Abstract
Dendritic cells (DCs) play an instrumental role in regulating tolerance to self-antigens and preventing autoimmunity. One mechanism by which “tolerogenic” DCs are established is through the inhibitory effects of apoptotic cells (ACs). Immature DCs encountering ACs are resistant to stimuli that activate and mature DCs. We have shown that the Mer receptor tyrosine kinase (MerTK) plays a key role in transducing inhibitory signals upon binding of ACs, which in turn involve the phosphatidylinositol 3-kinase (PI3K) pathway. Nevertheless, the molecular basis for AC-induced inhibition of DCs is ill defined. In the current study, the proximal signaling events induced by MerTK after AC binding were studied. AC treatment of bone marrow–derived or splenic DCs established a complex consisting of MerTK, the nonreceptor tyrosine kinase c-Src, the transcription factor STAT3, and PI3K. In contrast, AC treatment of DCs lacking MerTK expression failed to increase c-Src and STAT3 activation. In addition, the inhibitory effects of ACs were blocked by treating DCs with pharmacologic inhibitors or siRNA specific for c-Src and STAT3. These findings demonstrate that AC-induced inhibition of DCs requires MerTK-dependent activation of c-Src and STAT3, and provide evidence for novel roles for c-Src and STAT3 in the immunoregulation of DCs.
Introduction
Dendritic cells (DCs) play key roles in promoting proinflammatory responses against pathogenic microbes, in addition to establishing and maintaining tolerance to self-antigens.1,2 These disparate functions are regulated by multiple factors including the nature of the antigen. Apoptotic cells (ACs) that are continuously generated in the body can block the capacity of immature DCs to be activated and mature upon subsequent stimulation.3,4 The inhibitory effect of ACs is thought to be an important mechanism by which self-tolerance is initiated and maintained by DCs.5,6 However, the molecular basis for AC-induced inhibition of DCs remains ill defined.
A variety of receptors expressed by immature DCs such as αvβ5 integrin, CD36, complement receptor C1qR, the phosphatidylserine receptor, and the Mer tyrosine kinase (MerTK) contribute to AC binding and/or ingestion.7-10 MerTK belongs to the so-called “TAM” family of receptor tyrosine kinases, which includes Axl and Tyro3.11-13 Binding of ACs by MerTK is mediated by recognition of growth arrest–specific factor 6 (Gas6); Gas6 binds to phosphatidylserine expressed on the inverted plasma membrane of ACs.14 In addition to DCs, MerTK is expressed by macrophages (Møs), natural killer cells, natural killer T cells, B cells, and endothelial and epithelial subtypes.15-18 Expression of MerTK by Møs is required for efficient phagocytosis of ACs, and defective MerTK expression by retinal pigment epithelial cells leads to the accumulation of apoptotic photoreceptor outer segments and the development of a type of retinitis pigmentosa in rats, mice, and humans.9,19-21 Phagocytosis of ACs in these cell types is due to MerTK-dependent signaling events promoting cytoskeletal reorganization. MerTK contains 2 immunoglobulin-like and 2 fibronectin type III repeats, a transmembrane region, and an intracellular region containing multisubstrate docking sites that bind SH2-containing proteins such as the p85 subunit of phosphatidylinositol 3-kinase (PI3K) and the adapters Grb2 and Vav1.22,23 In various transformed and primary Møs, AC binding induces a series of MerTK-dependent signaling events, including tyrosine autophosphorylation of MerTK, activation of PLC-γ2, and induction of downstream PKC-dependent signals that regulate cytoskeletal reorganization.24-26
Recently, our group demonstrated that MerTK plays a critical role in mediating AC-induced inhibition of DCs. DCs lacking MerTK expression (MerTK−/−) are no longer sensitive to the inhibitory effects of ACs.10 In addition, autoimmune diabetes is exacerbated in MerTK−/− T-cell receptor transgenic nonobese diabetic (NOD) mice, because immature DCs are no longer inhibited by apoptotic β cells.6 The inhibitory effect of ACs is due mainly to MerTK-induced blockade of the NF-κB pathway in immature DCs. NF-κB is a transcription factor that regulates the expression of several genes that control activation, maturation, and the antigen-presenting function of DCs.7,10 In addition, PI3K activation via MerTK is necessary for AC-induced inhibition of NF-κB.10 The signaling pathway transduced by MerTK in DCs after AC binding nevertheless remains poorly defined.
The current study was carried out to define the proximal MerTK-dependent signaling events associated with AC-induced inhibition of DC activation and maturation. Herein we show that c-Src is required for DC inhibition by ACs, demonstrating a novel role for this nonreceptor tyrosine kinase in DC immunoregulation. Furthermore, we show that STAT3 activation in DCs is also essential for mediating the inhibitory effects of ACs.
Methods
Mice
NOD/LtJ and C57BL/6 (B6) mice were maintained and bred under specific pathogen-free conditions. The establishment of NOD.MerTK−/− and B6.MerTK−/− mice that lack MerTK expression has been previously described.10,27 All mouse procedures were approved by the Institutional Animal Care and Use Committee of the University of North Carolina at Chapel Hill.
Preparation of DCs
Bone marrow–derived DCs (BMDCs) and splenic DCs (sDCs) were prepared from male or female mice between 8 to 12 weeks of age as described.28
DC pretreatment with ACs or signaling molecule inhibitors
DCs were cultured in RPMI 1640 supplemented with 10% heat-inactivated fetal bovine serum, 50 μM 2-ME, 1× nonessential amino acids, 1 mM sodium pyruvate, 2 mM l-glutamine, 100 U/mL penicillin, and 100 μg/mL streptomycin (complete RPMI medium). To prepare ACs, thymocytes isolated from 4- to 6-week-old mice were adhered to plastic for 2 hours to remove antigen presenting cells, and either irradiated at 600 Gy and cultured in complete RPMI medium for 12 hours or incubated with 1 μM dexamethasone for 4 to 6 hours. Flow cytometry demonstrated more than 95% apoptotic and less than 5% necrotic thymocytes based on annexin-V and propidium iodide staining. DCs were cocultured with ACs at a ratio of 1:5 (DCs/ACs) for the indicated times, and stimulated with lipopolysaccharide (LPS).
In some experiments, DCs (3 × 106 cells per well) were treated with (1) STAT3 inhibitors Jsi124 (10 nM) or sttatic (20 nM), (2) c-Src inhibitors E804 (10 nM) or PP1 (20 nM), STAT1 inhibitor fludarabine (20 μM; Calbiochem), (3) PI3K inhibitors wortmannin (Wort; 200 nM) or Ly294002 (LY; 50 μM; Cell Signaling Technology), or (4) 10 μg/mL αMerTK antibody (Ab) or isotype control Ab (R&D Systems) for 1 hour before AC treatment or LPS stimulation.
EMSA and Western blotting
Nuclear and cytoplasmic extracts were prepared from DCs as described.29 Electrophoretic mobility shift assay (EMSA) was performed using 32P-labeled DNA probes containing NF-κB binding sites derived from MHC class I H2K promoter: 5′-CAGGCTGGGGATTCCCATCTCCACAGTTTCACTTC-3′.30 The sis-inducible element 5′-CTTCATTTCCCGTAAATCCCTAAAGCT-3′ was used to measure STAT3 DNA-binding activity. A double-stranded Sp1 DNA probe, 5′-ATTCGATCGGGGCGGGGCGAG-3′, was used as control. Bands were visualized using a phosphoimager (Molecular Dynamics). For the STAT3 supershift assay, Abs specific for STAT1 or STAT3 (2 μg; Santa Cruz Biotechnology) were added to nuclear extracts and incubated for 30 minutes at room temperature. For each treatment, at least 3 EMSAs were performed.
Western blotting was carried out as described.28 Notably, ACs were added to “untreated” control DC cultures immediately before preparation of the lysates. In this way, the possibility that increased protein expression after AC pretreatment of DCs is due to AC-derived proteins can be ruled out. Membranes were probed with Abs specific for IκBα, pSTAT3, pc-Src (Y416), pAKT (Thr308), AKT, and ptyrosine (pTyr; Cell Signaling Technology); MerTK (R&D Systems); STAT3 and c-Src (Santa Cruz Biotechnology); and β actin (Sigma-Aldrich). Binding of secondary HRP-labeled αgoat, αrabbit, or αmouse Abs (Santa Cruz Biotechnology) was analyzed using SuperSignal West Pico or West Dura Chemiluminescent Substrate (Pierce). Densitometry analysis was done using ImageJ software (National Institutes of Health) for Western blots.
Immunoprecipitation of MerTK
For “pull-down” experiments, DCs (107) were treated with ACs (1:5) for various times, chilled on ice, resuspended in 1 mL of the cell-permeable protein cross-linker dimethyl 3,3′-dithiopropionimidate dihydrochloride (Sigma-Aldrich) in PBS (2 mg/mL), and incubated at room temperature for 20 minutes. Whole-cell lysates were prepared, precleared with protein G–Sepharose beads, and MerTK immunoprecipitated using protein G–Sepharose beads precoated with αMerTK Ab.
siRNA transfection of BMDCs
BMDCs were transfected with siRNA oligos (60 nM) specific for STAT3, c-Src, or a scrambled control set of oligos (Santa Cruz Biotechnology) using Lipofectamine 2000 (Invitrogen) as per the manufacturer's instructions. Transfection efficiency was assessed using control siRNA labeled with fluorescein isothiocyanate (FITC), and measuring the frequency of FITC-positive DCs via flow cytometry. Typically, more than 85% of BMDCs were transfected under the conditions used.
c-Src kinase assay
BMDCs were incubated with ACs for the indicated times, and whole-cell lysates prepared. Lysates (1 mg) were precleared using protein G–Sepharose beads, and then incubated with 30 μg αc-Src Ab coupled to protein G–Sepharose beads for 2 to 3 hours at 4°C under rotation. Protein G–Sepharose beads were washed 4 times with radioimmunoprecipitation assay buffer, twice with kinase buffer (50 mM Hepes, pH 7.4, 5 mM MgCl2, 3 mM MnCl2), and resuspended in a final volume of 45 μL kinase buffer. The kinase reaction was carried out by addition of 1 mM ATP and 10 μg/sample of the c-Src kinase substrate Sam68 at 30°C for 10 minutes. Reactions were stopped with 4× Laemmli buffer, samples were resolved by 8% sodium dodecyl sulfate–polyacrylamide gel electrophoresis, and phosphorylation of Sam68 was measured via Western blotting using an αpTyr Ab; c-Src protein was detected with an αc-Src Ab.
Measurement of IL-12 production from DCs
BMDCs (0.5 × 106/well) were pretreated plus/minus ACs for 3 hours, washed, and stimulated with LPS (1 μg/mL) for 24 hours. Supernatants were assayed for IL-12p70 in triplicate using an enzyme-linked immunosorbent assay kit (BD Biosciences) following the manufacturer's instructions.
Results
STAT3 activation is necessary for MerTK-dependent AC-induced inhibition of DCs
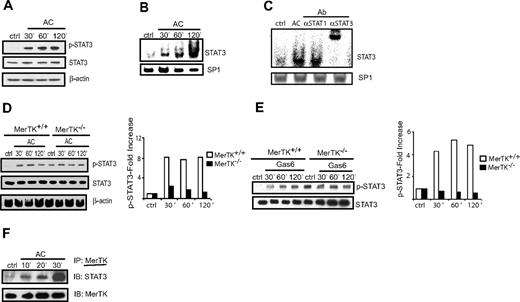
The intracellular domain of MerTK contains a binding motif (YPGV) for the STAT3 transcription factor. Because STAT3 signaling is typically associated with blocking DC activation,31-34 a role for this transcription factor in MerTK-dependent AC-induced inhibition of DCs was investigated. Initially, STAT3 activation was examined in MerTK+/+ BMDCs treated with ACs. As demonstrated in Figure 1A, a temporal increase in phosphorylation of STAT3 was detected in whole-cell lysates prepared from MerTK+/+ BMDCs after AC treatment. The level of STAT3 protein, however, remained unaltered (Figure 1A). Furthermore, STAT3 DNA-binding activity as measured by EMSA was also increased in AC-treated MerTK+/+ BMDCs (Figure 1B). Supershift analysis using αSTAT3 and αSTAT1 Abs demonstrated that the complex consisted of a STAT3 homodimer (Figure 1C). Next, sDCs were examined to rule out the possibility that AC-induced STAT3 activation was intrinsic to BMDCs. As seen in MerTK+/+ BMDCs, phosphorylated STAT3 (pSTAT3) levels increased in MerTK+/+ sDCs after AC treatment (Figure 1D). Notably, induction of pSTAT3 by AC treatment was MerTK dependent. Although higher constitutive levels of pSTAT3 relative to MerTK+/+ sDCs were detected, AC coculture failed to elevate pSTAT3 in MerTK−/− sDCs (Figure 1D). Similarly, pSTAT3 levels were increased in MerTK+/+ but not MerTK−/− sDCs treated with Gas6 (Figure 1E).
AC-induced STAT3 activation in DCs is MerTK dependent. MerTK+/+ BMDCs (A-C) or MerTK+/+ and MerTK−/− sDCs (D) were treated with ACs for the indicated times. pSTAT3, STAT3, and β actin were measured in whole-cell lysates via Western blot using the same membranes (A,D), or DNA-binding activity of nuclear STAT3 and SP1 determined by EMSA (B) and a supershift assay (C). (E) MerTK+/+ and MerTK−/− sDCs were treated with Gas6 (150 nM) for the indicated times, and pSTAT3 and STAT3 were measured in whole-cell lysates via Western blot using the same membrane. (D-E) Fold induction of pSTAT3 was determined by normalizing densitometric readings against control cultures. (F) MerTK+/+ BMDCs were incubated with ACs for the indicated times, MerTK was immunoprecipitated from whole-cell lysates, and Western blot membranes were probed for STAT3 and MerTK protein using the same membrane. Data are representative of a minimum of 3 experiments.
AC-induced STAT3 activation in DCs is MerTK dependent. MerTK+/+ BMDCs (A-C) or MerTK+/+ and MerTK−/− sDCs (D) were treated with ACs for the indicated times. pSTAT3, STAT3, and β actin were measured in whole-cell lysates via Western blot using the same membranes (A,D), or DNA-binding activity of nuclear STAT3 and SP1 determined by EMSA (B) and a supershift assay (C). (E) MerTK+/+ and MerTK−/− sDCs were treated with Gas6 (150 nM) for the indicated times, and pSTAT3 and STAT3 were measured in whole-cell lysates via Western blot using the same membrane. (D-E) Fold induction of pSTAT3 was determined by normalizing densitometric readings against control cultures. (F) MerTK+/+ BMDCs were incubated with ACs for the indicated times, MerTK was immunoprecipitated from whole-cell lysates, and Western blot membranes were probed for STAT3 and MerTK protein using the same membrane. Data are representative of a minimum of 3 experiments.
Immunoprecipitation experiments demonstrated a physical association between STAT3 and MerTK. MerTK+/+ BMDCs were treated with ACs for various times, MerTK was immunoprecipitated, and the resulting complexes were analyzed via Western blot. Immunoblots probed with αSTAT3 Ab demonstrated a temporal increase in STAT3 protein in AC-treated BMDCs; however, no significant change in the level of MerTK protein was observed over time (Figure 1F). These findings demonstrate that STAT3 is activated in a MerTK-dependent manner in DCs after AC binding.
STAT3 activation is required for AC-induced inhibition of NF-κB activation in DCs
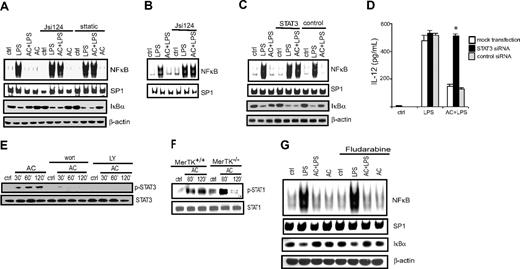
Previous work by our group demonstrated that the NF-κB pathway is an important target of AC-induced inhibition of DCs.10 Therefore a role for STAT3 in blocking NF-κB activation was assessed. MerTK+/+ BMDCs were pretreated with 2 different STAT3 inhibitors, Jsi124 or sttatic, cocultured with ACs, and then stimulated with LPS. As expected, NF-κB DNA binding and IκBα degradation as determined by EMSA and Western blot, respectively, were inhibited in LPS-stimulated MerTK+/+ BMDCs treated with ACs only (Figure 2A). In contrast, pretreatment with either Jsi124 or sttatic blocked the inhibitory effect of ACs on LPS-stimulated NF-κB activation (Figure 2A). Similarly, ACs no longer inhibited LPS-induced NF-κB DNA binding in MerTK+/+ sDCs pretreated with Jsi124 (Figure 2B).
STAT3 activation is necessary for AC-induced inhibition of NF-κB activation in DCs. MerTK+/+ BMDCs (A) or sDCs (B) were incubated with the STAT3 inhibitors Jsi124 (10 nM) or sttatic (20 nM) for 1 hour, cocultured with ACs or left untreated for 3 hours, and stimulated with LPS (50 ng/mL) for 0.5 hours. Nuclear NF-κB and SP-1 DNA-binding activity were determined via EMSA, and IκBα and β actin protein expression was detected by Western blot. (C-D) MerTK+/+ BMDCs were transfected with STAT3-specific or scrambled control siRNA, cocultured with ACs for 3 hours, stimulated with LPS, and either (C) nuclear NF-κB and SP-1 DNA-binding activity and IκBα or β actin protein was measured as described for panels A and B or (D) IL-12 secretion was measured by enzyme-linked immunosorbent assay; *P < 10−3, DCs treated with STAT3-specific siRNA versus mock-transfected DCs or DCs transfected with control siRNA (Student t test). Error bars indicate ± SEM. (E) MerTK+/+ BMDCs were pretreated with the PI3K inhibitors Wort and LY for 1 hour and incubated with ACs for the indicated times, and pSTAT3 and STAT3 protein was measured. (F) MerTK+/+ or MerTK−/− BMDCs were incubated with ACs for the indicated times, and p-STAT1 and STAT1 proteins were measured by Western blot. (G) MerTK+/+ BMDCs were treated with STAT1 inhibitor fludarabine (20 μM) for 1 hour, and cocultured with ACs for 3 hours or left untreated, and stimulated with LPS (50 ng/mL). Nuclear NF-κB or SP-1 DNA-binding activity, and IκBα and β actin protein were measured as described for panels A-B. Data are representative of a minimum of 3 experiments.
STAT3 activation is necessary for AC-induced inhibition of NF-κB activation in DCs. MerTK+/+ BMDCs (A) or sDCs (B) were incubated with the STAT3 inhibitors Jsi124 (10 nM) or sttatic (20 nM) for 1 hour, cocultured with ACs or left untreated for 3 hours, and stimulated with LPS (50 ng/mL) for 0.5 hours. Nuclear NF-κB and SP-1 DNA-binding activity were determined via EMSA, and IκBα and β actin protein expression was detected by Western blot. (C-D) MerTK+/+ BMDCs were transfected with STAT3-specific or scrambled control siRNA, cocultured with ACs for 3 hours, stimulated with LPS, and either (C) nuclear NF-κB and SP-1 DNA-binding activity and IκBα or β actin protein was measured as described for panels A and B or (D) IL-12 secretion was measured by enzyme-linked immunosorbent assay; *P < 10−3, DCs treated with STAT3-specific siRNA versus mock-transfected DCs or DCs transfected with control siRNA (Student t test). Error bars indicate ± SEM. (E) MerTK+/+ BMDCs were pretreated with the PI3K inhibitors Wort and LY for 1 hour and incubated with ACs for the indicated times, and pSTAT3 and STAT3 protein was measured. (F) MerTK+/+ or MerTK−/− BMDCs were incubated with ACs for the indicated times, and p-STAT1 and STAT1 proteins were measured by Western blot. (G) MerTK+/+ BMDCs were treated with STAT1 inhibitor fludarabine (20 μM) for 1 hour, and cocultured with ACs for 3 hours or left untreated, and stimulated with LPS (50 ng/mL). Nuclear NF-κB or SP-1 DNA-binding activity, and IκBα and β actin protein were measured as described for panels A-B. Data are representative of a minimum of 3 experiments.
To rule out nonspecific effects of the pharmacologic inhibitors on AC-induced inhibition of NF-κB activation, siRNA was used to block STAT3 expression. MerTK+/+ BMDCs were transfected with STAT3-specific or scrambled control siRNA. Greater than 85% of BMDCs were transfected under the conditions used based on flow cytometric analysis of BMDCs transfected with FITC-conjugated siRNA (data not shown). Furthermore, STAT3 protein expression was reduced approximately 80% in BMDCs transfected with STAT3-specific siRNA (supplemental Figure 1A, available on the Blood website; see the Supplemental Materials link at the top of the online article). As demonstrated in Figure 2C, AC inhibition of LPS-stimulated NF-κB DNA binding and IκBα protein degradation was effectively blocked in MerTK+/+ BMDCs transfected with STAT3-specific but not control siRNA. In addition, STAT3-specific siRNA blocked the inhibitory effect of ACs on LPS-induced IL-12 secretion (Figure 2D); expression of the IL-12p40 gene is dependent on NF-κB. To rule out the possibility that the role of STAT3 in AC-induced inhibition was intrinsic to the NOD genotype, BMDCs derived from B6 mice were pretreated with Jsi124, incubated with ACs, and then stimulated with LPS. Similar to results obtained with NOD DCs, Jsi124 efficiently blocked AC-induced inhibition of NF-κB DNA activity and IκBα degradation in B6 BMDCs stimulated with LPS (supplemental Figure 2A). These results demonstrate that STAT3 activation is necessary for AC-induced inhibition of the NF-κB pathway in DCs, and is independent of the genotype of DCs.
Our previous findings showed a key role for the PI3K pathway in mediating MerTK-dependent AC-induced inhibition of DCs.10 To determine whether PI3K activity was required for increased pSTAT3, the effect of the PI3K inhibitors LY and Wort on AC-induced STAT3 activation was investigated. MerTK+/+ BMDCs were pretreated with PI3K inhibitors and cultured with ACs, and pSTAT3 was measured via Western blot. As demonstrated in Figure 2E, phosphorylation of STAT3 was markedly reduced when PI3K activity was blocked. Inhibition of Janus kinases that activate STAT3 had no effect on AC-induced pSTAT3 (data not shown). Therefore, PI3K activity is required for STAT3 activation.
A recent study indicated that the TAM family member Axl induces DC suppression via STAT1 phosphorylation in a negative feedback loop after TLR signaling.35 To determine a potential role for STAT1 in AC-induced inhibition of DCs, MerTK+/+ and MerTK−/− BMDCs were treated with ACs and pSTAT1 was investigated. Wild-type BMDCs express both Axl and MerTK.10,35 As demonstrated in Figure 2F, pSTAT1 was induced by AC treatment in both MerTK+/+ and MerTK−/− BMDCs; the latter indicating that the increase in pSTAT1 was MerTK independent. Furthermore, pretreatment of MerTK+/+ BMDCs with 20 μM fludarabine, a STAT1 inhibitor, had no effect on AC-induced inhibition of NF-κB activation (Figure 2G). These results demonstrate that STAT3 but not STAT1 plays a key role in AC-induced inhibition of DCs.
c-Src is activated in DCs after MerTK binding of ACs
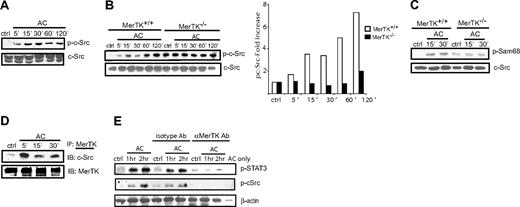
Binding of ACs by MerTK induces autophosphorylation, which may directly recruit and activate PI3K and downstream STAT3 (Figure 1). Alternatively, an additional tyrosine kinase(s) may be associated with proximal signaling that provides “specificity” to MerTK-induced events. One interesting candidate is the nonreceptor tyrosine kinase c-Src, which has been reported to play a role in DC activation, in addition to being involved in phosphorylation of both PI3K and STAT3.36-38 To test this possibility, AC-induced activation of c-Src was assessed in DCs. MerTK+/+ sDCs were cocultured with ACs for various times and phosphorylation of the c-Src tyrosine 416 (Tyr416) measured via Western blot. Activation of c-Src is associated with phosphorylation of Tyr416, which resides in the catalytic domain.39 pTyr416 was detected within 5 minutes after AC stimulation, and increased with time (Figure 3A). In contrast, no significant increase in the level of c-Src protein was detected (Figure 3A).
c-Src is activated in a MerTK-dependent manner upon AC treatment of DCs. (A-B) MerTK+/+ or MerTK−/− sDCs were incubated with ACs for the indicated times, and pc-Src and c-Src protein was measured by Western blot. Fold induction of pc-Src was determined by normalizing densitometric readings against control cultures. (C) MerTK+/+ BMDCs were treated with ACs, c-Src was immunoprecipitated from whole-cell lysates, and in vitro c-Src kinase activity and c-Src protein were measured by Western blot. (D) MerTK+/+ BMDCs were incubated with ACs for the indicated times, MerTK was immunoprecipitated from whole-cell lysates, and c-Src and MerTK protein were determined by Western blot. (E) MerTK+/+ BMDCs were pretreated with αMerTK or isotype Ab for 1 hour, and then incubated with ACs. Whole-cell lysates were examined via Western blot for pSTAT3, pc-Src, and β actin. Data are representative of a minimum of 3 experiments.
c-Src is activated in a MerTK-dependent manner upon AC treatment of DCs. (A-B) MerTK+/+ or MerTK−/− sDCs were incubated with ACs for the indicated times, and pc-Src and c-Src protein was measured by Western blot. Fold induction of pc-Src was determined by normalizing densitometric readings against control cultures. (C) MerTK+/+ BMDCs were treated with ACs, c-Src was immunoprecipitated from whole-cell lysates, and in vitro c-Src kinase activity and c-Src protein were measured by Western blot. (D) MerTK+/+ BMDCs were incubated with ACs for the indicated times, MerTK was immunoprecipitated from whole-cell lysates, and c-Src and MerTK protein were determined by Western blot. (E) MerTK+/+ BMDCs were pretreated with αMerTK or isotype Ab for 1 hour, and then incubated with ACs. Whole-cell lysates were examined via Western blot for pSTAT3, pc-Src, and β actin. Data are representative of a minimum of 3 experiments.
Next, whether induction of c-Src activity was MerTK dependent was ascertained. The level of constitutive pTyr416 was increased in sDCs lacking MerTK expression relative to wild-type sDCs (Figure 3B). However, the level of pc-Src was unaffected by AC coculture of MerTK−/− sDCs in contrast to MerTK+/+ sDCs (Figure 3B). Consistent with these results, c-Src kinase activity was increased in MerTK+/+ BMDCs stimulated with ACs based on in vitro phosphorylation of the c-Src substrate Sam68 (Figure 3C). On the other hand, no increase in c-Src kinase activity was observed in MerTK−/− BMDCs treated with ACs (Figure 3D). Notably, a temporal increase in c-Src protein complexed with MerTK was detected in ACs treated MerTK+/+ BMDCs (Figure 3D).
To confirm that AC-induced activation of c-Src in wild-type DCs was indeed MerTK dependent, the effect of a blocking αMerTK Ab on MerTK+/+ BMDCs was assessed. Phosphorylation of c-Src and STAT3 by ACs was significantly reduced in MerTK+/+ BMDCs pretreated with the αMerTK Ab but not the isotype control Ab (Figure 3E). Together, these results demonstrate that AC binding results in a MerTK–c-Src complex, and activation of c-Src.
AC-induced DC inhibition requires c-Src activation
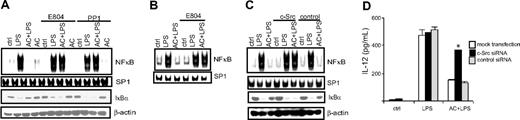
To test a functional role for c-Src in AC-induced inhibition of DCs, the effect of 2 c-Src inhibitors, E804 and PP1, was investigated. MerTK+/+ BMDCs were pretreated with E804 or PP1 and cocultured with ACs, and LPS-induced NF-κB activation was measured. Inhibition of c-Src with either E804 or PP1 effectively blocked the inhibitory effects of ACs on LPS-stimulated NF-κB DNA-binding activity and IκBα degradation in MerTK+/+ BMDCs derived from NOD (Figure 4A) or B6 (supplemental Figure 2B) mice. Similarly, AC-induced inhibition of NF-κB activation was blocked in MerTK+/+ sDCs pretreated with E804 (Figure 4B). Transfection of MerTK+/+ BMDCs with c-Src–specific but not control siRNA also significantly reduced c-Src protein expression (supplemental Figure 1B), and prevented AC-induced inhibition of NF-κB DNA-binding activity and IκBα degradation stimulated by LPS (Figure 4C). Furthermore, LPS-stimulated MerTK+/+ BMDCs transfected with c-Src–specific siRNA continued to secrete IL-12p70 despite coculture with ACs (Figure 4D). Together these results demonstrate that c-Src activation is necessary for AC-induced inhibition of DCs.
c-Src activation is required for AC-induced inhibition of DCs. MerTK+/+ BMDCs (A) or sDCs (B) were incubated with the c-Src inhibitors E804 (10 nM) or PP1 (20 nM) for 1 hour and cocultured with ACs for 3 hours or left untreated, and stimulated with LPS (50 ng/mL). Nuclear NF-κB or SP-1 DNA-binding activity was determined via EMSA, and IκBα and β actin protein measured by Western blot. (C-D) MerTK+/+ BMDCs were transfected with c-Src–specific or scrambled control siRNA, cocultured with ACs for 3 hours or left untreated, and stimulated with LPS (50 ng/mL for 0.5 hours or 1 μg/mL for 24 hours for IL-12 detection), and nuclear NF-κB and SP-1 DNA-binding activity and IκBα or β actin protein were examined (C), and IL-12p70 secretion was measured (D); *P < 10−3, DCs treated with c-Src–specific siRNA versus mock-transfected DCs or DCs transfected with control siRNA (Student t test). Data are representative of a minimum of 3 experiments. Error bars in panel D indicate ± SEM.
c-Src activation is required for AC-induced inhibition of DCs. MerTK+/+ BMDCs (A) or sDCs (B) were incubated with the c-Src inhibitors E804 (10 nM) or PP1 (20 nM) for 1 hour and cocultured with ACs for 3 hours or left untreated, and stimulated with LPS (50 ng/mL). Nuclear NF-κB or SP-1 DNA-binding activity was determined via EMSA, and IκBα and β actin protein measured by Western blot. (C-D) MerTK+/+ BMDCs were transfected with c-Src–specific or scrambled control siRNA, cocultured with ACs for 3 hours or left untreated, and stimulated with LPS (50 ng/mL for 0.5 hours or 1 μg/mL for 24 hours for IL-12 detection), and nuclear NF-κB and SP-1 DNA-binding activity and IκBα or β actin protein were examined (C), and IL-12p70 secretion was measured (D); *P < 10−3, DCs treated with c-Src–specific siRNA versus mock-transfected DCs or DCs transfected with control siRNA (Student t test). Data are representative of a minimum of 3 experiments. Error bars in panel D indicate ± SEM.
c-Src activation is upstream of PI3K and STAT3 activation
As demonstrated earlier inhibition of PI3K activity blocked STAT3 phosphorylation (Figure 2E), indicating that activation of PI3K was upstream of STAT3 phosphorylation. To determine whether c-Src activation was an event upstream of PI3K and STAT3 activation, the effect of inhibition of c-Src on the latter 2 signaling pathways was investigated. Initially, PI3K activity in vitro was assessed by measuring phosphorylation of AKT, a substrate of PI3K. As demonstrated in Figure 5A, AC-induced pAKT was blocked in MerTK+/+ BMDCs pretreated with the c-Src inhibitor E804. Similarly, AC-induced phosphorylation of STAT3 was reduced in E804 pretreated MerTK+/+ BMDCs (Figure 5B). Interestingly, immunoprecipitation of MerTK from AC-treated BMDCs demonstrated continued binding of the p85 subunit of PI3K and STAT3 to MerTK despite inhibition of c-Src by E804 incubation (Figure 5C). Finally, whether c-Src activation was required for MerTK phosphorylation was assessed. MerTK+/+ BMDCs were pretreated with E804 and incubated with ACs, and MerTK was then immunoprecipitated and pTyr was determined via Western blot. Phosphorylation of MerTK was induced by ACs regardless of whether MerTK+/+ BMDCs were treated with E804 (Figure 5D). Together these results demonstrate that c-Src activation is required for the subsequent activation but not the binding of PI3K and STAT3 to MerTK. Furthermore, autophosphorylation of MerTK after AC binding is independent of c-Src activity.
c-Src activation is an upstream event of PI3K and STAT3 activation. MerTK+/+ BMDCs were pretreated with E804 (10 nM) for 1 hour and incubated with ACs for indicated times. Whole-cell lysates were prepared to detect p-AKT, AKT, p-STAT3, and STAT3 via Western blot (A-B) or MerTK was immunoprecipitated with MerTK Ab and Western blots were probed for STAT3, PI3Kp85, p-Tyr, and MerTK (C-D).
c-Src activation is an upstream event of PI3K and STAT3 activation. MerTK+/+ BMDCs were pretreated with E804 (10 nM) for 1 hour and incubated with ACs for indicated times. Whole-cell lysates were prepared to detect p-AKT, AKT, p-STAT3, and STAT3 via Western blot (A-B) or MerTK was immunoprecipitated with MerTK Ab and Western blots were probed for STAT3, PI3Kp85, p-Tyr, and MerTK (C-D).
Discussion
The inhibitory effect of ACs on DCs is believed to be an important mechanism by which self-tolerance is maintained within the DC compartment.2,5,6 However, the molecular basis for AC-induced inhibition of DCs is poorly understood. The current study provides novel insight into the early DC signaling events elicited by ACs. Namely inhibition of DCs by ACs requires activation of c-Src and STAT3 in a MerTK-dependent manner.
Our finding that STAT3 is necessary for AC-induced inhibition establishes a new role for this transcription factor in DC immunoregulation. STAT3 was found physically associated with MerTK after AC or Gas6 treatment, consistent with a STAT3 binding motif found in the intracellular region of MerTK. In addition, DCs were made resistant to the inhibitory effects of ACs by blocking STAT3 signaling via siRNA and pharmacologic inhibitors. The latter is in agreement with a variety of studies demonstrating a general inhibitory role for STAT3 signaling in DC activation and maturation.31-34 The mechanism by which AC-induced pSTAT3 blocks the NF-κB pathway and subsequent DC activation and maturation after stimulation needs to be defined. Presumably pSTAT3 upon translocation to the nucleus regulates expression of genes encoding molecules that inhibit the NF-κB and other pathways required for DC activation and maturation. This model is consistent with our previous findings that the effect of ACs is blocked by pretreating DCs with transcription and translation inhibitors.10 Of note is that STAT3 regulates expression of suppressor of cytokine signaling 3, which is induced in DCs by Gas6 and blocks TLR and cytokine-receptor signaling.35,40,41
In contrast to our finding, Rothlin et al reported that STAT3 phosphorylation is not induced in BMDCs treated with Gas6.35 This discrepancy may be explained by a 3-fold lower concentration of Gas6 (eg, 50 nM) used to stimulate DCs by this group coupled with a reduced affinity of MerTK for Gas6 relative to Axl.42 Indeed, 50 nM Gas6 induced phosphorylation of STAT1 but not STAT3 (supplemental Figure 3A-B). The role of STAT3 in AC-induced inhibition appears to be DC specific. Similar to DCs, ACs inhibit activation and the effector function of Møs. However, the Ivashkiv group demonstrated that AC binding fails to phosphorylate STAT3 (and STAT1) in human and murine Møs (Tassiulas et al43 ). The requirement for STAT3 signaling in DCs but not Møs is not surprising because ACs regulate distinct signaling pathways in these 2 types of antigen-presenting cells.44 Most notable is the NF-κB pathway, which is blocked by AC binding in DCs but not Møs. Interestingly, the identity of the TAM receptor appears to determine which STAT molecule is activated in DCs. Rothlin et al showed that Axl physically associates with the type 1 IFN receptor and that this complex down-regulates TLR-induced signaling via STAT1 phosphorylation in a negative feedback loop.35 Although phosphorylation of STAT1 was detected in MerTK+/+ BMDCs treated with ACs (Figure 2F), STAT1 signaling was not required to mediate the inhibitory effects of ACs (Figure 2G). Furthermore, pSTAT1 was also detected in MerTK−/− BMDCs, suggesting that activation of STAT1 was induced by Axl signaling (Figure 2F). Together, these findings suggest that the respective TAM receptors not only signal via different pathways, but also perform distinct functions. MerTK plays an essential role in mediating the inhibitory effects of ACs, whereas Axl may serve as an important “feedback inhibitor” for TLR signaling.
Another key finding made in this study is that c-Src has a functional role in AC-induced immunoregulation of DCs. c-Src is widely known for regulating proliferation, differentiation, survival, and motility of a large number of cell types.45 Indeed dysregulation of this nonreceptor tyrosine kinase is typically linked to events driving tumorigenesis. On the other hand, only a few studies have demonstrated a role for c-Src in DC immunobiology. For instance, c-Src has been implicated in TLR3 and TLR4 signaling that leads to DC activation, and Src family tyrosine kinases such as Lyn are associated with DC development and maturation.36,46,47 In this study, we found that AC coculture induced a complex consisting of MerTK and c-Src. Currently, whether c-Src binds MerTK directly or associates with an adaptor molecule is not known. Nevertheless, a direct role for c-Src was demonstrated by experiments in which siRNA and pharmacologic inhibitors specific for c-Src blocked AC-induced inhibition of NF-κB activation and IL-12p70 secretion upon LPS stimulation. Recent work by the Birge group suggests that the effects of MerTK signaling on AC phagocytosis versus inhibition of the NF-κB pathway in human embryonic kidney and human Mø cell line transfectants are separable (Tibrewal et al26 ). Phagocytosis is regulated by autophosphorylation of Tyr867 in the protein tyrosine kinase domain of MerTK, however, suppression of NF-κB activation is independent of autophosphorylation of Tyr867 and other tyrosines.26 The latter suggests that an additional protein tyrosine kinase is required to mediate the inhibitory effect of ACs. Based on our findings, we argue that c-Src initiates signaling upon AC binding by MerTK that selectively blocks DC activation and maturation events.
Notably the context of STAT3 and c-Src activation determined the effect of these signaling molecules on DC activation and maturation. For instance, MerTK−/− DCs expressed higher constitutive levels of pSTAT3 and pc-Src compared with MerTK+/+ DCs. Nevertheless, MerTK−/− versus MerTK+/+ DCs in the absence of AC exhibit the same (1) cell-surface phenotype, (2) response to LPS- or CD40-mediated stimulation in terms of up-regulation of costimulatory molecules and MHC class II and cytokine secretion, and (3) capacity to stimulate T cells.6,10 Therefore, the inhibitory function of pSTAT3 and pc-Src on DCs is established in the context of AC-induced MerTK signaling. In other words, although pSTAT3 and pc-Src are constitutively higher in MerTK−/− versus MerTK+/+ DCs, it may be the dynamic change in these respective activities in the context of signals induced by AC binding that fully mediates the inhibitory cascade.
Based on our findings, we propose that AC binding by MerTK establishes a complex consisting of c-Src, PI3K, and STAT3. Recruitment of these molecules appears to be mediated by autophosphorylation of MerTK upon AC binding. For instance, blocking c-Src activation had no effect on MerTK phosphorylation or binding of PI3K and STAT3 to MerTK (Figure 5C-D). Furthermore, results indicate a sequence of c-Src→PI3K→STAT3 activation (Figures 2E, 5A-B). Studies have reported c-Src–mediated activation of PI3K in various cell types, and direct phosphorylation of the regulatory p85 subunit at Tyr688 of PI3K by Src family kinases such as Lck and Abl.48-51 In addition, activation of STAT3 has been shown to be a downstream event of c-Src–mediated signaling.38 On the other hand, only limited evidence indicates a role for PI3K-induced activation of STAT3.52-54 For instance, activation of STAT3 via acetylation of Lys685 has been shown to be a downstream event of PI3K/Akt activation after LIF or IL-6 treatment of various human transformed lines.53 The identity of an intermediate(s) between PI3K and STAT3 needs to be determined. Blocking the activation of mTOR, PLC-γ and PKC, which are possible candidates, had no effect on AC-induced inhibition of DCs (Z.Y. and R.T., unpublished results, February 2009).
In summary, the current study further defines the molecular basis for AC-induced inhibition of DCs by demonstrating novel roles for c-Src and STAT3. Targeting these molecules may provide one approach to effectively induce “tolerogenic” DCs for the purpose of immunotherapy of autoimmune diseases. Manipulating MerTK signaling in DCs residing in the thymus versus periphery would be predicted to have marked effects on the development of autoreactive T cells. For instance, NOD mice lacking MerTK expression fail to develop pathogenic β cell–specific T cells due to an enhanced capacity of thymic DCs to mediate negative selection of autoreactive thymocytes.55 Blocking the inhibitory function of MerTK and therefore increasing the stimulatory capacity of wild-type thymic DCs could provide a strategy to increase deletion of autoreactive thymocytes. On the other hand, inducing the inhibitory function of MerTK may limit the capacity of DCs residing in the periphery to promote the differentiation of pathogenic, autoreactive T effectors.6
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by grants from the Juvenile Diabetes Research Foundation (1-2005-984) and the National Institutes of Health (AI066075; R.T.). B.W. is a recipient of a Career Development Award from the American Diabetes Association.
National Institutes of Health
Authorship
Contribution: Z.Y. designed and performed research and wrote the paper; L.L. contributed to experimental design; G.K.M. and H.S.E contributed a key mouse model and to the writing of the paper; B.W. contributed to experimental design and analyses; and R.T. contributed to experimental design and analyses and the writing of the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Roland Tisch, Department of Microbiology and Immunology, Mary Ellen Jones Bldg, Rm 804, Campus Box No. 7290, University of North Carolina at Chapel Hill, Chapel Hill, NC 27599-7290; e-mail: rmtisch@med.unc.edu.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal