Abstract
Vascular endothelial growth factor (VEGF) is a potent angiogenic cytokine that also increases vascular permeability. Nitric oxide (NO) released from endothelial cells, after activation of endothelial NO synthase (eNOS), contributes to proangiogenic and permeability effects of VEGF. Angiopoietin-1 (Ang-1), via Tie2 receptors, shares many of the proangiogenic properties of VEGF on endothelial cells. However, in contrast to VEGF, Ang-1 protects blood vessels from increased plasma leakage, which contributes to their stabilization. Because eNOS-derived NO is central to increased permeability in response to VEGF, we investigated whether Ang-1 interferes with VEGF signaling to eNOS. We demonstrate that Ang-1 stimulation of endothelial cells inhibits VEGF-induced NO release and transendothelial permeability. In contrast to VEGF stimulation, Ang-1 causes a marked protein kinase C (PKC)–dependent increase in phosphorylation of eNOS on the inhibitory Thr497. Furthermore, using pharmacologic inhibitors, overexpression studies, and small interfering RNA-mediated gene silencing, we demonstrate that atypical PKCζ is responsible for phosphorylation of eNOS on Thr497 in response to Ang-1. In addition, PKCζ knockdown abrogates the capacity of Ang-1 to inhibit VEGF-induced NO release and endothelial permeability. Thus, inhibition of NO production by Ang-1, via phosphorylation of eNOS on Thr497 by PKCζ, is responsible, at least in part, for inhibition of VEGF-stimulated endothelial permeability by Ang-1.
Introduction
Nitric oxide (NO) produced by endothelial cells modulates many of the important proangiogenic properties of vascular endothelial growth factor (VEGF). Indeed, NO released from endothelial cells in response to VEGF stimulation, via activation of VEGF receptor-2 (VEGFR-2), is an essential regulator of postnatal angiogenesis. Direct support for the role of endothelial NO synthase (eNOS) in increased blood vessel permeability in response to VEGF was provided by the fact that eNOS-deficient mice display reduced albumin extravasation after subdermal VEGF administration.1 Similarly, blood vessels of tumors implanted onto eNOS-deficient mice are less leaky than vessels from tumors on wild-type (WT) animals.2 After VEGFR-2 activation, eNOS activity is increased by PI3-kinase–dependent activation of Akt, which results in phosphorylation of eNOS on Ser1177 (1179 in bovine eNOS) and leads to increased eNOS activity and NO release.3-5 Simultaneously, dephosphorylation of Thr497 of bovine eNOS is thought to release the inhibitory modulation afforded by basal phosphorylation of this residue.6-8 Protein kinase C (PKC) activity, possibly through PKCα,9 is thought to be responsible for basal phosphorylation of the Thr497 residue, and therefore, maintains eNOS in a less active state. However, it has also been suggested that the PKC isoforms α, δ, and ϵ are upstream of Akt activation and eNOS phosphorylation on Ser1177.10-12 Together, these posttranslational modifications contribute significantly to the positive and negative regulation of eNOS activity and consequently to NO release from endothelial cells after exposure to VEGF.
Angiopoietin-1 (Ang-1), through activation of the tyrosine kinase receptor Tie2, is also proangiogenic and leads to the stabilization of blood vessels, which is associated with decreased vascular permeability.13-16 Interestingly, Ang-1 and VEGF play separate, but essential roles in embryogenesis: VEGF acts early in vascular development by contributing to the formation of the initial vascular plexus, whereas Ang-1 acts during the subsequent remodeling of the vasculature into a hierarchical network of mature vessels.17 Consistent with their established roles in angiogenesis, VEGF and Ang-1 share common signaling pathways that lead to survival, proliferation, and migration of endothelial cells, but play antagonistic roles in the regulation of endothelial permeability.18-21
The cellular mechanisms by which Ang-1 counteracts increased transendothelial permeability stimulated by VEGF are still being defined. In the present study, we identify a clear relationship between inhibition of NO release by Ang-1 and inhibition of in vitro endothelial permeability stimulated by VEGF. We delineate a specific molecular mechanism in which atypical PKCζ is required downstream of Tie2 to phosphorylate eNOS on Thr497, which inactivates the enzyme, abrogates NO production, and inhibits endothelial permeability stimulated by VEGF.
Methods
Cell culture
Bovine aortic endothelial cells (BAEC) and bovine lung microvascular endothelial cells (BLMVEC), obtained from VEC Technologies, were cultured in Dulbecco modified Eagle medium (DMEM) supplemented with 10% fetal bovine serum (HyClone), 2.0 mM l-glutamine, 100 U/mL penicillin, and 100 μg/mL streptomycin. COS-7 cells were cultured in DMEM supplemented with 10% fetal bovine serum (Invitrogen), 2.0 mM l-glutamine, 100 U/mL penicillin, and 100 μg/mL streptomycin.
Reagents and antibodies
Recombinant human VEGF-A (VEGF in this study), obtained from the Biological Resources Branch Preclinical Repository of the National Cancer Institute Cancer Research and Development Center, and recombinant human Ang-1, obtained from R&D Systems, were used for cell stimulation throughout this study. PKC inhibitors, Bisindolylmalmeide I (Bis I), Gö6976, and myristoylated PKCζ-pseudosubstrate inhibitor were from Calbiochem. Fluorescein isothiocyanate (FITC)–labeled dextran (molecular mass 40 000 Da) was from Invitrogen. The NO synthase (NOS) inhibitor, Nω-nitro-l-arginine methyl ester hydrochloride (l-NAME) and phorbol 12-myristate 13-acetate (PMA) were from Sigma-Aldrich. The primary antibodies used were as follows: anti–VEGFR-2 (monoclonal antibody [mAb]), anti-Tie2 (polyclonal antibody [pAb]), and anti–PKCζΗ-1 (mAb) from Santa Cruz Biotechnology, and anti-eNOS (mAb) from BD Transduction Laboratories. Anti–p-Tyr783-phospholipase C (PLC)–γ1(pAb), PLC-γ1 (pAb), p-p44/42-extracellular signal–regulated kinase (Erk) (Thr202/Tyr204) (mAb), p44/42-Erk (Thr202/Tyr204) (pAb), p-Ser473-Akt (pAb), Akt (pAb), p-Thr497-eNOS (pAb), p-Ser1179-eNOS (pAb), pan-p-Ser660-PKCβ2 (pAb), p-Thr410- PKCζ (pAb), PKCα (pAb), and PKCδ (pAb) were all from Cell Signaling Technology, and anti-phosphotyrosine (4G10) was from Upstate Biotechnology.
Plasmids and transfections
Plasmids coding for bovine eNOS (in pcDNA3), human VEGFR-2 (in pRK7), and human Tie2 (in pcDNA3) used in this study were described elsewhere.22,23 Flag-PKCζ and kinase-dead PKCζ (in pCMV5) were generously provided by Dr Michel Cayouette (Institut de recherches cliniques de Montréal). Kinase-dead K855A-Tie2 was generated by site-directed mutagenesis (Stratagene) using the following forward primer: 5′-CGGATGGATGCTGCCATCGCAAGAATGAAAGAATATGCCTCC-3′. Bovine T497A and S1179D-eNOS expression plasmids were generously provided by Dr William C. Sessa (Yale University School of Medicine). T497A/S1179D-eNOS was generated using S1179D-eNOS as template and the following forward primer: 5′-GCATCACCAGGAAGAAGCCCTTTAAGGAAGTGGCC-3′. All constructs were sequenced to confirm the presence of the mutations. COS-7 cells, BLMVEC, and BAEC were transfected using Lipofectamine 2000, according to the manufacturer's protocol (Invitrogen).
Immunoprecipitations and immunoblotting
For immunoprecipitations, cells were solubilized with a lysis buffer containing 1% Nonidet P-40, 0.1% sodium dodecyl sulfate (SDS), 0.1% deoxycholic acid, 50 mM Tris (pH 7.4), 0.1 mM ethylenediaminetetraacetic acid (EDTA), 0.1 mM ethyleneglycoltetraacetic acid (EGTA), 20 mM sodium fluoride, 1 mM sodium pyrophosphate, and 1 mM sodium orthovanadate. Soluble proteins were incubated with primary antibodies (2 μg) at 4°C for 2 hours. Protein A-Sepharose (Sigma-Aldrich; 50 μL of a 50% slurry) was added and incubated for an additional hour. The immune complexes were precipitated by centrifugation, washed 3 times with lysis buffer, boiled in SDS sample buffer, separated by SDS–polyacrylamide gel electrophoresis, transferred onto a nitrocellulose membrane (Hybond ECL; Amersham), and Western blotted. Antibody detection was performed by a chemiluminescence-based detection system (ECL; Amersham) or by a LI-COR Odyssey infrared imaging system (LI-COR Biosciences) using Alexa 680– and Alexa 800–labeled secondary antibodies (Invitrogen).
NO release analysis
Cell medium was processed for the measurement of nitrite (NO2−), the stable breakdown product of NO in aqueous solution, by NO-specific chemiluminescence using a NO analyzer (NOA 280i; GE Ionics Instruments).24 For the measurement of accumulated NO production from transfected COS-7 cells, culture medium was collected 48 hours posttransfection and proteins were removed by ethanol precipitation. Agonist-stimulated NO production from BAEC and BLMVEC was performed on serum-starved cells (6 hours), 48 hours posttransfection. The medium was processed for measurement of nitrite levels 30 minutes after stimulation.
Endothelial permeability assay
Permeability across endothelial cell monolayers was measured using type I collagen–coated Transwell units (6.5 mm diameter, 3.0 μm pore size polycarbonate filter; Corning Costar). BAEC or BLMVEC cells were plated 48 hours after transfection at a density of 105 cells per well and were cultured for 3 to 4 days until the formation of a tight monolayer. Cells were serum-starved for 1 hour in DMEM containing 1% bovine serum albumin (BSA) before pretreatment with NOS inhibitors (30 minutes). VEGF (40 ng/mL) and/or Ang-1 (100 ng/mL) were added to the upper chambers in presence of 1 mg/mL FITC-labeled dextran (molecular mass: 40 kDa). Endothelial permeability was measured by collecting 50 μL sample from the lower compartment, which was diluted with 300 μL phosphate-buffered saline (PBS) and measured for fluorescence at 520 nm when excited at 492 nm with a Wallac Victor 3V spectrophotometer (PerkinElmer).
Immunofluorescence
BAEC were cultured on 0.1% gelatin-coated coverslips. Serum-starved cells were stimulated for 15 minutes with Ang-1 (100 ng/mL). Cells were then washed briefly with cold PBS and fixed for 15 minutes in PBS containing 3.5% paraformaldehyde. Cells were rinsed with PBS and permeabilized with 0.3% Triton in PBS for 5 minutes. Fixed cells were blocked with 1% BSA and then incubated for 1 hour with the primary antibodies in 0.1% BSA in PBS (mouse anti–eNOS at 1:200 and rabbit anti–p-Thr497-eNOS at 1:100). Bound primary antibodies were visualized after 1 hour of incubation using Alexa Fluor 488–labeled goat anti–rabbit (1:100) and Alexa Fluor 568–labeled goat anti–mouse (1:100; Invitrogen). Coverslips were mounted with Gel Mount (Biomeda) and observed using a Zeiss LSM 510 confocal laser-scanning microscope. Samples were viewed with a 63×/1.4 NA oil objective. Images were assembled using Adobe Photoshop CS.
Small interfering RNA–mediated gene silencing of PKCζ
PKCζ small interfering RNA (siRNA) as well as a nonsilencing control siRNA were obtained from Santa Cruz Biotechnology. The working concentration of siRNA duplexes applied was 35 μM. BLMVEC and BAEC were transfected with PKCζ siRNA or nonspecific control siRNA using Lipofectamine 2000 (Invitrogen) according to the manufacturer's instructions. Transfected cells were starved in serum-free medium (6 hours) 48 hours after transfections and then exposed to the indicated concentrations of Ang-1, VEGF, or both.
Statistical analysis
Values are reported as mean plus or minus SEM. Data were analyzed by analysis of variance, followed by Bonferroni post hoc test. A P value less than .05 was considered as statistically significant.
Results
Ang-1 prevents VEGF-induced endothelial permeability and NO release from endothelial cells
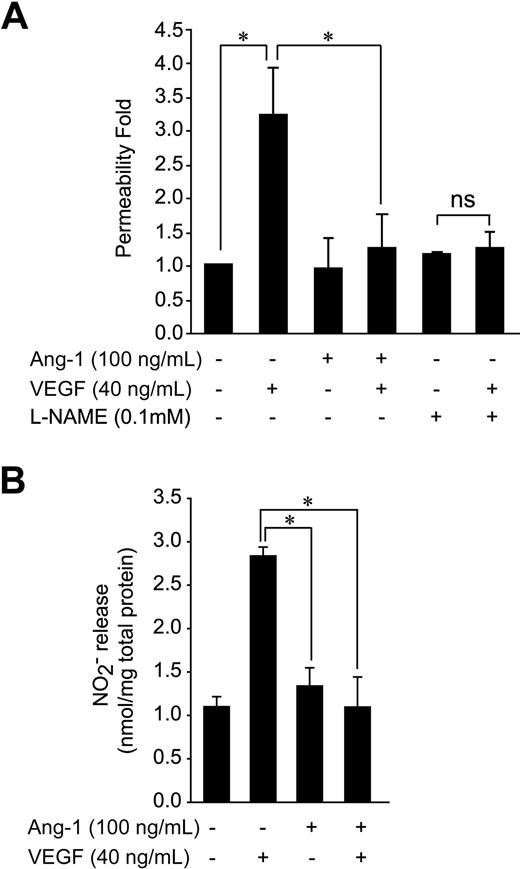
Stimulation of NO production by endothelial cells has been implicated in the induction of vascular permeability by VEGF.1 Because Ang-1 has been shown to inhibit vascular permeability stimulated by VEGF, we verified the possibility that Ang-1 decreases VEGF-induced endothelial permeability via inhibition of NO production. First, we show that the ability of VEGF to increase passage of FITC-labeled dextran (molecular mass: 40 kDa) across a monolayer of BAEC is completely blocked when cells are costimulated with Ang-1 (Figure 1A). Similarly, pretreatment of the BAEC monolayer with a NOS inhibitor, l-NAME, abolished the increase in endothelial permeability in response to VEGF (Figure 1A). To determine whether inhibition of VEGF-stimulated endothelial permeability by Ang-1 could result from an interference with NO production, we monitored the effect of Ang-1 on VEGF-stimulated NO release from BAEC. Similar to its effects on endothelial permeability, Ang-1 failed to induce NO production and, more importantly, inhibited NO production stimulated by VEGF (Figure 1B). These effects of Ang-1 on VEGF-induced endothelial permeability and NO release were also observed in cultured BLMVEC (supplemental Figure 1, available on the Blood website; see the Supplemental Materials link at the top of the online article). Taken together, these results demonstrate that Ang-1 may interfere with VEGFR-2–stimulated NO release from endothelial cells and suggest that this may contribute to the inhibition by Ang-1 of endothelial permeability stimulated by VEGF.
Ang-1 stimulation counteracts VEGF-induced transendothelial permeability and NO production. (A) Transendothelial permeability was determined by measuring the passage of FITC-dextran through a monolayer of BAEC. Passage of FITC-dextran was measured after exposure of BAEC to VEGF, Ang-1, or VEGF and Ang-1 in combination (at the indicated concentrations). In some cases, cells were pretreated with the NOS inhibitor, l-NAME, as indicated. The data represent permeability to FITC-dextran expressed as the mean fold increases (± SEM) with respect to untreated cells. (B) NO released in the culture medium of BAEC subjected to 30-minute stimulation with Ang-1 (100 ng/mL), VEGF (40 ng/mL), or both. Samples of culture medium were taken for nitrite quantification, as described in “NO release analysis.” Data are the average ± SEM of at least 4 experiments. *P < .05.
Ang-1 stimulation counteracts VEGF-induced transendothelial permeability and NO production. (A) Transendothelial permeability was determined by measuring the passage of FITC-dextran through a monolayer of BAEC. Passage of FITC-dextran was measured after exposure of BAEC to VEGF, Ang-1, or VEGF and Ang-1 in combination (at the indicated concentrations). In some cases, cells were pretreated with the NOS inhibitor, l-NAME, as indicated. The data represent permeability to FITC-dextran expressed as the mean fold increases (± SEM) with respect to untreated cells. (B) NO released in the culture medium of BAEC subjected to 30-minute stimulation with Ang-1 (100 ng/mL), VEGF (40 ng/mL), or both. Samples of culture medium were taken for nitrite quantification, as described in “NO release analysis.” Data are the average ± SEM of at least 4 experiments. *P < .05.
Ang-1 activates PKC independently of PLCγ
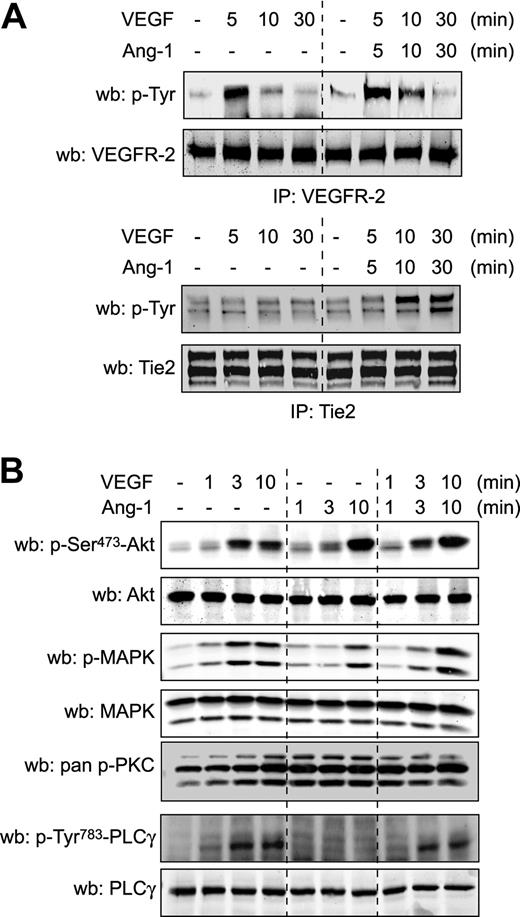
To decipher the molecular mechanisms underlying the inhibitory effect of Ang-1 against VEGF-induced NO production and vascular permeability, we examined whether VEGFR-2 activation by VEGF is altered by costimulation with Ang-1. Stimulation of endothelial cells with Ang-1, which caused tyrosine phosphorylation of Tie2 (supplemental Figure 2 bottom panel), did not affect the capacity of VEGF to induce autophosphorylation of VEGFR-2 (Figure 2A and supplemental Figure 2 top panels). Reciprocally, VEGF stimulation did not interfere with induction of Tie2 phosphorylation by Ang-1 (Figure 2A and supplemental Figure 2 bottom panels). These results suggest that Ang-1 acts downstream of VEGFR-2 activation to modulate VEGF-stimulated NO production and transendothelial permeability.
Ang-1 activates PKC independently of PLCγ, but does not inhibit VEGFR-2 activation and signaling. (A) BAEC were stimulated with VEGF (40 ng/mL), Ang-1 (100 ng/mL), or both simultaneously for the indicated times. VEGFR-2 and Tie2 tyrosine phosphorylation levels were monitored in VEGFR-2 and Tie2 immunoprecipitates (IP). Equal immunoprecipitation levels from BAEC lysates were confirmed by Western blot (wb). supplemental Figure 2 shows the complementary treatments and IPs. These experiments were repeated at least 3 times with identical results. (B) Activation of Akt, MAPK, PKC, and PLCγ, using the indicated phospho-specific antibodies, was monitored in BAEC stimulated with Ang-1 (100 ng/mL), VEGF (40 ng/mL), or both simultaneously for the indicated times. Equal protein loading was confirmed by reprobing the membranes with antibodies against total Akt, MAPK, and PLCγ. These experiments were repeated at least 3 times with similar results.
Ang-1 activates PKC independently of PLCγ, but does not inhibit VEGFR-2 activation and signaling. (A) BAEC were stimulated with VEGF (40 ng/mL), Ang-1 (100 ng/mL), or both simultaneously for the indicated times. VEGFR-2 and Tie2 tyrosine phosphorylation levels were monitored in VEGFR-2 and Tie2 immunoprecipitates (IP). Equal immunoprecipitation levels from BAEC lysates were confirmed by Western blot (wb). supplemental Figure 2 shows the complementary treatments and IPs. These experiments were repeated at least 3 times with identical results. (B) Activation of Akt, MAPK, PKC, and PLCγ, using the indicated phospho-specific antibodies, was monitored in BAEC stimulated with Ang-1 (100 ng/mL), VEGF (40 ng/mL), or both simultaneously for the indicated times. Equal protein loading was confirmed by reprobing the membranes with antibodies against total Akt, MAPK, and PLCγ. These experiments were repeated at least 3 times with similar results.
To determine VEGF-dependent signaling pathways that are affected by Ang-1, we investigated whether stimulation of BAEC with Ang-1 altered the ability of VEGF to activate Akt and mitogen-activated protein kinase (MAPK) pathways. VEGF or Ang-1 stimulation of BAEC induced phosphorylation of both Akt and Erk1/2. Simultaneous treatment of cells with VEGF and Ang-1 did not alter the kinetics of phosphorylation of both signaling pathways (Figure 2B). Next, we examined the effect of Ang-1 stimulation on PLCγ activation and show, as previously described, that Ang-1 stimulation fails to induce PLCγ phosphorylation.25 Furthermore, Ang-1 treatment of BAEC did not interfere with the capacity of VEGF to induce PLCγ phosphorylation (Figure 2B). These results are in line with previous reports showing that Ang-1, in contrast to VEGF, is unable to induce intracellular calcium mobilization in endothelial cells.25 Lastly, PKC activation was monitored using a pan phospho-PKC antibody that recognizes the activated status of classical and novel PKC isoforms (pan phospho-PKCβII-Ser660). Both VEGF and Ang-1 stimulation of BAEC markedly increased the phosphorylation levels of PKCs, with Ang-1 displaying more rapid activation kinetics. Interestingly, VEGF treatment in combination with Ang-1 also induced PKC phosphorylation with kinetics identical to Ang-1 stimulation alone. Because Ang-1 activates PKCs independently of PLCγ, this suggests that classical PKC isoforms, which are dependent on mobilization of intracellular calcium for their activation, are not implicated.
Phosphorylation of eNOS on Thr497 is required for eNOS inhibition by Tie2
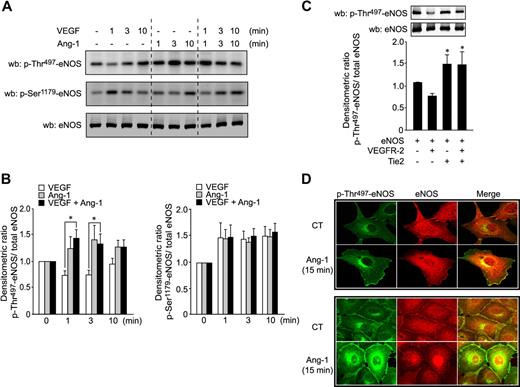
It is well established that PKC activity inhibits eNOS via phosphorylation of Thr497;7,8 thus, we examined the possibility that phosphorylation of eNOS by PKCs contributes to its inhibition by Ang-1. We first monitored phosphorylation of eNOS on Ser1179 and Thr497 residues in response to Ang-1 or VEGF stimulation. In agreement with the reported reciprocal regulation of eNOS activity by coordinated dephosphorylation of Thr497 and phosphorylation of Ser1179,7,8 stimulation of BAEC with VEGF caused a rapid decrease in Thr497 phosphorylation levels, which coincides with a marked increase in phosphorylation of Ser1179 of eNOS (Figure 3A-B). In contrast to VEGF, Ang-1 stimulation caused a rapid and marked increase in Thr497 phosphorylation of eNOS (Figure 3A-B). Interestingly, cotreatment of BAEC with VEGF and Ang-1 resulted in a significant increase in phosphorylation levels of Thr497. The costimulation completely abolished the dephosphorylation of Thr497 observed with VEGF stimulation alone. In contrast, Ser1179 phosphorylation was not significantly different for each of the agonists used alone (Figure 3B). To confirm the involvement of Tie2 in these Ang-1–mediated effects, we overexpressed Tie2 alone or in combination with VEGFR-2 in COS-7 cells and monitored phosphorylation levels of eNOS on Thr497 (Figure 3C). Similarly to stimulation of BAEC with VEGF, VEGFR-2 expression decreased the levels of eNOS phosphorylation on Thr497. In contrast, Tie2 overexpression, alone or in combination with VEGFR-2, significantly increased levels of Thr497 phosphorylation, indicating that Tie2 activity counteracts the effects of VEGFR-2 on the Thr497 residue of eNOS.
Ang-1 induces phosphorylation of eNOS on the inhibitory Thr497 site. (A) BAEC were stimulated with Ang-1 (100 ng/mL), VEGF (40 ng/mL), or both simultaneously for the indicated times. Cell lysates were probed for eNOS phosphorylation on Thr497 and Ser1179 using phospho-specific antibodies. Equal protein loading was confirmed by Western blotting for total eNOS. (B) Densitometric ratio of results presented in panel A of p-Thr497-eNOS (right panel) or p-Ser1179-eNOS (left panel) levels relative to total eNOS. Data were normalized with respect to control-unstimulated cells. Bar graph represents the average of 5 experiments expressed as mean ± SEM; *P < .05. (C) Tie2 counteracts the effects of VEGFR-2 on Thr497 residue of eNOS. Phosphorylation levels of eNOS on Thr497 were monitored in COS-7 cells transfected with eNOS plasmids in combination with expression vectors coding for VEGFR-2, Tie2, or both. Total cell lysates were analyzed for p-Thr497-eNOS and total eNOS. Bar graph shows the p-Thr497/total eNOS densitometric ratio expressed as mean ± SEM of 4 independent experiments; *P < .05 compared with phosphorylation levels of eNOS expressed with VEGFR-2. (D) Cellular localization of p-Thr497-eNOS in sparse (top panels) or confluent (bottom panels) BAEC after Ang-1 stimulation. BAEC were cultured on 0.1% gelatin-coated coverslips. Serum-starved cells were either untreated or treated with 100 ng/mL Ang-1 for 15 minutes. Cells were fixed, permeabilized, and incubated with anti–p-Thr497-eNOS (pAb; green) and anti-eNOS (mAb; red) primary antibodies. Immunofluorescent staining was performed as described in “Immunofluorescence” and observed by confocal microscopy (×63 objective).
Ang-1 induces phosphorylation of eNOS on the inhibitory Thr497 site. (A) BAEC were stimulated with Ang-1 (100 ng/mL), VEGF (40 ng/mL), or both simultaneously for the indicated times. Cell lysates were probed for eNOS phosphorylation on Thr497 and Ser1179 using phospho-specific antibodies. Equal protein loading was confirmed by Western blotting for total eNOS. (B) Densitometric ratio of results presented in panel A of p-Thr497-eNOS (right panel) or p-Ser1179-eNOS (left panel) levels relative to total eNOS. Data were normalized with respect to control-unstimulated cells. Bar graph represents the average of 5 experiments expressed as mean ± SEM; *P < .05. (C) Tie2 counteracts the effects of VEGFR-2 on Thr497 residue of eNOS. Phosphorylation levels of eNOS on Thr497 were monitored in COS-7 cells transfected with eNOS plasmids in combination with expression vectors coding for VEGFR-2, Tie2, or both. Total cell lysates were analyzed for p-Thr497-eNOS and total eNOS. Bar graph shows the p-Thr497/total eNOS densitometric ratio expressed as mean ± SEM of 4 independent experiments; *P < .05 compared with phosphorylation levels of eNOS expressed with VEGFR-2. (D) Cellular localization of p-Thr497-eNOS in sparse (top panels) or confluent (bottom panels) BAEC after Ang-1 stimulation. BAEC were cultured on 0.1% gelatin-coated coverslips. Serum-starved cells were either untreated or treated with 100 ng/mL Ang-1 for 15 minutes. Cells were fixed, permeabilized, and incubated with anti–p-Thr497-eNOS (pAb; green) and anti-eNOS (mAb; red) primary antibodies. Immunofluorescent staining was performed as described in “Immunofluorescence” and observed by confocal microscopy (×63 objective).
To investigate whether Thr497 phosphorylation in response to Ang-1 is localized intracellularly where eNOS has been shown to be activated, the Golgi and plasma membrane,26 we examined the cellular localization of p-Thr497-eNOS (green) and eNOS (red) by immunofluorescence confocal microscopy in sparse or confluent BAEC. Figure 3D shows that, in control-unstimulated conditions, phospho-specific eNOS antibody showed a relatively uniform staining throughout the cell. After Ang-1 stimulation for 15 minutes, p-Thr497-eNOS staining in BAEC is markedly increased at the plasma membrane and perinuclear level, where it colocalizes in part with eNOS staining (red). No staining was observed when cells were incubated with secondary antibodies alone (data not shown).
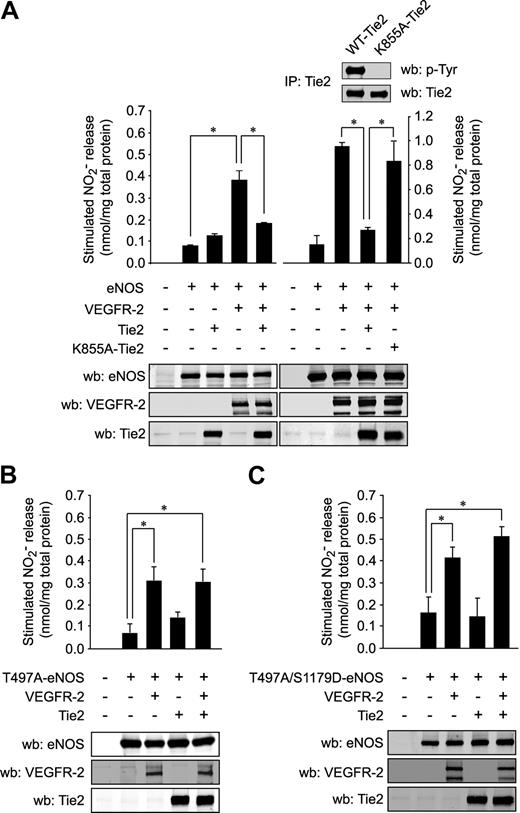
To verify that Tie2-mediated Thr497 phosphorylation of eNOS results in inhibition of NO release, we measured NO release from COS-7 cells transfected with eNOS in presence of VEGFR-2 and/or Tie2 (Figure 4A left panels). Expression of VEGFR-2 led to a marked increase in eNOS-derived NO production from transfected cells. Similarly to the stimulation of BAEC with VEGF and Ang-1, coexpression of Tie2 with VEGFR-2 resulted in a complete inhibition of eNOS activity and NO production (Figure 4A left panels). In a separate set of experiments, we confirmed that kinase activity of Tie2 is important for this inhibitory effect. COS-7 cells were again cotransfected with VEGFR-2 and WT Tie2 or a kinase-dead Tie2 construct (lysine 855 substituted for alanine; K855A-Tie2) (Figure 4A right panels). In contrast to WT Tie2, the inactive mutant K855A-Tie2 was unable to inhibit NO production stimulated by VEGFR-2 (Figure 4A right panels).
Phosphorylation of eNOS on Thr497 is required for inhibition of NO production by Tie2. (A) The effect of Tie2 on NO production stimulated by VEGFR-2 was measured in transfected COS-7 cells. Cells were cotransfected with expression vectors coding for eNOS in combination with VEGFR-2 and WT Tie2 (left panels) or in separate sets of experiments the kinase-dead K855A-Tie2 constructs (right panel). Levels of NO released in the medium after transfection were measured, as in Figure 1B. Expression levels of the transfected proteins were monitored by Western blotting (bottom panels). The inactivity of K855A-Tie2, in contrast to WT-Tie2, was determined by anti-phosphotyrosine Western blotting of Tie2 IPs (inset, left panel). NO released from COS-7 cells transfected with expression vectors coding for T497A-eNOS single mutant (B) or T497A/S1179D-eNOS double mutant (C), in combination with VEGFR-2, Tie2, or both, was measured by chemiluminescence, as described above. Equal transfection levels were monitored by Western blotting using anti–VEGFR-2, anti-Tie2, and anti-eNOS antibodies. Data are the average ± SEM of at least 4 experiments; *P < .05.
Phosphorylation of eNOS on Thr497 is required for inhibition of NO production by Tie2. (A) The effect of Tie2 on NO production stimulated by VEGFR-2 was measured in transfected COS-7 cells. Cells were cotransfected with expression vectors coding for eNOS in combination with VEGFR-2 and WT Tie2 (left panels) or in separate sets of experiments the kinase-dead K855A-Tie2 constructs (right panel). Levels of NO released in the medium after transfection were measured, as in Figure 1B. Expression levels of the transfected proteins were monitored by Western blotting (bottom panels). The inactivity of K855A-Tie2, in contrast to WT-Tie2, was determined by anti-phosphotyrosine Western blotting of Tie2 IPs (inset, left panel). NO released from COS-7 cells transfected with expression vectors coding for T497A-eNOS single mutant (B) or T497A/S1179D-eNOS double mutant (C), in combination with VEGFR-2, Tie2, or both, was measured by chemiluminescence, as described above. Equal transfection levels were monitored by Western blotting using anti–VEGFR-2, anti-Tie2, and anti-eNOS antibodies. Data are the average ± SEM of at least 4 experiments; *P < .05.
To confirm the obligatory role for Thr497 phosphorylation in the inhibitory effects of Tie2, we performed similar experiments in COS-7 cells cotransfected with eNOS constructs that are not phosphorylable at the Thr497 position (T497A-eNOS) along with Tie2 and/or VEGFR-2 and monitored NO production (Figure 4B). In contrast to cells expressing WT eNOS (Figure 4A), Tie2 failed to inhibit NO production when cotransfected with VEGFR-2 in T497A-eNOS–expressing cells (Figure 4B). Similarly, in a separate set of experiments, the inhibitory effects of Tie2 on VEGFR-2–induced NO release were not observed when both receptors were cotransfected with eNOS constructs that are phosphomimetic at the Akt phosphorylation site Ser1179 and not phosphorylable at Thr497 (T497A/S1179D-eNOS; Figure 4C). These data strongly suggest that phosphorylation of eNOS at the Thr497 position after Tie2 activation can potently block endothelial production of NO stimulated by VEGFR-2.
PKCζ is required for phosphorylation of eNOS on Thr497 in response to Ang-1
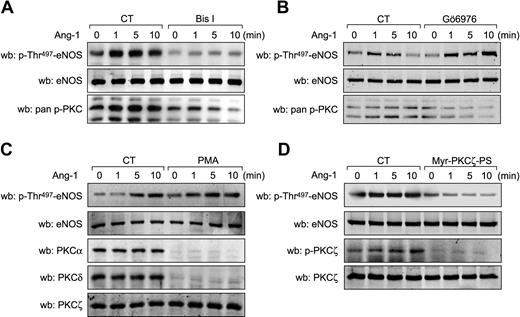
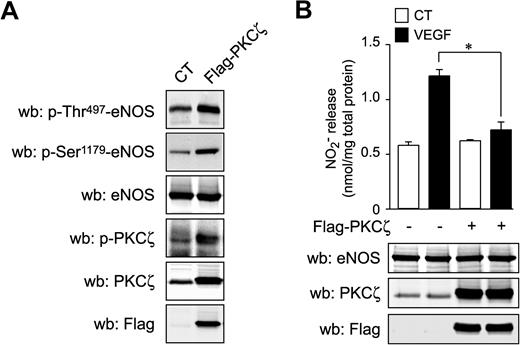
Our data demonstrate that Tie2 activation results in eNOS inhibition via phosphorylation of the putative PKC inhibitory phosphorylation site, Thr497, and suggest that activation of PKCs by Ang-1 is accomplished independently of intracellular calcium mobilization. To demonstrate the implication of PKCs in Ang-1 signaling to eNOS, we treated BAEC with Bis I, a wide spectrum PKC inhibitor, and monitored phosphorylation on Thr497 in response to Ang-1 stimulation (Figure 5A). Bis I pretreatment of cells significantly reduced Ang-1–induced phosphorylation of eNOS on Thr497, confirming that PKC activity is necessary for Thr497 phosphorylation in response to Ang-1 (Figure 5A). In contrast, pretreatment with a specific inhibitor of classical (calcium-dependent) PKC isozymes, Gö6976, did not prevent Ang-1–stimulated phosphorylation of Thr497 of eNOS (Figure 5B). This confirms that calcium-dependent classical PKCs are not involved in this phosphorylation event induced by Ang-1. Next, we attempted to further determine the PKC isoform responsible for Thr497 phosphorylation in response to Ang-1. BAEC and BLMVEC (data not shown) were treated with PMA for 24 hours to down-regulate all diacyl-glycerol–dependent novel and classical isoforms of PKC. As expected, PMA treatment drastically reduced the expression levels of PKCα (classical) and PKCδ (novel) without affecting levels of PKCζ (atypical) (Figure 5C). Interestingly, despite the absence of classical and novel isoforms, Ang-1 still induced a significant increase in Thr497 phosphorylation. This indicates that atypical PKC may be involved. Interestingly, when BAEC were pretreated with a cell-permeable myristoylated PKCζ pseudosubstrate inhibitor, Ang-1–induced phosphorylation on Thr497 of eNOS was significantly reduced (Figure 5D). In parallel to eNOS phosphorylation on Thr497, Ang-1 stimulation does induce PKCζ phosphorylation on Thr410, which is inhibited by the pseudosubstrate inhibitor (Figure 5D). To verify whether PKCζ could induce phosphorylation of eNOS on Thr497, we transiently expressed a Flag-tagged PKCζ construct (Flag-PKCζ) in BAEC and monitored Thr497 phosphorylation levels. Indeed, cells that overexpress PKCζ display a marked increase in Thr497 and PKCζ phosphorylation levels (Figure 6A). Interestingly, phosphorylation on Ser1179 was also increased in cells overexpressing PKCζ. However, VEGF-stimulated NO release was significantly inhibited in cells overexpressing PKCζ, demonstrating that eNOS activation was prevented by PKCζ-mediated phosphorylation of Thr497, which seems to prevail over the activating phosphorylation on Ser1179 (Figure 6B). Finally, to highlight the specific requirement of PKCζ kinase activity in these effects, we overexpressed a kinase-dead PKCζ construct in BAEC, which did not affect the basal phosphorylation levels of eNOS, but markedly inhibited Ang-1–stimulated phosphorylation of eNOS on Thr497 (supplemental Figure 3).
Inhibition of PKCζ prevents phosphorylation of eNOS on Thr497. (A) BAECs were pretreated with PKC inhibitors, (A) Bis I (0.5 μM) or (B) Gö6976 (0.5 μM) for 30 minutes before Ang-1 (100 ng/mL) stimulation for the indicated times. Cell lysates were probed for eNOS phosphorylation on Thr497 using phospho-specific antibodies. Equal protein loading was confirmed by Western blotting for total eNOS. Inhibition of PKC activity was confirmed by Western blotting using anti–pan-p-Ser660-PKCβ2 antibodies. (C) BAEC were treated for 24 hours with PMA (5 μM) to down-regulate classical and novel PKC isozymes. Cells were then stimulated with Ang-1 (100 ng/mL) for the indicated times. Membranes were probed with anti–p-Thr497-eNOS antibodies. Equal protein loading was confirmed by Western blotting for total eNOS. Cellular levels of PKC isozymes were confirmed by Western blotting using anti-PKCα, anti-PKCδ, and anti-PKCζ antibodies. (D) BAEC were treated with a myristoylated PKCζ-pseudosubstrate inhibitor (Myr-PKCζ-PS; 10 μM, 30 minutes) and stimulated for the indicated times with Ang-1 (100 ng/mL). Total cell lysates were analyzed for p-Thr497-eNOS and p-Thr410/403-PKCζ levels. Equal protein loading was confirmed by blotting for total eNOS and PKCζ. These experiments were repeated at least 3 times with similar results.
Inhibition of PKCζ prevents phosphorylation of eNOS on Thr497. (A) BAECs were pretreated with PKC inhibitors, (A) Bis I (0.5 μM) or (B) Gö6976 (0.5 μM) for 30 minutes before Ang-1 (100 ng/mL) stimulation for the indicated times. Cell lysates were probed for eNOS phosphorylation on Thr497 using phospho-specific antibodies. Equal protein loading was confirmed by Western blotting for total eNOS. Inhibition of PKC activity was confirmed by Western blotting using anti–pan-p-Ser660-PKCβ2 antibodies. (C) BAEC were treated for 24 hours with PMA (5 μM) to down-regulate classical and novel PKC isozymes. Cells were then stimulated with Ang-1 (100 ng/mL) for the indicated times. Membranes were probed with anti–p-Thr497-eNOS antibodies. Equal protein loading was confirmed by Western blotting for total eNOS. Cellular levels of PKC isozymes were confirmed by Western blotting using anti-PKCα, anti-PKCδ, and anti-PKCζ antibodies. (D) BAEC were treated with a myristoylated PKCζ-pseudosubstrate inhibitor (Myr-PKCζ-PS; 10 μM, 30 minutes) and stimulated for the indicated times with Ang-1 (100 ng/mL). Total cell lysates were analyzed for p-Thr497-eNOS and p-Thr410/403-PKCζ levels. Equal protein loading was confirmed by blotting for total eNOS and PKCζ. These experiments were repeated at least 3 times with similar results.
Overexpression of PKCζ counteracts VEGF-induced NO production. (A) BAEC were transfected with an empty vector or with a Flag-PKCζ expression vector, and total cell lysates were analyzed for p-Thr497-eNOS, p-Ser1179-eNOS, and p-Thr410/403-PKCζ. Transfection efficacy was verified using anti-Flag antibodies. Total eNOS and total PKCζ levels were monitored to confirm equal protein loading and overexpression, respectively. (B) BAEC were transfected with an empty vector or with a Flag-PKCζ expression vector. Serum-starved cells were stimulated with VEGF (40 ng/mL) for 30 minutes. Samples of culture medium were taken for nitrite quantification, as described in “NO release analysis.” Transfection efficacy was verified using anti-Flag antibodies. Total eNOS and total PKCζ levels were monitored to confirm equal protein loading and overexpression, respectively (bottom panels).
Overexpression of PKCζ counteracts VEGF-induced NO production. (A) BAEC were transfected with an empty vector or with a Flag-PKCζ expression vector, and total cell lysates were analyzed for p-Thr497-eNOS, p-Ser1179-eNOS, and p-Thr410/403-PKCζ. Transfection efficacy was verified using anti-Flag antibodies. Total eNOS and total PKCζ levels were monitored to confirm equal protein loading and overexpression, respectively. (B) BAEC were transfected with an empty vector or with a Flag-PKCζ expression vector. Serum-starved cells were stimulated with VEGF (40 ng/mL) for 30 minutes. Samples of culture medium were taken for nitrite quantification, as described in “NO release analysis.” Transfection efficacy was verified using anti-Flag antibodies. Total eNOS and total PKCζ levels were monitored to confirm equal protein loading and overexpression, respectively (bottom panels).
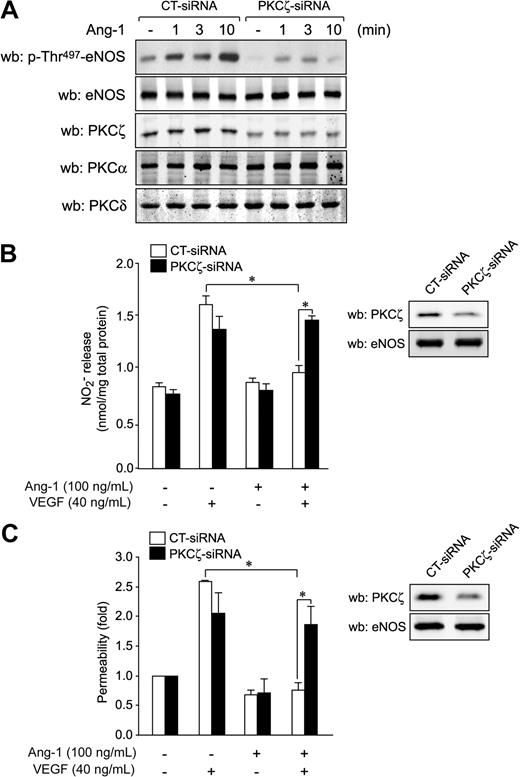
Next, we down-regulated PKCζ expression by siRNA to confirm its role in Ang-1–induced phosphorylation on Thr497. Transfection of siRNA against PKCζ in BAEC (Figure 7A) and in BLMVEC (supplemental Figure 4A) resulted in a reduction of PKCζ expression levels of more than 50% without affecting the expression of PKCα and δ. Down-regulation of PKCζ markedly reduced Ang-1–stimulated phosphorylation of eNOS on Thr497 in BAEC (Figure 7A) and in BLMVEC (supplemental Figure 4A). Finally, to establish a direct link between PKCζ activation by Ang-1 and inhibition of NO production and of transendothelial permeability, we determined whether knockdown of PKCζ by siRNA could interfere with the inhibitory effects of Ang-1. Gene silencing of PKCζ in BAEC (Figure 7B) and BLMVEC (supplemental Figure 4B) eliminated the counteracting effects of Ang-1 on VEGF-induced NO production (Figure 7B) without affecting VEGF-stimulated NO release (Figure 7B and supplemental Figure 4B). Similarly, knockdown of PKCζ abolished the inhibitory effects of Ang-1 on VEGF-stimulated endothelial permeability in BAEC (Figure 7C) and BLMVEC (supplemental Figure 4C). These results suggest that PKCζ activity is responsible for the inhibitory effects of Ang-1 on VEGF-induced endothelial permeability via inhibition of NO release from endothelial cells.
PKCζ knockdown reverses the inhibition by Ang-1 of eNOS, NO release, and endothelial permeability. (A) BAEC were transfected with siRNA targeted against PKCζ or with control siRNA. Forty-eight hours after transfections, cells were starved for 6 hours before Ang-1 stimulation (100 ng/mL) for the indicated times. Membranes were probed with antibodies against p-Thr497-eNOS and eNOS. PKCζ knockdown was confirmed by Western blotting (wb). Cellular levels of PKCα and PKCδ were not affected by PKCζ-siRNA. (B) BAEC were transfected with PKCζ-siRNA (35 μM) or control siRNA (35 μM). Forty-eight hours posttransfection, cells were starved for 6 hours before stimulation with VEGF (40 ng/mL), Ang-1 (100 ng/mL), or both for 30 minutes. Samples of culture medium were taken for the quantification of NO released, as described in “NO release analysis.” PKCζ knockdown was confirmed by Western blotting, and eNOS protein levels were monitored to confirm equal protein loading (inset). (C) Permeability to FITC-dextran was determined in confluent BAEC monolayers transfected with PKCζ-siRNA (35 μM) or control siRNA (35 μM) before stimulation with VEGF (40 ng/mL), Ang-1 (100 ng/mL), or both for 30 minutes. Data represent permeability to FITC-dextran expressed as the mean fold increase ± SEM with respect to untreated cells. PKCζ knockdown was confirmed by Western blotting, and eNOS protein levels were monitored to confirm equal protein loading (inset). *P < .05.
PKCζ knockdown reverses the inhibition by Ang-1 of eNOS, NO release, and endothelial permeability. (A) BAEC were transfected with siRNA targeted against PKCζ or with control siRNA. Forty-eight hours after transfections, cells were starved for 6 hours before Ang-1 stimulation (100 ng/mL) for the indicated times. Membranes were probed with antibodies against p-Thr497-eNOS and eNOS. PKCζ knockdown was confirmed by Western blotting (wb). Cellular levels of PKCα and PKCδ were not affected by PKCζ-siRNA. (B) BAEC were transfected with PKCζ-siRNA (35 μM) or control siRNA (35 μM). Forty-eight hours posttransfection, cells were starved for 6 hours before stimulation with VEGF (40 ng/mL), Ang-1 (100 ng/mL), or both for 30 minutes. Samples of culture medium were taken for the quantification of NO released, as described in “NO release analysis.” PKCζ knockdown was confirmed by Western blotting, and eNOS protein levels were monitored to confirm equal protein loading (inset). (C) Permeability to FITC-dextran was determined in confluent BAEC monolayers transfected with PKCζ-siRNA (35 μM) or control siRNA (35 μM) before stimulation with VEGF (40 ng/mL), Ang-1 (100 ng/mL), or both for 30 minutes. Data represent permeability to FITC-dextran expressed as the mean fold increase ± SEM with respect to untreated cells. PKCζ knockdown was confirmed by Western blotting, and eNOS protein levels were monitored to confirm equal protein loading (inset). *P < .05.
Discussion
It is well established that VEGF and Ang-1 share many functional properties that contribute to the induction of angiogenesis and vasculogenesis.14,27,28 However, in contrast to VEGF, Ang-1 enhances vascular maturation and stabilization and acts as an anti-inflammatory agent by modifying endothelial cell adhesion molecules and cell-to-cell junctions.18,29 Ang-1 is also known to maintain the integrity of the endothelial barrier against a variety of inflammatory challenges and to protect the vasculature from vascular leakage.15,16,30
In this study, we aimed at understanding how Ang-1 inhibits endothelial permeability induced by VEGF. Our results show that Ang-1 not only fails to increase endothelial permeability, but also inhibits VEGF-induced permeability. Interestingly, pharmacologic inhibition of eNOS with l-NAME completely abolished VEGF-stimulated passage of FITC-labeled dextran across a monolayer of endothelial cells (Figure 1). This confirms that eNOS and NO contribute to increased endothelial permeability stimulated by VEGF.1,31,32 Our results also provide evidence that Ang-1 interferes with VEGFR-2–stimulated NO release from endothelial cells, and that this effect is mediated by Tie2 (Figure 4A). Overall, this suggests that acute blockade of NO production by Ang-1 may contribute to the inhibition of endothelial permeability (Figure 1). Ang-1 induces proliferation, migration, and survival of endothelial cells.19,20,33 However, the effects of Ang-1 on eNOS activity remain controversial. Our study supports previous findings showing that short-term Ang-1 stimulation of endothelial cells is incapable of inducing NO-dependent increase of intracellular cGMP levels.34 Others have reported that some of the proangiogenic effects of Ang-1 are dependent on NO, and that long-term stimulation of endothelial cells does result in a modest, in comparison with VEGF, but significant increase in NO production.35-37 It is important to note that in these latter studies the angiopoietins used were engineered Ang-1/Ang-2 chimeras, and this may explain some of the differences observed. In addition, we have exposed BAEC to Ang-1 concentrations of up to 500 ng/mL for 24 hours without detecting any significant increase in NO levels (results not shown). Nonetheless, we cannot exclude the possibility that some of the in vivo proangiogenic effects of Ang-1 are dependent on eNOS and NO. However, these effects could result from a direct action of Ang-1 at the eNOS gene expression level rather than an acute activation of signaling pathways leading to eNOS activation and NO production.38,39 Interestingly, many studies, including ours, do show that Ang-1 does induce phosphorylation of eNOS on Ser1179 and that it is also a potent activator of Akt in endothelial cells.34,35,37 To explain the inability of Ang-1 to induce NO release, we examined the phosphorylation levels of the inhibitory Thr497 residue of eNOS. In contrast to VEGF, Ang-1 induces phosphorylation on Thr497, which contributes to eNOS inhibition regardless of the fact that Ang-1 also induces phosphorylation on Ser1179. This underscores the importance of a coordinated dephosphorylation of Thr497 and phosphorylation of Ser1179 for optimal eNOS activation. The consequence of Thr497 phosphorylation on eNOS activity has been previously revealed by the phosphomimetic mutation of Thr497 on eNOS to Asp, which generates a form of eNOS markedly less active than WT when challenged with agonists that are also capable of inducing phosphorylation on Ser1179.40 Our results confirm that increased phosphorylation on Thr497 in response to Ang-1 results in a less active eNOS, even though Ang-1 also induces phosphorylation on Ser1179. Thus, the observed increase in phosphorylation of eNOS at Thr497 after Tie2 activation by Ang-1 (Figure 3) explains the potent antagonistic effect of Ang-1 on VEGF-stimulated NO production. Furthermore, the mutation of Thr497 to alanine on eNOS proves that Thr497 phosphorylation is required for inhibition of NO production by Tie2. Indeed, in contrast to expression of WT-eNOS, NO release from VEGFR-2–stimulated T497A-eNOS–expressing cells is not inhibited by coexpression of Tie2, confirming the necessity of Thr497 phosphorylation for inhibition of NO release by Tie2 activation (Figure 4). Moreover, the dual mutation T497A/S1179D on eNOS also reveals that phosphorylation on Thr497 is essential for the inhibitory effects of Tie2 even in the context of a phosphomimetic mutation at the 1179 position. Interestingly, the double mutant still retains, to some extent, the capacity to be further activated by VEGFR-2. This indicates that cellular mechanisms other than phosphorylation by Akt, possibly intracellular calcium mobilization or changes in protein-protein interactions, are also involved for maximal activation of eNOS by VEGFR-2.3,41-45
Our results also indicate that PKC activity plays an important role in the signaling axis that leads to the modulation of eNOS activity by Ang-1. Interestingly, Ang-1 stimulation does not activate PLCγ, which confirms previous findings demonstrating that Ang-1 cannot elicit an increase in intracellular calcium.25 This also suggests that activation of PKCs in response to Ang-1 is achieved in a calcium-independent manner. Thus, the increase in eNOS phosphorylation levels on the putative inhibitory PKC site, Thr497, after Ang-1 stimulation is most probably dependent on activity of calcium-independent PKC isozymes. Moreover, Gö6976, a specific inhibitor of calcium-dependent classical PKCs, did not inhibit Thr497 phosphorylation of eNOS by Ang-1 (Figure 5). Importantly, Ang-1 stimulation still induced phosphorylation on Thr497 in cells in which classical and novel PKC isozymes were down-regulated by a prolonged exposure of cells to PMA. This allowed us to suggest that atypical PKCs might be involved in this effect of Ang-1. Finally, by combining experiments using cell-permeable peptide inhibitors (Figure 5), overexpression studies (Figure 6), and siRNA-mediated gene silencing (Figure 7), we identified atypical PKCζ as being important for Ang-1–mediated Thr497 phosphorylation. Importantly, inhibition of PKCζ expression abrogates the capacity of Ang-1 to interfere with VEGF-induced NO production and to increase endothelial permeability in vitro. Our results do not exclude the participation of the other atypical PKC isoform, PKCλ/ι, in Ang-1 signaling; however, we demonstrate that activation of PKCζ by Ang-1 seems to restrain NO production and may explain the protective effects of Ang-1 against eNOS-dependent increases in vascular permeability. Interestingly, when PKCζ is overexpressed in endothelial cells, VEGF-stimulated NO release is inhibited similar to a costimulation of cells with VEGF and Ang-1 (Figure 6). In addition, we demonstrate for the first time that Ang-1 stimulation induces the phosphorylation of endogenous PKCζ in endothelial cells (Figure 5D). It has previously been shown that thrombin-mediated endothelial permeability is prevented by dominant-negative PKCζ.46 Our results show that PKCζ opposes VEGF-stimulated NO release and endothelial permeability in vitro. It is an interesting possibility that PKCζ acts both as a positive and negative modulator of endothelial permeability, depending on the factor involved. Nonetheless, Ang-1 stimulation induces phosphorylation and activation of PKCζ in endothelial cells, and the full consequences of these remain to be investigated. Interestingly, atypical PKCs have been associated with the formation of tight junctions in polarized epithelial cells.47,48 The implication of atypical PKCζ in the stabilization of the endothelium in ways that do not involve NO is also very plausible and warrants more investigation.
The protective effects of Ang-1 against vascular leakage have been well documented. It also remains clear that multiple independent or interconnected signaling pathways are involved in these effects. For example, it has been elegantly demonstrated that Ang-1 inhibits vascular permeability through the sequestration of Src kinase via mDia, resulting in altered VEGF signaling at cell-to-cell junctions.18 In addition, c-Src has recently been shown to positively regulate Akt and eNOS activity in response to VEGF.45 However, our results show that Ang-1 does not influence VEGF-induced Akt and eNOS phosphorylation on Ser1179. This suggests that the inhibition of Src may act in parallel to the inhibition of eNOS for the blockade of endothelial permeability by Ang-1. In addition, it has also been shown that disruption of endothelial cell junctional complexes by VEGF is impaired by Ang-1 and that PKCβ inhibition blocks VEGF-mediated dissociation of β-catenin from VE-cadherin.29 Others have shown that the antipermeability effects of Ang-1 are due to the altered ability of VEGF to increase Ca2+ influx through TRPC1 channels in presence of Ang-1.25 Finally, it has been suggested that Ang-1 stimulation activates p190 RhoGAP, which results in RhoA inhibition and in stabilization of the endothelial monolayer.21 Our study now adds a new layer of regulation to this process by demonstrating that Ang-1 inhibits endothelial permeability by preventing the release of an essential modulator of vascular permeability in vitro and in vivo, NO. Overall, this demonstrates that multiple and essential signaling pathways do converge to provoke the stabilization of the endothelium. It remains to be shown whether some of these signaling pathways are specifically solicited in vivo in certain physiologic or pathologic conditions.
In summary, we provide in this study the first evidence that inhibition of endothelial permeability by Ang-1 results, at least in part, from the inhibition of NO release in response to VEGF, and that this is due to the phosphorylation of eNOS on the inhibitory Thr497 site by PKCζ.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Chantal Delisle for expert technical support and Daniela Baggio for secretarial assistance.
This work was supported by grants (MOP-86464 and MOP-53295) from the Canadian Institutes of Health Research to J.-P.G. M.O. was a recipient of a studentship from the Canadian Institutes of Health Research–funded Training Program in Cancer Research at Institut de Recherches Cliniques de Montréal. J.-P.G. holds a Tier 2 Canada Research Chair.
Authorship
Contribution: M.O. performed research, analyzed data, and wrote the paper; and J.-P.G. designed research, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Jean-Philippe Gratton, Institut de Recherches Cliniques de Montréal, 110 des Pins Ave West, Montreal, QC, H2W 1R7, Canada; e-mail: jean-philippe.gratton@ircm.qc.ca.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal