Abstract
Graft-versus-host disease (GVHD) is a major cause of morbidity and mortality in patients with hematologic malignancies undergoing allogeneic hematopoietic stem cell transplantation. Current treatment of GVHD relies on immunosuppressive regimens, considerably increasing the incidence of opportunistic infections. As T cells mediate both GVHD as well as protection against viral infections and the malignant disease, strategies to selectively target host-reactive T cells without impairing pathogen- and disease-specific immunity are highly warranted. Activation of T cells is accompanied by increased expression of the chaperone heat shock protein of 90 kDa (Hsp90), which stabilizes several key signaling pathways crucial for T-cell activation. In this study, selective targeting of Hsp90 in activated T lymphocytes with pharmacologic inhibitors already applied successfully in anticancer therapy resulted in induction of apoptosis predominantly in activated cells. Moreover, if T cells were stimulated with allogeneic dendritic cells, alloreactive T cells were selectively eliminated. In contrast, third party reactions including antiviral T-cell immunity were quantitatively and functionally fully preserved. These data suggest that Hsp90 represents a novel target for selective depletion of alloreactive T cells, and provide the rationale for application of Hsp90 inhibitors as potential approach to selectively prevent and treat GVHD in hematopoietic stem cell transplantation recipients without impairing pathogen- and disease-specific T-cell immunity.
Introduction
Allogeneic hematopoietic stem cell transplantation (HSCT) is often the only curative approach for patients with hematologic malignancies. Nevertheless, the success of HSCT is severely challenged by acute graft-versus-host disease (GVHD) mediated by host-reactive, donor-derived T cells.1-3 Although extensive posttransplant immunosuppressive therapy as well as rigorous T-cell depletion of the allograft help to reduce the incidence of acute GVHD, the thereby created immunodeficient environment favors recurrence of the malignant disease as well as infectious complications.3 As T-cell reconstitution is a key determinant to the outcome of HSCT, more specific approaches to selectively deplete host-reactive donor lymphocytes in stem cell allografts are highly warranted. Ex vivo stimulation of donor lymphocytes with host-derived antigen-presenting cells, followed by selective removal of the alloreacting T cells, can be achieved by using beads or antibodies against surface antigens of recent activation (eg, CD25, CD69, CD71, or CD137), photodynamic purging strategies targeting activation-based changes in p-glycoprotein, or the induction of anergy by costimulatory blockade.4,5 Although all these approaches could prove their experimental feasibility, clinical data on selective allodepletion are very limited and largely based on the use of anti-CD25 immunotoxins.6,7 Instability of surface marker expression has been postulated to be in part responsible for the appearance of residual GVHD after CD25-based selective allodepletion. Therefore, novel strategies are highly warranted to improve the outcome of selectively depleted allotransplants.
Activation and proliferation of T cells are orchestrated by signal transduction cascades involving the activation of several key signaling intermediates such as Raf-1, Akt, Lck, signal transducers and activators of transcription (STATs), and Janus kinases (Jaks).8 Many of the same signaling pathways are also active in malignant cells and are stabilized by the molecular chaperone heat shock protein of 90 kDa (Hsp90).9 The central role of Hsp90 for survival of tumor cells has been demonstrated with pharmacologic inhibitors that block the intrinsic adenosine triphosphatase activity of the chaperone, resulting in induction of apoptosis in various cancer cell types.10,11 Furthermore, treatment of cancer patients with the Hsp90 inhibitors 17-allylamino-17-demethoxygeldanamycin and 17-(dimethylaminoethylamino)-17-demethoxygeldanamycin(17-DMAG) has already shown promising results in phase I/II clinical trials.10,11 Thus, the underlying hypothesis of this study is that activated T cells, just as malignant cells, are especially dependent on Hsp90 function to stabilize signal transduction after T-cell activation by T-cell receptor engagement. In this study, we show that concomitant abrogation of multiple signaling pathways by blockage of Hsp90 can specifically eliminate alloreactive cells in primary human T-lymphocyte populations without impairing beneficial third party immunity such as T-cell responses to viral antigens.
Methods
Blood donors and cell culture
Blood from healthy donors was obtained after informed consent in accordance with the Declaration of Helsinki, under a protocol that received Institutional Review Board approval from Universitaetsklinikum Wuerzburg, and peripheral blood mononuclear cells (PBMC) were isolated via centrifugation in Biocoll Separating Solution (Biochrom). Cells were cultured in RPMI 1640 with l-glutamine, supplemented with 10% heat-inactivated, pooled human serum, and 100 U/mL penicillin-streptomycin (all from Invitrogen). Lymphocyte cultures were supplemented with 10 U/mL interleukin (IL)-2 (Proleukin; Chiron) every other day, and culture medium was replenished, as needed. CD3+, CD4+, or CD8+ T lymphocytes were isolated from whole PBMC by positive selection using magnetic cell-sorting techniques (Miltenyi Biotec).
Immunoblot analysis of Hsp90 protein levels and STAT5, STAT3, Akt, and extracellular signal-regulated kinase 1,2 phosphorylation
Hsp90α and β protein levels were determined in whole-cell lysates of isolated CD4+ and CD8+ T cells after activation with 10 μg/mL phytohemagglutinin (PHA; Sigma-Aldrich) and 50 U/mL IL-2 for 48 hours, and in untreated control cells or in CD25-positive and CD25-negative fractions of mixed lymphocyte reactions (MLRs) separated on day 6 of the culture using magnetic cell-sorting techniques (Miltenyi Biotec). Cell pellets were lysed and Western blot analysis was performed using antibodies directed against human Hsp90α and Hsp90β (AB3466 and AB3468; Chemicon International) and vinculin (c-25336; Santa Cruz Biotechnology), according to standard protocols. To determine phosphorylation of STAT3, STAT5, Akt, and extracellular signal-regulated kinase (ERK)1,2, isolated CD3+ T cells were preincubated with 5 μM 17-DMAG for 43 hours and subsequently activated with CD3/CD28 Dynabeads (Invitrogen) for 1 hour, according to manufacturer's instructions. Western blot analysis was performed after lysis of cell pellets using antibodies against total STAT5, STAT3, Akt (9363, 9132, and 9272; Cell Signaling Technology), or ERK1,2 (442675; Calbiochem), or antibodies specific for Tyr694-phosphorylated STAT5, Tyr705-phosphorylated STAT3, Ser473-phosphorylated Akt, or Thr202/Tyr204-phosphorylated ERK1,2 (9359, 9131, 4058, and 9101; Cell Signaling Technology). An anti–β-actin antibody (A5316; Sigma-Aldrich) was used to assess equal loading.
Assessment of apoptosis in resting and activated T lymphocytes
CD4+ and CD8+ T cells were activated with 10 μg/mL PHA for 48 hours, washed, and cultured in the presence of 0 to 10 μM 17-DMAG (ant-dgl-1; InvivoGen) for 68 hours. Apoptosis was assessed by flow cytometric analysis of annexin V/propidium iodide–stained cells (Abcam).
T-cell activation, MLRs, and 5-(and 6-)carboxyfluorescein diacetate succinimidyl ester dilution experiments
PBMC were 5-(and 6-)carboxyfluorescein diacetate succinimidyl ester (CFSE) labeled with Vybrant CFDA SE Cell Tracer Kit (Invitrogen) and activated either with 10 μg/mL PHA, CD3/CD28 Dynabeads, or allogeneic dendritic cells (DC). DC were generated within 3 days from whole PBMC, according to the protocol previously described by Dauer et al,12 and matured by addition of 25 ng/mL tumor necrosis factor-α, 5 ng/mL IL-1β, 10 ng/mL IL-6 (all R&D Systems), and 1 μg/mL prostaglandin E2 (Pharmacia) for 24 hours to the culture medium. MLRs were established by mixing CFSE-labeled PBMC with mature DC from an allogeneic donor in a ratio of 10:1. Cultures were either left untreated or treated with 10 μM 17-DMAG (administered 48 hours after culture initiation). Alternatively, CFSE-labeled PBMC were electroporated with negative control small interfering RNA (siRNA) or Hsp90β-specific siRNA, as described below, before addition of allogeneic DC. Flow cytometric analysis was performed 4 days after culture initiation. For some experiments, CD45RO-negative and CD45RO-postive T cells were separated using a naive CD8+ T-cell isolation kit (Miltenyi Biotec) before CFSE labeling. For other experiments, 10 μM 17-DMAG was added to MLRs 4 days after culture initiation, and the cells were stained with annexin V 40 hours after inhibitor treatment and analyzed flow cytometrically.
Targeting of Hsp90β-specific mRNA with siRNA and mRNA quantification by real-time polymerase chain reaction
Negative control siRNA (OR-0030-Neg05) or Hsp90β-specific siRNA (5′→3′-AUUCUUGUCGGCCUCAGCCdTdT; both from Eurogentec) was introduced into T cells by electroporation. The transfection protocol for primary human T cells was optimized with nerve growth factor receptor (NGFR)–coding mRNA, and cell viability and transfection efficiency were estimated by flow cytometric enumeration. For Hsp90β knockdown, 5 × 106 cells were preincubated on ice in 200 μL of OPTI-MEM I (Invitrogen) with 40 μM siRNA and electroporated using a Equibio EasyjecT plus device (450 μF, 350 V). After transfection, the T cells were either left untreated or activated with 10 μg/mL PHA for 24 hours. Knockdown was determined by real-time polymerase chain reaction (PCR) in a Bio-Rad DNA Engine Opticon TM2 device using a MESA GREEN quantitative PCR kit (Eurogentec) and the primers Hsp90 forward (5′→3′-GAGAGCCTGACAGACCC) and Hsp90 reverse (5′→3′-GCCCAATCATGGAGATGT). β-Microglobulin was used as reference gene (forward primer, 5′→3′-GGGTTTCATCCATCCGACAT and reverse primer, 5′→3′-GATGCTGCTTACATGTCTCGA).
Major histocompatibility complex–pentamer staining and interferon-γ enzyme-linked immunosorbent assay of MLRs
MLRs of PBMC from a cytomegalovirus (CMV)-seropositive donor and allogeneic, matured DC at a ratio of 10:1 were cultivated for 8 days either without inhibitor or in the presence of 5 μM 17-DMAG from day 2 to day 5 of the culture period. Antigen-specific T cells were quantified on day 6 with a major histocompatibility complex (MHC) class I pentamer specific for the CMV antigen pp65 (A*0201 NLVPMVATV; Proimmune). Recall responses of CMV-specific memory T cells were determined by restimulation of the cultures on day 8 with autologous monocytes pulsed with 1 μg/mL overlapping 15-mer CMVpp65 peptide pool (PepMix pp65; JPT Peptide Technologies) at a ratio of 5:1. Responses of alloreactive T cells were determined by restimulation with the same allogeneic, matured DC that initially triggered the alloresponse at a ratio of 10:1. Supernatants were collected after 24 hours, and sandwich enzyme-linked immunosorbent assay was performed using anti-interferon (IFN)-γ antibodies (M-700A and M-701B; Perbio Science), according to standard protocols.
Statistical analysis
Graphs and statistical analyses were performed with the use of Prism 5.01 for Windows software (GraphPad Software). P values up to .05 were considered significant.
Results
Inhibition of Hsp90 specifically induces apoptosis in activated T lymphocytes
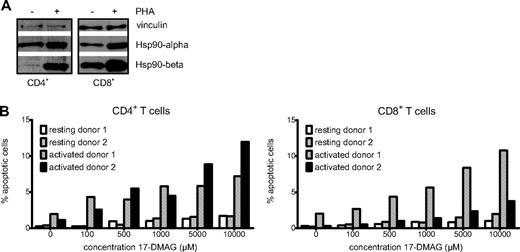
The molecular chaperone Hsp90 has been shown to stabilize many key signaling intermediates8 and to exert antiapoptotic effects in various cell types,13-15 strongly suggesting a similar function in primary human T lymphocytes. First, we addressed the question as to whether expression of both Hsp90 isoforms α and β in T lymphocytes is activation-dependent as in other cell types.16 Western blot experiments performed with isolated CD4+ and CD8+ T cells showed a strong up-regulation of both Hsp90α as well as Hsp90β after activation with PHA for 48 hours. Of note, the up-regulation of Hsp90β was more pronounced (Figure 1A). This finding is in good accordance with previous observations showing induction of Hsp90 expression by mitogens and IL-2, a key mediator of T-cell signaling.16 Interestingly, CD4+ lymphocytes display a stronger induction of Hsp90 protein expression after activation than CD8+ cells.
The Hsp90 chaperone is up-regulated upon T-lymphocyte activation and necessary for survival of activated cells. (A) Western blot analysis showing Hsp90α and β protein levels of resting and PHA-activated CD4+ and CD8+ T lymphocytes with vinculin as loading control. (B) Diagrammatic representation of the proportion of apoptotic cells in resting and activated CD4+ and CD8+ T-cell cultures of 2 donors treated with increasing concentrations of the Hsp90 inhibitor 17-DMAG.
The Hsp90 chaperone is up-regulated upon T-lymphocyte activation and necessary for survival of activated cells. (A) Western blot analysis showing Hsp90α and β protein levels of resting and PHA-activated CD4+ and CD8+ T lymphocytes with vinculin as loading control. (B) Diagrammatic representation of the proportion of apoptotic cells in resting and activated CD4+ and CD8+ T-cell cultures of 2 donors treated with increasing concentrations of the Hsp90 inhibitor 17-DMAG.
This observed up-regulation of Hsp90 protein levels induced by T-cell activation and the known antiapoptotic function of Hsp90 suggested that Hsp90 blockade could potentially differentially affect viability of activated compared with resting cells. To investigate whether activated T lymphocytes are more prone to apoptotic cell death in the presence of an Hsp90 inhibitor than resting cells, we performed annexin V/propidium iodide stainings of isolated CD4+ and CD8+ lymphocytes treated with increasing concentrations of 17-DMAG (Figure 1B). Whereas inhibitor treatment had only little effect on cell viability in resting lymphocytes, cells activated with PHA exhibited distinctly higher apoptosis rates. Furthermore, flow cytometric analysis of T cells stained with antibody for the lymphocyte activation marker CD25 and annexin V revealed that the activated T-cell population with high CD25 expression is clearly reduced by inhibitor treatment. In contrast, CD25-low or -negative lymphocytes are much less sensitive to 17-DMAG (data not shown). Thus, targeting Hsp90 results in an increased rate of apoptosis, especially in activated T cells.
Hsp90 inhibition impairs T-cell activation-induced signal transduction
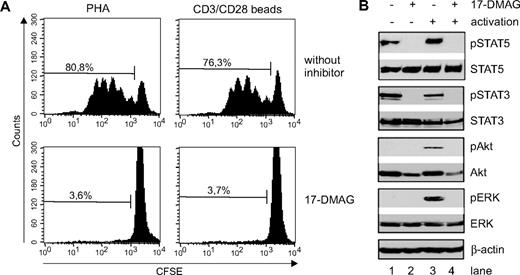
Next, we analyzed the role of the Hsp90 chaperone function for the signal transduction in activated primary T cells. T-cell activation-induced signaling is initiated by the concomitant stimulation of both the T-cell receptor CD3 and the coreceptor CD28. Therefore, T cells were activated with CD3/CD28 beads instead of mitogens to mimic physiologic stimulation and subsequently treated with 17-DMAG. To ensure that proliferation of the CD3/CD28-activated T cells is also inhibited by Hsp90 blockade, side-by-side comparison of mitogen and CD3/CD28-activated T cells was performed. As shown in Figure 2A, both activation protocols led to substantial proliferation, as measured by CSFE dilution. Treatment of both cultures resulted in equal reduction of CSFEdim cells, demonstrating that CD3/CD28-activated T cells are as prone to 17-DMAG effects as mitogen-activated T cells.
Hsp90 inhibition abrogates T-cell proliferation and phosphorylation of STAT5, STAT3, Akt, and ERK1,2 in activated primary CD3+ T lymphocytes. (A) CFSE dilution after 4 days in T-cell cultures activated either with PHA or CD3/C28 microbeads in control cultures or cultures treated with the Hsp90 inhibitor 17-DMAG. (B) Primary human CD3+ T cells were either left untreated (lanes 1,3) or treated with 17-DMAG (lanes 2,4) for 43 hours before activation with CD3/CD28 Dynabeads for 1 hour (lanes 3-4), and analyzed by Western blot. The protein expression levels of phosphorylated (Y694) and total STAT5, phosphorylated (Y705) and total STAT3, phosphorylated (Ser473) and total Akt, and phosphorylated (Thr202/Thr204) and total ERK1,2 are shown. Staining of β-actin was used as loading control.
Hsp90 inhibition abrogates T-cell proliferation and phosphorylation of STAT5, STAT3, Akt, and ERK1,2 in activated primary CD3+ T lymphocytes. (A) CFSE dilution after 4 days in T-cell cultures activated either with PHA or CD3/C28 microbeads in control cultures or cultures treated with the Hsp90 inhibitor 17-DMAG. (B) Primary human CD3+ T cells were either left untreated (lanes 1,3) or treated with 17-DMAG (lanes 2,4) for 43 hours before activation with CD3/CD28 Dynabeads for 1 hour (lanes 3-4), and analyzed by Western blot. The protein expression levels of phosphorylated (Y694) and total STAT5, phosphorylated (Y705) and total STAT3, phosphorylated (Ser473) and total Akt, and phosphorylated (Thr202/Thr204) and total ERK1,2 are shown. Staining of β-actin was used as loading control.
To address the question as to which key signaling pathways are stabilized by Hsp90 and abrogated by 17-DMAG treatment, we analyzed the phosphorylation of STAT5, STAT3, ERK1,2, and Akt in CD3/CD28-activated T cells either treated with or without the Hsp90 inhibitor 17-DMAG. Western blot analyses revealed the basal and the phosphorylated protein levels of STAT5, STAT3, ERK1,2, and Akt (Figure 2B). T-cell activation did not alter basal protein expression levels of STAT5, STAT3, ERK1,2, and Akt, but induced phosphorylation of ERK1,2 and Akt, increased phosphorylation of STAT5, and slightly reduced STAT3 phosphorylation, indicating the involvement of STAT5, ERK1,2, and Akt, but not STAT3 in T-cell activation-induced signaling. Interestingly, inhibition of Hsp90 by treatment with 17-DMAG completely abrogated the phosphorylation of STAT5, STAT3, and ERK1,2, and reduced both basal and phosphorylation levels of Akt. Thus, pharmacologic targeting of Hsp90 effectively abrogates phosphorylation of key signaling components, leading to impairment of the respective T-cell activation-induced signaling pathways (Jak/STAT5, Ras/mitogen-activated protein kinase kinase 1,2/ERK1,2, and the phosphatidylinositol 3-kinase/Akt).
Hsp90 inhibition abrogates proliferation of alloreactive T cells
In the next set of experiments, we addressed the question as to whether T cells activated by allogeneic stimulator cells can be specifically eliminated by Hsp90 blockade. At first, we confirmed that T cells activated by allogeneic stimulation up-regulated both Hsp90α and β subunit. Alloreactive T cells were identified by initiating a MLR and isolating the alloreactive T cells based on their CD25 expression (Figure 3A-B). To analyze the effect of Hsp90 inhibition on proliferation of alloreactive T cells, we stimulated CFSE-labeled PBMC with allogeneic irradiated DC in a ratio PBMC:DC of 10:1. As shown in Figure 3D, standard MLR conditions resulted in a robust alloresponse, with multiple cell divisions detectable at day 4. In contrast, in cultures treated with 17-DMAG, a significant T-cell proliferation was not detectable. Statistical analysis of a total of 9 separate experiments showed a significant (P < .001) reduction in alloreactivity (Figure 3E). Reduction was seen in all experiments using different HLA-mismatched pairs. Analysis of the minimal duration of inhibitor treatment necessary for abrogation of an alloresponse demonstrated that presence of 17-DMAG for 24 hours was sufficient to block proliferation of alloreactive T cells completely (data not shown). Furthermore, annexin V staining showed that alloreactive T cells that initially proliferated after contact with allogeneic DC rapidly undergo apoptosis after addition of Hsp90 inhibitor (Figure 3C).
Inhibition of Hsp90 function with 17-DMAG predominantly induces apoptosis in alloreactive, proliferating T cells in MLRs. (A) CFSEdim proliferating T cells in MLRs show up-regulation of the T-cell activation marker CD25. (B) CD25-positive activated T cells and CD25-negative resting cells of a mixed lymphocyte reaction were separated with microbeads, and the levels of Hsp90α and Hsp90β were determined by Western blot. (C) A mixed lymphocyte reaction was pulsed with 17-DMAG 4 days after culture initiation, and apoptosis was determined by annexin V staining 40 hours after inhibitor treatment. (D) CFSE dilution after 4 days in MLRs either left untreated or treated with the Hsp90 inhibitor 17-DMAG. (E) Summary of depletion efficacy in 9 experiments: proliferative fraction of MLRs with and without 17-DMAG after stimulation with allogeneic DC. The displayed P value was obtained using a 2-tailed paired t test.
Inhibition of Hsp90 function with 17-DMAG predominantly induces apoptosis in alloreactive, proliferating T cells in MLRs. (A) CFSEdim proliferating T cells in MLRs show up-regulation of the T-cell activation marker CD25. (B) CD25-positive activated T cells and CD25-negative resting cells of a mixed lymphocyte reaction were separated with microbeads, and the levels of Hsp90α and Hsp90β were determined by Western blot. (C) A mixed lymphocyte reaction was pulsed with 17-DMAG 4 days after culture initiation, and apoptosis was determined by annexin V staining 40 hours after inhibitor treatment. (D) CFSE dilution after 4 days in MLRs either left untreated or treated with the Hsp90 inhibitor 17-DMAG. (E) Summary of depletion efficacy in 9 experiments: proliferative fraction of MLRs with and without 17-DMAG after stimulation with allogeneic DC. The displayed P value was obtained using a 2-tailed paired t test.
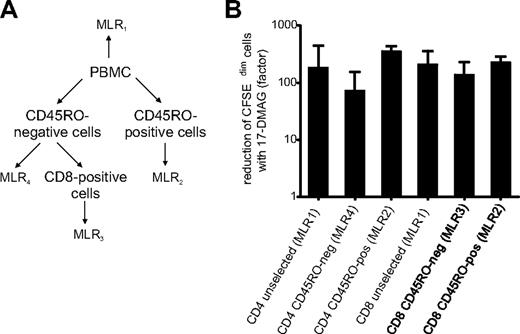
Alloreactive T cells can belong to different T-cell compartments, such as the naive CD4+ or CD8+ T-cell population, as well as the CD4+ or CD8+ memory/effector population. To determine whether all T-cell compartments can initiate an alloreaction and are equally susceptible to 17-DMAG treatment, CD45R0-positive and -negative CD4+ and CD8+ T cells were separately tested in MLRs (Figure 4A). As shown in Figure 4B, the alloreactive T cells in the bulk T-cell population as well as in the different T-cell compartments are equally affected by 17-DMAG treatment, with no significant difference between the different T-cell fractions.
Hsp90 inhibitor treatment reduces the proliferative fraction in CD45RO-positive memory as well as CD45RO-negative naive CD4+ and CD8+ T cells. (A) Separation of CD45RO-positive and CD45RO-negative T cells and setup of 4 separate MLRs. (B) Reduction of CFSEdim proliferating cells of the MLRs shown in panel A by Hsp90 inhibitor treatment.
Hsp90 inhibitor treatment reduces the proliferative fraction in CD45RO-positive memory as well as CD45RO-negative naive CD4+ and CD8+ T cells. (A) Separation of CD45RO-positive and CD45RO-negative T cells and setup of 4 separate MLRs. (B) Reduction of CFSEdim proliferating cells of the MLRs shown in panel A by Hsp90 inhibitor treatment.
Genetic targeting of Hsp90 by siRNA also reduces alloreactivity
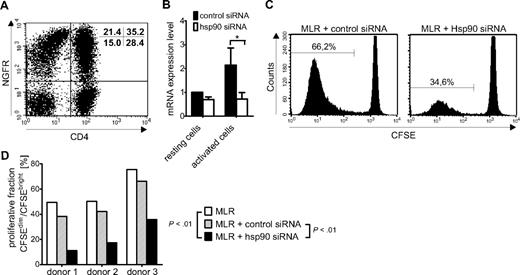
As Hsp90 inhibitors have also recently been reported to possess the capacity to markedly affect DC maturation, antigen uptake, and function,17 we designed a second set of experiments to exclude that the reported DC dysfunction entailed by Hsp90 blockage may be the underlying reason for the observed MLR inhibition. To address this question, we selectively targeted Hsp90 function in the T-lymphocyte population with Hsp90-specific siRNA. Because the Hsp90β isoform is distinctly more up-regulated than the α isoform after T-lymphocyte activation (Figures 1A and 3B) and only genetic knockdown of the β isoform in myeloma cells showed a significant effect,18 we used Hsp90β-specific siRNA to block Hsp90 function. The transfection protocol for primary human T lymphocytes was first optimized and evaluated by electroporation of T cells with NGFR-specific mRNA and flow cytometric determination of cell viability and NGFR expression, showing a transfection efficiency of 59% for CD8+ T cells and 55% for CD4+ T cells with low cell toxicity (Figure 5A). T cells transfected with control siRNA or Hsp90β-specific siRNA using the optimized transfection protocol were either left untreated or activated with PHA for 24 hours. Transfection with Hsp90-specific siRNA resulted in moderate down-regulation of Hsp90β predominantly in the activated T cells, as determined by real-time PCR in 3 donors (Figure 5B). Despite the only moderate down-regulation of Hsp90β expression, which is probably associated with relatively high residual activity of the Hsp90 chaperone, a significant reduction of proliferating alloreactive T cells in MLR cultures was observed in comparison with T cells transfected with negative control siRNA (Figure 5C). Statistical analysis of 3 independent experiments demonstrated that both the comparison between MLR or MLR plus control siRNA and MLR plus Hsp90 siRNA were significant in the reduction of proliferating alloreactive T cells (Figure 5D). We therefore assume that the absence of T-cell proliferation in MLRs treated with 17-DMAG is not due to inadequate stimulation of alloreactive T cells by Hsp90 inhibitor-affected DC, but rather is caused by specific interruption of Hsp90 function in the stimulated alloreactive T-cell population.
Hsp90 knockdown by siRNA reduces proliferation in mixed lymphocyte reactions. (A) Flow cytometric analysis of CD3+ T cells electroporated with NGFR-specific mRNA to estimate transfection efficiency in primary human lymphocytes. Transfection efficiency was 59% for CD8+ cells and 55% for CD4+ cells. (B) Genetic knockdown of Hsp90β mRNA with siRNA in resting or activated CD3+ T cells, as determined by real-time PCR. (C) CFSE dilution after 4 days in MLRs of PBMC electroporated either with control siRNA or Hsp90β-specific siRNA. (D) Summary of depletion efficacy in 3 experiments: proliferative fraction of MLRs transfected with control siRNA or Hsp90-specific siRNA. Displayed P values were obtained using a 2-tailed paired t test.
Hsp90 knockdown by siRNA reduces proliferation in mixed lymphocyte reactions. (A) Flow cytometric analysis of CD3+ T cells electroporated with NGFR-specific mRNA to estimate transfection efficiency in primary human lymphocytes. Transfection efficiency was 59% for CD8+ cells and 55% for CD4+ cells. (B) Genetic knockdown of Hsp90β mRNA with siRNA in resting or activated CD3+ T cells, as determined by real-time PCR. (C) CFSE dilution after 4 days in MLRs of PBMC electroporated either with control siRNA or Hsp90β-specific siRNA. (D) Summary of depletion efficacy in 3 experiments: proliferative fraction of MLRs transfected with control siRNA or Hsp90-specific siRNA. Displayed P values were obtained using a 2-tailed paired t test.
PBMC depleted of alloreactive cells by Hsp90 blockade exhibit functional virus-specific responses
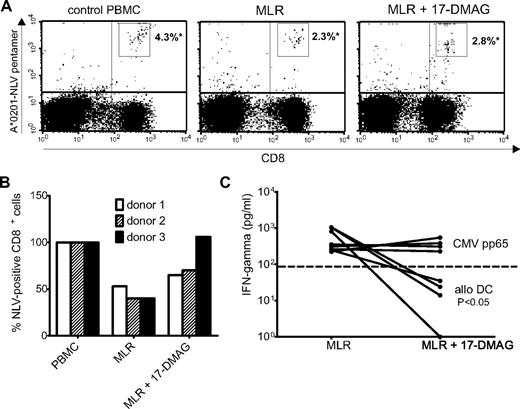
Current treatment of GVHD frequently results in a sharp increase in morbidity and mortality due to infectious complications. The applied immunosuppressive procedures such as high dose steroids generally lead to a critical impairment of innate as well as adaptive cellular immunity. Thus, novel GVHD prevention and treatment strategies should entail the capacity to spare pathogen-specific immunity and selectively eradicate alloreactive T cells. As cytomegalovirus (CMV) has been identified as one of the most prevalent pathogens causing severe infections after allogeneic transplantation, we first identified donors with measurable numbers of CMVpp65-specific CD8+ T cells by screening a pool of potential HLA-A*0201+ donors with a MHC class I pentamer specific for the CMVpp65-derived epitope NLV bound to the MHC class I molecule HLA-A*0201. PBMC from donors with high frequencies of HLA-A*0201–restricted CMVpp65-specific CD8+ T cells were then stimulated for 6 days with allogeneic DC in the presence or absence of 17-DMAG. To address whether viral immunity was preserved, CMV-specific CD8+ T cells were again visualized with the same MHC class I pentamer after stimulation. As shown in Figure 6A-B, CMVpp65-specific CD8+ T cells were readily detected at similar frequencies in both the untreated as well as 17-DMAG–treated cultures. To assess the ability of CMVpp65-specific T cells to respond to antigen after treatment with Hsp90 inhibitor, pretreated as well as untreated cultures were subject to rechallenge with autologous monocytes pulsed with a CMVpp65 peptide pool. To demonstrate successful depletion of alloreactive T cells, cultures were also rechallenged with the same allogeneic DC that were used to induce the alloresponse. Supernatant was harvested, and the amount of secreted IFN-γ was quantified by enzyme-linked immunosorbent assay. As expected, the 17-DMAG–treated cultures displayed no relevant IFN-γ production upon re-exposure to allo-DC, whereas IFN-γ was easily detected in untreated cultures (Figure 6C). However, untreated as well as inhibitor-pretreated cultures responded equally strong to rechallenge with the CMVpp65-specific peptide pool, demonstrating that the resting lymphocytes surviving inhibitor treatment, including memory T cells specific for CMVpp65, are not compromised in their viability and function by previous treatment with Hsp90 inhibitor.
PBMC depleted of alloreactive cells by Hsp90 inhibitor display functional virus-specific responses. (A) Quantification of CMV-specific CD8+ T cells in PBMC and untreated or Hsp90 inhibitor-treated MLRs of a CMV-seropositive donor using a MHC class I pentamer specific for a CMVpp65 epitope (A*0201-NLV; *numbers indicate percentages of pentamer-specific CD8+ cells). (B) Percentage of CMV NLV-pentamer–specific CD8+ cells of PBMC and untreated or 17-DMAG–treated MLRs of 3 different donors (percentage of NLV-specific CD8+ cells in untreated PBMC = 100%). (C) Summary of depletion efficacy in 4 experiments: quantification of supernatant IFN-γ levels after restimulation of untreated or 17-DMAG–treated MLRs of CMV-seropositive donors with the same allogeneic DC triggering the alloresponse or autologous monocytes pulsed with a CMVpp65 peptide pool. Displayed P values were obtained using a 2-tailed paired t test.
PBMC depleted of alloreactive cells by Hsp90 inhibitor display functional virus-specific responses. (A) Quantification of CMV-specific CD8+ T cells in PBMC and untreated or Hsp90 inhibitor-treated MLRs of a CMV-seropositive donor using a MHC class I pentamer specific for a CMVpp65 epitope (A*0201-NLV; *numbers indicate percentages of pentamer-specific CD8+ cells). (B) Percentage of CMV NLV-pentamer–specific CD8+ cells of PBMC and untreated or 17-DMAG–treated MLRs of 3 different donors (percentage of NLV-specific CD8+ cells in untreated PBMC = 100%). (C) Summary of depletion efficacy in 4 experiments: quantification of supernatant IFN-γ levels after restimulation of untreated or 17-DMAG–treated MLRs of CMV-seropositive donors with the same allogeneic DC triggering the alloresponse or autologous monocytes pulsed with a CMVpp65 peptide pool. Displayed P values were obtained using a 2-tailed paired t test.
Discussion
The results presented in this work indicate that Hsp90 blockade could indeed be a feasible novel target to selectively deplete alloreactive donor lymphocytes after transplantation without impairing third party immunity. Potential applications may therefore be upfront depletion of alloreactive T cells by short ex vivo incubation of donor lymphocytes with recipient-derived antigen-presenting cells in the presence of 17-DMAG or other Hsp90 inhibitors. Alternatively, Hsp90 inhibitors could also be administered to HSCT patients on the onset of GVHD. The latter approach could even ameliorate disease more potently, as host-DC, which seem to be primarily responsible for the induction of GVHD,19,20 are also highly susceptible to Hsp90 blockage.17 In addition, T cells activated by antigens exclusively expressed in nonhematopoietic tissues such as skin, gut, or liver could be eliminated by direct administration of Hsp90 inhibitors in vivo.5 Irrespectively, both strategies would not impair pathogen-specific immunity, as predominantly activated alloreactive T cells are prone to apoptosis upon treatment. In addition, it appears that all subpopulations of potential alloreactive T cells are equally susceptible to blockade of Hsp90. Together, these strategies may therefore result in a reduction of long-lasting immunosuppressive therapy, and thus lead to a faster immune reconstitution of the HSCT recipients with a reduction of infectious disease-related morbidity and mortality as well as improved control of the malignant disease.
Our observation that T-lymphocyte viability, predominantly after activation, is highly dependent on elevated Hsp90 levels is in good accordance with previous observations showing induction of Hsp90 expression by mitogens and IL-2, a key mediator of T-cell signaling.16 This finding raised the question as to whether Hsp90 might be critically involved in T-cell activation-induced signaling. T-cell activation is initiated by the concomitant stimulation of both the T-cell receptor CD3 and the coreceptor CD28, and involves intermediates such as Raf-1, Akt, Jak, and STAT5,8 which have previously been shown to be dependent on appropriate Hsp90 chaperone function in various mammalian cell types.21-26 In this study, we could show that signaling through the Jak/STAT5, Ras/ERK1,2, and phosphatidylinositol 3-kinase/Akt signaling pathways, which are activated in T cells after combined stimulation with anti-CD3 and anti-CD28 beads, was indeed effectively abrogated by inhibition of Hsp90. Taken together, the interplay of signaling pathways and Hsp90-chaperoning activity facilitates function and survival of activated T cells, and pharmacologic targeting of Hsp90 activity might therefore be an effective attempt to induce cell death in activated T cells.
The beneficial graft-versus-leukemia (GvL) effect of allogeneic donor lymphocyte transfusions is probably based on the same basic principles as and closely linked with the development of GVHD.27 Therefore, the drawbacks of unselected T-cell depletion are increased rates of infectious complications and recurrence of the malignant disease.28 However, as there is evidence that different T-cell clones are capable of distinguishing between GvL and GVHD antigens,29 approaches such as selective allodepletion may overcome these limitations. Furthermore, Hsp90 blockade has been shown to have a direct antitumor effect on many hematologic malignancies such as multiple myeloma18 and chronic lymphocytic leukemia,30 malignancies that are subject to allogeneic transplantation. Therefore, Hsp90 inhibitors may compensate for partial loss of GvL effects in the allodepletion procedure, if administered in vivo during the onset of GVHD by not only eliminating alloreactive T-cell clones, but also contributing to eradication of residual malignant cells and thereby protecting from disease relapse. In addition, if removal of GVHD clones by the selective allodepletion was complete, this approach promises transplantation without additional immunosuppression, thereby enhancing residual GvL activities normally compromised by GVHD prophylaxis. In this study, we provide for the first time the proof of the experimental feasibility of Hsp90-based allodepletion. Despite these encouraging results obtained using different functional assays measuring proliferation and cytokine secretion, it should be emphasized that they cannot fully predict the clinical outcome, as discussed by Mielke et al.31 Therefore, we will further study this novel selective allodepletion approach in a clinical trial of allotransplantation. As the presence of GVHD and infectious episodes do correlate, we cannot fully exclude the possibility that Hsp90-targeted therapy may also have a negative impact on the allograft′s ability to combat infections by targeting activated, pathogen-specific T-cell responses.
Taken together, Hsp90 inhibitors may be a useful tool for both ex vivo and in vivo selective allodepletion in patients with hematologic malignancies undergoing allogenic hematopoetic stem cell transplantation. This novel pathway may improve the outcome after allogeneic HSCT by decreasing the incidence and severity of GVHD through selective allodepletion of donor-derived, host-reactive T cells, as well as clearance of residual host DC and malignant cells.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Gabriele Kuntz and Heike Lorentz for technical assistance.
This work was supported in part by Deutsche Forschungsgemeinschaft (SFB 479; C12) and in part by Deutsche Krebshilfe (107715 Ba).
Authorship
Contribution: C.S., S.L., J.D., and F.R. designed, performed, and analyzed experiments; M.C. designed, performed, and analyzed the experiment in Figure 2B; R.C.B. and H.E. analyzed the research; S.M. and M.S.T. designed and analyzed the research; and C.S., S.M., and M.S.T. wrote the paper.
Conflict-of-interest disclosure: S.M. has received funding from KIADIS Pharma. The remaining authors declare no competing financial interests.
Correspondence: Max S. Topp, Medizinische Klinik und Poliklinik II, Universitaetsklinikum Wuerzburg, Klinikstr. 6-8, Wuerzburg D-97072, Germany; e-mail: Topp_M@medizin.uni-wuerzburg.de.
References
Author notes
*S.M. and M.C. contributed equally to this work.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal