achieve a CCyR have an excellent survival (∼ 90%).6 Approximately 60% of patients treated with IM on the International Randomized Study of Interferon versus STI-571 (IRIS) trial remain in CCyR on IM after 6 years of therapy.7
2. Still, some patients become resistant and progress
A minority of chronic-phase cases may demonstrate primary resistance to IM therapy, become resistant to therapy after an initial response, or progress to advanced-phase disease. Relapse often stems from a point mutation in the Abl tyrosine kinase domain, which affects TKI binding and Abl inhibition. It appears that the number of patients who progress to advanced-phase disease has declined over the follow-up period of the IRIS trial, suggesting that chronic-phase patients treated early enough in their disease course may have the molecular events responsible for progression effectively blocked.7
3. Imatinib resistance can be effectively treated
In cases of inadequate primary responses, loss of response, or progression, other treatment alternatives, such as second-generation TKIs (dasatinib or nilotinib), or allogeneic transplantation, are useful. For example, CCyR can be achieved in approximately 50% of patients with IM resistance, and transplantation is effective in more than 75% of chronic-phase cases.8 Therapy with any modality is less successful for those patients in advanced-phase disease compared with chronic phase, forming the logic for early detection of impending relapse and progression.
4. Monitoring endpoints correlate with long-term outcomes
The achievement of a CCyR is a treatment endpoint clearly highly correlated with long-term outcome. Molecular monitoring using PCR strategies is a more sensitive assay that can measure the depth of disease burden in CCyR cases and may identify patients at higher risk of resistance.
In sum, the majority of chronic-phase CML cases will do great on IM therapy; a proportion of patients will become resistant; approximately half of those will obtain a CCyR with second-generation TKI, although the durability of these responses is unclear. Given that all therapies for CML work better in chronic- rather than advanced-phase disease, early identification of relapse or progression could increase the chance that alternative treatments will be effective.
Monitoring methods and response definitions
The unique t(9;22) reciprocal translocation forming the Ph chromosome forms the basis of monitoring in CML. Because all CML cells will harbor the Ph, but normal hematopoietic cells should not, the Ph is a unique genetic fingerprint for CML detection. Conventional metaphase cytogenetics looks for the Ph and can detect other chromosomal changes associated with advanced-phase disease (Table 1). Molecular cytogenetics using fluorescence in situ hybridization (FISH) is a more sensitive method to detect the fusion BCR-ABL gene and has the advantage of routinely interrogating 50 to 200 metaphase or interphase cells, although additional chromosomal changes cannot be detected unless specific probes for those abnormalities are added to the FISHing trip. The most sensitive approach to detect CML is the RT-PCR of the chimeric BCR-ABL mRNA, which can detect one CML cell in approximately 100 000 to 1 million cells. The assay has well-documented pitfalls, mostly revolving around the complexity and the fact that there is little standardization across laboratories. On an extremely positive note, peripheral blood can be used instead of bone marrow for monitoring.
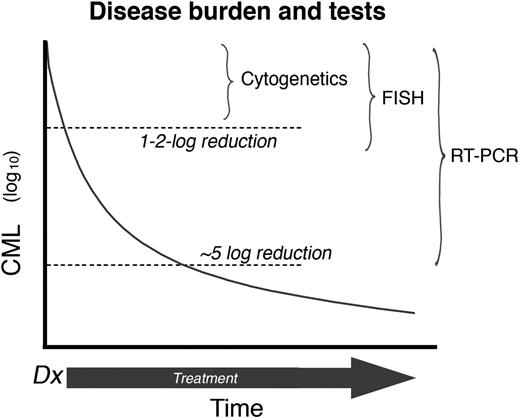
In a newly diagnosed CML patient, TKI therapy progressively reduces the disease burden. Therefore, as the number of leukemia cells decrease, the sensitivity of the techniques used to effectively monitor the disease must increase accordingly (Figure 1). Hematologic response, defined as the normalization of peripheral blood counts, is the first level of response observed with IM treatment (usually within 1-3 months after the start of treatment in chronic-phase CML) and is the earliest monitoring point. The next level of determination is a cytogenetic response, which is determined by bone marrow metaphase chromosome analysis (using at least 20 metaphases). The level of cytogenetic response is based on the number of Ph+ metaphases: complete (CCyR; no Ph+ metaphases), partial (PCyR; 1%-34% Ph+ metaphases), or minor (35%-90% Ph+ metaphases). After a CCyR, residual leukemia can be detected using an RT-PCR assay. Molecular responses are defined by the magnitude of reduction in BCR-ABL transcripts from a standardized value (rather than a single patient's original level).9 A major molecular response (MMR) is defined as a more than 3-log reduction in BCR-ABL/control gene ratio. The criteria for monitoring patients receiving TKIs are summarized in the European LeukemiaNet and National Comprehensive Cancer Network guidelines.10,11
Disease burden and tests. The reduction of CML burden and the sensitivity of assays (plot not to scale). Thus, routine cytogenetics will fail to detect the Ph (a CCyR) after a 1 to 2 log reduction in CML burden. The detection limit of RT-PCR is approximately a 5 to 6 log reduction of disease burden. Professional illustration by Marie Dauenheimer.
Disease burden and tests. The reduction of CML burden and the sensitivity of assays (plot not to scale). Thus, routine cytogenetics will fail to detect the Ph (a CCyR) after a 1 to 2 log reduction in CML burden. The detection limit of RT-PCR is approximately a 5 to 6 log reduction of disease burden. Professional illustration by Marie Dauenheimer.
The clinical significance of monitoring
In treating CML, we have these “ground truths”: (1) very effective first-line TKI therapy for chronic-phase disease, (2) effective second-line therapy for those who cannot tolerate or have a suboptimal response to initial therapy, (3) sensitive measures to monitor disease, and (4) published guidelines that suggest what are reasonable treatment milestones and monitoring strategies. What could go wrong?
There are several excellent manuscripts that detail responses to TKI and the rationale for monitoring.10,12-16 Rather than repeat the content of these fine papers, I will instead focus on my views of monitoring in CML using examples of frequently asked questions (FAQs) posed to me by phone, e-mail, or while waiting in the interminably long Starbucks line at the American Society of Hematology annual meeting.
FAQ 1: Why do I need to perform a bone marrow aspirate at diagnosis?
It is tempting to simply diagnose CML based on clinical features, a peripheral blood white blood cell count, and a peripheral blood PCR or FISH assay. It is true that a patient who initially presents without much in the way of constitutional symptoms, peripheral blasts, splenomegaly, etc, will probably be in chronic phase. However, given that TKI therapy is far more effective in chronic- than advanced-phase disease and that transplantation would be a definite consideration for a patient with advanced-phase disease, it is prudent to have a firm staging before initiating therapy, and this includes a bone marrow for morphology and cytogenetics. Although it is tempting to use FISH as the only measure of CML, FISH is limited because it will only identify the Ph, but not other cytogenetic lesions that suggest advanced-phase disease.
FAQ 2: I have a patient with a normal white blood cell count after several months of therapy. Is there any reason to get another bone marrow?
Cytogenetic response provides the strongest measure of treatment success. For example, cytogenetic response after 6 months of therapy is strongly associated with the probability of subsequently achieving a CCyR by 2 years of therapy. Patients with no response, minor response, or partial cytogenetic response had a 15%, 50%, and 80% chance, respectively, of obtaining a CCyR after 2 years of imatinb therapy in the IRIS trial of newly diagnosed CML.17 At the 12-month mark, patients with either no or minor cytogenetic response have less than 20% chance of eventually achieving a CCyR, compared with 50% for those with a partial cytogenetic response. Information from a bone marrow within the 6-month mark is useful for predicting long-term response and treatment planning.
There is some controversy in regard to the optimal timing to achieve a CCyR. Some analyses suggest that patients who take 18 months to reach a CCyR enjoy the same outcome as those patients who obtain a CCyR at 12 months.18 However, this observation does not help the clinician or patient without a CCyR at the 12-month mark. Consider this analogy: imagine a contest where swimmers of various abilities are asked to swim across a shark-infested pool. The “time doesn't matter” argument would point out that the swimmers that made it across the pool in one piece have similar outcomes from the time they leave the pool, no matter whether they are fast or slow swimmers. However, this argument ignores the ugly fate of the slow swimmers who don't manage to make it across the pool. Similarly, comparing the outcome of patients who obtain an early CCyR with those who obtain a late CCyR ignores the fate of the patients trying to get to a late CCyR response, who may progress on the way.
FAQ 3: Why should I monitor after CCyR?
The science and art of molecular monitoring by quantitative PCR (QPCR) are continuously undergoing fine-tuning, given the well-documented issues of assay variability, needs for standardization, and the development of new, better, faster assays. None of these potential limitations substantially undermines the potential clinical utility of QPCR. For all the potential caveats, the measure of BCR-ABL transcript by QPCR is a very sensitive and powerful measure of disease burden. The level of QPCR is especially important in cases that have achieved a CCyR. In the IRIS trial, patients who did not achieve a CCyR had a risk of progression of approximately 25% compared with patients who achieved a CCyR, and had a less than 3-log reduction or more than 3-log reduction in BCR-ABL by 12 months. At 54 months of follow-up, progression-free survival for patients who never achieved a CCyR, had a less than 3-log reduction in BCR-ABL at 12 months, or had a more than 3-log reduction at 12 months, was 72%, 89%, and 97%, respectively.9 A 5-year update of the data confirms the excellent prognosis associated with an MMR.18 For patients who achieved CCyR and a MMR at 18 months, no patient progressed to accelerated or blast phase by 60 months of follow-up. The rate of progression for those that had a CCyR and a less than 3 log reduction in BCR-ABL was only 3%. Subsequent studies have confirmed the IRIS PCR data and demonstrate that patients with a deeper molecular response at the time of initial CCyR, or a more than 3-log reduction of BCR-ABL during CCyR, have very low odds of progression and a superior progression-free survival compared with patients with an inferior response.19-23
Early monitoring after starting IM therapy may also be useful in predicting response. The rate BCR-ABL decline in the initial 2 to 3 months of IM is a strong predictor of subsequent response, as patients with less than 1-log reduction after 3 months had a 13% probability of ever achieving a MMR after 2.5 years of follow-up, compared with more than 70% in patients with more than 1-log response.19 Cortes et al found that patients who have a less than 1-log reduction after 3 months of imatinib therapy had a 55% chance of ever achieving a MMR at 2 years, compared with those with a more than 1-log or more than 2-log reduction, in whom a MMR was achieved in 84% and 95%, respectively.20
Thus, QPCR of BCR-ABL is a very convenient and powerful method to forecast future response to TKI in chronic-phase patients.
FAQ 4: What should I do with a rising BCR-ABL PCR?
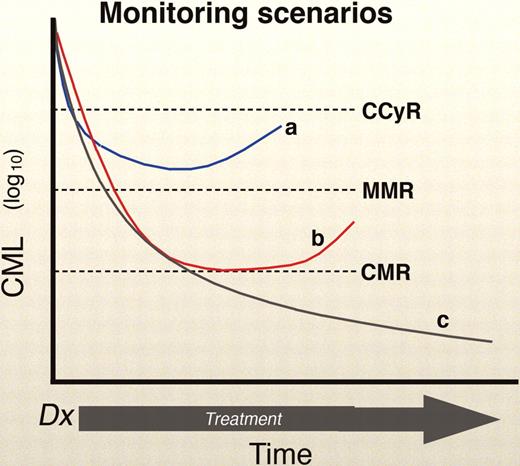
The BCR-ABL QPCR may rise in a patient for a number of reasons. One possibility relates to compliance, especially in the context of an expensive drug and a patient with a good molecular response (a situation where the temptation to enjoy a “drug holiday” is strong). Second, the assay may “wobble” because of sampling error (especially when the patient enjoys a very low tumor burden) or the variability in the test itself. In most laboratories, however, a 5- to 10-fold change in the QPCR is probably “real.” However, it is not known how BCR-ABL levels vary in patients naturally over time while on TKI therapy. CML is known to have cyclic oscillations, with peaks and troughs occurring at even 1- to 2-month intervals, and this has not been studied in cases with residual disease.24-26 Of course, the last and most worrisome, possible explanation for an increase in BCR-ABL is impending relapse (Figure 2).
Monitoring scenarios. The “a” curve shows a patient in a CCyR, but without a MMR, with a rising BCR-ABL. This is a worrisome case that demands close follow-up (Figure 3). Curve “b” show a patient with a CCyR and a MMR, with a rising BCR-ABL. This case requires follow-up, but not necessarily major concern, unless the rise continues, especially with a resistant mutation. Lastly, curve “c” shows a luck patient with the best of circumstances: a CCyR, MMR, and a stable or declining BCR-ABL. Professional illustration by Marie Dauenheimer.
Monitoring scenarios. The “a” curve shows a patient in a CCyR, but without a MMR, with a rising BCR-ABL. This is a worrisome case that demands close follow-up (Figure 3). Curve “b” show a patient with a CCyR and a MMR, with a rising BCR-ABL. This case requires follow-up, but not necessarily major concern, unless the rise continues, especially with a resistant mutation. Lastly, curve “c” shows a luck patient with the best of circumstances: a CCyR, MMR, and a stable or declining BCR-ABL. Professional illustration by Marie Dauenheimer.
There are several lines of evidence suggesting that a truly rising BCR-ABL deserves concern. First, several studies have shown that a rising BCR-ABL is associated with a greater increase of the acquisition of an Abl point mutation and resistance.27-29 In addition, loss of MMR is associated with an increased risk of relapse and a lower disease-free survival.28,30 Nonetheless, not all patients with a rise in BCR-ABL, or a detectable mutation, inevitably relapse.31-33 So, in the face of a rising BCR-ABL, does one ignore it, panic, or do something in between?
The latter strategy is the most practical and healthy option for both patient and doctor. A reasonable first action is to repeat the test, eg, in a month. If it is still increased (or increasing), then mutation testing should be undertaken (see “FAQ 5”). The next response depends on how high the BCR-ABL level has risen. For example, a rise from the lowest levels of detection (0.0001%) to a value even 50 times higher would still be well with the range of a MMR. However, a patient who begins at the MMR and rises above that level is certainly heading toward cytogenetic relapse, and here a bone marrow aspirate looking for cytogenetic reoccurrence would be warranted.
FAQ 5: When should I do mutation testing (and what do I do with the answer)?
Despite the success story of IM in chronic-phase CML, resistance to IM still occurs and indeed is the rationale behind frequent monitoring. A large proportion of these relapses are secondary to point mutations in the Abl gene, which blunt the ability of IM to inhibit the aberrant BCR-ABL kinase activity.20-23 A few facts about Abl mutations put mutation screening in context. First, the prevalence of Abl mutations increases with the “disease time”: that is, rare in newly diagnosed chronic-phase and increasing with late chronic-phase and advanced-phase disease (ie, with increasing Sokal score).27-29 Thus, Abl mutations occur as part of the natural history of CML, rather than a merely a manifestation of selective pressure from TKI therapy. Several studies have demonstrated that these mutations are associated with both an increase in loss of cytogenetic response and progression to advanced-phase disease.27-29 However, in some cases, particularly in those patients with a low disease burden, mutations can be detected, yet remain at a low level and do not cause problems.
The screening of mutations is limited by the sensitivity of the available assays. QPCR is uniquely sensitive because it is amplifying a chimeric mRNA not found in normal cells. The detection of a single point mutation in the tyrosine kinase domain of BCR-ABL against a background of wild type BCR-ABL is obviously a much more difficult task. The most common method of direct nucleotide sequencing can detect an Abl tyrosine kinase domain mutation if it composes 10% to 20% of the total BCR-ABL sampled population. Other assays done in the research setting can improve the sensitivity by 10-fold or more.
The relatively poor sensitivity of these assays makes it difficult to identify point mutations early in the course of therapy. The routine screening of chronic-phase patients with a CCyR will rarely (< 5%) detect a mutation; and although the subsequent risk of relapse in these rare patient is approximately 4-fold higher than those without mutation, such screening appears rather costly for the potential benefit. However, frequent monitoring by QPCR can detect populations at a higher risk for relapse and mutation. Branford et al showed that 61% of patients with a more than 2-fold increase in BCR-ABL had detectable mutations compared with 0.6% of patients with stable or decreasing BCR-ABL.27 The caveat here is that few laboratories can equal this degree of precision; moreover, one should use caution and reason concerning the “2-fold” rule because an increase from a QPCR-negative status to a level of 0.0001% would be an infinite increase in BCR-ABL but would should not cause much worry. Thus, screening for mutations would be reasonable in any advanced-phase patient, chronic-phase patients not achieving cytogenetic milestones, and patients with rising BCR-ABL, especially those nearing or passing the MMR level.
It is doubtful that a continuously rising BCR-ABL would suddenly reverse course without some sort of remedy. It is also true that there is no evidence that early intervention of a rising BCR-ABL, before cytogenetic relapse, alters the long-term outcome of the patient. This issue is being pursued in clinical trials. Lastly, chronic-phase patients who develop a mutation have an increase risk of progression and, indeed, have many molecular characteristics similar to advanced-phase disease. Thus, on the practical side, a steadily rising BCR-ABL with an accompanying mutation that predicts relative or absolute indifference to IM (eg, T315I or E255V) can reasonably trigger a change in treatment strategy, even before the patient crosses into cytogenetic relapse (Figure 3). At the very least, patients with rising BCR-ABL levels and mutations need a discussion about the availability of other tyrosine kinases, both approved and in trial; in addition, human leukocyte antigen typing of the patient and family should be started, and referral to a transplantation specialist might be a fine idea to begin contingency planning.
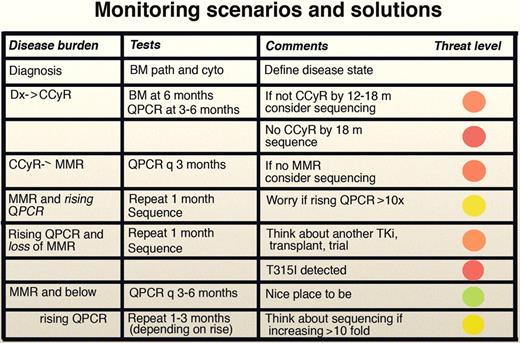
Monitoring scenarios and solutions. The concept of the “threat level” is adopted from Homeland Security. Thus, red is the most worrisome, followed by orange, yellow, and green. Professional illustration by Marie Dauenheimer.
Monitoring scenarios and solutions. The concept of the “threat level” is adopted from Homeland Security. Thus, red is the most worrisome, followed by orange, yellow, and green. Professional illustration by Marie Dauenheimer.
FAQ 6: How often do I need to test? And until when—forever?
The set guidelines of the European LeukemiaNet and the National Cancer Care Network suggest peripheral blood testing every 3 months for QPCR.10,11 On a practical side, however, if a patient has been in a MMR (or, better yet, a complete molecular remission) for months, one can probably get away with testing every 6 months. If there is a significant change in BCR-ABL level (negative to positive, or an increase in > 2- to 5-fold in patients with detectable disease), then moving back to more frequent testing is prudent.
FAQ 7: These PCR tests are hard to understand and compare. When are things going to get better?
QPCR tests for BCR-ABL suffer from a lack of standardization. Minor and major technical differences may occur from laboratory to laboratory; the use of a ratio to report the result (BCR-ABL transcript number/control gene) complicates matters because various laboratories use various control genes (hence, the denominator, and thus the ratio, change). Thus, the same sample may get different ratios at different laboratories. This is especially problematic in the United States, where many laboratories are available, laboratory use may vary as insurance contracts are changed, etc. Moreover, there are several concepts of BCR-ABL reporting that are often misunderstood. The near-mythical concept of the MMR was originally described as a 3-log reduction in BCR-ABL level from a median level of an aggregate of diagnostic samples. Thus, each laboratory's (and each clinical study's) MMR value may vary because it will be based on a different population. Thankfully, there is a movement to standardize BCR-ABL testing and reporting; a standardized International Scale has been proposed by the National Institutes of Health Consensus Group, which uses the baseline values defined in the IRIS trial to represent 100% and fixes a 3-log reduction from the standardized baseline (MMR) at 0.10%.15
Another complicated issue is the issue of a negative test. Obviously, on the surface, one would rather be negative for BCR-ABL than positive, but negative QPCR assays can result from (1) a relatively insensitive assay, (2) a degraded sample, or (3) actual disease burden below the level of detection, with an adequate sample. Only the last should be greeted with enthusiasm. The implications of a “complete molecular remission,” defined by the negative QPCR in a sensitive assay, with strict controls as to RNA quality, is currently under investigation in clinical trials.
In conclusion, the treatment of CML has undergone a radical change in a very short time. Only a decade ago, patients with the disease would be ushered to transplantation ASAP. With the success of IM, some clinicians have flipped all the way to the most nihilistic treatment strategies, using the “fire and forget” approach of simply giving IM, and occasionally after peripheral blood counts, hoping for the IM to do its magic. A more enlightened approach can use molecular testing to quickly gauge treatment efficacy, predict outcomes, and plan contingencies. Doctors and patients should view monitoring as a sensitive gauge that helps to develop plans and amend them, rather than a simple “idiot light” that reads green (calm) or red (panic). CML has led the way in many facets of leukemia translational medicine, and the development of molecular monitoring is just the latest in that trend. Molecular monitoring, warts or not, is a powerful tool that is here to stay in CML, and what researchers, clinicians, and patients learn here will soon be incorporated in the strategies of other leukemia treatments.
Acknowledgments
First and foremost, I apologize for any blunders or oversights in the paper. Any errors are entirely mine. On the other hand, I want to acknowledge the efforts of my friends and colleagues, near and far, whose work formed the basis of any value of the manuscript.
Authorship
Contribution: J.P.R. was the sole author of this paper.
Conflict-of-interest disclosure: J.P.R. has served on advisory boards for Novartis and BMS and has received laboratory research contracts from both companies.
Correspondence: Jerald P. Radich, Clinical Research Division, Fred Hutchinson Cancer Research Center, 1100 Fairview Ave N, Seattle, WA 98109; e-mail: jradich@fhcrc.org.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal