Abstract
Up to now, no lentiviral vector (LV) tool existed to govern efficient and stable gene delivery into quiescent B lymphocytes, which hampers its application in gene therapy and immunotherapy areas. Here, we report that LVs incorporating measles virus (MV) glycoproteins, H and F, on their surface allowed transduction of 50% of quiescent B cells, which are not permissive to VSVG-LV transduction. This high transduction level correlated with B-cell SLAM expression and was not at cost of cell-cycle entry or B-cell activation. Moreover, the naive and memory phenotypes of transduced resting B cells were maintained. Importantly, H/F-LVs represent the first tool permitting stable transduction of leukemic cancer cells, B-cell chronic lymphocytic leukemia cells, blocked in G0/G1 early phase of the cell cycle. Thus, H/F-LV transduction overcomes the limitations of current LVs by making B cell–based gene therapy and immunotherapy applications feasible. These new LVs will facilitate antibody production and the study of gene functions in these healthy and cancer immune cells.
Introduction
B lymphocytes are attractive targets for gene therapy of genetic diseases associated with B-cell dysfunction. In addition, long-lasting transgene expression in B cells is of particular interest for immunotherapy by its potential to induce specific immune activation or tolerance. Moreover, efficient delivery of genes or shRNAs for gene expression knock-down into primary human B lymphocytes would allow us to study gene functions in these cells
However, up to now, long-term gene transfer into primary human B cells has been notoriously difficult,1-3 and lentiviral vector (LV) transduction of truly quiescent B cells has not yet been reported. It is now generally accepted that efficient lentiviral transduction of hematopoietic stem cells and T cells requires a minimal stimulation with cytokines or other factors leading to entry of the cells into the G1b phase of the cell cycle.4-8 Yet primary B cells remain very poorly transducible with VSVG-pseudotyped LVs even when stimulated into proliferation by crosslinking of CD40 in the presence of various cytokines.1-3 Such vectors allow only efficient transduction of primary B cells on coculture with murine EL-4 B5 thymoma cells as helper T cells. However, this system led to strong proliferation and plasmocytic differentiation of all human B-cell subsets. More recently, efficient transduction of B cells was reported when they were cultured in the presence of CpG DNA and cytokines or using CD20 scFv-displaying LVs inducing stimulation and strong proliferation.9-11
Here, we demonstrate that LVs incorporating Edmonston measles virus (MV) glycoproteins hemagglutinin (H) and fusion protein (F) on their surface, named H/F-LVs,12 were able to transduce completely quiescent B cells in the absence of any exogenous stimulus. These novel gene transfer vehicles transduced unstimulated B cells (> 50%) without inducing activation, cell-cycle entry, or change in B-cell phenotype. Of utmost importance, these H/F-LVs allowed also high-level gene transfer into B-cell chronic lymphocytic leukemia (B-CLL) cells, a target that up to now was not permissive to lentiviral transduction.1
Methods
Plasmids and constructs
Antibodies
Anti–hCD150-PE (anti-SLAM) antibody was purchased from eBioscience. Anti–hCD46-PE, anti–hCD3-APC, anti–hCD19-APC, anti–hCD27-APC, anti–hCD69-PE, anti–hCD71-PE, and anti–hCD86-PE antibodies were purchased from BD Biosciences.
Production of LVs and titering
Production and titering was performed as described.12 Briefly, self-inactivating HIV-1–derived vectors were generated by transient transfection of 293T cells. For codisplay of the different H and F gps, 3 μg of each envelope plasmid was transfected. Viral supernatant was harvested 48 hours after transfection. Low-speed concentration of the vectors was performed by overnight centrifugation of the viral supernatant at 3000g at 4°C. To determine transduction efficiency and infectious titers of HIV vectors, serial dilutions of vector preparations were added to 293T cells. The infectious titers are expressed as 293T transducing units per milliliter (TU/mL).
Primary cells
Blood samples were obtained from healthy adult donors or patients with B-CLL after informed consent in accordance with the Declaration of Helsinki. Peripheral blood mononuclear cells (PBMCs) were isolated on a Ficoll-gradient (Sigma-Aldrich). CD19+ B cells and CD3+ T cells were purified by negative selection using the Rosette tetrameric complex system (StemCell Technologies).14 Purity of isolated B and T cells was monitored using anti-hCD19APC and anti-hCD3APC antibodies, respectively, and was analyzed by fluorescence-activated cell sorting (FACSCalibur; BD Biosciences). Donors give consent to use their blood specimen for research purposes, through deposit at the tumor bank of the Hospices Civils de Lyon, approved by French ethics laws.
Transduction assays
PBMCs, B lymphocytes, and T lymphocytes from healthy donors and B-CLL cells were isolated and cultured in RPMI 1640 medium (Gibco BRL Invitrogen) supplemented with 10% FCS and penicillin/streptomycin. Cells were immediately seeded for transduction or prestimulated with Staphylococcus aureus Cowan (SAC; 0.001%; Calbiochem)/IL-2 (1 ng/mL; Sigma-Aldrich) for B cells or with anti-hCD3 (1 μg/mL)/anti-hCD28 (1 μg/mL)/IL-2 (1 ng/mL) for T cells, as described previously.7 Briefly, 1E5 cells were seeded in 48-well plates, and concentrated vector was added at indicated doses. The percentage of GFP+ cells was determined by FACS 72 hours after transduction. Incubation with AZT 10 μM 2 hours before transduction was performed as described.15
Transduced T-cell cultures were continued in RPMI supplemented with rhIL-7, replenished every 3 days, and harvested for FACS analysis, quantitaive polymerase chain reaction (Q-PCR), and Alu-PCR. B cells were transferred to MS5 cell monolayer in RPMI supplemented with 10% AB serum, 5% FCS, 50 ng/mL rhSCF, 10 ng/mL rhIL-15, and 5 ng/mL rhIL-2, and medium was refreshed every 4 days. For Q-PCR and Alu-PCR detection, CD19+ cells were sorted at day 12 of culture on MS5 using a FACSAria Cell Sorter (BD Biosciences).
VSVG-H/F cotransduction assay
1E5 freshly isolated B or T cells were transduced with either mRFP-encoding VSVG-LVs, H/F-LVs coding for GFP, or a mixture of both vectors at indicated vector doses. At day 2 or 3 after transduction for B and T cells, respectively, the percentage of mRFP+ cells versus the percentage of GFP+ cells was measured by FACS. T cells were prestimulated for 24 hours with anti-hCD3/anti-hCD28/rhIL2, and B cells were prestimulated with SAC/IL-2 for 2 days before transduction, following the same protocol as for quiescent cells.
Time-course assay
1E5 quiescent T and B cells were seeded on 48-well plates in RPMI complete medium and transduced with either VSVG-LVs or H/F-LVs at a multiplicity of infection (MOI) of 30 and 10, respectively. DNA extraction was performed at 10 minutes, 12 hours, and 48 hours after contact with the vectors.
DNA extraction and Q-PCR
DNA was extracted using the QIAamp DNA mini kit (QIAGEN) or with NucleoSpin Tissue XS (Macherey-Nagel) for less than 5E4 cells. Total LTR DNA content was detected with the following primers for total-LTR sequences: Fwd LTR-2, 5′gcctcaataaagcttgccttga3′, and Rev LTR-2, 5′ggcgccactgctagagatttt3′. For 2-LTR circles determination the primer Fwd LTR-2 was used in combination with Rev LTR-8: 5′tcccaggctcagatctggtctaac3′. Primers, Fwd-G 5′gacagtcagccgcatcttctt3′ and Rev-G 5′agttaaaagcagccctggtga3′, were used to amplify a sequence of GAPDH housekeeping gene. Q-PCR was performed in the Applied 7000 cycler (Applied Biosystems Inc). In each well a final volume of 20 μL was prepared containing 10 μL Mastermix SUPER MIX with ROX (Invitrogen), 400 nM of each primer, and 10 ng DNA and water. The PCR conditions were as follows: 50°C, 2 minutes/95°C, 10 minutes/40 times (95°C, 115 seconds; 60°C, 1 minute), and a dissociation stage of 95°C, 15 seconds/60°C, 20 seconds/95°C, 15 seconds. For absolute copy number determination, a standard curve was prepared for each sequence by performing serial dilutions of purified pHIV-GFP (for total LTR), purified PCR product from Fwd-LTR2 and Rev-8 (for 2-LTR circles), and serial dilutions of total DNA from Raji cells for GAPDH. Number of copies per cell was calculated by dividing the absolute number of the target sequence by the housekeeping gene in each sample duplicate.
Alu-PCR
A first PCR step was performed on 500 ng genomic DNA with the primer Alu-s (5′TCCCAGCTACTGGGGAGGCTGAGG3′) and the primer 5NC2-as (5′GAGTCCTGCGTCGAGAGAG3′). The PCR conditions were denaturation and activation of the enzyme at 95°C for 10 minutes followed by 30 cycles of denaturation at 94°C for 1 minute, annealing at 57°C for 1 minute, and extension at 72°C for 1 minute. Then, 10 μL of the first PCR product were subjected to a second PCR with the primers Fwd LTR-2 and Rev LTR-2 (see previous paragraph). The conditions for this PCR are identical to the first step of the Alu-PCR, except for the annealing temperature which is 55°C and in the number of cycles (in this case 17 cycles). PCR products were loaded on 1% agarose gels, and the band intensity was measured in a UV transilluminator (Gene Flash; Syngene BIO Imaging) and using the Gene Tools Analyses software (Version 3.08E; Syngene). The housekeeping gene GAPDH was amplified as loading control.
PY/7-AAD cell-cycle analysis
Cell-cycle analysis was performed by staining DNA and RNA with 7-amino-actinomycin-D (7AAD) and pyronin Y, respectively, as previously described.7
Results
H/F-LVs allow efficient transduction of quiescent human B cells
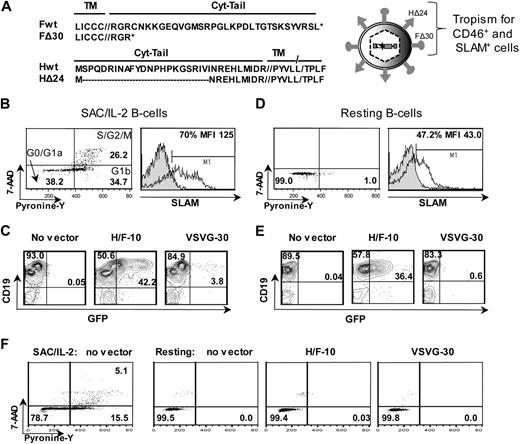
We previously generated high-titer LVs pseudotyped with MV glycoproteins (gps), H and F, from Edmonston strain (Figure 1A). These H/F-LVs allowed transduction of completely quiescent human T cells, whereas VSVG-LVs remained inefficient.12
The H/F-displaying lentiviral vectors efficiently transduce quiescent and proliferating B cells. (A) Schematic representation of the lentiviral vectors displaying hemagglutinin (H) and fusion (F) glycoproteins derived from Edmonston strain MV. The combination of the cytoplasmic-tail mutants of MV glycoproteins, HΔ24 and FΔ30, allowed efficient coincorporation on the LV surface, resulting in high-titer HIV vectors (hereafter named H/F-LVs). These H/F-LVs conserved the parental tropism as confirmed by transduction of CD46+ and SLAM+ cell lines.12 (B) Adult B cells were prestimulated for 24 hours with SAC/IL-2, and cell-cycle progression was monitored by simultaneously visualizing RNA (pyronin-Y) and DNA content (7-AAD; left). The percentages of cells in the G0/G1a, G1b, and S/G2/M phase of the cell cycle are indicated in the dot plot. Surface staining of SLAM by FACS is shown (right, open histogram). Closed histograms represent staining with IgG isotype controls. The percentage and mean fluorescence intensity (MFI) of SLAM-expressing cells are indicated. (C) Percentage of GFP measured by FACS at day 2 after transduction of SAC/IL-2 prestimulated B cells transduced with H/F-LVs (MOI = 10) and VSVG-LVs (MOI = 30; representative data of 5 experiments). (D) Adult B cells were verified for their quiescent state on isolation by PY/7-AAD staining as in panel B, and surface staining of SLAM by FACS is shown. (E) Percentage of GFP expression and (F) pyronin-Y/ 7AAD cell-cycle analysis at day 2 after transduction of adult resting B cells transduced as in panel C (representative data of 5 experiments).
The H/F-displaying lentiviral vectors efficiently transduce quiescent and proliferating B cells. (A) Schematic representation of the lentiviral vectors displaying hemagglutinin (H) and fusion (F) glycoproteins derived from Edmonston strain MV. The combination of the cytoplasmic-tail mutants of MV glycoproteins, HΔ24 and FΔ30, allowed efficient coincorporation on the LV surface, resulting in high-titer HIV vectors (hereafter named H/F-LVs). These H/F-LVs conserved the parental tropism as confirmed by transduction of CD46+ and SLAM+ cell lines.12 (B) Adult B cells were prestimulated for 24 hours with SAC/IL-2, and cell-cycle progression was monitored by simultaneously visualizing RNA (pyronin-Y) and DNA content (7-AAD; left). The percentages of cells in the G0/G1a, G1b, and S/G2/M phase of the cell cycle are indicated in the dot plot. Surface staining of SLAM by FACS is shown (right, open histogram). Closed histograms represent staining with IgG isotype controls. The percentage and mean fluorescence intensity (MFI) of SLAM-expressing cells are indicated. (C) Percentage of GFP measured by FACS at day 2 after transduction of SAC/IL-2 prestimulated B cells transduced with H/F-LVs (MOI = 10) and VSVG-LVs (MOI = 30; representative data of 5 experiments). (D) Adult B cells were verified for their quiescent state on isolation by PY/7-AAD staining as in panel B, and surface staining of SLAM by FACS is shown. (E) Percentage of GFP expression and (F) pyronin-Y/ 7AAD cell-cycle analysis at day 2 after transduction of adult resting B cells transduced as in panel C (representative data of 5 experiments).
Here, we sought to evaluate the performance of these H/F-LVs on primary human B cells, a very important target. B cells are refractory to gene transfer mediated by VSVG-LVs, even after a strong stimulation through the B-cell receptor (BCR).1-3 Therefore, freshly isolated B cells were prestimulated through the BCR for 24 hours. This brief stimulation resulted in B cell–cycle entry into the G1b phase of the cell cycle and induced proliferation with the percentage of cells in the S/G2/M phase always exceeding 20%, as determined by PY/7-AAD staining. Of note, this was accompanied by an up-regulation of the MV hSLAM receptor (Figure 1B). B cells were transduced with H/F-LVs at an MOI of 10, yielding transduction of up to 50%, whereas transduction levels of VSVG-LVs at high vector doses (MOI = 30) did not reach greater than 4% (Figure 1C). Thus, H/F-LVs are highly superior over VSVG-LVs for transduction of proliferating B cells.
Because we previously showed that H/F-LVs transduced quiescent T cells with high efficiency,12 we hypothesized that these vectors might be capable of transducing quiescent B cells. Quiescent B cells were isolated by negative selection to avoid activation. Before transduction, we confirmed by PY/7AAD cell-cycle staining that cells resided in the G0/G1a cell-cycle phase and detected SLAM expression on 30% to 50% of these cells (Figure 1D).
Remarkably, in the absence of any exogenous stimulus, H/F-LVs transduced 35% to 50% of the quiescent B-cell population without inducing cell-cycle entry, whereas VSVG-LVs did not transduce these cells at all (Figure 1E-F). This high transduction level induced by H/F-LVs did not have a negative effect on cell survival compared with control B cells or cells incubated with VSVG-LV, kept in the absence of any cytokines (percentage of cell survival, H/F-LV = 32.3% ± 5.3% vs VSVG-LV = 31.9% ± 5.2% and control = 35.6% ± 3.7%; P = .39; n = 5).
To exclude pseudo-transduction, we incubated quiescent B cells with H/F-LVs and VSVG-LVs at MOIs of 10 and 30, respectively, in the presence or absence of the reverse transcriptase inhibitor AZT. We detected up to 65-fold inhibition of transduction in the presence of AZT (Figure 2A). An identical picture was seen for quiescent T cells (supplemental Figure 1A, available on the Blood website; see the Supplemental Materials link at the top of the online article). Moreover, we found that H/F-LVs produced with a nonfunctional integrase (INT-D116A mutant) resulted in reduced transduction of quiescent B cells [H/F-LV = 35.7% vs H/F-LV(INT-D116A) = 7.0%; Figure 2B]. Interestingly, the nonfunctional integrase H/F-LVs gave only a global shift of B cells to a slightly higher GFP fluorescence [MFI, H/F-LV(INT-D116A) = 23 vs H/F-LV = 220; Figure 2B]. We found similar results for T-cell transduction (supplemental Figure 1B). To confirm long-term transduction, we incubated quiescent B cells for 24 hours with H/F-LVs and VSVG-LVs at an MOI of 10 and 30, respectively. Next, we continued the culture for 12 days on MS5 stroma cells. In the B lymphocytes we detected a stable transduction over time, but we observed consistently a slightly lower transduction of long-term cultured T cells compared with B cells (percentage of transduction: T cells = 30.3% ± 6.7%; B cells = 40.4% ± 16.0%; Figure 2C vs supplemental Figure 1C). To show LV provirus integration into the host genome, DNA from H/F-LV– and VSVG-LV–transduced quiescent B and T cells was extracted at day 12 after transduction and analyzed using a nested Alu-PCR detection method.3 Interestingly, only B and T cells transduced with the H/F-LVs showed a clear specific Alu-PCR signal, which was not detectable for VSVG-LV transduction (Figure 2C insert; supplemental Figure 1C). Thus, H/F-LVs induced easily detectable and true integration of the transgene, indicating genuine transduction of quiescent B and T cells.
H/F-LVs allow genuine transduction of quiescent B cells. (A) Quiescent adult B cells were transduced with H/F-LVs (MOI = 10) or VSVG-LVs (MOI = 30) in the absence or presence of AZT. The dot plots show the percentage of GFP in the CD19+ population at day 2 after transduction. (B) Cells were transduced with an H/F-LV encoding a nonfunctional integrase (INT-D116A) or a wild-type integrase (INT-wt) at an MOI of 10. GFP expression and MFI are shown for day 2 after transduction. (C) Quiescent B cells were transduced with H/F-LVs (MOI = 10) or VSVG-LVs (MOI = 30). At day 2 after transduction, part of the B cells was continued on a monolayer of MS5 cells. GFP-expression in the B-cell (CD19+) population is indicated for day 2, day 6, and day 12 after transduction as analyzed by FACS (means ± SD; n = 3). A nested Alu-PCR on the DNA of transduced and nontransduced sorted CD19+ cells at day 12 of culture on MS5 cells was performed using specific primers (see “Alu-PCR”). The PCR-specific band corresponding to integrated vector sequence of 121 base pair is detected only in H/F-transduced B cells (graph inset).
H/F-LVs allow genuine transduction of quiescent B cells. (A) Quiescent adult B cells were transduced with H/F-LVs (MOI = 10) or VSVG-LVs (MOI = 30) in the absence or presence of AZT. The dot plots show the percentage of GFP in the CD19+ population at day 2 after transduction. (B) Cells were transduced with an H/F-LV encoding a nonfunctional integrase (INT-D116A) or a wild-type integrase (INT-wt) at an MOI of 10. GFP expression and MFI are shown for day 2 after transduction. (C) Quiescent B cells were transduced with H/F-LVs (MOI = 10) or VSVG-LVs (MOI = 30). At day 2 after transduction, part of the B cells was continued on a monolayer of MS5 cells. GFP-expression in the B-cell (CD19+) population is indicated for day 2, day 6, and day 12 after transduction as analyzed by FACS (means ± SD; n = 3). A nested Alu-PCR on the DNA of transduced and nontransduced sorted CD19+ cells at day 12 of culture on MS5 cells was performed using specific primers (see “Alu-PCR”). The PCR-specific band corresponding to integrated vector sequence of 121 base pair is detected only in H/F-transduced B cells (graph inset).
H/F-LVs conserve the phenotype of transduced B cells
For most of the gene therapy applications it is indispensable that the initial phenotype of the cells is not altered by the transduction protocol. Indeed, H/F-LV transduction did not induce up-regulation of activation markers (CD69, CD71) on primary B cells (Figure 3A; supplemental Figure 2). Importantly, no phenotypic switch of naive (CD27−) to memory (CD27+) B cells was observed after transduction with H/F-LVs (Figure 3A). Note that the B-7 molecule, CD86, is a costimulatory molecule present on B cells. In view of the importance of CD86 for T- to B-cell interaction, we confirmed that contact with the H/F-LVs did not induce any change in CD86 expression on the cell surface (Figure 3A).
H/F-LVs conserve the phenotype of transduced B cells and transduce B cells preferentially over T cells. (A) Resting B cells were transduced with H/F-LVs and VSVG-LVs at an MOI of 10 and 30, respectively, or incubated without vector. Surface staining of activation markers (CD69, CD71) for day 2 after transduction is shown. Surface expression of CD86, naive (CD27−) and memory B-cell (CD27+) subsets was detected by FACS analysis (mean ± SD; n = 4). (B) The surface expression of the 2 measles receptors, hCD46 and hSLAM, was detected on nontransduced, H/F-LV– and VSVG-LV–transduced purified B cells 2 days after transduction (mean ± SD; n = 6). (C) Human PBMCs were isolated by a Ficoll gradient and transduced with either H/F-LVs or VSVG-LVs at identical vector doses as in panel A. Surface expression of SLAM receptor on adult B cells (CD19+) and T cells (CD3+) present in the PBMC population is shown directly after isolation (day 0) and after transduction with H/F-LVs or VSVG-LVs (day 3). At day 3 after transduction GFP expression was determined in the B cells (CD19+) and T cells (CD3+) present in the PBMC population by FACS analysis. These data are representative of 3 experiments.
H/F-LVs conserve the phenotype of transduced B cells and transduce B cells preferentially over T cells. (A) Resting B cells were transduced with H/F-LVs and VSVG-LVs at an MOI of 10 and 30, respectively, or incubated without vector. Surface staining of activation markers (CD69, CD71) for day 2 after transduction is shown. Surface expression of CD86, naive (CD27−) and memory B-cell (CD27+) subsets was detected by FACS analysis (mean ± SD; n = 4). (B) The surface expression of the 2 measles receptors, hCD46 and hSLAM, was detected on nontransduced, H/F-LV– and VSVG-LV–transduced purified B cells 2 days after transduction (mean ± SD; n = 6). (C) Human PBMCs were isolated by a Ficoll gradient and transduced with either H/F-LVs or VSVG-LVs at identical vector doses as in panel A. Surface expression of SLAM receptor on adult B cells (CD19+) and T cells (CD3+) present in the PBMC population is shown directly after isolation (day 0) and after transduction with H/F-LVs or VSVG-LVs (day 3). At day 3 after transduction GFP expression was determined in the B cells (CD19+) and T cells (CD3+) present in the PBMC population by FACS analysis. These data are representative of 3 experiments.
H/F-LVs can use hCD46 and hSLAM molecules as entry receptors.12 We found that levels of CD46 expression were not affected on B cells after H/F-LV transduction. Of note, SLAM-receptor was down-regulated 14-fold on B cells after H/F-LV transduction compared with SLAM expressed on VSVG-LV transduced or untransduced B cells (percentage of SLAM, H/F-LV = 2.8% ± 1.5% vs VSV-G-LV = 39.3% ± 5.5% and control = 41.3% ± 6.2%; Figure 3B). SLAM expression returned to initial levels at day 6 after transduction (data not shown). Notably, SLAM expression levels are higher on B cells than on T cells after purification as well as in the context of total PBMCs (Figure 3C; day 0). To address the question of whether SLAM expression could be determining tropism and/or entry of H/F-LVs, we performed transduction assays in total human PBMCs. In this case an identical vector dose is available per B and T cell in this cell mixture. H/F-LVs showed a clear preference for transduction of B cells compared with T cells (Figure 3C). Importantly, the fact that SLAM expression is higher on B cells than on T cells (47.7% in B cells; 26.6% in T cells) as well as the down-regulation observed in H/F-LV–transduced cells (1.2% in B cells; 0.4% in T cells; Figure 3C) suggests an implication of this receptor in binding or entry or both binding and entry of H/F-LVs into these cells.
We conclude that H/F-LVs not only conserve B-cell phenotype, but they show a preference for gene transfer into quiescent human B cells over T cells in agreement with higher SLAM expression on the B-cell population.
H/F-LV transduction does not facilitate VSVG-LV transduction of quiescent B and T cells
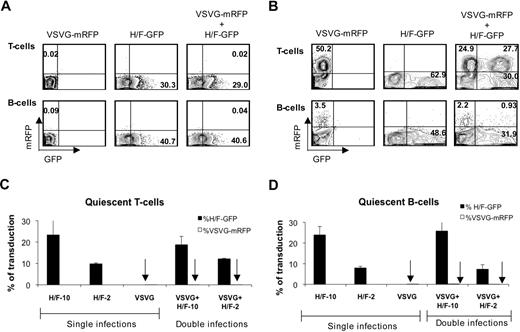
We sought to verify whether contact of quiescent lymphocytes with H/F-LVs could facilitate VSVG-LV transduction, because of a possible induction of transient cell activation or cell-cycle entry. Therefore, we designed a transduction protocol in which quiescent B and T cells were transduced with VSVG-LVs expressing red fluorescent protein (mRFP) or with H/F-LVs expressing GFP or with both types of LVs, allowing us to distinguish easily between transduction levels resulting from either LV pseudotype in a cotransduction assay.
Quiescent T and B cells were transduced with VSVG-LVs (MOI = 50), whereas incubation with H/F-LVs was performed at MOIs of 10 or 2, and transduction levels obtained were compared for the different vector pseudotypes. Cotransduction of quiescent T or B cells with both vector types conferred greater than 30% H/F-LV transduction, whereas VSVG-LVs did not allow efficient transduction (Figure 4A). Thus, no increase in the VSV-G-LV transduction level of quiescent T and B cells was observed when H/F-LVs were present compared with a transduction with VSVG-LVs alone (Figure 4A,C-D). Of note, VSVG-LVs (MOI = 50) alone were capable of transducing T-cell receptor–stimulated T cells, but this transduction level was not increased in cotransduction assays with H/F-LVs (Figure 4B). As expected, we achieved with VSVG-LVs barely 3.5% of transduction in SAC/IL-2–stimulated B cells. This transduction level was not increased because of the presence of H/F-LVs (Figure 4B). These results strongly suggest that the 2 vector types exploit different entry mechanisms in quiescent lymphocytes and that H/F-LV entry does not trigger or facilitate VSVG-LV entry.
H/F-LV transduction does not facilitate VSVG-LV transduction. (A) Freshly isolated adult quiescent T and B cells were transduced with VSVG-LVs coding for the monomeric red fluorescent protein (VSVG-mRPF) at an MOI of 50, H/F-LV coding for GFP (H/F-GFP) at an MOI of 10, or with both vectors simultaneously. At day 3 after transduction mRFP versus GFP expression was measured by FACS. (B) T cells were preactivated through the T-cell receptor by incubation with anti-CD3 and anti-CD28 antibodies in the presence of rIL-2, whereas B cells were preactivated with SAC and rIL-2 for 24 hours before they were subjected to the same transduction protocol as indicated in panel A. (C) Freshly isolated adult quiescent T cells were transduced with H/F-LVs and VSVG-LVs as indicated in panel A. Percentages of mRFP+ versus GFP+ cells from single transductions or double transductions are indicated as determined by FACS (mean ± SD; n = 4). (D) Freshly isolated adult quiescent B cells were subjected to the same protocol as indicated in panel A. The percentage of mRFP versus GFP expression from single transductions or double transductions are indicated (mean ± SD; n = 4).
H/F-LV transduction does not facilitate VSVG-LV transduction. (A) Freshly isolated adult quiescent T and B cells were transduced with VSVG-LVs coding for the monomeric red fluorescent protein (VSVG-mRPF) at an MOI of 50, H/F-LV coding for GFP (H/F-GFP) at an MOI of 10, or with both vectors simultaneously. At day 3 after transduction mRFP versus GFP expression was measured by FACS. (B) T cells were preactivated through the T-cell receptor by incubation with anti-CD3 and anti-CD28 antibodies in the presence of rIL-2, whereas B cells were preactivated with SAC and rIL-2 for 24 hours before they were subjected to the same transduction protocol as indicated in panel A. (C) Freshly isolated adult quiescent T cells were transduced with H/F-LVs and VSVG-LVs as indicated in panel A. Percentages of mRFP+ versus GFP+ cells from single transductions or double transductions are indicated as determined by FACS (mean ± SD; n = 4). (D) Freshly isolated adult quiescent B cells were subjected to the same protocol as indicated in panel A. The percentage of mRFP versus GFP expression from single transductions or double transductions are indicated (mean ± SD; n = 4).
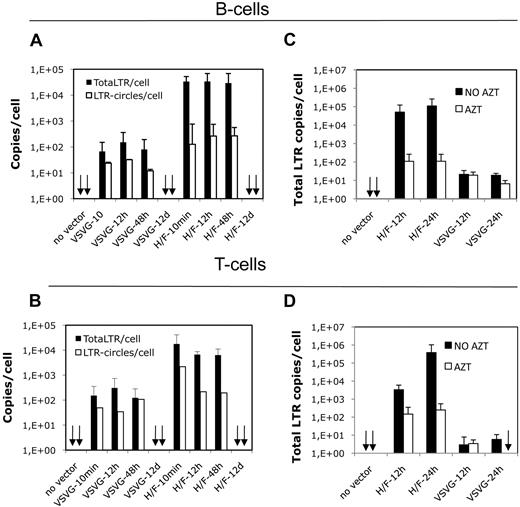
High transduction levels of H/F-LV–transduced B and T cells correlate with high levels of reverse-transcribed vector DNA
Viral reverse-transcribed cDNA coexists under the form of linear integrated and nonintegrated DNA, 1-LTR and 2-LTR circles. These 1-LTR and 2-LTR circles can serve as a measure for nuclear entry of viral DNA.3 To elucidate the mechanism of action that allows H/F-LVs to transduce quiescent B and T cells, we determined the presence of total reverse-transcribed proviral (total-LTR) and the amount of 2-LTR circles (LTR-circles). Quiescent B and T cells were transduced with H/F-LVs and VSVG-LVs at MOIs of 10 and 30, respectively. The kinetics of total-LTR products and 2-LTR circles was monitored at 10 minutes, 12 hours, 48 hours, and 12 days after transduction. It is important to point out that the content of total-LTR and 2-LTR circles is extremely high in quiescent B cells transduced with H/F-LVs (2.9 × 104 total-LTR/cell and 2.7 × 102 2-LTR circles/cell; Figure 5A). In contrast, up to 48 hours, the kinetics showed for VSVG-LV counterpart transductions only very low levels of total-LTR and 2-LTR circles. We detected at 48 hours a level of total-LTRs per cell in the VSVG-LV transduced B-cell population that was 360 times lower compared with H/F-LV–transduced B cells, coinciding with a VSVG-LV transduction efficiency that never exceeded 1% (H/F-LV = 2.9 × 104 total-LTR copies/cell vs VSVG-LV = 8 × 101 total-LTR copies/cell; Figures 5A, 1E). 2-LTR circles were also decreased in VSVG-transduced B cells (Figure 5A). A quite similar picture as for B cells was found for H/F-LV versus VSVG-LV transduction of quiescent T cells, but a slightly lower content of total-LTR products was detected in the latter population (Figure 5B). A strong reduction in total-LTR content of H/F-LV–transduced B and T cells in the presence of AZT was detected. At 24 hours T cells showed up to 1500-fold less total-LTR copies per cell on RT inhibition, whereas B cells showed 1000-fold reduction, confirming that this LTR-specific Q-PCR signal came from retrotranscribed proviral DNA (Figure 5C-D). On long-term culture (12 days) total-LTR content decreased up to 0.3 copies/cell in B and T cells, and 2-LTR circles were no longer detectable (Figure 5A-B). Summarizing, the high H/F-LV transduction efficiency correlates with nuclear entry of high amounts of reverse-transcribed vector DNA.
H/F-LV transduction of quiescent lymphocytes induces high levels of reverse-transcribed viral DNA. (A) Quiescent B and T cells were transduced with H/F and VSVG pseudotyped LVs at an MOI of 10 and 30, respectively. A time-course assay was performed to determine total-LTR content per cell and 2-LTR circles per cell present in VSVG-LV– and H/F-LV–transduced quiescent B cells at 10 minutes, 12 hours, 48 hours, and 12 days after transduction. The specific primers used are described in “DNA extraction and Q-PCR.” The total-LTR copies per cell and number of 2-LTR circles per cell are indicated in the y-axis (mean ± SD; n = 3). (B) Time-course assay for transduction of quiescent T cells was performed as in panel A. The total-LTR copies per cell and number of 2-LTR circles per cell are indicated in the y-axis (mean ± SD; n = 3). (C) Quiescent adult B cells were transduced with H/F-LVs (MOI = 10) or VSVG-LVs (MOI = 30) in the absence or presence of AZT. Total-LTR copies per cell present in VSVG-LV– and H/F-LV–transduced quiescent B cells at 12 and 24 hours is shown. The number of total-LTR copies per cell is indicated in the y-axis (mean ± SD; n = 3). (D) Time-course assay for transduction of quiescent T cells was performed as in panel C.
H/F-LV transduction of quiescent lymphocytes induces high levels of reverse-transcribed viral DNA. (A) Quiescent B and T cells were transduced with H/F and VSVG pseudotyped LVs at an MOI of 10 and 30, respectively. A time-course assay was performed to determine total-LTR content per cell and 2-LTR circles per cell present in VSVG-LV– and H/F-LV–transduced quiescent B cells at 10 minutes, 12 hours, 48 hours, and 12 days after transduction. The specific primers used are described in “DNA extraction and Q-PCR.” The total-LTR copies per cell and number of 2-LTR circles per cell are indicated in the y-axis (mean ± SD; n = 3). (B) Time-course assay for transduction of quiescent T cells was performed as in panel A. The total-LTR copies per cell and number of 2-LTR circles per cell are indicated in the y-axis (mean ± SD; n = 3). (C) Quiescent adult B cells were transduced with H/F-LVs (MOI = 10) or VSVG-LVs (MOI = 30) in the absence or presence of AZT. Total-LTR copies per cell present in VSVG-LV– and H/F-LV–transduced quiescent B cells at 12 and 24 hours is shown. The number of total-LTR copies per cell is indicated in the y-axis (mean ± SD; n = 3). (D) Time-course assay for transduction of quiescent T cells was performed as in panel C.
Novel H/F-LVs permit efficient gene transfer into B-CLL cells
B-CLL is a cancer in which B cells are blocked into the G0/early G1 phase of the cell cycle16 (Figure 6A) and accumulate in the periphery. They have strongly reduced responses to proliferating stimuli, and no method for a stable transduction of these cells has been reported.1 First, we confirmed that freshly isolated B-CLL cells expressed hCD46 and SLAM17 receptors (Figure 6A; data not shown), which led us to suggest that H/F-LVs would transduce these cells. Indeed, the H/F-LVs transduced B-CLL cells very efficiently in contrast to classical VSVG-LVs that remained inefficient (Figure 6B). As detected for normal B cells (Figure 3B), H/F-LV transduction of B-CLL cells coincided with SLAM down-regulation (Figure 6C). At day 12 of culture of the H/F-LV–transduced B-CLL cells on MS5 stroma, we demonstrated that stable transduction persisted (data not shown). We obtained high-level gene transfer, ranging from 20% to 45%, in quiescent B-CLL cells from 3 different patients without inducing entry into the G1b phase of the cell cycle (Figure 6D,G). As for healthy B cells, no toxic effect on cell survival of H/F-LV–transduced B-CLL cells was detected compared with the controls (percentage of cell survival, H/F-LV = 33% ± 11% vs VSVG-LV = 38% ± 15% and control = 40% ± 13%; P = .55; n = 6).
Novel H/F-LVs allow efficient gene transfer into quiescent and stimulated B-CLL cells. (A) B-CLL cells were verified for their cell-cycle state by simultaneously visualizing RNA content (pyronin-Y) and DNA content (7-AAD; left). SLAM surface staining of B-CLL cells on isolation was analyzed by FACS. Closed histograms represent staining with IgG isotype controls. The percentage of SLAM-expressing cells and MFI are indicated. Resting B-CLL cells were transduced with H/F-LVs and VSVG-LVs at an MOI of 10 and 50, respectively. At day 2 after transduction, GFP expression (B) and SLAM expression (C) were detected by FACS analysis. (D) Transduction of freshly isolated B-CLL cells from 3 different donors is shown. BCLL-1 and BCLL-3 cells were transduced with 3 different VSVG-LV and H/F-LV vector preparations as indicated, whereas for BCLL-2 only 1 vector prep was used for each vector type. (E) B-CLL cells were prestimulated for 48 hours with SAC/IL-2, and cell-cycle progression was monitored by pyronin-Y/7-AAD staining (left). Surface staining of SLAM by FACS is shown (right, open histogram). Closed histograms represent staining with IgG isotype controls. (F) SAC/IL-2 prestimulated B-CLL cells were transduced with H/F-LVs and VSVG-LVs at an MOI of 10 and 50, respectively. The plots show GFP expression of CD19+-transduced cells at day 2 after transduction. (G) Resting B-CLL cells were transduced with H/F-LVs and VSVG-LVs at an MOI of 10 and 50, respectively. At day 2 of transduction, B-CLL cells were verified for their cell-cycle state by simultaneously visualizing RNA content (pyronin-Y) and DNA content (7-AAD).
Novel H/F-LVs allow efficient gene transfer into quiescent and stimulated B-CLL cells. (A) B-CLL cells were verified for their cell-cycle state by simultaneously visualizing RNA content (pyronin-Y) and DNA content (7-AAD; left). SLAM surface staining of B-CLL cells on isolation was analyzed by FACS. Closed histograms represent staining with IgG isotype controls. The percentage of SLAM-expressing cells and MFI are indicated. Resting B-CLL cells were transduced with H/F-LVs and VSVG-LVs at an MOI of 10 and 50, respectively. At day 2 after transduction, GFP expression (B) and SLAM expression (C) were detected by FACS analysis. (D) Transduction of freshly isolated B-CLL cells from 3 different donors is shown. BCLL-1 and BCLL-3 cells were transduced with 3 different VSVG-LV and H/F-LV vector preparations as indicated, whereas for BCLL-2 only 1 vector prep was used for each vector type. (E) B-CLL cells were prestimulated for 48 hours with SAC/IL-2, and cell-cycle progression was monitored by pyronin-Y/7-AAD staining (left). Surface staining of SLAM by FACS is shown (right, open histogram). Closed histograms represent staining with IgG isotype controls. (F) SAC/IL-2 prestimulated B-CLL cells were transduced with H/F-LVs and VSVG-LVs at an MOI of 10 and 50, respectively. The plots show GFP expression of CD19+-transduced cells at day 2 after transduction. (G) Resting B-CLL cells were transduced with H/F-LVs and VSVG-LVs at an MOI of 10 and 50, respectively. At day 2 of transduction, B-CLL cells were verified for their cell-cycle state by simultaneously visualizing RNA content (pyronin-Y) and DNA content (7-AAD).
Alternatively, we stimulated the B-CLL cells through the BCR receptor, inducing cell-cycle entry into the G1b phase and up-regulation of SLAM surface expression (Figure 6E). Surprisingly, this allowed a transduction of 80% of the B-CLL population, whereas high doses (MOI = 50) of VSVG-LVs did not transduce these cells efficiently (Figure 6F).
In summary, this novel H/F lentivector system allowed stable gene transfer not only into normal quiescent B cells but also into quiescent and proliferating B-CLL cells.
Discussion
This is the first demonstration of a new lentivirus-derived tool that can efficiently transduce quiescent B cells in the absence of any other stimulus and this without inducing cell-cycle progression. These H/F-LVs achieved 50% transduction of resting adult B cells when counterpart classical VSVG-LVs remained refractory. The naive phenotype of the H/F-LV–transduced B cells was conserved, and no up-regulation of any activation marker was detected. No differential expression of the B7 molecule, CD86, was detected, implying that B-T interactions should stay intact. Of utmost importance, H/F-LVs are also the first tool allowing stable transduction of B-CLL cells, one of the most prominent leukemic cancers.18
Up to now gene transfer into primary human B cells has been notoriously difficult,1-3 and only very complex coculture systems or strong BCR activation or both allowed efficient LV transduction.1,10,19 In 2 reports LVs were developed displaying a CD20 scFv to target gene transfer to B cells.9,11 However, binding of the CD20-targeted vectors to B cells results in a proliferative stimulus through clustering of the BCR.20,21 In contrast, the H/F-LVs described here do not induce activation or cell entry of resting B cells and allowed very high levels of gene delivery without affecting B-cell phenotype. This is in agreement with our previous results showing that these vectors efficiently transduce resting T cells without inducing cell entry or phenotypic changes.12
Likewise, up to now, gene transfer into B-CLL cells was not satisfactory. Recombinant adenoviral vector transduction was inefficient because B-CLL cells lack the adenoviral receptor.22-24 Vectors based on recombinant adenoassociated virus mediated sufficient transgene expression in B-CLL cells; however, this needed a complex coculture with a feeder cell layer expressing CD40L.19,25 In contrast, the H/F-LV system allowed efficient and stable transduction of B-CLL cells without stimulation or need of helper cells. Finally, once stimulated through the BCR, close to 80% of the B-CLL cells were stably transduced, whereas a classical VSVG-LV did not allow efficient transduction.
Interestingly, we have shown that H/F-LV contact with the target B or T cells does not trigger or facilitate VSVG-LV entry, strongly suggesting that the 2 different vector pseudotypes exploit different entry mechanisms in these cells. Previously, we suggested that primary quiescent T cells need to express SLAM receptor to allow H/F-LV transduction.12 Here, this notion is reinforced because in a mixed PBMC population in which B cells express higher amounts of SLAM receptor than T cells, the former are indeed consistently better transduced by H/F-LVs, despite equivalent CD46 expression. Moreover, our current data suggest that both hCD46 and hSLAM receptors need to be engaged for binding or entry or both to allow efficient H/F-LV transduction of quiescent B cells as well as T cells (C.F., C.C., F.-L.C., and E.V., unpublished data, May 2009). B-CLL cells arrested in G0/early G1 phase of the cell cycle show a hCD46/hSLAM expression profile typical of resting B cells, allowing their H/F-LV transduction. After BCR stimulation, greater than 80% of transduction was achieved with these vectors in agreement with SLAM up-regulation in 90% of the B-CLL cells. Binding of H gps to SLAM/CD46 receptors on quiescent cells could activate the uncoating process of incoming viral particles. Alternatively, H/F-LVs might avoid interaction with postentry restriction factors26 using specific cell entry mechanisms, triggered by interaction through SLAM/CD46. It will be of importance to elucidate the processes facilitating quiescent cell transduction by H/F-LVs.
Finally, these H/F-LVs represent a new tool allowing highly stable gene transfer of primary quiescent B cells, T cells, and cancer B cells that now makes it possible to study with ease gene function and therapeutic gene transfer in these cells. In addition, H/F-LVs may facilitate production of monoclonal antibodies by allowing efficient transduction of primary memory B cells with BCL6 and BCL-XL genes inducing immortalization.27 These novel vectors may allow expression of costimulatory molecules such as CD40L or B7 molecules in nondividing tumor B cells such as B-CLL cells to elicit immune responses against gene-modified and unmodified cells.24,28 Thus, H/F-LVs may open the way to improved genetic vaccination strategies against cancer or infectious or autoimmune diseases, as well as gene therapy of genetic diseases.28-30
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Nicolas Rachinel for his help with B-CLL cells.
This work was supported by grants from the Agence Nationale pour la Recherche contre le SIDA et les Hépatites Virales (ANRS), the Agence Nationale de la Recherche (ANR), the European Research Council (ERC-2008-AdG-233130-HEPCENT), and the European Community (LSHB-CT-2004-005242 CONSERT and FP7-HEALTH-2007-B/222878 PERSIST). C.F. is supported by an ANRS postdoctoral fellowship.
Authorship
Contribution: C.F. and E.V. designed and performed research, analyzed data, and wrote the paper; C.C., C.L., and D.N. performed research; S.J.R., A.M., and K.-W.P. contributed vital new reagents; G.S. provided the B-CLL cells; and F.-L.C. designed research, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: François-Loïc Cosset, EVIR, Inserm U758, ENS de Lyon, 46 Allée d'Italie, 69364 Lyon Cedex 07, France; e-mail: flcosset@ens-lyon.fr; or Els Verhoeyen, EVIR, Inserm U758, ENS de Lyon, 46 Allée d'Italie, 69364 Lyon Cedex 07, France; e-mail: els.verhoeyen@ens-lyon.fr.
References
Author notes
F.-L.C. and E.V. contributed equally to this study.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal