Chronic granulomatous disease (CGD) involving either the membrane or the cytosolic components of the phagocyte NADPH oxidase results from defective generation of superoxide and downstream reactive oxygen species (ROS). The hallmark of the disorder is the predisposition to recurrent bacterial and fungal infections from catalase positive organisms. This is attributable to the lack of a functional NADPH oxidase. Independently, the disorder is also characterized by overexuberant sterile inflammation and a risk for autoimmune disorders, such as inflammatory bowel disease, rheumatoid arthritis, discoid lupus erythematosus, and chorioretinitis.1 In hematoxylin and eosin–stained sections from patients, macrophages may contain a golden pigment and often form diffuse granulomata, giving CGD its descriptive name.
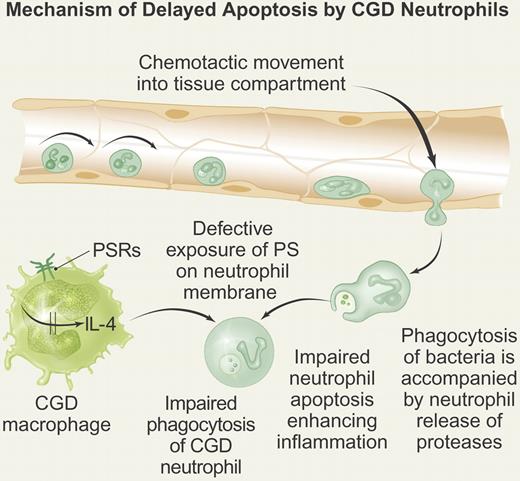
Neutrophils from CGD patients are able to ingest microorganisms and efficiently dispose of catalase negative bacteria but there is evidence of delayed apoptosis.2 The long-lived neutrophils express reduced level of phosphatidylserine (PS), resulting in their deficient recognition by macrophages.3 Both delayed neutrophil apoptosis and deficient PS exposure result from the loss of NADPH oxidase function. The failure to clear the CGD neutrophils leads to prolonged release of intracellular constituents, such as serine proteases that contribute to the ongoing inflammation (see figure).
Mechanism of delayed CGD neutrophil apoptosis. CGD neutrophils undergo normal rolling and adhesion onto activated endothelium. The neutrophils then undergo diapedesis in response to chemotactic stimuli into tissue. The neutrophils are able to ingest bacteria but manifest impaired apoptosis accompanied by prolonged release of proteases into tissue from primary granules, thereby generating sterile inflammation. The CGD macrophage fails to ingest the CGD neutrophil because of reduced exposure of phosphatidylserine (PS) on the neutrophil membrane. In turn, the failure to express normal amounts of PS leads to failure to activate phosphatidylserine receptors (PSRs) on the CGD macrophage, leading to attenuated production of endogenous IL-4, a required cytokine necessary to initiate stimulus-response coupling required for macrophage activation. Professional illustration by Paulette Dennis.
Mechanism of delayed CGD neutrophil apoptosis. CGD neutrophils undergo normal rolling and adhesion onto activated endothelium. The neutrophils then undergo diapedesis in response to chemotactic stimuli into tissue. The neutrophils are able to ingest bacteria but manifest impaired apoptosis accompanied by prolonged release of proteases into tissue from primary granules, thereby generating sterile inflammation. The CGD macrophage fails to ingest the CGD neutrophil because of reduced exposure of phosphatidylserine (PS) on the neutrophil membrane. In turn, the failure to express normal amounts of PS leads to failure to activate phosphatidylserine receptors (PSRs) on the CGD macrophage, leading to attenuated production of endogenous IL-4, a required cytokine necessary to initiate stimulus-response coupling required for macrophage activation. Professional illustration by Paulette Dennis.
In a set of novel experiments by Fernandez-Boyanapalli et al, CGD macrophages were shown to be impaired in their ability to recognize and clear apoptotic cells in a process independent of NADPH oxidase activity.4 Previous studies have shown that activation of wild-type macrophages with exogenous interleukin (IL)–4, IL-13, IL-10, or macrophage colony-stimulating factor (M-CSF) enhances their ability to ingest apoptotic cells.5,–7 In this study, the lack of endogenous production of only IL-4 resulted in defective stimulus-response coupling by X-linked CGD macrophages compared with wild-type macrophages. Other investigations have shown that exogenous IL-4 coordinately stimulates both 12/15-lipoxygenase activity and up-regulates peroxisome proliferator-activated receptor (PPAR)γ expression and/or activity.8 In turn, PPARγ is required for efficient removal of apoptotic cells by macrophages. The authors observed that use of rosiglitazone, a PPARγ activator, could normalize the capacity of X-linked CGD macrophages to ingest apoptotic cells. Furthermore, the authors newly described that IL-4 could directly increase 12/15-lipoxygenase activity, leading to 12-HETE production upstream of PPARγ and thereby signaling PPARγ activation. This novel pathway critical to wild-type macrophage activation is required for ingesting apoptotic neutrophils. In contrast, the pathway is defective in the X-linked CGD macrophage.
One of the biochemical hallmarks of the CGD neutrophil is the deficient expression of PS, which is required to activate macrophages.9 The authors show that injection of PS in vivo restored IL-4–dependent X-linked CGD macrophages and the ability of the macrophage to clear apoptotic cells. The implications from this work suggest that the CGD neutrophil remains long-lived in the tissue milieu because of its own intrinsic inability to generate free radicals and to be cleared efficiently by CGD macrophages deficient in IL-4 production. What remains to be clarified is how the defective macrophage function contributes to the formation of the granulomata, the hallmark of the disorder, and predisposes the host to autoimmune syndromes.
Conflict-of-interest disclosure: The author declares no competing financial interests. ■

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal