Abstract
Ninety-one children and adolescents 18 years or younger after allogeneic hematopoietic stem cell transplantation (HSCT) for relapsed or refractory Hodgkin lymphoma (HL) were analyzed. Fifty-one patients received reduced intensity conditioning (RIC); 40 patients received myeloablative conditioning (MAC). Nonrelapse mortality (NRM) at 1 year was 21% (± 4%), with comparable results after RIC or MAC. Probabilities of relapse at 2 and 5 years were 36% (± 5%) and 44% (± 6%), respectively. RIC was associated with an increased relapse risk compared with MAC; most apparent beginning 9 months after HSCT (P = .01). Progression-free survival (PFS) was 40% (± 6%) and 30% (± 6%) and overall survival (OS) was 54% (± 6%) and 45% (± 6%) at 2 and 5 years, respectively. Disease status at HSCT was predictive of PFS in multivariate analysis (P < .001). Beyond 9 months, PFS after RIC was lower compared with MAC (P = .02). Graft-versus-host disease did not affect relapse rate and PFS. In conclusion, children and adolescents with recurring HL show reasonable results with allogeneic HSCT. Especially patients allografted in recent years with good performance status and chemosensitive disease show highly encouraging results (PFS: 60% ± 27%, OS: 83% ± 15% at 3 years). Because relapse remains the major cause of treatment failure, additional efforts to improve disease control are necessary.
Introduction
Children and adolescents with localized and advanced Hodgkin lymphoma (HL) have an excellent prognosis with overall survival rates exceeding 90%.1-5 In this age group, even patients with recurring disease have a fair chance of cure using various risk-stratified approaches.6 Autologous hematopoietic stem cell transplantation (HSCT) is increasingly used as salvage therapy also in children7,8 with poor risk features, although the 2 randomized studies demonstrating superior event-free survival (EFS) and progression-free survival (PFS) after high-dose therapy (HDT) and HSCT included only adult patients.9,10
Allogeneic HSCT for HL first reported in the 1980s11,12 allowed for disease control in patients with relapsed or otherwise refractory disease.13,14 Its widespread use, however, has been hindered by a prohibitively high rate of nonrelapse mortality (NRM), caused mainly by acute as well as chronic graft-versus-host disease (GVHD) and/or severe infectious complications.14-16 More recently, reduced-intensity conditioning (RIC) was introduced to ameliorate NRM while maintaining the graft-versus-lymphoma (GVL) effect.17-21
Information regarding the role of allogeneic HSCT for HL in the pediatric population is very limited. Children undergoing allogeneic HSCT have been occasionally included in series of adult patients,13,14,22,23 whereas exclusively pediatric series were limited to fewer than 10 patients.24
We performed an analysis of pediatric and adolescent patients reported to the European Group for Blood and Marrow Transplantation (EBMT) Registry who underwent allogeneic HSCT for relapsed and refractory HL.
Methods
Patients, transplantation characteristics, and definitions
All children and adolescents up to the age of 18 years at allogeneic HSCT with biopsy-confirmed HL who were reported to EBMT were included in this analysis. EBMT is a voluntary organization that comprises nearly 550 transplantation centers. Members are required to report all consecutive HSCTs including a follow-up once a year. Data for this study were obtained from the lymphoma registry files and were verified and extended by a specific questionnaire sent to all participating transplantation centers to obtain missing data. Patients were allografted between 1987 and 2005 in 69 transplantation centers. Patients undergoing allogeneic HSCT as part of a planned tandem auto-allo program were not included. Key data of the patient cohort are given in Table 1. Data were analyzed as of May 2007.
Characteristics of 91 children and adolescents undergoing allogeneic HSCT for relapsed and refractory Hodgkin lymphoma
| Parameter . | Availableno. ofpatients . | Wholeseries, n = 91 . | Conditioning regimen . | P . | |
|---|---|---|---|---|---|
| MAC, n = 40 . | RIC, n = 51 . | ||||
| Period of allogeneic HSCT (%) | 91 | <.001 | |||
| 1987-2001 | 44 (48) | 28 (70) | 16 (31) | ||
| 2002-2005 | 47 (52) | 12 (30) | 35 (69) | ||
| Male sex, % | 91 | 52 (57) | 25 (62) | 27 (53) | NS |
| Median age at diagnosis, y (range) | 91 | 13 (2-17) | 12 (2-17) | 14 (4-16) | NS |
| Stage III/IV at diagnosis (%) | 54 | 38 (70) | 21 (78) | 17 (63) | NS |
| Median time from diagnosis to allogeneic HSCT, mo (range) | 91 | 26 (6-105) | 23 (7-93) | 31 (6-105) | .02 |
| 4 or more lines of prior treatment before allogeneic HSCT (%) | 66 | 31 (47) | 8 (26) | 23 (66) | .001 |
| Prior failed autologous HSCT (%) | 91 | 40 (44) | 8 (20) | 32 (63) | <.001 |
| Median time of autologous to allogeneic HSCT, mo (range) | 37 / 50 | 14 (3-38) | 13 (8-31) | 15 (3-38) | NS |
| Median age at allogeneic HSCT, y (range) | 91 | 16 (4-18) | 14 (4-18) | 17 (8-18) | .01 |
| Disease status at allogeneic HSCT (%) | 91 | NS | |||
| CR | 24 (26) | 13 (32) | 11 (22) | ||
| PR/sensitive relapse | 30 (33) | 11 (28) | 19 (37) | ||
| Refractory relapse/progression | 32 (35) | 14 (35) | 18 (35) | ||
| Untested relapse/progression | 5 (6) | 2 (6) | 3 (6) | ||
| Chemosensitivity at HSCT (%) | 91 | NS | |||
| Sensitive disease | 54 (59) | 24 (60) | 30 (59) | ||
| Refractory disease/untested | 37 (41) | 16 (40) | 19 (41) | ||
| Karnofsky/Lansky score of 80% or less | 60 | 12 (20) | 2 (11) | 10 (24) | NS |
| Donor type (%) | 91 | NS | |||
| HLA-identical sibling* | 58 (64) | 28 (70) | 30 (59) | ||
| Other matched related | 3 (3) | 2 (5) | 1 (2) | ||
| Matched unrelated | 18 (20) | 4 (10) | 14 (27) | ||
| Mismatched related | 7 (8) | 4 (10) | 3 (6) | ||
| Mismatched unrelated | 5 (5) | 2 (5) | 3 (6) | ||
| Donor female/patient male (%) | 89 | 20 (23) | 13 (33) | 7 (14) | NS |
| Patient and donor CMV-negative (%) | 52 | 19 (36) | 10 (37) | 9 (36) | NS |
| Stem cell source, BM/PBSC, %† | 91 | 50/50 | 67/33 | 36/64 | .006 |
| Ex vivo T-cell depletion (%) | 91 | 9 (11) | 6 (16) | 3 (6) | NS |
| TBI included in conditioning (%) | 91 | 27 (30) | 16 (40) | 11 (22) | NS |
| Median follow-up of surviving patients, mo (range) | 91 | 21 (6-154) | 43 (6-154) | 16 (6-92) | .005 |
| Parameter . | Availableno. ofpatients . | Wholeseries, n = 91 . | Conditioning regimen . | P . | |
|---|---|---|---|---|---|
| MAC, n = 40 . | RIC, n = 51 . | ||||
| Period of allogeneic HSCT (%) | 91 | <.001 | |||
| 1987-2001 | 44 (48) | 28 (70) | 16 (31) | ||
| 2002-2005 | 47 (52) | 12 (30) | 35 (69) | ||
| Male sex, % | 91 | 52 (57) | 25 (62) | 27 (53) | NS |
| Median age at diagnosis, y (range) | 91 | 13 (2-17) | 12 (2-17) | 14 (4-16) | NS |
| Stage III/IV at diagnosis (%) | 54 | 38 (70) | 21 (78) | 17 (63) | NS |
| Median time from diagnosis to allogeneic HSCT, mo (range) | 91 | 26 (6-105) | 23 (7-93) | 31 (6-105) | .02 |
| 4 or more lines of prior treatment before allogeneic HSCT (%) | 66 | 31 (47) | 8 (26) | 23 (66) | .001 |
| Prior failed autologous HSCT (%) | 91 | 40 (44) | 8 (20) | 32 (63) | <.001 |
| Median time of autologous to allogeneic HSCT, mo (range) | 37 / 50 | 14 (3-38) | 13 (8-31) | 15 (3-38) | NS |
| Median age at allogeneic HSCT, y (range) | 91 | 16 (4-18) | 14 (4-18) | 17 (8-18) | .01 |
| Disease status at allogeneic HSCT (%) | 91 | NS | |||
| CR | 24 (26) | 13 (32) | 11 (22) | ||
| PR/sensitive relapse | 30 (33) | 11 (28) | 19 (37) | ||
| Refractory relapse/progression | 32 (35) | 14 (35) | 18 (35) | ||
| Untested relapse/progression | 5 (6) | 2 (6) | 3 (6) | ||
| Chemosensitivity at HSCT (%) | 91 | NS | |||
| Sensitive disease | 54 (59) | 24 (60) | 30 (59) | ||
| Refractory disease/untested | 37 (41) | 16 (40) | 19 (41) | ||
| Karnofsky/Lansky score of 80% or less | 60 | 12 (20) | 2 (11) | 10 (24) | NS |
| Donor type (%) | 91 | NS | |||
| HLA-identical sibling* | 58 (64) | 28 (70) | 30 (59) | ||
| Other matched related | 3 (3) | 2 (5) | 1 (2) | ||
| Matched unrelated | 18 (20) | 4 (10) | 14 (27) | ||
| Mismatched related | 7 (8) | 4 (10) | 3 (6) | ||
| Mismatched unrelated | 5 (5) | 2 (5) | 3 (6) | ||
| Donor female/patient male (%) | 89 | 20 (23) | 13 (33) | 7 (14) | NS |
| Patient and donor CMV-negative (%) | 52 | 19 (36) | 10 (37) | 9 (36) | NS |
| Stem cell source, BM/PBSC, %† | 91 | 50/50 | 67/33 | 36/64 | .006 |
| Ex vivo T-cell depletion (%) | 91 | 9 (11) | 6 (16) | 3 (6) | NS |
| TBI included in conditioning (%) | 91 | 27 (30) | 16 (40) | 11 (22) | NS |
| Median follow-up of surviving patients, mo (range) | 91 | 21 (6-154) | 43 (6-154) | 16 (6-92) | .005 |
BM indicates bone marrow; CMV, cytomegalovirus; CR, complete remission; HSCT, hematopoietic stem cell; MAC, myeloablative conditioning; NS, not significant; PBSC, peripheral blood stem cell; PR, partial remission; RIC, reduced-intensity conditioning; and TBI, total body irradiation.
One patient was grafted from a syngeneic donor.
One patient received BM and PBSCs.
Conditioning regimens were categorized as myeloablative (MAC, 40 patients) or reduced intensity (RIC, 51 patients) according to the definition of EBMT.25 MAC regimens included combinations of cyclophosphamide (100-200 mg/kg) with high-dose busulfan (16 mg/kg; 3 patients) or total-body irradiation (TBI, 8-12 Gy; 7 patients). TBI was combined with other drugs, such as etoposide or cytosine-arabinoside in 6 patients, whereas the remaining 24 patients received other chemotherapeutic regimens. Most of the patients in the RIC cohort received fludarabine-based regimens in combination with melphalan (70-140 mg/m2), busulfan (8-10 mg/kg), cyclophosphamide (60-120 mg/kg), thiotepa (5-10 mg/kg; 26 patients), or low-dose TBI (2-4 Gy) in 8 patients. Seventeen patients received other combinations.
Anti–thymocyte globuline or anti–lymphocyte globuline was given in 37% of the patients, 8% of the patients received alemtuzumab for in vivo T-cell depletion, and in vitro T-cell depletion was performed in 11% of cases. GVHD prophylaxis was heterogeneous but used cyclosporine A alone in 19 cases, combined with short-course methotrexate (23 cases), mycophenolate mofetil (6 cases), or steroids (4 patients).
Statistical analysis
Patient and transplant characteristics were compared between groups using the chi-square test or Fisher exact test for categoric variables and the t test or Mann-Whitney U test for continuous variables. The probabilities of PFS and overall survival (OS) were calculated from the time of transplantation using the Kaplan-Meier estimator, and compared by the 2-tailed log-rank test. The occurrence of neutrophil recovery, acute GVHD, chronic GVHD, NRM, and disease progression after HSCT was calculated using cumulative incidence estimates, taking into account the competing risk structure, and compared by univariate Cox regression models. The impact of acute grade II-IV GVHD or chronic GVHD on the outcome after transplantation was investigated introducing GVHD as a time-dependent covariate.
The following covariates were analyzed in univariate analysis: patient sex, patient age at HSCT (continuous and up to 14 years vs older than 14 years), year of HSCT (up to 2001 vs 2002-2005), time interval between diagnosis and HSCT (continuous covariate and < 36 months vs≥ 36 months), number of prior treatment lines before HSCT (up to 3 vs 4 or more), prior autologous HSCT (yes vs no), disease status at HSCT (chemosensitive vs chemorefractory disease), performance status at HSCT, type of conditioning regimen (RIC vs MAC), anti–thymocyte globuline in the conditioning regimen, TBI in the conditioning regimen (independent of RIC or MAC procedures), type of donor, recipient-donor sex match (female donor to male patient vs others), cytomegalovirus (CMV) risk group (donor and recipient seronegative vs others), stem cell source, ex vivo T-cell depletion, and in vivo T-cell depletion.
Potential prognostic factors for NRM, relapse/progression, PFS, and OS were investigated by Cox regression multivariate analyses, using a backward-stepping procedure. To avoid loss of information, a category for “unknown” was included in the Cox model for some risk factors. The assumption of proportional hazards for each factor in the Cox model was tested using time-dependent covariates. When this indicated differential effects over time (nonproportional hazards), models were constructed breaking the posttransplantation time course into 2 periods, using the most appropriate breakpoint. Sex of the patient, age at HSCT, number of prior treatment lines, prior failed autologous HSCT, year of allogeneic HSCT, disease status at allogeneic HSCT, performance status at allogeneic HSCT, type of donor, type of conditioning regimen, CMV risk group, and stem cell source were also used as covariates for NRM. The impact of sex on NRM was investigated by univariate and multivariate analyses in the whole series and splitting the population in patients up to 14 years and older than 14 years. Sex of the patient, age at HSCT, number of prior treatment lines, prior failed autologous HSCT, year of allogeneic HSCT, disease status at allogeneic HSCT, performance status at allogeneic HSCT, type of donor, type of conditioning regimen, stem cell source, and T-cell depletion were variables used for the multivariate analysis of relapse or progression. Sex of the patient, age at HSCT, number of prior treatment lines, prior failed autologous HSCT, year of allogeneic HSCT, disease status at allogeneic HSCT, performance status at allogeneic HSCT, type of donor, type of conditioning regimen, CMV risk group, stem cell source, and T-cell depletion were variables considered for both OS and PFS. All P values are 2-sided.
OS was defined as time from transplantation to last contact or death from any cause and was measured in months. PFS was defined as time to relapse or progression, death, or last contact. Relapse rate was defined as time to disease recurrence or progression. NRM was defined as time to death not related to disease progression or relapse. Complete remission (CR) was defined as disappearance of all measurable disease irrespective of the method used for disease detection. Partial remission (PR) was defined as disease reduction by more than 50% without reaching a CR. Stable disease was defined as response rate between 25% and 50%. Progression was defined as disease recurrence or appearance of new symptoms within 3 months after HSCT. Patients were considered as having sensitive disease at HSCT, if they had achieved CR or PR before HSCT, whereas refractory disease was diagnosed in patients who had no objective response or had proved refractory to salvage therapy before allogeneic HSCT. Acute and chronic GVHD were classified according to standard criteria.26,27
SPSS Version 14.0 was used for all statistical analyses with the exception of the cumulative incidence analyses, which were performed with NCSS97 (Number Cruncher Statistical System).
Results
Ninety-one patients with a median age of 13.5 years at diagnosis (range, 2.2-17.9 years) and 16.6 years at transplantation (range, 4.2-18.9 years) were analyzed. There were more males than females, and 70% of patients had shown advanced stages at diagnosis. Comparing patients who received MAC with RIC, the latter group had a longer time interval between diagnosis and allogeneic HSCT, had failed more lines of therapy including HDT and autologous HSCT, and was significantly older than patients who underwent transplantation after conventional conditioning (Table 1). No significant differences existed in the percentages of patients grafted in CR, PR, refractory disease, or untreated relapse and the performance status at the time of transplantation. In addition, the percentages of patients with human leukocyte antigen (HLA)–identical sibling donors, other matched related or unrelated donors, as well as mismatched donors were not significantly different. Not surprisingly, patients with reduced-intensity conditioning underwent transplantation more recently and preferentially received mobilized peripheral blood stem cells (PBSCs).
Hematopoietic recovery
Five patients were reported as primary graft failures. Four of them had been allografted with bone marrow stem cells after RIC regimens. Causes of death were NRM in 3 patients and early progression in 2 patients.
The cumulative incidence of neutrophil recovery (> 0.5 × 109/L) for all patients was 80% at 21 days, 88% at 28 days, and 93% at 100 days. The median time to neutrophil recovery was 16 days (range, 5-46 days), median time to platelet recovery (> 20 × 109/L) was 22 days (range, 7-80 days), and median time to platelet recovery (> 50/nL) was 28 days (range, 12-142 days). There was no statistical difference in neutrophil recovery between patients after MAC and RIC regimens (cumulative incidence at day +28, 90% vs 87%, respectively). Patients after RIC had a more rapid platelet recovery (> 20 × 109/L and > 50 × 109/L) than those after MAC (16 days vs 33 days [P = .007] and 20.5 days vs 35 days [P = .01], respectively). Patients who received PBSCs had a significantly shorter time to engraftment than those who received bone marrow (cumulative incidence at day +28, 94% vs 84%; P = .004).
Nonrelapse mortality
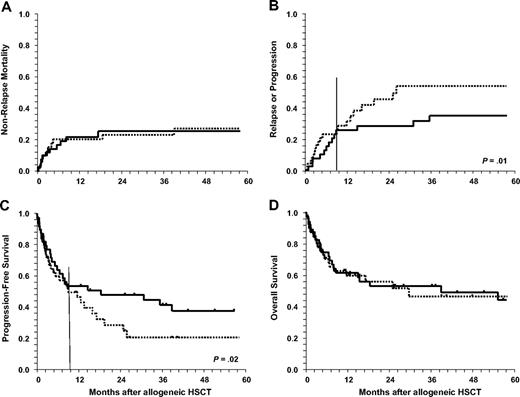
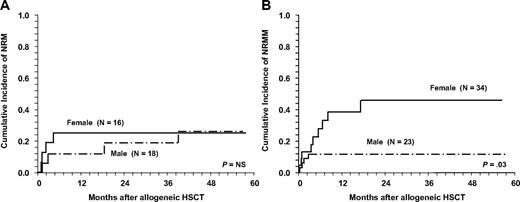
A total of 21 patients (23%) died after allogeneic HSCT without suffering a relapse or progression after transplantation. Ten patients were not evaluable due to early death, 8 patients had achieved a CR, and 3 patients were in PR or had minimal response after HSCT. Cumulative incidences for NRM at 1, 2, and 5 years were 21% (± 4%), 24% (± 5%), and 26% (± 5%), respectively. The main causes of NRM were infections (7 patients), infections in combination with GVHD (7 patients), and organ toxicity (7 patients). There was no difference in terms of NRM for patients with matched related versus unrelated donors (12 of 58 patients vs 3 of 18 patients, respectively) but HLA disparity between donor and recipient increased NRM significantly (Tables 2–3). NRM was not statistically significant between patients receiving MAC compared with RIC (Figure 1A). This observation was also true for patients who failed a prior autologous HSCT after HDT. There was a trend for increased NRM in patients with refractory disease and untested relapse (RR = 2.2; 95% confidence interval [CI]: 0.9-5.4; P = .06). Considering the year of HSCT, no significant difference was found between patients allografted up to 2001 and later for the whole group (P = .2). Cumulative incidence for NRM in RIC patients at 2 years was 26% and 27% for the 2 time periods. In contrast, the risk decreased for patients in the MAC group (29% for 28 patients who underwent transplantation up to 2001, compared with 9% in 12 patients allografted from 2002 onward, P = .02). Of note, female sex was correlated with higher NRM in both univariate and multivariate analyses even after adjusting for cofactors (Tables 2–3). This difference was apparent in patients older than 14 years (Figure 2B), whereas in the younger population up to 14 years no difference was seen (Figure 2A).
Univariate analysis of factors associated with adverse outcome in 91 pediatric and adolescent patients undergoing allogeneic transplantation for recurring Hodgkin lymphoma
| Variable . | Type . | P . |
|---|---|---|
| Nonrelapse mortality | ||
| Sex | Female vs male | .03 |
| Donor relation | Mismatched vs matched | .05 |
| Status at HSCT | Refractory vs sensitive | .09 |
| Performance status | Poor vs good | .09 |
| CMV status donor/recipient | Neg/neg vs others | .10 |
| Relapse rate | ||
| Performance status | Poor vs good | .002 |
| Status at HSCT | Refractory vs sensitive | .008 |
| Type of conditioning* | Reduced intensity vs myeloablative | .01 |
| Progression-free survival | ||
| Status of HSCT | Refractory vs sensitive | .001 |
| Performance status | Poor vs good | .001 |
| Type of conditioning | Reduced intensity vs myeloablative | .02 |
| Sex | Female vs male | .04 |
| CMV status of donor/recipient | Neg/neg vs others | .05 |
| Overall survival | ||
| Status at HSCT | Refractory vs sensitive | .01 |
| Performance status | Poor vs good | .01 |
| CMV status of donor/recipient | Neg/neg vs others | .02 |
| Time to allogeneic HSCT | < 36 mos vs ≥ 36 mos | .04 |
| Sex | Female vs male | .04 |
| Variable . | Type . | P . |
|---|---|---|
| Nonrelapse mortality | ||
| Sex | Female vs male | .03 |
| Donor relation | Mismatched vs matched | .05 |
| Status at HSCT | Refractory vs sensitive | .09 |
| Performance status | Poor vs good | .09 |
| CMV status donor/recipient | Neg/neg vs others | .10 |
| Relapse rate | ||
| Performance status | Poor vs good | .002 |
| Status at HSCT | Refractory vs sensitive | .008 |
| Type of conditioning* | Reduced intensity vs myeloablative | .01 |
| Progression-free survival | ||
| Status of HSCT | Refractory vs sensitive | .001 |
| Performance status | Poor vs good | .001 |
| Type of conditioning | Reduced intensity vs myeloablative | .02 |
| Sex | Female vs male | .04 |
| CMV status of donor/recipient | Neg/neg vs others | .05 |
| Overall survival | ||
| Status at HSCT | Refractory vs sensitive | .01 |
| Performance status | Poor vs good | .01 |
| CMV status of donor/recipient | Neg/neg vs others | .02 |
| Time to allogeneic HSCT | < 36 mos vs ≥ 36 mos | .04 |
| Sex | Female vs male | .04 |
Factors with a P value <.1 are listed.
CMV indicates cytomegalovirus; HSCT, hematopoietic stem cell transplantation; neg, negative; RIC, reduced-intensity conditioning; and vs, versus.
Higher relapse and progression rate for patients after RIC starting 9 months after HSCT.
Multivariate analysis of adverse prognostic factors in allogeneic HSCT for pediatric Hodgkin lymphoma associated with increased NRM, relapse rate, PFS, and OS
| . | RR . | CI . | P . |
|---|---|---|---|
| Nonrelapse mortality | |||
| Female vs male sex | 2.9 | 1.1-7.7 | .02 |
| Mismatched vs matched donor | 3.1 | 1.0-9.9 | .05 |
| Relapse rate | |||
| Poor vs good performance status | 3.2 | 1.2-8.4 | .02 |
| Refractory vs sensitive disease | 2.1 | 1.0-4.4 | .04 |
| Reduced vs myeloablative conditioning* | |||
| First 9 mos after HSCT | No differences | ||
| More than 9 mos after HSCT | 4.4 | 1.0-19.0 | .05 |
| Progression-free survival | |||
| Refractory vs sensitive disease | 2.8 | 1.6-4.9 | < .001 |
| CMV positivity in donor and/or recipient | 2.9 | 1.3-6.5 | .01 |
| Mismatched vs matched donor | 2.6 | 1.1-5.8 | .02 |
| Reduced vs myeloablative conditioning* | |||
| First 9 months after HSCT | No differences | ||
| More than 9 months after HSCT | 3.8 | 1.1-11.5 | .02 |
| Year of HSCT, before 2002 vs 2002-2005 | 2.0 | 1.0-4.2 | .05 |
| Overall survival | |||
| Refractory vs sensitive disease | 2.9 | 1.6-5.6 | .001 |
| CMV positivity in donor and/or recipient | 4.6 | 1.7-12.5 | .003 |
| Mismatched vs matched donor | 2.9 | 1.1-7.4 | .03 |
| . | RR . | CI . | P . |
|---|---|---|---|
| Nonrelapse mortality | |||
| Female vs male sex | 2.9 | 1.1-7.7 | .02 |
| Mismatched vs matched donor | 3.1 | 1.0-9.9 | .05 |
| Relapse rate | |||
| Poor vs good performance status | 3.2 | 1.2-8.4 | .02 |
| Refractory vs sensitive disease | 2.1 | 1.0-4.4 | .04 |
| Reduced vs myeloablative conditioning* | |||
| First 9 mos after HSCT | No differences | ||
| More than 9 mos after HSCT | 4.4 | 1.0-19.0 | .05 |
| Progression-free survival | |||
| Refractory vs sensitive disease | 2.8 | 1.6-4.9 | < .001 |
| CMV positivity in donor and/or recipient | 2.9 | 1.3-6.5 | .01 |
| Mismatched vs matched donor | 2.6 | 1.1-5.8 | .02 |
| Reduced vs myeloablative conditioning* | |||
| First 9 months after HSCT | No differences | ||
| More than 9 months after HSCT | 3.8 | 1.1-11.5 | .02 |
| Year of HSCT, before 2002 vs 2002-2005 | 2.0 | 1.0-4.2 | .05 |
| Overall survival | |||
| Refractory vs sensitive disease | 2.9 | 1.6-5.6 | .001 |
| CMV positivity in donor and/or recipient | 4.6 | 1.7-12.5 | .003 |
| Mismatched vs matched donor | 2.9 | 1.1-7.4 | .03 |
Those factors differing with P ≤ .05 are shown.
CI indicates confidence interval; CMV, cytomegalovirus; HSCT, hematopoietic stem cell transplantation; and RR, relative risk.
The covariate “type of conditioning” significantly influenced relapse rate and PFS starting at 9 months after allogeneic HSCT.
Outcome after allogeneic HSCT for pediatric HL according to conditioning regimen intensity (MAC vs RIC). Myeloablative (MAC) is indicated by solid line and reduced intensity (RIC) by dotted line. (A) Cumulative incidence for nonrelapse mortality. (B) Relapse rate is significantly increased after RIC (P = .01) from 9 months on. (C) Progression-free survival is lower (P = .02) from 9 months on after RIC. (D) Overall survival shows no significant differences between MAC and RIC regimens. HL indicates Hodgkin lymphoma; and HSCT, hematopoietic stem cell transplantation.
Outcome after allogeneic HSCT for pediatric HL according to conditioning regimen intensity (MAC vs RIC). Myeloablative (MAC) is indicated by solid line and reduced intensity (RIC) by dotted line. (A) Cumulative incidence for nonrelapse mortality. (B) Relapse rate is significantly increased after RIC (P = .01) from 9 months on. (C) Progression-free survival is lower (P = .02) from 9 months on after RIC. (D) Overall survival shows no significant differences between MAC and RIC regimens. HL indicates Hodgkin lymphoma; and HSCT, hematopoietic stem cell transplantation.
Cumulative incidences for NRM in children and adolescents according to sex and age. (A) No differences between girls (solid line) and boys (dashed line) in patients aged 14 years or younger. (B) Girls older than 14 years are at increased risk for nonrelapse mortality (NRM) compared with boys.
Cumulative incidences for NRM in children and adolescents according to sex and age. (A) No differences between girls (solid line) and boys (dashed line) in patients aged 14 years or younger. (B) Girls older than 14 years are at increased risk for nonrelapse mortality (NRM) compared with boys.
Acute and chronic GVHD
Data on acute GVHD were available for 85 patients (2 patients died early and data are missing on 4 patients). Forty patients (47.1%) did not develop acute GVHD. Grade I GVHD was present in16 patients (18.8%) and grade II to IV GVHD developed in21 patients (24.7%; grade II: 11 patients, grade III: 6 patients, grade IV: 4 patients). No significant impact of acute GVHD on NRM, relapse rate, PFS, or OS was found.
Of 62 patients at risk for spontaneous chronic GVHD (alive without relapse or disease progression and no donor lymphocyte infusion [DLI] at day +100), data were available in 53 cases. Nineteen patients (35.8%) developed chronic GVHD. Eight of 19 patients developed limited and 9 patients experienced extensive chronic GVHD (unknown in 2 cases). Eight (42.1%) of 19 patients with chronic GVHD are alive compared with 21 (61.8%) of 34 patients without chronic GVHD. The occurrence of chronic GVHD was associated with increased NRM (P = .01) and a trend for lower OS (P = .08), whereas relapse rate and PFS were similar with or without chronic GVHD.
Relapse and progression
Relapse or progression after allogeneic HSCT occurred in 33 patients (36.3%) after a median time of 6 months (range, < 1-36 months). Only 9 of these patients were alive at last contact, whereas 24 patients had died. Probability of relapse or progression at 2 and 5 years was 36% (± 5%) and 44% (± 6%), respectively. Disease and performance status at HSCT significantly influenced disease recurrence in univariate as well as in multivariate analyses (Tables 2–3). The year of HSCT was not associated with relapse.
Response to transplantation and outcome
At day +100, 79 patients were evaluable for disease response. Forty-seven patients (59.5%) had achieved a CR and 17 patients (21.5%) had achieved a PR, accounting for an overall response rate of 81%. A minimal response was seen in 3 patients, whereas 12 patients experienced disease recurrence or progression. With a median follow up of 21 months (range, 6-154 months) for surviving patients, 46 patients were alive at last contact, whereas 45 patients had died.
The probability of PFS at 2 and 5 years was 40% (± 6%) and 30% (± 6%), respectively (Figure 1C). Disease status at HSCT turned out as the strongest factor associated with PFS in univariate (Table 2; P = .001) and multivariate (Table 3; P < .001) analyses. In univariate analysis, patients with poor performance status at HSCT (P = .001) also had a lower PFS. Patients who had undergone HSCT before 2002 had a worse outcome than those allografted in recent years (RR = 2.0; CI: 1.0-4.2; P = .05). PFS in patients who received transplants from matched unrelated donors was not significantly different from that of HLA-identical siblings. This finding was confirmed in patients allografted after 2001 (P = .3). The use of mismatched donors significantly reduced PFS after HSCT.
Probability of OS at 2 and 5 years was 54% (± 6%) and 41% (± 6%), respectively. Refractory disease and poor performance status at HSCT were the strongest adverse factors in univariate analysis (P = .01 each, Table 2), but CMV status, time to HSCT, and sex were also significant factors. In multivariate analysis, refractory disease (P = .001), CMV status (donor/recipient other than negative/negative) (P = .003), and mismatched transplants (P = .03) were independent adverse risk factors (Table 3). Female sex (P = .06) and HSCT before 2002 (P = .07) showed a trend for inferior outcome.
Of note, the 26 patients with sensitive disease and good performance status who underwent transplantation between 2002 and 2005 showed a PFS of 60% (95% CI: 33%-87%) and OS of 83% (95% CI: 67%-98%), respectively, at 3 years (Figure 3). Fifteen of these patients (58% of the group) had previously failed autologous HSCT.
OS and PFS in 26 patients with good risk features. Patients allografted since 2001 with chemosensitive disease and good performance status at the time of allogeneic HSCT show a favorable outcome (overall survival [OS], solid line; progression-free survival [PFS], dotted line).
OS and PFS in 26 patients with good risk features. Patients allografted since 2001 with chemosensitive disease and good performance status at the time of allogeneic HSCT show a favorable outcome (overall survival [OS], solid line; progression-free survival [PFS], dotted line).
Conditioning regimen
In the early posttransplantation period, there was no difference in terms of relapse rate between patients receiving MAC or RIC. Accordingly, PFS with regard to conditioning regimen intensity was the same in the 2 groups. Starting at 9 months, however, it became apparent that the relapse risk was significantly higher in patients who had received RIC (Figure 1B; P = .01). In addition, from 9 months onward, PFS was lower in patients after RIC (Figure 1C; P = .02). Thus, the type of conditioning regimen had a significant impact on PFS that was time dependent (Tables 2–3). The type of conditioning (MAC versus RIC) did not affect OS (Figure 1D). When analyzing the impact of the conditioning regimen on outcome in relation to the year of HSCT, no significant differences were observed for PFS and relapse at 2 and 5 years, within the RIC cohort, respectively. Before 2002 (n = 16), at 2 and 5 years, PFS was 27.5% and 20.6%, respectively, compared with 28.9% and 21.7%, respectively, thereafter (n = 35), respectively. For the 28 patients who underwent transplantation up to 2001, these figures were 46.4% and 35.7%, respectively, in the MAC group and 55% at both time points for those 12 patients who underwent HSCT from 2002 onward (P = NS). Cumulative incidence of relapse in the RIC patients before 2002 was 46.9% and 53.8% at 2 and 5 years, respectively, compared with 44.1% and 51.3%, respectively, in patients who underwent transplantation thereafter (P = NS). For patients allografted after MAC, the percentages were 25% and 32.1% before 2002 versus 35.8% since then, respectively (P = NS).
Donor lymphocyte infusion
Twelve (15.6%) of 77 patients with pertinent information received DLI. The reason for DLI administration was disease recurrence in 7 cases, persistent disease in 2 patients, and preemptive disease in 3 patients. One patient treated for relapse/progression after HSCT is alive in CR 1 year later, 5 patients did not respond, and response is unknown in 1 case. Both patients treated for disease persistence around day +100 after HSCT showed no response and progressed after DLI. One of 3 patients who received preemptive DLI is alive and in CR 2 years after HSCT, whereas the other 2 patients experienced disease progression after DLI.
Discussion
This analysis reports the largest series of children and adolescents allografted for relapsed and refractory HL. Survival rates of 54% at 2 years and 45% at 5 years appear promising in a cohort of patients, most of whom had failed multiple of the otherwise highly successful pediatric salvage protocols, including autologous HSCT in almost half of them. Patients with good performance status and treatment-sensitive disease who underwent transplantation since 2001 achieved an OS of 83% and PFS of 60% at 3 years, which is particularly encouraging. OS and PFS of 45% and 30%, respectively, at 5 years reported for the whole group under study compare favorably to survival rates reported for adults after RIC. The series of adult patients recently published by Sureda et al reported OS and PFS rates of 22% and 20%, respectively, after conventional conditioning, and 28% and 18%, respectively, after RIC at 5 years.23 The largest series of adult patients with HL who received an allogeneic transplantation after reduced-intensity conditioning was recently reported by Robinson et al.28 In 285 patients, these authors reported NRM of 21.1% at 3 years and OS and PFS rates of 43% and 25% at 3 years, respectively. Thus, OS and more importantly PFS for the children and adolescents reported here seem substantially better than in adults. Although we realize that known and unknown differences in patient characteristics and transplantation modalities may have biased findings from all 3 studies, an OS of 45% at 5 years for a group of patients with no viable alternative is remarkable and should play a role in decision-making for individual young adults and children with relapsed or refractory HL. These results are even more encouraging taking into consideration that the good-risk patients (good performance status and sensitive disease at the time of transplantation), of whom more than 50% had previously failed an autologous HSCT, showed a PFS of 60%.
What other lessons may be learned from this analysis? NRM has been one of the major problems in any series of patients allografted for HL. The 21% NRM at 1 year for the patients reported here compares favorably to previous adult series reporting on outcomes after MAC.14-16 After RIC, NRM was 19.5% in the largest series of adult patients reported by EBMT28 but was significantly higher in adults receiving MAC (46%).23 We did not observe a significant difference in NRM for children and adolescents who underwent transplantation after MAC or RIC (Figure 1A), but relapses were increased after RIC compared with MAC, and PFS was better for patients having received MAC. Differences between MAC and RIC became significant only when the time periods beyond 9 months were considered. It should be mentioned that patients in the 2 groups were not completely balanced for treatment before allogeneic HSCT. However, the disease status before transplantation was comparable. Because relapse now is the major problem after allogeneic transplantation for HL in pediatric as well as in adult patients, whereas NRM was no worse after MAC in these younger patients, it may be wise to use myeloablative or “intermediate” conditioning at least in those children and adolescents who arrive at the transplantation center in good performance status but with multiply relapsed or refractory disease. Alternatively, other attempts to debulk the tumor before HSCT—using aggressive salvage therapy or HDT—should be considered.
Since it was documented that GVL effects could be generated by cytokine activation after autologous HSCT,29 it seems reasonable to assume that better efficacy might be obtained after allogeneic HSCT when donor lymphocytes can be induced to become more alloreactive. From this perspective, more studies are necessary to confirm the potential role of posttransplantation immunotherapy using DLI to activate the GVL effects.
In this study we were unable to demonstrate a significant effect of chronic GVHD on the risk of relapse and on PFS. In contrast, Sureda et al23 showed that adult patients developing GVHD had a significantly reduced risk of relapse and a trend for better PFS. In the large study by Robinson et al, chronic GVHD showed a trend to lower relapse rates but did not impact on PFS or OS. Development of chronic GVHD by 9 months after transplantation, however, reduced relapse rates significantly.28 We can only speculate why these observations could not be confirmed in this analysis on younger patients. It is well known that the risk to develop GVHD is age dependent with children and adolescents running a lower risk than adults. In addition, the number of patients overall and the number of patients developing acute and/or chronic GVHD was relatively low in this cohort, and the methods used for GVHD prophylaxis might have been too diverse to demonstrate a significant correlation between GVHD and relapse rate.
The impact of DLI in this cohort of patients is difficult to judge. Whereas DLI after in vivo T-cell depletion seems to induce remissions quite frequently indicating a significant graft-versus-Hodgkin effect,21 patients receiving full marrow or PBSC grafts respond less favorably,28 shedding some doubt on the assumption that DLI will be of great help to induce further remissions in patients with HL who received T-cell replete transplants.
In summary, we show that allogeneic transplantation is a viable treatment strategy for children and adolescents with relapsed HL who failed most if not all other options available. Prospective trials for children, adolescents, and young adults with HL will be mandatory to better define the optimal time point for allogeneic HSCT, as well as the best conditioning regimen and way of GVHD prophylaxis.
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
Presented in part in abstract form at the 49th annual meeting of the American Society of Hematology, Atlanta, GA, December 10, 2007.30
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgment
We acknowledge the kind support of all physicians and data managers of the participating transplantation centers.
Authorship
Contribution: A.C., A.S., and N.S. designed the study, collected and analyzed the data, and wrote the paper; A.C., D.D., J.S., I.B., A.P., S.M., S.S., J.-H.D., M.J.C., M. Sarhan., R.F.W., M. Suttorp, G.D., A.S., and N.S. provided study material and included patients; and C.C. analyzed the data and performed statistical analyses. All authors have read and approved the final version of the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
A complete list of EBMT centers that participated in this joint study by the Lymphoma and Pediatric Working Parties appears in the supplemental Appendix (available on the Blood website; see the Supplemental Materials link at the top of the online article).
Correspondence: Alexander Claviez, Department of Pediatrics and Bone Marrow Transplant Unit, University Hospital of Schleswig-Holstein Campus Kiel, Schwanenweg 20, 24105 Kiel, Germany; e-mail: a.claviez@pediatrics.uni-kiel.de.

![Figure 3. OS and PFS in 26 patients with good risk features. Patients allografted since 2001 with chemosensitive disease and good performance status at the time of allogeneic HSCT show a favorable outcome (overall survival [OS], solid line; progression-free survival [PFS], dotted line).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/114/10/10.1182_blood-2008-11-189399/4/m_zh89990939950003.jpeg?Expires=1769409256&Signature=JFHRGCJ49Eu1OoaH5hK3WBKPtoW~bIMpg5WTyqb9jtwEzovEASK38u--lGokGe1MCl0eedPzx6PkJFGN7kCUyS~Xuc4lkCF7R7u~x8mB8F8KeTBrI-XQISHFqMgwQhxeZxRsUTOV66zdOtplIfN6K4LVyUcywoZP5fZ2m32R3eM2WXMwIXeMNZzq109zQuZPllRHKFvZFgyGzaVifnYXTH2bgry0GgTSTtQUKxM1c2EDtsunKlCFjxDiD2OSo1I-1eykSy-Ho4vUbE6ECZY79~FgniHS4mqgd3BbOhZ4HyfA12orHYLVE0Ab3JEFv0WaE2yVo7jUljlvd~oBOkGkvQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)