Abstract
In multiple myeloma (MM), the impact of complete response (CR) could be shown only after introduction of high-dose therapy plus autologous stem cell transplantation (ASCT). In the context of ASCT, achieving CR (negative immunofixation and normal bone marrow) or at least very good partial response is associated with longer progression-free survival and in most studies longer survival. With novel agents, high CR rates are achieved and this prognostic impact of CR is being shown as well, both in relapsed and in newly diagnosed MM. However the benefit of CR achievement depends on the type of treatment and is not identical for all patients. In elderly patients, treatments inducing more CR may be more toxic. Although CR achievement is necessary in patients with poor-risk disease, it might not be as critical for long survival in more indolent MM. CR achievement is not the only objective of treatment because it is possible to further improve the depth of response and the outcome by continuing treatment after CR achievement. Finally, there are several levels of CR and in the future it will be necessary to confirm the prognostic impact of immunophenotypic or molecular CR or of CR defined by imaging procedures.
Introduction
In many hematologic malignancies, there is a correlation between the achievement of complete response (CR) and the outcome. This correlation has been shown for many years in acute leukemias or in high-grade lymphomas. In more chronic malignancies, such as follicular lymphomas or chronic lymphocytic leukemia, it was difficult to obtain CR with conventional chemotherapy. The correlation between a better quality of response and a better disease control was shown only when recent therapeutic improvements increased the CR rate.1-3
In multiple myeloma (MM), the CR rate was very low with conventional chemotherapy and the majority of clinical studies failed to show a significant influence of the degree of response on survival.4-6 The survival benefit for patients achieving CR could be demonstrated only in one large study from the Eastern Cooperative Oncology Group on 628 patients.7 In fact, the prognostic impact of CR achievement was considered mostly after introduction of high-dose therapy (HDT) plus autologous stem cell transplantation (ASCT).8,9 Compared with conventional chemotherapy, HDT increased not only the response rate but also the CR rate, which translated to at least a longer progression-free survival (PFS) in most randomized studies.10 More recently, high CR rates were obtained using novel therapies and the prognostic impact of CR achievement is being shown in this setting as well.11,12
Definition of complete response
When HDT was introduced in the treatment of MM, CR was first defined as the disappearance of the M-component on serum and/or urine electrophoresis with 5% or fewer plasma cells on bone marrow aspiration.13
Later, when large series of patients treated with HDT supported by ASCT became available, it appeared that it was possible to obtain negative immunofixation, which is more sensitive than electrophoresis. More recently, it has been shown that serum-free light chain assay allows for quantitative monitoring of MM patients, especially in oligosecretory MM.14 The different techniques used to define CR are shown in Table 1.
Techniques to measure response to treatment in multiple myeloma
| . | Comments . |
|---|---|
| Currently used techniques | |
| Bone marrow aspiration |
|
| Bone marrow biopsy |
|
| Serum/24-h urine electrophoresis (agarose gel or capillary zone) |
|
| Immunofixation (serum/urine) |
|
| Serum free light chain assay (and k/λ ratio) |
|
| Techniques under evaluation | |
| Imaging techniques (MRI, PET scan) |
|
| Bone marrow immunochemistry/fluorescence |
|
| Bone marrow immunophenotyping (multiparameter flow cytometry) |
|
| Bone marrow molecular remission assessment (allelic-specific oligonucleotide RQ-PCR) |
|
| . | Comments . |
|---|---|
| Currently used techniques | |
| Bone marrow aspiration |
|
| Bone marrow biopsy |
|
| Serum/24-h urine electrophoresis (agarose gel or capillary zone) |
|
| Immunofixation (serum/urine) |
|
| Serum free light chain assay (and k/λ ratio) |
|
| Techniques under evaluation | |
| Imaging techniques (MRI, PET scan) |
|
| Bone marrow immunochemistry/fluorescence |
|
| Bone marrow immunophenotyping (multiparameter flow cytometry) |
|
| Bone marrow molecular remission assessment (allelic-specific oligonucleotide RQ-PCR) |
|
MRI indicates magnetic resonance imaging; PET, positron emission tomography; RQ-PCR, real-time quantitative–polymerase chain reaction.
Two classifications of response to treatment are currently used. According to the European Group for Blood and Marrow Transplantation (EBMT) criteria for evaluating response, CR is defined by a negative immunofixation plus a normal bone marrow evaluation.15 Patients with a normal electrophoresis but a positive immunofixation are considered as having near-CR (n-CR).16 Because n-CR is sometimes difficult to assess, in the case of very small spikes (Figure 1A-B) or in the case of post-ASCT oligoclonal gammopathies17 (Figure 1C), the Intergroupe Francophone du Myelome introduced the concept of very good partial remission (VGPR).18 In the International Myeloma Working Group (IMWG) uniform criteria that have been adapted from the EBMT criteria and are currently proposed as a new standard,19 n-CR and VGPR have been brought together in the VGPR category and the level of stringent CR has been introduced. Currently used definitions of CR are shown in Table 2.
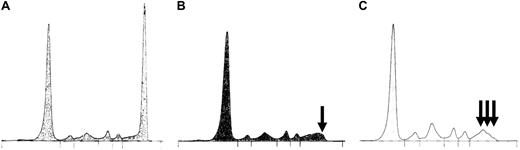
Complete response assessment by electrophoresis: examples of pitfalls. Patient with an initial spike in the gammaglobulins region (M-IgG) (A) was first considered in n-CR (positive immunofixation and normal electrophoresis). The tracing was reviewed by an independent expert committee who noticed the persistence of a small spike (B,  ) and reclassified this case as VGPR. (C) Post-ASCT oligoclonal gammopathy (
) and reclassified this case as VGPR. (C) Post-ASCT oligoclonal gammopathy (
 ). Because one of the small spikes corresponded to the same isotype as the original M-component, this patient was considered in VGPR.
). Because one of the small spikes corresponded to the same isotype as the original M-component, this patient was considered in VGPR.
Complete response assessment by electrophoresis: examples of pitfalls. Patient with an initial spike in the gammaglobulins region (M-IgG) (A) was first considered in n-CR (positive immunofixation and normal electrophoresis). The tracing was reviewed by an independent expert committee who noticed the persistence of a small spike (B,  ) and reclassified this case as VGPR. (C) Post-ASCT oligoclonal gammopathy (
) and reclassified this case as VGPR. (C) Post-ASCT oligoclonal gammopathy (
 ). Because one of the small spikes corresponded to the same isotype as the original M-component, this patient was considered in VGPR.
). Because one of the small spikes corresponded to the same isotype as the original M-component, this patient was considered in VGPR.
Current definition of complete remission
| . | EBMT criteria,* Bladé et al15 . | IMWG uniform criteria,† Durie et al19 . |
|---|---|---|
| sCR | ND | CR plus normal FLC ratio Absence of clonal plasma cells by Immunochemistry or fluorescence |
| CR |
| Same definition |
| VGPR | ND | Serum and urine M protein detectable by IFX but not on electrophoresis or 90% or greater reduction in serum M protein plus urine M-protein < 100 mg per 24 h |
| . | EBMT criteria,* Bladé et al15 . | IMWG uniform criteria,† Durie et al19 . |
|---|---|---|
| sCR | ND | CR plus normal FLC ratio Absence of clonal plasma cells by Immunochemistry or fluorescence |
| CR |
| Same definition |
| VGPR | ND | Serum and urine M protein detectable by IFX but not on electrophoresis or 90% or greater reduction in serum M protein plus urine M-protein < 100 mg per 24 h |
sCR indicates stringent CR; ND, not defined; CR, complete response; FLC, free light chain; IFX, immunofixation; and VGPR, very good partial response.
Maintained at least 6 weeks.
Two consecutive assessments before any new therapy.
Correlation between the depth of response and the outcome
The comparison of studies in which the prognostic impact of the depth of response has been evaluated is not that easy, because of different definitions of CR. In some studies CR has been stringently defined, whereas in others CR represents the addition of patients with CR and with near-CR. Recently, the achievement of at least VGPR according to IMWG uniform criteria has been more frequently used because it appeared as a simple and yet robust surrogate marker of the outcome and because it concerns more patients than CR, even with modern treatments using novel agents with or without ASCT.20 Another issue is the type of comparison: in some studies patients with CR (or CR plus VGPR) are compared with patients with partial remission, whereas in others they are compared with patients without CR (including patients with stable or progressive disease). Obviously the difference in favor of CR is more marked if patients with progressive disease are included in the comparator arm. Moreover, some studies were prospective and included homogeneously treated patients; others were retrospective analysis of patients treated more heterogeneously. Finally, some investigators consider that landmark analysis should be performed to correctly assess the impact of CR achievement and avoid biases related to the time necessary to achieve CR.15,21 .
Despite these concerns, the overall message is that there is a statistical correlation between maximal response and outcome. In the HDT plus ASCT paradigm, although some of the initial studies on small number of patients failed to show a correlation between CR achievement and a better outcome,22,23 the majority of studies on single15,24-29 or double ASCT20,21,30-32 in newly diagnosed patients demonstrated that achieving CR or at least VGPR was associated with a longer PFS and usually a longer overall survival (OS; Table 3). A recent review of retrospective and prospective studies on almost 5000 patients treated with HDT indicated a highly significant association between maximal response and long-term outcome.33 This correlation was also found in large series including relapsed patients.34,35
Impact of maximal response on the outcome in newly diagnosed patients treated with high-dose therapy
| Author . | No. of patients . | Comparison . | EFS/PFS . | OS . |
|---|---|---|---|---|
| Single ASCT | ||||
| Björkstrand et al24 | 130 | R | NA | OS benefit (CR vs non-CR) |
| Attal et al18 | 200 | P | NA | OS benefit (≥ VGPR vs PR) |
| Lahuerta et al25 | 344 | R | EFS benefit (CR vs n-CR, VGPR and PR; no difference between n-CR, VGPR and PR | OS benefit (CR vs n-CR VGPR, PR; no difference between n-CR, VGPR, PR) |
| Child et al26 | 407 | P | NA | OS benefit (CR vs PR) |
| Alvares et al27 | 387 | R | PFS benefit (CR vs non-CR) | OS benefit (CR vs non-CR) |
| O'Shea et al28 | 211 | R | EFS benefit (CR vs PR) | OS benefit (CR vs PR) |
| Lenhoff et al29 | 247 | P | EFS benefit (CR vs non-CR) | No OS benefit (CR vs non-CR) |
| Double ASCT | ||||
| Barlogie et al21 | 231 | P | EFS benefit (CR vs PR) | No OS benefit (CR vs PR) |
| Attal et al30 | 399 | P | NA | OS benefit (≥ VGPR) |
| Cavo et al31 | 321 | P | EFS benefit (CR plus n-CR) | OS benefit (CR plus n-CR) |
| Sonneveld et al32 | 303 | P | EFS and PFS benefit (CR) | No OS benefit of CR |
| Harousseau et al20 | 802 | P | EFS benefit (≥ VGPR vs PR) | OS benefit (≥ VGPR vs PR) |
| Author . | No. of patients . | Comparison . | EFS/PFS . | OS . |
|---|---|---|---|---|
| Single ASCT | ||||
| Björkstrand et al24 | 130 | R | NA | OS benefit (CR vs non-CR) |
| Attal et al18 | 200 | P | NA | OS benefit (≥ VGPR vs PR) |
| Lahuerta et al25 | 344 | R | EFS benefit (CR vs n-CR, VGPR and PR; no difference between n-CR, VGPR and PR | OS benefit (CR vs n-CR VGPR, PR; no difference between n-CR, VGPR, PR) |
| Child et al26 | 407 | P | NA | OS benefit (CR vs PR) |
| Alvares et al27 | 387 | R | PFS benefit (CR vs non-CR) | OS benefit (CR vs non-CR) |
| O'Shea et al28 | 211 | R | EFS benefit (CR vs PR) | OS benefit (CR vs PR) |
| Lenhoff et al29 | 247 | P | EFS benefit (CR vs non-CR) | No OS benefit (CR vs non-CR) |
| Double ASCT | ||||
| Barlogie et al21 | 231 | P | EFS benefit (CR vs PR) | No OS benefit (CR vs PR) |
| Attal et al30 | 399 | P | NA | OS benefit (≥ VGPR) |
| Cavo et al31 | 321 | P | EFS benefit (CR plus n-CR) | OS benefit (CR plus n-CR) |
| Sonneveld et al32 | 303 | P | EFS and PFS benefit (CR) | No OS benefit of CR |
| Harousseau et al20 | 802 | P | EFS benefit (≥ VGPR vs PR) | OS benefit (≥ VGPR vs PR) |
Summary of largest studies (> 100 patients).
EFS indicates event-free survival; PFS, progression-free survival; OS, overall survival; ASCT, autologous stem cell transplantation; R, retrospective; CR, complete response; P, prospective; VGPR, very good partial response; PR, partial response; n-CR, near complete response; and NA, not available.
The better quality of response and especially the higher CR rate with HDT is the most likely explanation for the superiority of HDT compared with conventional chemotherapy. In 6 randomized trials comparing conventional chemotherapy and HDT, the higher CR or CR plus VGPR rate in the HDT arm translated to a longer PFS15,26,36-39 (although the PFS benefit was not significant in one trial due to a small number of patients39 ). In only one study was there no significant increase in the CR rate and consequently no difference in PFS and OS between HDT and conventional chemotherapy40 (Table 4).
Randomized studies comparing conventional chemotherapy versus high-dose therapy
| Author (reference) . | No. of patients . | Age, y . | Median follow up . | CR rate, % . | Median EFS, mo . | Median OS, mo . | |||
|---|---|---|---|---|---|---|---|---|---|
| CC . | HDT . | CC . | HDT . | CC . | HDT . | ||||
| Attal et al18 | 200 | < 65 | 7 y | 5* | 22* | 18* | 28* | 44* | 57* |
| Fermand et al36 | 190 | 55-65 | 56 mo | 5* | 19* | 19* | 24* | 50 | 55 |
| Bladé et al39 | 164 | < 65 | 44 mo | 11* | 30* | 33 | 42 | 66 | 61 |
| Palumbo et al37 | 195 | < 70 | 39 mo | 6* | 25* | 15.6* | 28* | 42* | 58* |
| Child et al26 | 407 | < 65 | 42 mo | 8* | 44* | 19* | 31* | 42* | 54* |
| Fermand et al38 | 190 | 55-65 | 10 y | 20 CR + VGPR* | 48 CR + VGPR* | 19† | 25† | 48 | 48 |
| Barlogie et al40 | 516 | ≤ 70 | 76 mo | 15 | 17 | 7 y 14% | 7 y 17% | 7 y 38% | 7 y 38% |
| Author (reference) . | No. of patients . | Age, y . | Median follow up . | CR rate, % . | Median EFS, mo . | Median OS, mo . | |||
|---|---|---|---|---|---|---|---|---|---|
| CC . | HDT . | CC . | HDT . | CC . | HDT . | ||||
| Attal et al18 | 200 | < 65 | 7 y | 5* | 22* | 18* | 28* | 44* | 57* |
| Fermand et al36 | 190 | 55-65 | 56 mo | 5* | 19* | 19* | 24* | 50 | 55 |
| Bladé et al39 | 164 | < 65 | 44 mo | 11* | 30* | 33 | 42 | 66 | 61 |
| Palumbo et al37 | 195 | < 70 | 39 mo | 6* | 25* | 15.6* | 28* | 42* | 58* |
| Child et al26 | 407 | < 65 | 42 mo | 8* | 44* | 19* | 31* | 42* | 54* |
| Fermand et al38 | 190 | 55-65 | 10 y | 20 CR + VGPR* | 48 CR + VGPR* | 19† | 25† | 48 | 48 |
| Barlogie et al40 | 516 | ≤ 70 | 76 mo | 15 | 17 | 7 y 14% | 7 y 17% | 7 y 38% | 7 y 38% |
Significant.
Borderline significance.
y indicates years; CR, complete response; EFS, event-free survival; OS, overall survival; CC, conventional chemotherapy; HDT, high-dose therapy; mo, months; and VGPR, very good partial response.
The use of novel agents (thalidomide, bortezomib, lenalidomide) is also associated with a higher response rate, including CR or VGPR, compared with conventional treatment. In the setting of relapsed MM, large randomized trials comparing dexamethasone with either bortezomib or lenalidomide plus dexamethasone showed a significant superiority of the novel agent in terms of time to progression and OS, which was associated with a higher response rate and a higher CR rate.41-43 Subanalysis of the bortezomib and the lenalidomide arms confirmed that the CR achievement in these arms was associated with a significantly longer time to next therapy, PFS, and even OS with lenalidomide-dexamethasone.11,12
In another large randomized trial comparing bortezomib alone and the combination of bortezomib plus pegylated liposomal doxorubicin, the longer PFS in the combination arm (9.3 months vs 6.5 months) might be related to a significantly higher CR plus VGPR rate (27% vs 19%), although there was no subgroup analysis of the effect of response on outcome.44
For newly diagnosed patients, 2 published randomized trials have shown that compared with melphalan-prednisone (MP), the combination of MP plus thalidomide yielded superior PFS.45,46 . In both studies, the CR and the CR plus VGPR rates were superior as well in the combination arm. In the Vista randomized trial, the combination of MP with bortezomib was superior to MP for all efficacy parameters, including the CR rate.47 In the bortezomib arm, the CR rate was 30% (EBMT criteria), which is quite comparable with CR rates achieved with HDT. Therefore it was possible to assess the impact of CR achievement in this arm, and again patients achieving CR had significantly longer PFS that patients achieving only PR.48 However, the better tumor reduction obtained in the bortezomib arm did not translate yet into an OS advantage, due to a short follow-up and an effective salvage in the MP arm.
Is the benefit of CR achievement identical in all patients?
CR achievement is usually associated with a longer PFS compared with PR in all situations where the CR rate is high enough (HDT, novel agents both in the relapse setting and in newly diagnosed patients). However, the benefit in OS is not that clear.
First, in some studies, patients achieving CR had a longer PFS but no improvement of OS compared with patients achieving less than VGPR.21,29,32 Moreover, in some randomized trials, although in the best arm, the CR rate improvement was associated with a PFS prolongation, the OS was not significantly different either in the ASCT setting31,32,36,38 or with novel agents.49,50 This is often explained by a better salvage in the control arm. Another explanation might be that, at the time of publication, the follow-up time was too short to show significant differences in OS. For instance, in the first publication of the randomized Total Therapy 2 program from the Arkansas group, the addition of thalidomide throughout the whole treatment (induction, consolidation, and maintenance) yielded significantly more CR and longer PFS.49 But there was no OS benefit in the thalidomide arm due to a better salvage therapy with longer survival after relapse in the no-thalidomide arm. However, with longer follow-up, the OS curves diverged after 5 years and OS became better in the thalidomide arm, especially in patients with cytogenetic abnormalities.51
The impact of CR achievement also depends on the type of treatment
The best example is the Vista trial.47 The median CR duration was 24 months in the 111 patients achieving CR with melphalan, prednisone, bortezomib versus only 12.8 months in the 13 patients achieving CR with MP. This probably means that the quality of CR achieved with melphalan, prednisone, bortezomib was better than CR achieved with MP. Another example is the IFM94 trial comparing single and double ASCT.30 Although on an intent-to-treat analysis the event-free survival and OS were significantly longer in the double ASCT arm, there was no significant difference in the CR plus VGPR rate between the 2 arms. The survival benefit might be related to an increased number of deeper responses in the more intensive arm, and when focusing on patients who did actually undergo double transplantation, the CR plus VGPR rate was indeed significantly superior in the double transplantation arm (63% vs 49%).
The impact of CR depends on the age of patients
In elderly patients, there are several studies showing that a given treatment increases the CR rate without improving even PFS. This was the case in the ASCT setting in the IFM99-06 published by the IFM.46 In this 3-arm randomized trial, the CR plus VGPR rate achieved in the intensive arm (with 2 courses of intermediate-dose melphalan supported by ASCT) was comparable with that achieved in the MP-thalidomide arm (43% and 47%, respectively) and significantly better than that achieved in the MP arm (7%). Yet, PFS was significantly superior in the MP-thalidomide arm than in the intensive arm (median PFS, 27.5 months vs 19.4 months) and there was no difference between the intensive arm and the MP arm (17.8 months). This could partly be explained by a poor feasibility of the intensive arm in this population of patients aged 65 to 75 years because only 65% could actually receive the whole planned treatment. The same was true in the randomized trial comparing thalidomide-dexamethasone and MP in elderly patients.52 The thalidomide-dexamethasone yielded superior response rate including CR rate (26% vs 13%) but there was no significant difference in PFS due to a higher toxic death rate, particularly in patients older than 75 years with poor performance status, and due to a poorer compliance to treatment in the thalidomide-dexamethasone arm. Therefore in elderly or frail patients, the use of more effective but more aggressive treatments may induce more frequent and more severe toxicities, which may overcome the potential benefit of CR achievement. This result has been previously observed in other malignancies. For instance, treatment of elderly patients with acute myeloid leukemia with conventional cytotoxic chemotherapy induced more CR than low-dose cytarabine but OS was not superior due to higher toxic death rate.53
Finally, the impact of CR depends on the clinical presentation and on the biology of MM
According to the Arkansas group's experience with tandem ASCT, patients with history of previous monoclonal gammopathy or of smoldering MM may have extended survival without achieving CR. Patients presenting initially with a smoldering course had substantially lower CR rates than the remainders but the median survival of the 2 groups was identical.54 This observation should however be reanalyzed in the context of the recent publication showing that a monoclonal gammopathy precedes MM in most patients.55 In addition, when examining CR rates and survival according to gene expression profiles defining monoclonal gammopathy of unknown significance (MGUS)–like versus non–MGUS-like MM, survival was identical despite a lower CR rate in the former group.56 This could mean that with intensive treatment, a MGUS-like status can be re-established in these patients with destruction of the more proliferative clone but with the persistance of a subclone of slowly dividing cells that are resistant to therapy. Similarly, in the IFM99 trials all patients received double ASCT.20 In International Staging System (ISS) stages 2 and 3, patients achieving at least VGPR had significantly longer PFS and OS than patients achieving only PR, whereas in patients with ISS stage 1, there was no significant impact of CR or VGPR achievement. These results suggest that CR or VGPR achievement may not be critical for long OS in patients with indolent or nonaggressive forms of MM. On the contrary, patients with t(4;14) or with del(17p) have a poor prognosis after ASCT57-60 even with tandem ASCT.61 These patients may actually achieve CR but relapses are usually very rapid.60 Therefore the benefit of CR has been questioned in this subgroup of aggressive MM. Other aggressive forms of MM can be defined by a rapid decrease of the initial tumor burden, suggesting a high proliferative activity.61 However, both the Arkansas and the IFM groups have shown that CR (or CR plus VGPR) achievement is useful in poor-risk MM.20,62 In the IFM99 trials, median PFS and OS were significantly better in patients with t(4;14) and/or del(17p) achieving at least VGPR (respectively, 2.1 years and 4.8 years) than in patients with only PR (respectively, 1.2 years and 2.1 years.20 Analysis of the intensive programs at Arkansas University suggested that stringently defined CR was critical for EFS and OS only in a small subgroup of patients with poor prognosis, as defined by gene expression profiling.63,64 These findings could mean that in more aggressive MMs, which are usually characterized by a more proliferative clone, elimination of this clone is necessary to obtain prolonged response.
Is CR achievement the major objective of treatment in MM?
One frequently asked question is whether it is necessary to continue treatment once CR is achieved, or, in other words, what is the impact of consolidation of maintenance treatments? The first aspect of this question is the role of HDT as consolidation treatment in patients who obtained CR with induction therapy. In the literature there are conflicting reports. In some studies, there was no significant survival benefit for patients achieving CR before ASCT compared with patients achieving CR only after ASCT20,65,66 as if the potential benefit of initial therapy was overcome by the power of HDT. In other studies, the outcome was better for patients achieving CR before ASCT.24,27 This discrepancy could be explained partly by the low CR rate obtained with conventional chemotherapy induction regimens. With novel agents, the CR/VGPR rate has increased dramatically and the impact of CR achievement before ASCT, if any, will be easier to demonstrate.
The objective of consolidation/maintenance could be to improve the quality of CR by further decreasing the number of residual tumor cells. Before the introduction of novel agents, CR was obtained mostly with HDT and it was probably difficult to further improve the level of CR because the number of active drugs was limited. The only way was to repeat HDT. However, the impact of the second ASCT has been a matter of debate. Whereas the Arkansas group stated that performing 2 ASCTs in a timely fashion was an important prognostic factor,21,67 subanalysis of the IFM and the Italian randomized trials evaluating double ASCT showed that in patients achieving CR or VGPR after the first ASCT, there was no OS benefit from the second.30,31
The role of post-ASCT maintenance is not yet clarified. Two randomized studies have shown that post-ASCT treatment with thalidomide increases the CR plus VGPR rate, the PFS, and the OS,68,69 but in one of them the survival benefit was observed only in patients who were not at least in VGPR after ASCT.68 This could mean that the most important role of this type of maintenance is not to control disease by reducing minimal residual tumor but more simply to obtain more CR.
However, more recently, a larger use of novel agents in the post-ASCT phase of treatment suggests that it is possible not only to further increase the proportion of CR patients70 but also to further decrease the tumor cell burden in patients who already have obtained CR or VGPR. For instance, the Italian group has shown that in patients achieving at least VGPR after ASCT, it is possible to improve the level of response and obtain molecular remission using consolidation with thalidomde-bortezomib-dexamethasone.71 Therefore, in the era of novel therapies, achieving CR should not be the only objective, because it might be possible to prolong CR by improving the depth of response using these agents.
Which level of response is necessary?
The current definitions of CR are based on serologic and cytologic results. The most convenient level is VGPR that is simply defined by 90% reduction of the M-component in the serum (or less than 100 mg in the 24-hour urines). This level is currently frequently used because it has been repeatedly associated with a better PFS and OS in the ASCT setting15,20,30 and because it may apply to more patients than CR even in the era of novel agents.20 With HDT, CR was achieved in less than half of the patients, not more than 25% to 30% of cases with single ASCT and 30% to 45% with double ASCT, whereas the addition of CR plus VGPR concerned 50% to 55% of cases.10 The introduction of novel therapies increases the CR rates. In frontline therapy, combinations of MP with thalidomide, bortezomib, or lenalidomide yielded CR rates of 13% to 33% but CR/VGPR was achieved in 41% to 48% of patients.45-47,72 With prolonged treatment with the combination of lenalidomide plus dexamethasone, CR was achieved in 39% of cases, and 74% of patients had at least a 90% decrease of the M-component.73 The same is true in the context of ASCT plus novel agents. Induction treatments with thalidomide or bortezomib plus dexamethasone yield 6% to 19% CR and 19% to 46% CR/VGPR before ASCT.74-76 With the addition of a third agent, post-ASCT CR rates may reach 15% to 40%, whereas CR/VGPR rates are 50% to 75%.77-80 However, it is clear that compared with VGPR, the CR level is associated with a better outcome.25,81 Currently, CR defined by the absence of M-component on immunofixation and a normal bone marrow negative is the most widely accepted level. Serum free-light chain assay, which has been introduced more recently, may help to assess response in patients with oligo or nonsecretory MM.82 The IMWG has introduced the concept of stringent CR defined by a normal bone marrow by immunocytochemistry or immunofluorescence plus a normal serum free-light chain kappa-lambda ratio,19 but this has not yet been evaluated in large multicentric studies.
An important aspect is that measurement of the M-component or of the serum-free light chains only reflects the product of the secreting clone and probably not all malignant cells are secretory.
Other methods may better evaluate the tumor cell compartment. Suppression of karyotype abnormalities as evaluated by cytogenetic monitoring may predict superior outcome,83 as in acute leukemias. However, interest in this technique is doubtful because standard karyotype is informative in only 30% of patients in MM.
Molecular remissions are associated with long-term remissions and possibly with cure.84,85 This level has been achieved almost exclusively in the context of allogeneic stem cell transplantation in a very small subset of patients. However, in the near future, molecular remissions might be achieved in more patients with the combination of HDT and novel agents.71 This procedure is cumbersome, expensive, and currently available only in a limited number of specialized centers.
Compared with molecular remission, assessment of immunophenotypic CR by multiparameter flow cytometry is slightly less specific and sensitive but is applicable in more patients and less time-consuming.86 In the experience of investigators from Spain, in the context of HDT, achievement of immunophenotypic remission is the most relevant prognostic factor for PFS in multivariate analysis.87 Within the group of patients with negative immunofixation, the absence of residual disease by multiparameter flow cytometry was associated with a significantly better outcome.
Because more profound responses are likely to occur with new therapeutic strategies, it will be necessary to evaluate the clinical relevance of these levels of CR in future clinical trials.
Finally, the observation of persistant focal lesions on magnetic resonance imagery in CR patients also indicates that serologic methods are not sensitive enough to precisely evaluate the residual disease.88 An alternative explanation might be that these focal lesions, which may be the first site of recurrence, contain cells that are nonsecretory (possibly myeloma stem cells). Therefore, a more precise approach of the CR status might require an imaging technique (magnetic resonance imagery or positron emission tomography–computed tomography scan) in addition to serologic, cytologic, and possibly immunophenotypic analysis.
Conclusions
Like in other chronic hematologic malignancies (chronic lymphocytic leukemia or indolent lymphomas), CR achievement might not be the principal objective of treatment in elderly and frail patients or in patients with ISS stage 1 disease, especially when the treatment is potentially toxic
In all other patients, CR achievement is not just a cosmetic89 finding and is statistically correlated with a better outcome. It is a valid surrogate marker of the treatment efficacy and should be one objective of any therapeutic strategy, even in patients with poor-risk disease. However, the final objective is not only to achieve CR but also to maintain CR by further reducing the tumor cell mass including cells that do not secrete the M-component.90
Future studies are needed to determine the optimal way to define CR in the era of novel therapies, which, in addition to cytotoxic agents, do increase the proportion and the duration of CR. One can imagine that in the near future it will be possible to obtain deeper CR and that the objective of treatment will be to obtain phenotypic responses as in chronic lymphocytic leukemia91 or even molecular remission as in chronic myeloid leukemia.92 Monitoring of the CR level would then help to define therapeutic strategies and to modify or prolong treatments when the required level is not (or no longer) obtained.
Authorship
Contribution: J.-L.H., M.A., and H.A.-L. wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Jean-Luc Harousseau, Centre René Gauducheau, Bd Jacques Monod 44850, St Herblain, France; e-mail jl-harousseau@nantes.fnclcc.fr.