Abstract
Macrophages as inflammatory cells are involved in the pathogenesis of atherosclerosis that today is recognized as an inflammatory disease. Activation of coagulation leads to the late complication of atherosclerosis, namely atherothrombosis with its clinical manifestations stroke, unstable angina, myocardial infarction, and sudden cardiac death. Thus inflammation and coagulation play fundamental roles in the pathogenesis of atherosclerosis. We show that the coagulation enzyme thrombin up-regulates oncostatin M (OSM), a pleiotropic cytokine implicated in the pathophysiology of vascular disease, in human monocyte-derived macrophages (MDMs) up to 16.8-fold. A similar effect was seen in human peripheral blood monocytes and human plaque macrophages. In MDMs, the effect of thrombin on OSM was abolished by PPACK and mimicked by a PAR-1–specific peptide. Thrombin induced phosphorylation of ERK1/2 and p38 in MDMs. The ERK1/2 inhibitor PD98059 blocked the effect of thrombin on OSM production in MDMs, whereas the p38 inhibitor SB202190 had no effect. Thrombin induced translocation of c-fos and c-jun to the nucleus of MDMs. Using OSM promoter–luciferase reporter constructs transfected into MDMs, we show that a functional AP-1 site is required for promoter activation by thrombin. We present another link between coagulation and inflammation, which could impact on the pathogenesis of atherosclerosis.
Introduction
Macrophages as inflammatory cells, which produce an array of inflammatory mediators, growth factors, and proteases are critically involved in the pathogenesis of atherosclerosis that today is recognized as an inflammatory disease.1,2 Rupture of advanced atherosclerotic lesions leads to activation of the coagulation cascade, resulting in thrombin generation and subsequently in atherothrombosis with its clinical complications such as stroke, unstable angina, myocardial infarction, and sudden cardiac death, which are the most common causes of morbidity and mortality in the Western world today.3-8
The central coagulation enzyme thrombin acts as a proinflammatory mediator and is chemotactic for monocytes and stimulates their proliferation and phagocytic activity.9-13 In monocytes and macrophages, thrombin induces the production of inflammatory cytokines with well-established roles in cardiovascular disease such as interleukin-1 (IL-1), monocyte chemoattractant protein-1, and IL-6, a member of the IL-6 family of cytokines.14-18
In this paper we have addressed the question whether thrombin affects the expression of yet another member of the IL-6 family of cytokines, which is produced mainly by macrophages, namely oncostatin-M (OSM) in these cells. This pleiotropic cytokine, which plays a critical role in numerous physiologic and pathophysiologic processes including inflammation, hematopoiesis, tissue remodeling, development, and cell growth, has been implicated recently in the pathophysiology of cardiovascular disease.19-24 In vascular smooth muscle cells, OSM induces the expression of matrix metalloproteinase 9, the protease inhibitor plasminogen activator inhibitor-1, and alkaline phosphatase and stimulates the proliferation of these cells.25-28 Thus OSM seems to be involved in progression, matrix remodeling, and calcification of atherosclerotic lesions. Furthermore, OSM has been implicated in plaque thrombogenicity by stimulating tissue factor expression in smooth muscle cells.29 In endothelial cells, OSM induces the production of monocyte chemoattractant protein-1, thus presumably facilitating monocyte migration to the lesion area, and down-regulates tissue-type PA and up-regulates plasminogen activator inhibitor-1 in these cells thereby creating an antifibrinolytic prothrombotic milieu.30,31 Furthermore, OSM is thought to contribute to plaque destabilization by inducing vascularization of the lesion due to its angiogenic properties and OSM has been found in human aortic aneurysms.32-34
Thus, it was the aim of this study to investigate whether thrombin regulates the expression of OSM in human macrophages.
Methods
Materials
Human α-thrombin (2000 U/mg) obtained from Sigma-Aldrich was used in all experiments described in this paper. Recombinant hirudin, human serum, purified bovine serum albumin, and polymyxin B (PMX-B) were also purchased from Sigma-Aldrich. Human serum is a pooled serum from human male AB plasma and was endotoxin tested and sterile filtered by Sigma-Aldrich. It was heat inactivated at 60°C for 60 minutes before use. Human α-thrombin (3800 U/mg) used an alternative preparation of thrombin in experiments depicted in Figure 1, and active site–blocked human FPRck-thrombin was purchased from Haematologic Technologies. Hirulog (bivalirudin) was a gift from Nycomed. Ultraculture medium was provided as a serum-free mixture of DMEM/F12 supplemented with insulin, transferrin, and bovine serum proteins by the manufacturer and was obtained from BioWhittaker. SB202190 and PD98059 were purchased from Calbiochem/Merck. d-Phe-Pro-Arg-chloromethylketone (PPACK) was purchased from Calbiochem. PAR-1 peptide (sequence: Tyr-Asn-Ser-Ser-Gly-Gln-Leu-Met-Ala-Ser-Lys-Met-Asp-Thr-Cys-Ser-Ser-Asn-Leu-Asn-Asn-Ser-Ile-Tyr-Lys-Lys-Leu-Leu-Thr), PAR-3 peptide (sequence: Cys-Leu-Asp-Pro-Phe-Leu-Tyr-Phe-Leu-Met-Ser-Lys-Thr-Arg-Asn-His-Ser-Thr-Ala-Tyr-Leu-Thr-Lys), and PAR-4 peptide (sequence: Cys-Ser-Pro-Gly-Asp-Thr-Val-Ala-Ser-Lys-Ala-Ser-Ala-Glu-Gly-Gly-Ser-Arg-Gly-Met-Gly-Thr-His-Ser-Ser-Leu-Leu-Gln) were purchased from Phoenix Pharmaceuticals.
Cell culture
Peripheral blood mononuclear cells from healthy volunteers were isolated using a Ficoll-Hypaque density gradient (Amersham Biosciences). Positive isolation of peripheral blood monocytes (PBMs) from peripheral blood mononuclear cells was performed using a magnetic-activated cell sorting monocyte isolation kit (Miltenyi Biotec) using CD14 antibodies conjugated to magnetic beads and a column placed in the magnetic field of a magnetic-activated cell sorting separator (Miltenyi Biotec).35,36 Purity of these preparations of PBMs was more than 90% as determined by fluorescence-activated cell sorting analysis using CD14 antibodies.36 Macrophage differentiation of these cells was performed as described by us before.35 Macrophage phenotype was confirmed by immunofluorescence staining using a CD68 antibody after culture of the cells on coverslips for 10 days. More than 90% of the cells stained positive for the macrophage marker CD68. Less than 2% of cells in all experiments stained positive for trypan blue (data not shown).
Isolation of plaque macrophages
Human plaque macrophages were isolated from atherosclerotic plaques from carotid arteries obtained from 3 patients undergoing carotid endarterectomy as described by us before using collagenase digestion of the plaque material followed by positive selection of CD14+ cells using CD14 antibodies conjugated to magnetic beads.37 All subjects were white and did not suffer from diabetes mellitus, acute infection, cancer, or any other consuming disease. All human material was obtained and processed according to the recommendations of the hospital's Ethics Committee and Security Board, which included obtaining informed consent in accordance with the Declaration of Helsinki.
Treatment of cells with α-thrombin
Before all experiments described in this section, monocytes, macrophages, and plaque macrophages were seeded into 48-well dishes at a density of 105 cells/well and were incubated for 24 hours in serum-free Ultraculture medium supplemented with antibiotics and 0.1% bovine serum albumin. Thereafter 250 μL fresh medium was added to the wells with or without α-thrombin at concentrations indicated, and the cells were incubated at 37°C for the time periods indicated. At the end of the incubation period, conditioned media from these cultures were collected and stored at −80°C. Before the experiments, α-thrombin was tested for lipopolysaccharide (LPS) contamination using the Coatest LPS kit from Kabi Diagnostica. No LPS could be detected in the α-thrombin preparations using this assay (detection limit, 0.05 ng/mL).
Quantification of OSM
OSM antigen in conditioned media was determined by a specific enzyme-linked immunosorbent assay ([ELISA]; human OSM ELISA; R&D Systems) using monoclonal antibodies.
Determination of the phosphorylation of MAPKs
For determination of the phosphorylation of mitogen-activated protein kinases (MAPKs), we used a Proteome Profiler Human Phospho-MAPK Array (R&D Systems), which uses capture and control antibodies immobilized in duplicate on nitrocellulose membranes. Cell lysates, prepared according to the manufacturer's instructions, were incubated with the membranes and after binding of phosphorylated and unphosphorylated kinases, unbound material was washed away. A cocktail of phospho-site–specific biotinylated antibodies was used to detect phosphorylated kinases via streptavidin–horseradish peroxidase and chemiluminescence. Quantification was performed using image analysis software (GelEval V1.22; FrogDance Software).
Analysis of specific binding of AP-1 to DNA
Nuclear extracts of MDMs were prepared using a nuclear extract kit (Active Motif) according to the manufacturer's instructions. c-fos and c-jun binding to their consensus oligonucleotide was determined using the ELISA-based Trans-AM AP-1 kit (Active Motif) according to the manufacturer's instructions. Specificity of c-fos and c-jun binding, respectively, was confirmed by incubation of nuclear extracts with the immobilized AP-1 consensus-binding probe in the presence of excess wild-type or mutated oligonucleotide (data not shown).
mRNA purification
Cells were stimulated as described in “Treatment of cells with α-thrombin,” the supernatant was removed, and mRNA was isolated using QuickPrep Micro mRNA Purification Kit (Amersham Biosciences) according to the manufacturer's instructions.
Real-time PCR
Real-time polymerase chain reaction (RealTime-PCR) was performed using LightCycler TaqMan Master (Roche) according to the manufacturer's instructions. Primers were designed using the Roche Universal Probe Library Assay Design Center (http://www.universalprobelibrary.com): GAPDH (forward primer: 5′-agccacatcgctcagacac-3′, reverse primer: 5′-gcccaatacgaccaaatcc-3′, UPL probe no. 60), OSM (forward primer: 5′-agtaccgcgtgctccttg-3′, reverse primer: 5′-ccctgcagtgctctctcagt-3′, UPL probe no. 50, amplicon size [bp]: 126), PAR-1 (forward primer: 5′-tcagaagatgcctccggata-3′, reverse primer: 5′-cacagatgggacaaagagtgtc-3′, UPL probe no. 17, amplicon size [bp]: 60). The amplification conditions consisted of an initial incubation at 95°C for 10 minutes, followed by 45 cycles of 95°C for 10 seconds, 63°C for 20 seconds, and 72°C for 6 seconds, and a final cooling to 40°C. Data were analyzed using LightCycler Software Version 3.5 (Roche).
Transfection of macrophages
MDMs were transfected with chimeric OSM promoter–luciferase reporter constructs as described recently.38 A 304-bp, a 194-bp, a 109-bp, and a 304-bp construct with a mutated AP-1 site were used, respectively.39 Transfection of MDMs and luciferase assays were carried out as described by Ma et al.39 Briefly, MDMs were seeded into 48-well plates at a concentration of 100000 cells/well and transfection was performed using jetPEI-Macrophage (Polyplus-transfection) according to the manufacturer's instructions 24 hours before stimulation with α-thrombin. For determination of transfection efficiency, the expression plasmid pRL (sea pansy Renilla reniformis gene under control of an SV40 promoter; Promega) was used as an internal control, and total transfection efficiency was determined by cotransfection of the cells with a phMGFP Vector (Promega). For all transient transfection studies, the dual luciferase reporter assay system (Promega) was used.
Statistical analysis
Data were compared by ANOVA. A P value less than .05 was considered significant.
Results
Thrombin stimulates OSM expression in human monocyte-derived macrophages, human peripheral blood monocytes, and human plaque macrophages
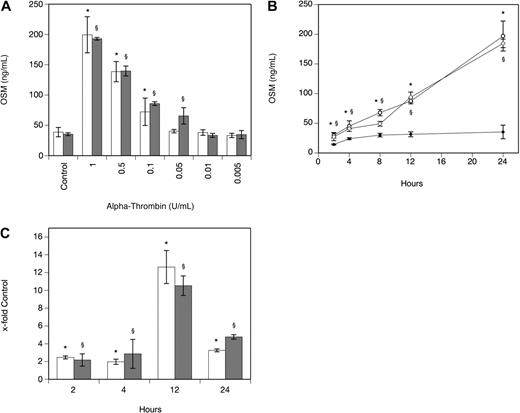
As can be seen from Table 1, α-thrombin significantly increased OSM production after 24 hours in human MDMs isolated from 4 different donors that also expressed PAR-1. When human PBMs were incubated with 1 U/mL α-thrombin for 24 hours, OSM production increased 7.6-fold (P < .001). In human plaque macrophages, α-thrombin at 1 U/mL significantly increased OSM-mRNA production after 3 hours by 9.3-fold (P < .001). In MDMs, the effect of α-thrombin on OSM production was dose dependent with maximum stimulation seen at a concentration of 1 U/mL α-thrombin (Figure 1A). When the cells were incubated in the absence and presence of α-thrombin at a concentration of 1 U/mL for 2, 4, 8, 12, and 24 hours, a significant increase in OSM at 2, 4, 8, 12, and 24 hours was observed compared with values determined in untreated cells (Figure 1B). mRNA levels specific for OSM were significantly increased after 2-, 4-, 12-, and 24-hour treatment of the cells with 1 U/mL α-thrombin compared with untreated control cells (Figure 1C). When these experiments were performed with a different preparation of human α-thrombin, similar results were obtained (Figure 1A-C). To rule out possible LPS effects on OSM production in these cells, α-thrombin was boiled before addition to MDMs and cells were preincubated with PMX-B to block LPS activity. Boiled α-thrombin did not affect OSM synthesis in these cells, whereas preincubation of macrophages with PMX-B did not affect the ability of α-thrombin to stimulate OSM production (data not shown).
Effect of thrombin on OSM production in human monocyte-derived macrophages isolated from 3 different donors
| . | OSM . | PAR-1 mRNA . | |
|---|---|---|---|
| Control, ng/mL . | α-thrombin, ng/mL . | ||
| Donor 1 | 48.5 ± 15.7 | 144.4 ± 13.9* | 1 |
| Donor 2 | 38.7 ± 7.7 | 199.5 ± 29.8* | 2.4 |
| Donor 3 | 20.3 ± 3.6 | 418.5 ± 36.3* | 5.9 |
| Donor 4 | 38.8 ± 6.6 | 651.5 ± 58.6* | 50 |
| . | OSM . | PAR-1 mRNA . | |
|---|---|---|---|
| Control, ng/mL . | α-thrombin, ng/mL . | ||
| Donor 1 | 48.5 ± 15.7 | 144.4 ± 13.9* | 1 |
| Donor 2 | 38.7 ± 7.7 | 199.5 ± 29.8* | 2.4 |
| Donor 3 | 20.3 ± 3.6 | 418.5 ± 36.3* | 5.9 |
| Donor 4 | 38.8 ± 6.6 | 651.5 ± 58.6* | 50 |
OSM antigen was determined with a specific ELISA as described in “Quantification of OSM” in MDMs incubated for 24 hours with or without α-thrombin (1 U/mL). Values are given in nanograms per milliliter and represent mean values ± SD of 3 independent determinations.
PAR-1 mRNA levels, normalized according to the respective GAPDH mRNA levels, were determined as described in “Real-time PCR” in cells without addition of α-thrombin. Values are given as x-fold of mRNA levels of donor 1 that showed the lowest PAR-1 expression and thus was arbitrarily set to 100%.
OSM indicates oncostatin M.
P < .001.
Thrombin increases OSM production and expression in human monocyte-derived macrophages. (A-B) MDMs were incubated with α-thrombin obtained from Sigma-Aldrich (A: □; B: ○) and α-thrombin obtained from Haematologic Technologies (A: ▩; B:  ) at indicated concentrations or for indicated time periods in the absence (B: ●) or presence of α-thrombin (1 U/mL). Conditioned media of such treated cells were collected and OSM antigen was determined as described in “Quantification of OSM.” Values are given in nanograms per milliliter and represent mean values ± SD of 3 independent determinations. Experiments were performed 3 times with MDMs obtained from 3 different donors with similar results. A representative experiment is shown. (C) MDMs were incubated with or without α-thrombin obtained from Sigma-Aldrich (□) and α-thrombin obtained from Haematologic Technologies (▩; 1 U/mL) for the indicated time period. mRNA was prepared and analyzed by RealTime-PCR with specific primers for OSM and GAPDH as described in “Real-time PCR.” OSM mRNA levels were normalized according to the respective GAPDH mRNA levels. Values are given as x-fold of control, which was set as 100%, and represent mean values ± SD of 3 independent determinations. Experiments were performed 2 times with MDMs obtained from 2 different donors with similar results. A representative experiment is shown. *P < .001 (α-thrombin; Sigma-Aldrich); §P < .001 (α-thrombin; Haematologic Technologies).
) at indicated concentrations or for indicated time periods in the absence (B: ●) or presence of α-thrombin (1 U/mL). Conditioned media of such treated cells were collected and OSM antigen was determined as described in “Quantification of OSM.” Values are given in nanograms per milliliter and represent mean values ± SD of 3 independent determinations. Experiments were performed 3 times with MDMs obtained from 3 different donors with similar results. A representative experiment is shown. (C) MDMs were incubated with or without α-thrombin obtained from Sigma-Aldrich (□) and α-thrombin obtained from Haematologic Technologies (▩; 1 U/mL) for the indicated time period. mRNA was prepared and analyzed by RealTime-PCR with specific primers for OSM and GAPDH as described in “Real-time PCR.” OSM mRNA levels were normalized according to the respective GAPDH mRNA levels. Values are given as x-fold of control, which was set as 100%, and represent mean values ± SD of 3 independent determinations. Experiments were performed 2 times with MDMs obtained from 2 different donors with similar results. A representative experiment is shown. *P < .001 (α-thrombin; Sigma-Aldrich); §P < .001 (α-thrombin; Haematologic Technologies).
Thrombin increases OSM production and expression in human monocyte-derived macrophages. (A-B) MDMs were incubated with α-thrombin obtained from Sigma-Aldrich (A: □; B: ○) and α-thrombin obtained from Haematologic Technologies (A: ▩; B:  ) at indicated concentrations or for indicated time periods in the absence (B: ●) or presence of α-thrombin (1 U/mL). Conditioned media of such treated cells were collected and OSM antigen was determined as described in “Quantification of OSM.” Values are given in nanograms per milliliter and represent mean values ± SD of 3 independent determinations. Experiments were performed 3 times with MDMs obtained from 3 different donors with similar results. A representative experiment is shown. (C) MDMs were incubated with or without α-thrombin obtained from Sigma-Aldrich (□) and α-thrombin obtained from Haematologic Technologies (▩; 1 U/mL) for the indicated time period. mRNA was prepared and analyzed by RealTime-PCR with specific primers for OSM and GAPDH as described in “Real-time PCR.” OSM mRNA levels were normalized according to the respective GAPDH mRNA levels. Values are given as x-fold of control, which was set as 100%, and represent mean values ± SD of 3 independent determinations. Experiments were performed 2 times with MDMs obtained from 2 different donors with similar results. A representative experiment is shown. *P < .001 (α-thrombin; Sigma-Aldrich); §P < .001 (α-thrombin; Haematologic Technologies).
) at indicated concentrations or for indicated time periods in the absence (B: ●) or presence of α-thrombin (1 U/mL). Conditioned media of such treated cells were collected and OSM antigen was determined as described in “Quantification of OSM.” Values are given in nanograms per milliliter and represent mean values ± SD of 3 independent determinations. Experiments were performed 3 times with MDMs obtained from 3 different donors with similar results. A representative experiment is shown. (C) MDMs were incubated with or without α-thrombin obtained from Sigma-Aldrich (□) and α-thrombin obtained from Haematologic Technologies (▩; 1 U/mL) for the indicated time period. mRNA was prepared and analyzed by RealTime-PCR with specific primers for OSM and GAPDH as described in “Real-time PCR.” OSM mRNA levels were normalized according to the respective GAPDH mRNA levels. Values are given as x-fold of control, which was set as 100%, and represent mean values ± SD of 3 independent determinations. Experiments were performed 2 times with MDMs obtained from 2 different donors with similar results. A representative experiment is shown. *P < .001 (α-thrombin; Sigma-Aldrich); §P < .001 (α-thrombin; Haematologic Technologies).
Thrombin-induced OSM production in human monocyte-derived macrophages is mediated by PAR-1
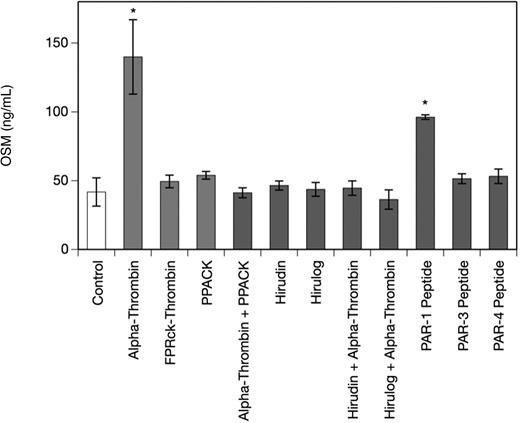
To determine the specificity of the α-thrombin–induced increase in OSM production in MDMs, we studied the effect of PAR-1–, PAR-3–, and PAR-4–specific peptides and the thrombin inhibitors PPACK, hirudin, and hirulog as well as active site–blocked human FPRck-thrombin in human MDMs. As can be seen from Figure 2 a PAR-1–specific peptide at a concentration of 10 μM significantly increased OSM production after 24 hours up to 2.5-fold in MDMs, whereas PAR-3– and PAR-4–specific peptides at concentrations of 10 μM each had no significant effect on OSM production in these cells (1.2-fold and 1.3-fold increase over control, respectively). When the cells were treated with FPRck-thrombin (0.2 μg/mL), no effect on OSM production in these cells was observed, whereas catalytically active α-thrombin significantly up-regulated OSM in MDMs. Pretreatment of the MDMs with PPACK (1 μM), hirudin (1 U/mg), or hirulog (1 μg/mL)40 for 1 hour at 37°C completely abolished the effect of α-thrombin on OSM production.41,42 PPACK, hirudin, or hirulog alone had no effect on OSM in MDMs (Figure 2).
Thrombin and PAR-1–specific peptide increase OSM production, whereas PPACK, hirudin, and hirulog inhibit the effect of α-thrombin on OSM in human monocyte-derived macrophages. MDMs were incubated with α-thrombin (1 U/mL), FPRck-thrombin (0.2 μg/mL), PAR-1–specific peptide (10 μM), PAR-3–specific peptide (10 μM) or PAR-4–specific peptide (10 μM) for 24 hours or preincubated with PPACK (1 μM), hirudin (1 U/mL), or hirulog (1 μg/mL) for 1 hour before addition of α-thrombin (1 U/mL) for 24 hours. Conditioned media of such treated cells were collected and OSM antigen was determined as described in “Quantification of OSM.” Values are given in nanograms per milliliter and represent mean values ± SD of 3 independent determinations. Experiments were performed 2 times with MDMs obtained from 2 different donors with similar results. A representative experiment is shown. *P < .001.
Thrombin and PAR-1–specific peptide increase OSM production, whereas PPACK, hirudin, and hirulog inhibit the effect of α-thrombin on OSM in human monocyte-derived macrophages. MDMs were incubated with α-thrombin (1 U/mL), FPRck-thrombin (0.2 μg/mL), PAR-1–specific peptide (10 μM), PAR-3–specific peptide (10 μM) or PAR-4–specific peptide (10 μM) for 24 hours or preincubated with PPACK (1 μM), hirudin (1 U/mL), or hirulog (1 μg/mL) for 1 hour before addition of α-thrombin (1 U/mL) for 24 hours. Conditioned media of such treated cells were collected and OSM antigen was determined as described in “Quantification of OSM.” Values are given in nanograms per milliliter and represent mean values ± SD of 3 independent determinations. Experiments were performed 2 times with MDMs obtained from 2 different donors with similar results. A representative experiment is shown. *P < .001.
To rule out a possible unspecific inhibitory effect of PPACK on α-thrombin–induced up-regulation of OSM, cells that were preincubated with PPACK for 1 hour at 37°C were stimulated for 24 hours at 37°C with rhC5a (0.5 μM), which has been previously shown by us to significantly up-regulate OSM production in MDMs.38 When MDMs were incubated with rhC5a alone, a significant increase in OSM production compared with untreated control cells was observed. Pretreatment of the cells with PPACK did not affect this rhC5a-induced OSM up-regulation in MDMs (OSM in nanograms per milliliter per 24 hours: control: 41.8 ± 10.2; rhC5a: 372.6 ± 29.2; rhC5a + PPACK: 381.0 ± 16.5 ng/mL; P < .05).
Thrombin-induced OSM production in human monocyte-derived macrophages is mediated by ERK1/2 and AP-1
To determine the effect of α-thrombin on the MAPK system in MDMs, a Proteome Profiler Array was performed. As can be seen from Figure 3A, α-thrombin induced phosphorylation of extracellular signal-regulated kinase (ERK1/2) and p38 time dependently, with a significant increase after 25 minutes. Treatment of the cells with the ERK1/2 inhibitor PD98059 or the p38 inhibitor SB202190 at a concentration of 30 μM, respectively, blocked the α-thrombin–induced phosphorylation of their respective kinases completely (Figure 3B). As can be seen from Figure 3C, pretreatment of MDMs with 30 μM PD98059 efficiently blocked the effect of α-thrombin on OSM production in these cells, whereas pretreatment with 30 μM SB202190 had no effect on OSM production in MDMs. In the absence of α-thrombin, neither of the inhibitors affected basal OSM production in these cells. As can be seen from Figure 4A, α-thrombin also induced translocation of the AP-1 subunits c-fos and c-jun to the nucleus of MDMs time dependently, with a maximum of 3.3-fold (c-fos) and 2.5-fold (c-jun) increase, respectively, over control reached after 120 minutes.
Thrombin induces phosphorylation of ERK1/2 and p38. (A-B) MDMs were incubated with α-thrombin (1 U/mL) for indicated time periods or preincubated for 30 minutes with the ERK1/2 inhibitor PD98059 (30 μM) or with the p38 inhibitor SB202190 (30 μM) and thereafter treated with α-thrombin (1 U/mL) for 25 minutes. Phosphorylation of the respective kinases was determined as described in “Determination of the phosphorylation of MAPKs.” Values are given in mean density and represent mean values ± SD of 3 independent determinations. Experiments were performed 2 times with MDMs obtained from 2 different donors with similar results. A representative experiment is shown. (C) MDMs were preincubated for 30 minutes with the ERK1/2 inhibitor PD98059 (30 μM) or with the p38 inhibitor SB202190 (30 μM), and thereafter treated with α-thrombin (1 U/mL) for 24 hours. Conditioned media of such treated cells were collected and OSM antigen was determined as described in “Quantification of OSM.” Values are given in nanograms per milliliter and represent mean values ± SD of 3 independent determinations. Experiments were performed 2 times with MDMs obtained from 2 different donors with similar results. A representative experiment is shown. *P < .001 (ERK1); §P < .001 (ERK2); #P < .001 (p38).
Thrombin induces phosphorylation of ERK1/2 and p38. (A-B) MDMs were incubated with α-thrombin (1 U/mL) for indicated time periods or preincubated for 30 minutes with the ERK1/2 inhibitor PD98059 (30 μM) or with the p38 inhibitor SB202190 (30 μM) and thereafter treated with α-thrombin (1 U/mL) for 25 minutes. Phosphorylation of the respective kinases was determined as described in “Determination of the phosphorylation of MAPKs.” Values are given in mean density and represent mean values ± SD of 3 independent determinations. Experiments were performed 2 times with MDMs obtained from 2 different donors with similar results. A representative experiment is shown. (C) MDMs were preincubated for 30 minutes with the ERK1/2 inhibitor PD98059 (30 μM) or with the p38 inhibitor SB202190 (30 μM), and thereafter treated with α-thrombin (1 U/mL) for 24 hours. Conditioned media of such treated cells were collected and OSM antigen was determined as described in “Quantification of OSM.” Values are given in nanograms per milliliter and represent mean values ± SD of 3 independent determinations. Experiments were performed 2 times with MDMs obtained from 2 different donors with similar results. A representative experiment is shown. *P < .001 (ERK1); §P < .001 (ERK2); #P < .001 (p38).
Thrombin induces translocation of c-fos and c-jun to the nucleus and activates the OSM promoter via a putative AP-1 transcription factor binding motif in human monocyte-derived macrophages. (A) MDMs were incubated for 30, 60, 90, and 120 minutes at 37°C with α-thrombin (1 U/mL). Thereafter, nuclear extracts were prepared from these cells and levels of c-fos and c-jun in these extracts were determined as described in “Analysis of specific binding of AP-1 to DNA.” Values are given as x-fold control and represent mean values ± SD of 3 independent determinations performed for 3 different donors. (B) MDMs were transfected with OSM promoter deletion mutant constructs and with a promoter construct with a mutated AP-1 binding motif indicated by X as described in “Transfection of macrophages.” Twenty-four hours after transfection, cells were incubated for 24 hours in the presence (▩) or absence (□) of α-thrombin (1 U/mL). Luciferase activity was determined as described in “Transfection of macrophages” and is given in percentage of control. Values are given in percentage of control and represent mean values ± SD of 3 determinations. Experiments were performed 2 times with MDMs obtained from 2 different donors with similar results. A representative experiment is shown. *P < .001 (c-fos); §P < .001 (c-jun).
Thrombin induces translocation of c-fos and c-jun to the nucleus and activates the OSM promoter via a putative AP-1 transcription factor binding motif in human monocyte-derived macrophages. (A) MDMs were incubated for 30, 60, 90, and 120 minutes at 37°C with α-thrombin (1 U/mL). Thereafter, nuclear extracts were prepared from these cells and levels of c-fos and c-jun in these extracts were determined as described in “Analysis of specific binding of AP-1 to DNA.” Values are given as x-fold control and represent mean values ± SD of 3 independent determinations performed for 3 different donors. (B) MDMs were transfected with OSM promoter deletion mutant constructs and with a promoter construct with a mutated AP-1 binding motif indicated by X as described in “Transfection of macrophages.” Twenty-four hours after transfection, cells were incubated for 24 hours in the presence (▩) or absence (□) of α-thrombin (1 U/mL). Luciferase activity was determined as described in “Transfection of macrophages” and is given in percentage of control. Values are given in percentage of control and represent mean values ± SD of 3 determinations. Experiments were performed 2 times with MDMs obtained from 2 different donors with similar results. A representative experiment is shown. *P < .001 (c-fos); §P < .001 (c-jun).
Transcriptional activation of OSM promoter by thrombin requires AP-1 sequence
To identify functional cis-acting elements in the OSM promoter that are required for α-thrombin–induced gene expression, a 304-bp wild-type, a 194-bp wild-type, a 109-bp wild-type, and a 304-bp OSM promoter construct with a mutated AP-1 binding site, respectively, were transfected into MDMs.39 Luciferase analysis demonstrated that the minimum promoter sequence required for α-thrombin–induced OSM expression in MDMs resides within 194 bp. Stimulation with α-thrombin at a concentration of 1 U/mL resulted in significant increase of luciferase activity when cells were transfected with 304-bp and the 194-bp constructs. Further deletion to 109 bp decreased luciferase activity to the control level of unstimulated cells. Similarly, when cells were transfected with the 304-bp construct containing a mutated AP-1 binding site, luciferase activity in α-thrombin–stimulated cells was reduced to values seen in cells transfected with the same construct but incubated in the absence of thrombin, demonstrating that a functional AP-1 binding site in the OSM promoter is required for its transcriptional activation by α-thrombin (Figure 4B).
Discussion
Here we present evidence for the first time that in human peripheral blood monocytes, in human monocyte-derived macrophages, and in human plaque macrophages the coagulation factor thrombin up-regulates the expression of the inflammatory mediator OSM. In the latter cells, due to the small cell number that could be isolated from the atherosclerotic plaque, only mRNA for OSM levels could be determined using sensitive RealTime-PCR. The low cell number did not allow OSM protein measurements by ELISA. The macrophage cytokine OSM secreted also by T cells, eosinophils, and neutrophils and originally described as a regulator of cell growth has been more recently shown to be also implicated in the pathogenesis of cardiovascular disease.22 It should be emphasized that the expression of OSM and its receptor has been shown recently to be increased in the atherosclerotic vessel wall in mice.43
Upon further analysis we show here that in human monocyte–derived macrophages the stimulating effect of thrombin on OSM synthesis was concentration and time dependent. Maximum effects were observed with 1 U/mL thrombin, and a significant increase in OSM production by macrophages was seen after 8 and 24 hours of treatment with thrombin. The lowest thrombin concentration resulting in a significant increase in OSM production by macrophages in our experiments was 0.05 U/mL. These results were also reflected on the level of mRNA expression, as macrophages treated with thrombin expressed significantly higher levels of OSM-specific mRNA compared with untreated macrophages. These results were reproducible with 2 different preparations of human thrombin. It should be noted that thrombin concentrations shown here to significantly stimulate OSM production by macrophages in our experiments are lower than average thrombin levels reported to be generated in human plasma.44 A possible contamination of thrombin used in our experiments with LPS could be ruled out by the fact that boiled thrombin did not stimulate OSM production in macrophages, whereas PMX-B did not affect thrombin's ability to induce OSM in these cells. We could also show that macrophages isolated from various donors expressed PAR-1. With respect to individual differences between macrophages isolated from different donors in the response to thrombin treatment, a trend could be observed showing that macrophages with higher expression levels of PAR-1 responded to thrombin treatment with a more pronounced increase in OSM production. However, this has to be interpreted with caution, as the number of individual donors tested was low. Pretreatment of cells with PPACK, a potent inhibitor of thrombin, in human macrophages completely abolished the thrombin-induced expression of OSM, whereas treatment of the cells with a PAR-1–specific peptide, but not with a PAR-3– and PAR-4–specific peptide, mimicked the effect of thrombin on OSM production. Thus we conclude that the interaction of thrombin with the receptor PAR-1, which belongs to the 7-transmembrane receptor subfamily of G-coupled receptors, is needed to induce OSM production in macrophages. This conclusion was further supported by the fact that active site–blocked human FPRck-thrombin did not up-regulate OSM production in these cells. Furthermore, it should be noted that PPACK did not block up-regulation of OSM production in macrophages treated with C5a, which has been recently shown by us to be a strong inducer of OSM in these cells.38
In agreement with others, we could show that thrombin caused phosphorylation of ERK1/2 and p38 in these cells and that phosphorylation of these kinases was blocked by the ERK1/2 inhibitor PD98059 and by the p38 inhibitor SB202190.45,46 Furthermore, we showed that in macrophages only PD98059 blocked thrombin-induced up-regulation of OSM, whereas SB202190 had no effect, indicating that phosphorylation of ERK1/2 was critical for the observed effect. It is known that phosphorylation of ERK1/2 leads to activation of c-jun and c-fos.47 In support of this notion, we could show that thrombin induced the translocation of the AP-1 subunits c-fos and c-jun to the nucleus of macrophages. Because an AP-1 site has been identified in the human OSM promoter, we transfected human macrophages with a series of different OSM promoter deletion mutant constructs and a promoter construct with a mutated AP-1 binding motif.39 Only macrophages transfected with promoter constructs containing the native AP-1 site responded to thrombin treatment with increased promoter activity, whereas macrophages transfected with constructs lacking the AP-1 site or containing the mutated AP-1 site did not respond to thrombin treatment. Thus we provide evidence that the thrombin-induced increase in OSM production in macrophages involves AP-1 activation.
Accumulating evidence supports the notion that inflammation and coagulation play key roles in the pathogenesis of atherosclerosis and in particular in the development of its late complication namely atherothrombosis. Thereby inflammation leads to activation of the coagulation system on the one hand but coagulation also affects the inflammatory milieu on the other hand.7 The coagulation enzyme thrombin has also been shown to act as a proinflammatory mediator and thus seems to be a central player in the cross talk between the 2 systems.9,10,46 Among other proinflammatory effects, thrombin is chemotactic for monocytes, stimulates their proliferation and phagocytic activity, and in monocytes and macrophages up-regulates inflammatory cytokines linked to cardiovascular disease.11-18
In conclusion, we report here for the first time that in human peripheral blood monocytes, in human plaque macrophages, and in human monocyte-derived macrophages thrombin up-regulates the expression of oncostatin M and that in the latter cell type this effect is mediated via activation of AP-1. Our results lend further support to the hypothesis that cross talk between coagulation and inflammation is critically involved in the development and progression of atherosclerosis.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by grants from the Austrian Heart Foundation and from the Hans and Blanca Moser Foundation, by the Fund for the Promotion of Scientific Research (grant number S9409-B11), by the Ludwig Boltzmann Cluster for Cardiovascular Research, and by the Association for the Promotion of Research in Arteriosclerosis, Thrombosis, and Vascular Biology.
Authorship
Contribution: S.P.K. designed the research, performed experiments, and wrote the paper; W.S.S., K.M.K., C.K., and G.R. performed experiments; A.A. and G.W.H. analyzed data; M.H. and R.d.M. performed experiments and analyzed data; Y.M. contributed vital new reagents; G.M. and K.H. designed the research; and J.W. designed the research and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Johann Wojta, Department of Internal Medicine II, Medical University of Vienna, Waehringer Guertel 18-20, A-1090 Vienna, Austria; e-mail: johann.wojta@meduniwien.ac.at.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal