Abstract
Current treatment strategies for Hodgkin lymphoma result in excellent survival but often confer significant long-term toxicity. We designed ABVE-PC (doxorubicin, bleomycin, vincristine, etoposide, prednisone, cyclophosphamide) to (1) enhance treatment efficacy by dose-dense drug delivery and (2) reduce risk of long-term sequelae by response-based reduction of cumulative chemotherapy. Efficient induction of early response by dose-dense drug delivery supported an early-response–adapted therapeutic paradigm. The 216 eligible patients were younger than 22 years with intermediate- or high-risk Hodgkin lymphoma. ABVE-PC was administered every 21 days. Rapid early responders (RERs) to 3 ABVE-PC cycles received 21 Gy radiation to involved regions; RER was documented in 63% of patients. Slow early responders received 2 additional ABVE-PC cycles before 21 Gy radiation. Five-year event-free-survival was 84%: 86% for the RER and 83% for the slow early responders (P = .85). Only 1% of patients had progressive disease. Five-year overall survival was 95%. With this regimen, cumulative doses of alkylators, anthracyclines, and epipodophyllotoxins are below thresholds usually associated with significant long-term toxicity. ABVE-PC is a dose-dense regimen that provides outstanding event-free survival/overall survival with short duration, early-response–adapted therapy. This trial was registered at www.clinicaltrials.gov as #NCT00005578.
Introduction
Hodgkin lymphoma (HL) was one of the first malignancies for which curative chemotherapeutic regimens were developed. MOPP (nitrogen mustard, vincristine, procarbazine, prednisone) and ABVD (doxorubicin, bleomycin, vinblastine, dacarbazine) have been mainstays of therapy for decades.1-3 Despite late sequelae (infertility, second malignancy, cardiopulmonary toxicity) and knowledge that total dose and rate of drug delivery impact treatment efficacy,3-5 the adequacy of these low-intensity regimens deterred efforts to improve them.
In 1997, we developed a novel dose-dense chemotherapy regimen, ABVE-PC (doxorubicin, bleomycin, vincristine, etoposide, prednisone, cyclophosphamide), with the goal of enhancing tumor cytotoxicity and inducing rapid tumor responses. This regimen modified ABVD by (1) substituting therapeutically equivalent vincristine6 for vinblastine to avoid myelosuppression, (2) replacing dacarbazine with etoposide to limit delays and reductions in therapy, (3) adding cyclophosphamide and prednisone to enhance efficacy, and (4) increasing dose density by the use of filgrastim to deliver chemotherapy every 3 weeks. ABVE had previously been shown to be effective in low-stage HL.7 The dose of each chemotherapeutic drug in ABVE-PC was specifically selected to limit the cumulative dose below recognized thresholds for known long-term toxicities.
Evidence from our previous trials in advanced HL8 showed that rapid early response (RER) was predictive of event-free survival (EFS), whereas later response (at completion of chemotherapy) was not predictive. This supported the premise that RER was a measure of chemosensitivity. On this basis, we hypothesized that early response assessments would permit patient-specific determination of the cumulative chemotherapy needed. Dose density (dose/week) was maximized using ABVE-PC. Cumulative doses were reduced both by the design of the regimen, as previously discussed, and by limiting the therapy duration to 3 (RER) or 5 slow early responders (SER) cycles. Although concurrently designed regimens also intensified dose density,9,10 they did not tailor cumulative therapy by early response.
We report the results of treating intermediate and advanced HL with dose-dense ABVE-PC and 21 Gy radiation according to a response-based treatment paradigm. Our goal was to achieve excellent treatment efficacy with reduced cumulative therapy, thereby limiting the potential for long-term toxicity.
Methods
This study was conducted at Pediatric Oncology Group institutions from June 1997 through October 2001. Written informed consent was obtained according to institutional guidelines and in accordance with the Declaration of Helsinki, and all studies were approved by the US Department of Health and Human Services.
Patients
Eligible patients were younger than 22 years with either intermediate-risk (IB, IIA/IIIA1 with large mediastinal adenopathy or IIIA2) or high-risk (IIB, IIIB, IV) biopsy-proven classic HL (excluding lymphocyte predominant HL). Centralized pathology review was required.
Systemic “B” symptoms were recurrent fever (> 38.3°C), unintentional weight loss (> 10% body weight), or recurrent, drenching night sweats. Large mediastinal adenopathy (LMA) was defined by a ratio (the mediastinal thoracic ratio [MTR]) of the mediastinal mass diameter divided by the transthoracic diameter at the dome of the diaphragm that was more than 0.33 on posterior-anterior chest radiograph. Staging included bone marrow biopsy, gallium scan, bone scan, and computerized tomography (CT) of the neck, chest, abdomen, and pelvis. Abdominal lymph nodes of more than 1 cm diameter were considered positive unless proven pathologically negative.
Chemotherapy
Patients received ABVE-PC every 21 days as follows:
Doxorubicin 30 mg/m2 intravenously, days 0 and 1;
Bleomycin 10 IU/m2 intravenously/subcutaneously, days 0 and 7 (postamendment: day 0 dose reduced to 5 IU/m2);
Vincristine 1.4 mg/m2 intravenously, days 0 and 7 (maximum, 2.8 mg);
Etoposide 75 mg/m2 intravenously, days 0 to 4;
Prednisone 40 mg/m2 orally, days 0 to 9 (postamendment: administered days 0-7);
Cyclophosphamide 800 mg/m2 intravenously, day 0;
Dexrazoxane (DRZ) 300 mg/m2 intravenously, days 0, 1, and 7 (randomized to receive/not receive); and
Filgrastim 5 μg/kg intravenously/subcutaneously (day 5 until neutrophil recovery; held day 7).
Radiation (21 Gy) was given to regional fields.
Subsequent chemotherapy cycles began on day 21 if the absolute phagocyte count (APC; total neutrophils, bands, and monocytes per cubic millimeter) was 750/mm3 or more. Toxicities were evaluated by Common Toxicity Criteria 2.0.11 Grade 3 indicated severe toxicity; grade 4, life-threatening toxicity; and grade 5, lethal toxicity. Full-dose chemotherapy was mandated unless (1) bleomycin was discontinued for a predicted carbon monoxide diffusing capacity of less than 50%, (2) incapacitating peripheral neuropathy (extensive weakness, severe paresthesia, or severe ileus) necessitated stopping vincristine until grade 3 symptoms resolved, or (3) a 20% reduction of doxorubicin and etposide doses was mandated for therapeutic delays exceeding 1 week in duration resulting from an APC less than 750/mm3 or grade 4, nonherpetic mucositis. Grade 4 typhlitis (in patients receiving DRZ) led to an amendment that reduced day 0 bleomycin to 5 units/m2 and prednisone to 7 days for the final 35 patients (regardless of DRZ randomization).
Radiation
Radiation (21 Gy) was administered sequentially in 14 fractions of 1.5 Gy to mantle, abdomen, or pelvic regions if involved by HL. Pericardial effusions, lung disease, or pericardial involvement were treated with 10.5 Gy. Real-time review and treatment modification by the Quality Assurance Review Committee ensured compliance with protocol-specified radiation treatment. Institutions were mandated to send prechemotherapy and postchemotherapy imaging as well as proposed radiation portals to the Quality Assurance Review Committee before beginning radiation treatments. Any deviation from protocol specifications was communicated immediately to the institutional radiation oncologist so that modifications could be made in real time.
Response criteria definitions: early response measurement
RER.
Reduction of 50% or more of sum of the products of the perpendicular diameters of measurable lesions (SPPD), MTR of 0.33 or less, and negative gallium scan after 3 chemotherapy cycles.
SER.
Failure to achieve RER.
Response criteria definitions: overall response measurement
Complete response (CR).
Disappearance of active HL (gallium negative, ≥ 70% decrease in SPPD, and negative bone marrow or bone scan if initially positive).
Partial response.
Decrease of 50% or more in SPPD, not a CR.
No response.
Less than 50% decrease in SPPD.
Progressive disease.
Increase of 25% or more in SPPD or appearance of new lesions.
Patients were removed from protocol therapy for (1) progressive disease, (2) no response after 5 cycles of ABVE-PC, (3) persistent/recurrent disease after therapy completion, (4) intolerable toxicity not amenable to dose modification, or (5) new malignancy or death.
Treatment protocol
Early response was evaluated after 3 ABVE-PC cycles. Those with RER then received involved region radiation. SERs received 2 additional ABVE-PC (5 total) followed by radiation. Three-cycle, end-of-chemotherapy, and postradiation response assessments included chest radiograph, CT scan, and gallium scan. Previously positive bone scans and marrow biopsies were repeated.
Patients were randomly assigned to receive or not to receive DRZ to evaluate its effect as a protectant from anthracycline-induced cardiac and bleomycin-induced pulmonary toxicities. The results of this randomization in regard to long-term cardiopulmonary toxicity will be reported separately. The acute toxicity, response assessments, and efficacy data are reported here.
Statistical methods
Efficacy was compared with the 80% 3-year EFS of P8725, the prior advanced HL trial. The futility monitoring rule was designed as follows: if 33 or fewer failures were observed in the first 165 patients (with ≥ 3 years of follow-up), then the lower bound of a one-sided 95% confidence interval on the EFS estimate was more than 75%.
The DRZ randomization affected accrual goals. Based on the initial prediction of 50% SER, accrual of 156 patients would have ensured sufficient patients treated with 5 ABVE-PC cycles to evaluate cardiopulmonary protection by DRZ. The low SER rate necessitated an increase in planned accrual. Additional high-risk patients were accrued to achieve sufficient SER, resulting in a greater proportion of high-risk patients than would have been expected.
Kaplan-Meier12 methodology was used to compute EFS and overall survival (OS) estimates. EFS was measured from treatment start until relapse, progressive disease, second malignancy, death, or last contact with SEs reported according to Peto and Peto.13 Survival estimates are quoted at the 5-year time point unless otherwise stated. The log-rank test14 was used to investigate differences in survival curves, with P values less than .05 considered statistically significant. Overall reduction in potential for toxicity is measured by consideration of overall cytotoxic agent exposure compared with conventional regimens. Analysis for this report was performed based on the data available in the Children's Oncology Group (COG) as of April 19, 2005.
Results
Patients
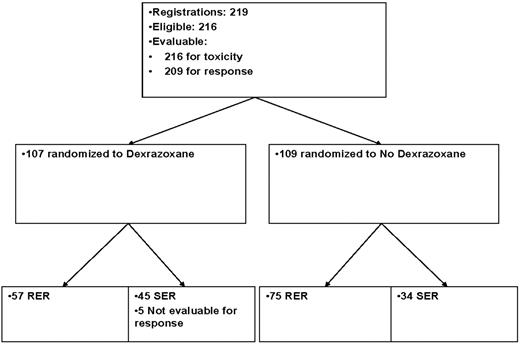
Of the 219 patients enrolled, 216 were eligible (Table 1; Figure 1). Three patients were ineligible because of incorrect disease staging. The mean age was 14 years (range, 4-21 years). There were 76 females and 140 males. Centralized pathology review identified 185 nodular sclerosis (NS; 86%), 19 mixed cellularity (MC; 9%), 3 lymphocyte depleted, and 9 with subtype not classified (3%). Distribution by stage was: stage I (n = 1), stage II (n = 81), stage III (n = 52), stage IV (n = 70), and unknown (n = 12).
Patient characteristics by histologic subtype
| Characteristic . | Overall, n . | Nodular sclerosis, n . | Mixed cellularity, n . | Lymphocyte depleted, n . | Not classified, n . |
|---|---|---|---|---|---|
| No. of patients | 216 | 185 | 19 | 3 | 9 |
| Age | |||||
| 10 years or less | 26 | 18 | 4 | 0 | 4 |
| 11-15 years | 122 | 106 | 11 | 1 | 4 |
| 16-20 years | 68 | 61 | 4 | 2 | 1 |
| Male sex | 140 | 113 | 17 | 3 | 7 |
| Stage | |||||
| IB*/II | 82 | 76 | 3 | 0 | 3 |
| III | 52 | 41 | 8 | 2 | 1 |
| IV | 70 | 58 | 7 | 1 | 4 |
| Unknown | 12 | 10 | 1 | 0 | 1 |
| Response to 3 cycles | 209 | 180 | 19 | 3 | 7 |
| Not RER | 77 | 67 | 6 | 0 | 4 |
| RER/CR | 88 | 75 | 9 | 2 | 2 |
| RER/less than CR | 44 | 38 | 4 | 1 | 1 |
| Unknown | 7 | 5 | 0 | 0 | 2 |
| Characteristic . | Overall, n . | Nodular sclerosis, n . | Mixed cellularity, n . | Lymphocyte depleted, n . | Not classified, n . |
|---|---|---|---|---|---|
| No. of patients | 216 | 185 | 19 | 3 | 9 |
| Age | |||||
| 10 years or less | 26 | 18 | 4 | 0 | 4 |
| 11-15 years | 122 | 106 | 11 | 1 | 4 |
| 16-20 years | 68 | 61 | 4 | 2 | 1 |
| Male sex | 140 | 113 | 17 | 3 | 7 |
| Stage | |||||
| IB*/II | 82 | 76 | 3 | 0 | 3 |
| III | 52 | 41 | 8 | 2 | 1 |
| IV | 70 | 58 | 7 | 1 | 4 |
| Unknown | 12 | 10 | 1 | 0 | 1 |
| Response to 3 cycles | 209 | 180 | 19 | 3 | 7 |
| Not RER | 77 | 67 | 6 | 0 | 4 |
| RER/CR | 88 | 75 | 9 | 2 | 2 |
| RER/less than CR | 44 | 38 | 4 | 1 | 1 |
| Unknown | 7 | 5 | 0 | 0 | 2 |
Stage IB, n = 1.
Treatment assignments and numbers of patients included in the analysis.
Response after 3 cycles was evaluable in 209 patients. Seven patients were inevaluable for response resulting from toxicity (n = 5), treatment refusal (n = 1), and early death (n = 1).
Dose density
The median time from initial treatment to completion of the third cycle was 8.7 (± 1.3) weeks and to completion of the fifth cycle was 16.0 (± 2.5) weeks. Comparison to planned durations (9 and 15 weeks) confirms minimal therapeutic delays. Myeloid recovery by day 21 occurred in most cycles, virtually eliminating drug dose reductions and maximizing dose density. Chemotherapy dose reductions for toxicity were made in fewer than 3% of patients.
The dose densities of chemotherapy agents used in this study exceeded those of most regimens, but cumulative doses of chemotherapy were often significantly lower (Table 2). For ABVE-PC, the doxorubicin dose density was 20 mg/m2 per week and 0.93 mg/m2 per week for vincristine. With a maximum vincristine dose of 2.8 mg allowed on ABVE-PC rather than the usual maximum of 2.0 mg, the vincristine dose density of ABVE-PC decreased to less than 0.93 mg/m2 per week only when the body surface area (BSA) exceeded 2.0 m2. On other trials, the vincristine dose density shown in Table 2 began to decrease when the BSA exceeded 1.4 m2. The differential dose density of vincristine on this regimen versus other regimens was incrementally larger for patients with a BSA of 1.4 to 2.0 m2.
Chemotherapy
| Chemotherapy . | Dose density per week . | Cumulative dose . | |||||
|---|---|---|---|---|---|---|---|
| ABVE-PC . | ABVD2 . | Escalated BEACOPP8 . | ABVE-PC (3 cycles) . | ABVE-PC (5 cycles) . | ABVD20 . | Escalated BEACOPP8 . | |
| Doxorubicin, mg/m2 | 20 | 12.5 | 11.7 | 180 | 300 | 300-400 | 280 |
| Bleomycin, units/m2 | 5 | 5 | 3.3 | 45 | 75 | 120-150 | 80 |
| Vincristine, mg/m2 | 0.93 | 0 | 0.46 | 8.4 | 14 | 0 | 16 |
| Etoposide, mg/m2 | 125 | 0 | 200 | 1125 | 1875 | 0 | 4800 |
| Cyclophosphamide, mg/m2 | 267 | 0 | 417 | 2400 | 4000 | 0 | 10 000 |
| Prednisone, mg/m2 | 133 | 0 | 187 | 1200 | 2000 | 0 | 4480 |
| Procarbazine, mg/m2 | 0 | 0 | 233 | 0 | 0 | 0 | 5600 |
| Vinblastine, mg/m2 | 0 | 3 | 0 | 0 | 0 | 72-96 | 0 |
| DTIC, mg/m2 | 0 | 188 | 0 | 0 | 0 | 4500-6000 | 0 |
| Chemotherapy . | Dose density per week . | Cumulative dose . | |||||
|---|---|---|---|---|---|---|---|
| ABVE-PC . | ABVD2 . | Escalated BEACOPP8 . | ABVE-PC (3 cycles) . | ABVE-PC (5 cycles) . | ABVD20 . | Escalated BEACOPP8 . | |
| Doxorubicin, mg/m2 | 20 | 12.5 | 11.7 | 180 | 300 | 300-400 | 280 |
| Bleomycin, units/m2 | 5 | 5 | 3.3 | 45 | 75 | 120-150 | 80 |
| Vincristine, mg/m2 | 0.93 | 0 | 0.46 | 8.4 | 14 | 0 | 16 |
| Etoposide, mg/m2 | 125 | 0 | 200 | 1125 | 1875 | 0 | 4800 |
| Cyclophosphamide, mg/m2 | 267 | 0 | 417 | 2400 | 4000 | 0 | 10 000 |
| Prednisone, mg/m2 | 133 | 0 | 187 | 1200 | 2000 | 0 | 4480 |
| Procarbazine, mg/m2 | 0 | 0 | 233 | 0 | 0 | 0 | 5600 |
| Vinblastine, mg/m2 | 0 | 3 | 0 | 0 | 0 | 72-96 | 0 |
| DTIC, mg/m2 | 0 | 188 | 0 | 0 | 0 | 4500-6000 | 0 |
DTIC indicates dacarbazine.
Rapid response
Of 209 patients evaluated for response, 132 (63%) were RER. Only 2 patients (< 1%) had progressive disease on this regimen (Table 3). Of the RER, 116 (88%) followed protocol, receiving 3 ABVE-PC cycles and 21 Gy radiation (Table 3). Sixteen RER received 2 additional cycles of ABVE-PC, usually because of misunderstanding of RER criteria. RER was achieved in 67% of intermediate HL (IIA/IIIA1 with bulk or all IIIA2) and 61% of high-risk HL (all IIB, IIIB, or IV) patients (P = .41). Analyses comparing patients achieving RER for “A” versus “B” symptoms, NS versus MC HL, and LMA versus no LMA did not reveal statistically significant differences (P = .87, .59, and .65, respectively). The proportions of patients with RER for DRZ versus no DRZ were 56% versus 69% (P = .07, not statistically significant).
Response
| . | Overall, N . | RERs, n (%) . | CR after chemotherapy, n (%) . | CR after radiation, n (%) . |
|---|---|---|---|---|
| All patients | 216 | 132 (63) | 134 (64) | 180 (90) |
| Evaluable | 209 | 209 | 200 | |
| Intermediate risk | 53 | 35 (67) | 33 (66) | 44 (96) |
| Stage IB | 1 | 1 | 1 | 1 |
| Stage IIA LMA | 34 | 22 | 19 | 28 |
| Stage IIIA | 18 | 12 | 13 | 15 |
| High risk | 163 | 97 (61) | 101 (64) | 136 (88) |
| Stage IIB | 49 | 34 | 33 | 43 |
| Stage IIIB | 37 | 27 | 24 | 32 |
| Stage IVA | 26 | 13 | 16 | 21 |
| Stage IVB | 51 | 23 | 28 | 40 |
| “B” symptoms | ||||
| No | 78 | 46 (62) | 47 (65) | 62 (91) |
| Yes | 138 | 86 (63) | 87 (64) | 118 (89) |
| LMA | ||||
| Yes | 125 | 79 (64) | 75 (63) | 103 (90) |
| No | 79 | 47 (61) | 53 (70) | 67 (89) |
| Dexrazoxane | ||||
| Yes | 107 | 57 (56)* | 60 (60) | 87 (86) |
| No | 109 | 75 (69) | 74 (70) | 93 (94) |
| . | Overall, N . | RERs, n (%) . | CR after chemotherapy, n (%) . | CR after radiation, n (%) . |
|---|---|---|---|---|
| All patients | 216 | 132 (63) | 134 (64) | 180 (90) |
| Evaluable | 209 | 209 | 200 | |
| Intermediate risk | 53 | 35 (67) | 33 (66) | 44 (96) |
| Stage IB | 1 | 1 | 1 | 1 |
| Stage IIA LMA | 34 | 22 | 19 | 28 |
| Stage IIIA | 18 | 12 | 13 | 15 |
| High risk | 163 | 97 (61) | 101 (64) | 136 (88) |
| Stage IIB | 49 | 34 | 33 | 43 |
| Stage IIIB | 37 | 27 | 24 | 32 |
| Stage IVA | 26 | 13 | 16 | 21 |
| Stage IVB | 51 | 23 | 28 | 40 |
| “B” symptoms | ||||
| No | 78 | 46 (62) | 47 (65) | 62 (91) |
| Yes | 138 | 86 (63) | 87 (64) | 118 (89) |
| LMA | ||||
| Yes | 125 | 79 (64) | 75 (63) | 103 (90) |
| No | 79 | 47 (61) | 53 (70) | 67 (89) |
| Dexrazoxane | ||||
| Yes | 107 | 57 (56)* | 60 (60) | 87 (86) |
| No | 109 | 75 (69) | 74 (70) | 93 (94) |
Percentages were calculated excluding patients whose data were unknown. RER is defined by gallium negativity and 50% reduction in 2-dimensional tumor size after 3 cycles of chemotherapy. CR is defined by CT-based response of 70% or more at the end of chemotherapy and after radiation; those not achieving this anatomic response often had residual fibrosis rather than active disease.
P = .07 for difference in RERs in dexrazoxane vs no dexrazoxane.
EFS and OS
The 5-year EFS was 84% (± 3%; Figure 2A): 84% (± 5%) for intermediate-risk HL and 85% (± 3%) for high-risk HL (P = .87; Figure 2B). The median follow-up time observed for patients who did not have an event was 5.2 years. Few relapses occurred beyond 3 years after enrollment, so additional survival follow-up would not have significantly changed the EFS or OS. There was no statistical difference in EFS for 3 versus 5 cycles of chemotherapy (84% [± 3%] vs 86% [± 4%], P = .85) or for RER versus SER (86% [± 3%] vs 83% [± 4%], P = .59; Figure 2C). After excluding the 16 RERs who received 5 cycles, the EFS of RER versus SER did not significantly change (P = .66). The 5-year EFS was 84% (± 4%) for NS HL versus 95% (± 5%) for the 8% of patients with MC HL (P = .22). Use of DRZ did not affect EFS.
Event-free and overall survival. (A) Overall EFS and OS curves for 216 HL patients treated on P9425. (B) EFS curves for intermediate-risk versus high-risk patients (P = .87). (C) EFS curves for rapid early responders versus slow early responders (P = .81).
Event-free and overall survival. (A) Overall EFS and OS curves for 216 HL patients treated on P9425. (B) EFS curves for intermediate-risk versus high-risk patients (P = .87). (C) EFS curves for rapid early responders versus slow early responders (P = .81).
The EFS differed between those with LMA versus those without LMA (80% [± 4%] vs 91% [± 3%], respectively, P = .03, Table 4). The 3-year EFS for stage IV was lower than for lesser stage HL (77.8% [± 5.0%] vs 92.1% [± 2.3%]; P = .015). There was no difference in EFS between RER who had also achieved CR after 3 cycles versus those who had not (negative gallium with tumor reduction that was > 50% but ≤ 70%; EFSCR = 89.7% [± 3.3%] vs EFS<CR = 90.9% [± 4.5%], P = .99).
Five-year EFS
| . | No. of patients . | All, % . | RER, % . | SER, % . |
|---|---|---|---|---|
| All | 216 | 84 (± 3) | 86 (± 3) | 83 (± 4) |
| Intermediate risk | 53 | 84 (± 5) | 82 (± 6) | 88 (± 8) |
| Stage IB* | 1 | — | — | — |
| Stage IIA | 34 | 78 (± 8) | — | — |
| Stage IIIA | 18 | 94 (± 5) | — | — |
| High risk | 163 | 85 (± 3) | 88 (± 3) | 82 (± 5) |
| Stage IIB | 49 | 92 (± 4) | — | — |
| Stage IIIB | 37 | 92 (± 6) | — | — |
| Stage IVA | 26 | 81 (± 8) | — | — |
| Stage IVB | 51 | 74 (± 6) | — | — |
| “B” symptoms | ||||
| Yes | 78 | 83 (± 4) | 84 (± 6) | 83 (± 7) |
| No | 138 | 85 (± 3) | 87 (± 4) | 84 (± 5) |
| LMA | ||||
| Yes | 125 | 80 (± 4) | 81 (± 5) | 80 (± 6) |
| No | 79 | 91 (± 3) | 94 (± 4) | 86 (± 6) |
| Dexrazoxane | ||||
| Yes | 107 | 83 (± 4) | 89 (± 4) | 78 (± 6) |
| No | 109 | 86 (± 3) | 84 (± 4) | 91 (± 5) |
| . | No. of patients . | All, % . | RER, % . | SER, % . |
|---|---|---|---|---|
| All | 216 | 84 (± 3) | 86 (± 3) | 83 (± 4) |
| Intermediate risk | 53 | 84 (± 5) | 82 (± 6) | 88 (± 8) |
| Stage IB* | 1 | — | — | — |
| Stage IIA | 34 | 78 (± 8) | — | — |
| Stage IIIA | 18 | 94 (± 5) | — | — |
| High risk | 163 | 85 (± 3) | 88 (± 3) | 82 (± 5) |
| Stage IIB | 49 | 92 (± 4) | — | — |
| Stage IIIB | 37 | 92 (± 6) | — | — |
| Stage IVA | 26 | 81 (± 8) | — | — |
| Stage IVB | 51 | 74 (± 6) | — | — |
| “B” symptoms | ||||
| Yes | 78 | 83 (± 4) | 84 (± 6) | 83 (± 7) |
| No | 138 | 85 (± 3) | 87 (± 4) | 84 (± 5) |
| LMA | ||||
| Yes | 125 | 80 (± 4) | 81 (± 5) | 80 (± 6) |
| No | 79 | 91 (± 3) | 94 (± 4) | 86 (± 6) |
| Dexrazoxane | ||||
| Yes | 107 | 83 (± 4) | 89 (± 4) | 78 (± 6) |
| No | 109 | 86 (± 3) | 84 (± 4) | 91 (± 5) |
Values are mean (± SE).
— indicates not analyzed.
Did not report EFS for stage IB because only 1 patient was in the stratum.
OS at 5 years was 95% (± 2%) and did not differ between RER versus SER, 3 versus 5 cycles or MC versus NS (P = .25, .99, and .39, respectively).
Toxicity
Toxicity was evaluable in 214 patients (Table 5). Enhanced hematologic toxicity was anticipated with dose density. Unexpectedly, higher grade hematologic toxicity occurred more frequently in those receiving versus not receiving DRZ and was associated with increased risk of infection and sepsis. Acute grade 3/4 pulmonary toxicity also occurred more often with DRZ (P = .005). Because pulmonary toxicity usually occurred early and in association with grade 4 infection, it was unlikely to be attributable to bleomycin-induced pulmonary injury.
Grade 3 and 4 toxicity with or without dexrazoxane
| . | Without dexrazoxane . | With dexrazoxane . | ||
|---|---|---|---|---|
| Count (n = 108) . | Incidence, % . | Count (n = 106) . | Incidence, % . | |
| ANC grade 4* | 84 | 77.8 | 99 | 93.4 |
| ANC grade 3 | 9 | 8.3 | 1 | 0.9 |
| Platelets* | 32 | 29.6 | 77 | 72.6 |
| Hemoglobin* | 44 | 40.7 | 64 | 60.4 |
| Thrombosis* | 1 | 0.9 | 4 | 3.8 |
| Infection, not otherwise specified/unknown* | 48 | 44.4 | 75 | 70.8 |
| Sepsis* | 9 | 8.4 | 18 | 17.0 |
| Typhlitis | 9 | 8.4 | 3 | 2.8 |
| Pulmonary*† | 3 | 2.8 | 13 | 12.3 |
| Cardiac function | 2 | 1.9 | 0 | 0 |
| Peripheral nervous system/central nervous system‡ | 3/0 | 2.8/0 | 2/1 | 1.9/0.9 |
| Stomatitis | 31 | 28.7 | 30 | 28.3 |
| Vomiting/nausea | 10 | 9.3 | 10 | 9.4 |
| Allergic reaction | 2 | 1.9 | 7 | 6.6 |
| Secondary malignancy | 1 | 0.9 | 3 | 2.8 |
| . | Without dexrazoxane . | With dexrazoxane . | ||
|---|---|---|---|---|
| Count (n = 108) . | Incidence, % . | Count (n = 106) . | Incidence, % . | |
| ANC grade 4* | 84 | 77.8 | 99 | 93.4 |
| ANC grade 3 | 9 | 8.3 | 1 | 0.9 |
| Platelets* | 32 | 29.6 | 77 | 72.6 |
| Hemoglobin* | 44 | 40.7 | 64 | 60.4 |
| Thrombosis* | 1 | 0.9 | 4 | 3.8 |
| Infection, not otherwise specified/unknown* | 48 | 44.4 | 75 | 70.8 |
| Sepsis* | 9 | 8.4 | 18 | 17.0 |
| Typhlitis | 9 | 8.4 | 3 | 2.8 |
| Pulmonary*† | 3 | 2.8 | 13 | 12.3 |
| Cardiac function | 2 | 1.9 | 0 | 0 |
| Peripheral nervous system/central nervous system‡ | 3/0 | 2.8/0 | 2/1 | 1.9/0.9 |
| Stomatitis | 31 | 28.7 | 30 | 28.3 |
| Vomiting/nausea | 10 | 9.3 | 10 | 9.4 |
| Allergic reaction | 2 | 1.9 | 7 | 6.6 |
| Secondary malignancy | 1 | 0.9 | 3 | 2.8 |
Significant grade 3 toxicities that occurred in more than 5% of patients, all grade 4 toxicities, and specific targeted toxicities (pulmonary, cardiac, peripheral nervous system) are included above. Except as specified (eg, ANC), the numbers represent combined grade 3 and grade 4 toxicities.
ANC indicates absolute neutrophil count.
Statistically significantly different (log-rank test, P ≤ .05).
Includes diffusion capacity for carbon monoxide, vital capacity, pulmonary/functional and oxygen saturation.
Central nervous system includes mood, cortical, and cerebellar.
Among 107 patients receiving DRZ, 3 second malignant neoplasms occurred, including acute myelogenous leukemia (AML) with t(10,17), AML with monosomy 7, and osteosarcoma. One patient of the 109 who did not receive DRZ acquired AML.A patient with preexisting seizures and severe developmental delay experienced central nervous system thrombosis and a vegetative state after ABVE-PC and DRZ.
From March 1997 through October 1999, 177 patients were entered on study. Accrual was temporarily closed (October 1999 to May 2000) because 3 patients receiving DRZ had grade 4 typhlitis; 2 required surgical intervention. Grade 3 typhlitis (2-3 days of abdominal pain, rapid antibiotic response) was reported in 3 with DRZ and 3 without DRZ. Enrollment resumed with modifications of bleomycin dose and prednisone duration (“Chemotherapy”). Three additional episodes of typhlitis (2 grade 3, 1 grade 4) occurred postamendment on the DRZ arm. Grade 3/4 typhlitis occurred more frequently with versus without DRZ (9 vs 3 cases), although the difference did not reach conventionally defined, statistical significance (P = .06).
Discussion
This dose-dense, early-response–based treatment paradigm provides patient-specific therapeutics within the context of a clinical trial. Our prior trials8 showed that early response predicted EFS, but end-of-treatment response did not. Because early response reflects the complex interplay between tumor, host, and chemotherapeutic factors, or “chemosensitivity,” we hypothesized that it could be used as the basis for tailoring individual treatment. We also increased chemotherapy dose density based on previous data,3-5 suggesting that enhanced drug delivery improves outcome.
Our treatment paradigm for advanced HL relied on 2 treatment principles: (1) dose density enhances therapeutic efficacy, and (2) RER is evidence of chemosensitivity and can serve as a basis for reduction of therapy. With ABVE-PC, we were able to deliver effective chemotherapy with dose density of doxorubicin and vincristine that exceeded even escalated BEACOPP9 (Table 2) while simultaneously reducing cumulative chemotherapy exposure for all patients (particularly the 63% RER).
Dose density
Our dose-dense regimen (ABVE-PC) supports a substantial reduction of cumulative therapy, limiting potential for late sequelae, even after 5 cycles of ABVE-PC. Procarbazine was not used in this regimen because of its significant effect on gonadal function. Male sterility is common with even low-dose procarbazine therapy,15 but probably not with the cumulative cyclophosphamide dose (2.4-4 g/m2) of ABVE-PC. Infertility in females occurs with standard-dose procarbazine16 but is doubtful in young women receiving the cyclophosphamide doses used in this study.17 Cumulative doxorubicin doses of 180 mg/m2 for RER and 300 mg/m2 for SER (mean, 230 mg/m2) represent a significant reduction of exposure compared with the doxorubicin doses in escalated BEACOPP8 (280 mg/m2) and ABVD18 (300-400 mg/m2). This would be expected to reduce long-term cardiac risk.19
The outcome is improved even in comparison with the 8 cycles of alternating ABVD/MOPP and low-dose radiation of our previous trial reported by Weiner et al (P8725).8 Comparable results with escalated BEACOPP9 rely on higher cumulative doses as well as the inclusion of procarbazine. Comparing aggregated results (Table 4) from this study using ABVE-PC with the COPP/ABV-based therapy,20 we found that, although EFS was similar for stage IV HL, the patients with stages IIB/IIIA HL fared significantly better on ABVE-PC than on COPP/ABV (3-year EFS of 95% vs 82%), despite the reduction of cumulative chemotherapy. Patients with IIIB/IVB HL also had higher EFS (82% [± 5%] with P9425 [ABVE-PC] vs 72% [± 5%] on CCG5942 [COPP/ABV/cytarabine/etoposide]; Table 6). Dose-dense delivery of chemotherapy may have improved the efficacy of treatment.
Comparison of outcomes
| Study . | Stages* . | Regimen . | Radiation . | 5-year EFS . |
|---|---|---|---|---|
| Pediatric | ||||
| Schwartz (P9425; current study) | II (LMA/B), III, IV | ABVE-PC3 (RER); 5(SER) | 21 Gy | 84%82% ± 5.3% (4 y) for IIIB/IVB |
| Dorffel et al22 | TG2: IIEA/B, IIIATG3: IIEB, IIIEA/B, IV | 2 OEPA (males)2 OPPA (females)2 or 4 COPP (TG2 or 3)79% had RT | CR: no RTPR: 20-35 Gy | 79%91% |
| Weiner et al8 (POG 8725) | II (LMA or B), III (not III1S), IV | 4 MOPP/4 ABVDalternating(RT: ± if CR) | 21 GyNo radiation | 7878 |
| Nachman et al20 (CCG 5942) | I/II adverse, III, IV | II, III: 6 COPP/ABVIV: 2 COPP, ABV, cytarabine, etoposide (± RT if CR) | 21 GyNo radiation | 88 (3 y)82 (3 y)72% ± 5.3% (4 y) for IIIB/IVB |
| Hudson et al21 | III, IV | 6 VAMP/COP | CR: 15 Gy; PR: 25.5 Gy (assessed after 2 cycles) | 68% |
| Adult | ||||
| Diehl et al9 | IIB or IIIA with risk factors, IIB, IV | BEACOPP escalated | 30 Gy to bulk disease and residual disease | 87% FFP |
| Horning et al10 | III, IV or locally extensive mediastinal stage I or II | Stanford V (12 wk) | Bulk disease or macroscopic splenic disease | 89% FFP |
| Gobbi et al23 | IIB, III, IV | ABVDStanford V | 36 Gy to initial bulk disease in CR or 42 Gy to any persistent disease | 85% FFP73% FFP |
| Canellos et al18 | IIIA2, IIIB, IV or relapse after RT | ABVD (6-8)6MOPP/6ABVD | Not included | 61% FFP64% FFP |
| Aleman et al24 | III, IV | 6-8 MOPP/ABV | CR: 16-24 Gy vs no RTPR: 30 Gy | 84%79%79% |
| Study . | Stages* . | Regimen . | Radiation . | 5-year EFS . |
|---|---|---|---|---|
| Pediatric | ||||
| Schwartz (P9425; current study) | II (LMA/B), III, IV | ABVE-PC3 (RER); 5(SER) | 21 Gy | 84%82% ± 5.3% (4 y) for IIIB/IVB |
| Dorffel et al22 | TG2: IIEA/B, IIIATG3: IIEB, IIIEA/B, IV | 2 OEPA (males)2 OPPA (females)2 or 4 COPP (TG2 or 3)79% had RT | CR: no RTPR: 20-35 Gy | 79%91% |
| Weiner et al8 (POG 8725) | II (LMA or B), III (not III1S), IV | 4 MOPP/4 ABVDalternating(RT: ± if CR) | 21 GyNo radiation | 7878 |
| Nachman et al20 (CCG 5942) | I/II adverse, III, IV | II, III: 6 COPP/ABVIV: 2 COPP, ABV, cytarabine, etoposide (± RT if CR) | 21 GyNo radiation | 88 (3 y)82 (3 y)72% ± 5.3% (4 y) for IIIB/IVB |
| Hudson et al21 | III, IV | 6 VAMP/COP | CR: 15 Gy; PR: 25.5 Gy (assessed after 2 cycles) | 68% |
| Adult | ||||
| Diehl et al9 | IIB or IIIA with risk factors, IIB, IV | BEACOPP escalated | 30 Gy to bulk disease and residual disease | 87% FFP |
| Horning et al10 | III, IV or locally extensive mediastinal stage I or II | Stanford V (12 wk) | Bulk disease or macroscopic splenic disease | 89% FFP |
| Gobbi et al23 | IIB, III, IV | ABVDStanford V | 36 Gy to initial bulk disease in CR or 42 Gy to any persistent disease | 85% FFP73% FFP |
| Canellos et al18 | IIIA2, IIIB, IV or relapse after RT | ABVD (6-8)6MOPP/6ABVD | Not included | 61% FFP64% FFP |
| Aleman et al24 | III, IV | 6-8 MOPP/ABV | CR: 16-24 Gy vs no RTPR: 30 Gy | 84%79%79% |
FFP indicates failure from progression; RT, radiation therapy.
A: no symptoms of fever, night sweats, weight loss; and B: symptoms of fever, night sweats, weight loss.
Reported trials for intermediate- and high-risk HL from the Stanford/St Jude Consortium/Dana-Farber consortium, and the German Pediatric Hodgkin trials did not use dose-dense delivery of chemotherapy, although they limited cumulative doses.21,22 For the former group, the efficacy of these regimens for stage III/IV HL was unsatisfactory (Table 6; 68.4% 5-year EFS).21 Early results reported by the GPOH-HD 95 show an efficacy that is similar to ours, although the intermediate- and high-risk groups (TG2 and TG3) continue to receive 6 to 9 g/m2 of procarbazine with its significant impact on fertility and possibly on secondary malignancy.22 A legacy Children's Cancer Group trial has reported excellent early efficacy of response-based BEACOPP.25 Even those patients whose therapy was modified after achieving CR with 4 BEACOPP (adding 4 ABVD for females or 2 ABVD with low-dose radiation therapy for males) still received procarbazine and were exposed to a higher cumulative doxorubicin dose than early responders treated on P9425.
Survivorship data note significant risk for cardiac dysfunction19,26 in the decades after anthracycline therapy, with enhanced risk engendered by mantle radiation. Although we had hoped to abrogate cardiac risk with DRZ, the unexpected hematopoietic/infectious toxicity in the DRZ arm made its use inadvisable for this population. We have opted to limit doxorubicin dose to 150 to 200 mg/m2 in ongoing COG HL trials.
Acute toxicity was not excessive with the non-DRZ regimen. ABVE-PC without DRZ is now the backbone therapy for intermediate- and high-risk HL in the COG. We think that the dose density of this regimen enhances efficacy and thereby supports an overall reduction in cumulative chemotherapy, particularly for the RER.
Early-response–based therapy
Our treatment paradigm was unique at its inception with its focus on early response after 9 weeks, measured to detect primary chemosensitivity. This approach diverges from the traditional “response at the end of chemotherapy” used to determine need for additional chemotherapy20,27 or radiation therapy8,20-22,25,27 (Table 6). Dorffel et al assessed response at 8 weeks but only in the lowest-risk population.22 In higher-risk patients, the decision to radiate was determined after 4 or 6 cycles of chemotherapy based on initial group. Hudson et al used response after 2 cycles to determine radiation dose (15 Gy vs 25.5 Gy) in high-risk patients21 ; 5-year EFS was only 68% in the stage III and IV patients. The German Hodgkin Study Group found that response assessment after 4 cycles of BEACOPP did not have prognostic impact.28 Carde et al recently reviewed early response to chemotherapy, noting that determination of “early response” varies widely in studies, both in time and methodology of “early” assessment and in the impact of the preceding therapy.28
The definition of RER took into consideration the known occurrence of residual fibrosis that limits ability to discern CR (traditionally termed “conditional CR”). We used both functional (gallium) and anatomic (CT) imaging to assess early response, requiring a completely negative gallium scan but only a 50% reduction in 2-dimensional tumor size. Because no difference was found between the EFS of RER who achieved versus those who did not achieve CR at the end of therapy, our approach for defining RER appears sufficiently rigorous; that is, a gallium-negative residual mass did not impact outcome. Positron emission tomography (PET) scanning was not yet readily available when the study was designed. It is now recommended29 for response assessment because it allows more precise identification, compared with gallium, of a cohort without metabolically active disease. PET scans are being directly compared with CT scans in ongoing COG trials. Our study also did not include central review of early response, an approach we currently use in ongoing trials. Both central review and use of PET probably improve the accuracy of response assessment and thereby further enhance the outcomes achieved with response-based therapy.
It is possible that better response assessment will allow us to further reduce therapy for good responders. Although recent pediatric trials8,20,22,25,27 have attempted to identify patients who may not need radiation therapy, Nachman et al20 and Dorffel et al22 found a reduction in EFS for those who did not receive radiation. The former trial20 randomized patients to receive or not receive radiation based on achieving CR; it had to be stopped early because of the reduction in EFS. In the latter study by the GPOH-HD,22 radiation was given only to those with a partial response; their outcome was better than that of the CR patients (21% of the entire cohort) who did not get radiation. Although OS has not been affected in the most recent updates of these trials, curative salvage therapy usually requires stem cell transplantation or high-dose radiation. Either of these approaches would be expected to have greater long-term risks than low-dose, limited-field radiation; these risks must be considered in any decision to eliminate radiation. Two pediatric trials successfully treated patients without radiation if they had achieved a CR at the end of therapy8 or after 4 cycles of therapy.25 Both regimens included procarbazine and required 8 cycles of chemotherapy.
We did not attempt to eliminate radiation in this study. Long-term risks of radiation therapy include secondary malignancy, hypothyroidism, myocardial ischemia, hypoplasia, and sterility,30-39 but referenced studies primarily report outcomes of children who received more than 30 Gy radiation. There are only limited data on long-term outcomes of radiation therapy in those who have received only 15 to 25 Gy. A study by van Leeuwen et al33 found that there was no increase in relative risk (RR) of breast cancer after HL in those treated with low-dose radiation (4-23.2 Gy) versus those receiving less than 4 Gy (RR = 1.2, P = .77). However, the risk of secondary breast cancer was increased for those receiving 24 to 38.2 Gy (RR = 4.91, P = .04) or more than 38.5 Gy (RR = 8.18, P = .01). Some have advocated for gender-specific therapeutics in which females would received higher doses of alkylating agents to avoid radiation, whereas males would conversely receive radiation in place of sterility-inducing alkylating agents.25 Because toxicities of radiation, such as myocardial ischemia, may affect males more than females, we seek to limit radiation exposure of both males and females. The radiation dose for all patients on this trial was limited to 21 Gy. Recent modeling by Hodgson et al shows the profound reduction in risk associated with lower-dose and limited fields.40
We now hypothesize that early response to chemotherapy may identify the patients with tumors that are sufficiently chemosensitive so as to be treated without radiation. Current COG trials evaluate the elimination of radiation therapy in early responders. We recognize, however, that radiation remains a highly effective modality of treatment that is needed in specific higher-risk cohorts to safely limit cumulative chemotherapy. Further limitation of field to the involved nodal areas may further reduce risk.
In conclusion, we have successfully achieved 84% 5-year EFS and 95% 5-year OS with a dose-dense, early-response-based treatment algorithm that minimized cumulative therapy. Only 9 weeks of chemotherapy was required in 63% of patients and 15 weeks in the remaining 37%, consolidated with 21 Gy regional radiation.
The proportions of intermediate- and high-risk patients were not representative of HL in the population; there is an enhanced proportion of high-risk patients. This is a result of closure of the intermediate-risk stratum earlier than closure of the high-risk stratum. The overall results of this study are therefore even more robust than the general results might suggest.
Dose-dense ABVE-PC is shown to support induction of early response and tailoring of therapy to individual need. It simultaneously provides high efficacy and reduces the cumulative doses of chemotherapy and radiation below thresholds usually associated with long-term adverse sequelae. As a dose-dense chemotherapy regimen that supports an early-response–based treatment algorithm, we think that ABVE-PC may represent a significant advance in the treatment of HL.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by the Chair's Grant U10 CA98543 and the Statistics and Data Center (Grant U10 CA98413) of the Children's Oncology Group from the National Cancer Institute, National Institutes of Health, Bethesda, MD. A complete listing of grant support for research conducted by CCG and POG before initiation of the COG grant in 2003 is available online at http://www.childrensoncologygroup.org/admin/grantinfo.htm.
A complete list of the Children's Oncology Group participants appears in the supplemental Appendix (available on the Blood website; see the Supplemental Materials link at the top of the online article).
National Institutes of Health
Authorship
Contribution: C.L.S., L.S.C., R.E.H., S.E.L., C.S.T., and A.C. designed research; C.L.S., L.S.C., D.V., W.B.L., R.E.H., R.S., S.E.L., C.S.T., P.A.d.A., and A.C. performed research; C.L.S., L.S.C., D.V., W.B.L., R.S., P.A.d.A., and A.C. analyzed and interpreted data; D.V., W.B.L., and R.S. performed statistical analysis; and C.L.S., L.S.C., D.V., W.B.L., S.E.L., P.A.d.A., and A.C. wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Cindy L. Schwartz, Hasbro Children's Hospital, Alpert Medical School of Brown University, 593 Eddy St, MP117, Providence, RI 02906; e-mail: Cindy_Schwartz@Brown.edu.