Abstract
Carcinoembryonic antigen cell adhesion molecule-1 (CEACAM1) is a surface glycoprotein expressed on various blood cells, epithelial cells, and vascular cells. CEACAM1 possesses adhesive and signaling properties mediated by its intrinsic immunoreceptor tyrosine-based inhibitory motifs that recruit SHP-1 protein-tyrosine phosphatase. In this study, we demonstrate that CEACAM1 is expressed on the surface and in intracellular pools of platelets. In addition, CEACAM1 serves to negatively regulate signaling of platelets by collagen through the glycoprotein VI (GPVI)/Fc receptor (FcR)–γ-chain. ceacam1−/− platelets displayed enhanced type I collagen and GPVI-selective ligand, collagen-related peptide (CRP), CRP-mediated platelet aggregation, enhanced platelet adhesion on type I collagen, and elevated CRP-mediated alpha and dense granule secretion. Platelets derived from ceacam1−/− mice form larger thrombi when perfused over a collagen matrix under arterial flow compared with wild-type mice. Furthermore, using intravital microscopy to ferric chloride-injured mesenteric arterioles, we show that thrombi formed in vivo in ceacam1−/− mice were larger and were more stable than those in wild-type mice. GPVI depletion using monoclonal antibody JAQ1 treatment of ceacam1−/− mice showed a reversal in the more stable thrombus growth phenotype. ceacam1−/− mice were more susceptible to type I collagen–induced pulmonary thromboembolism than wild-type mice. Thus, CEACAM1 acts as a negative regulator of platelet-collagen interactions and of thrombus growth involving the collagen GPVI receptor in vitro and in vivo.
Introduction
In vivo, several negative regulatory mechanisms have been described that down-modulate platelet-collagen interactions. These include prostacylin and nitric oxide released from endothelium and the immunoglobulin (Ig)–immunoreceptor tyrosine-based inhibitory motif (ITIM) superfamily member, platelet endothelial cell adhesion molecule–1 (PECAM-1) that serves an autoregulatory role when platelets come into contact with each other after exposure to collagen.1-4 Given the importance of collagen in promoting platelet adhesion and thrombus formation, particularly involving glycoprotein VI (GPVI)/Fc receptor (FcR)–γ-chain signaling, it is essential that natural inhibitors that contribute to autoregulatory mechanisms that modulate signaling between collagen and the GPVI/FcRγ chain are characterized in platelets.
While the ITIM-bearing receptor regulation of immunoreceptor tyrosine-based activation motif (ITAM)–associated pathways has been well described in leukocytes, they are poorly defined in platelets. This concept is further complicated by the observation that Ig-ITIM–bearing receptors have the capacity to bind multiple phosphatases including SHP-1, SHP-2, and SHIP to initiate signaling attenuation.5 However, there is also evidence that Ig-ITIM bearing receptors may differentially associate with distinct phosphatases depending on the cellular context and activation status of cells.6,7
These inhibitory mechanisms are further complicated by the fact that ITAM-bearing receptors can mediate inhibitory ITAM signaling under defined conditions using either the DAP12 adaptor or FcRγ chain.8,9 This switch between activating and inhibitory ITAM signaling is controlled by the avidity with which the ligand cross-links the receptor. It appears that low-avidity ligand interactions result in inhibitory signaling,possibly involving a phosphatase, and high-avidity ligand interactions produce activating ITAM-mediated signaling.8,9 Therefore, it is important to dissect out the respective mechanisms of activation and inhibition in platelets.
As platelets lack the prototypic inhibitory Ig-ITIM superfamily member FcγRIIb, there has been a prevailing view that platelet endothelial cell adhesion molecule–1 (PECAM-1) represents the sole surrogate Ig-ITIM superfamily member that serves a negative regulatory role.1,2 However, based on several studies, it would appear that platelets contain multiple Ig-ITIM superfamily members that are likely to have a modulatory role in regulating platelet-collagen interactions. Several reports suggest that platelets contain other Ig-ITIM superfamily members, including G6B and TREM (triggering receptors expressed on myeloid cells)–like transcript-1 (TLT-1), that may attenuate platelet function.10,11 In immunologic systems, SHP-1 protein-tyrosine phosphatase is classified as a negative regulator, while in collagen-GPVI platelet responses, it has been proposed to act as a positive regulator.12 Given this controversy, it is important that we study respective Ig-ITIM superfamily members that are linked to distinct protein-tyrosine phosphatases such as SHP-1 in platelets to determine their relative importance in regulating platelet thrombus formation.
Carcinoembryonic antigen-related cell adhesion molecule 1 (CEACAM1/CD66a/Bgp) is a member of the CEACAM family of genes that is a type I-transmembrane receptor broadly expressed on a wide range of cells including immune, hematologic, epithelial, and endothelial cells.13 CEACAM1 is the only CEACAM superfamily member expressed in rodents and humans. It shares some similarities with PECAM-1 in its capacity to mediate homophilic and heterophilic ligands (CEA and CEACAM6) but generally has a preference toward homophilic CEACAM1 interactions.13 CEACAM1 is composed of an N-terminal IgV-like domain that is responsible for homophilic adhesion, followed by 1 to 3 IgC-like domains in its extracellular structure. In addition, alternative mRNA splicing gives rise to different isoforms that differ in the length of their cytoplasmic domain, including the long ITIM-containing isoform (CEACAM1-L; 70-73 amino acids) or a short ITIM-less isoform (CEACAM1-S; 10-12 amino acids).13 The long form of CEACAM1 protein has 2 ITIMs that preferentially recruit SHP-1 protein-tyrosine phosphatase and, to a lesser extent, SHP-2 protein-tyrosine phosphatase.14,15 Based on recent studies in T cells, CEACAM1 functions as a negative regulator via its recruitment and activation of SHP-1 protein-tyrosine phosphatase.16 However, little is known about the presence and functional role of CEACAM1 in regulating platelet-collagen interactions and thrombus formation in vitro and in vivo. The only exception was a report by Hansson et al in 1990 that suggested that C-CAM (old terminology for CEACAM1) was present in the intracellular compartment of rat platelets.17
In the current study, we provide the first report that CEACAM1 serves as a negative regulator of platelet-collagen interactions, thrombus growth in vitro and in vivo, and susceptibility to type I collagen-induced pulmonary thromboembolism. Based on these studies, we propose CEACAM1 as a new negative regulator in platelets that serves an autoregulatory role when platelets come into contact with each other after exposure to collagen.
Methods
Antibodies
Rabbit anti–mouse CEACAM1 has been previously described.18 Anti-rabbit fluorescein isothiocyanate (FITC) was purchased from Dako (Botany, Australia). Anti–mouse GPVI,19 anti–mouse GPIbα/IX/V complex,20 anti–mouse integrin α2β1,21 anti–mouse CD9,22 and anti–mouse GPVI (JAQ1)23 were purchased or obtained from Emfret Analytics (Würzburg, Germany). Anti–mouse integrin αIIbβ3,24 anti–mouse CD4425 and HRP-conjugated anti–phosphotyrosine RC20 antibody were obtained from BD Biosciences Pharmingen (Franklin Lakes, NJ). Anti–mouse “390” PECAM-1 antibody was provided by Dr Steve Albelda (Philadelphia, PA).26 PLCγ2 and ERK-2 antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA).
Mice
The generation of ceacam1−/− mice and breeding on C57BL/6 background has been described.18 Wild-type and ceacam1+/− mice were age- and sex-matched C57BL/6. These mice were housed in the Burnet Institute at Austin Animal House and the St Michael's Hospital Research Vivarium. All procedures were approved by the Austin Health Animal Ethics committee no. 2005:2098 and no. 2005:02354, and the Animal Care Committee of St Michael's Hospital.
Preparation of mouse platelets
Platelet-rich plasma (PRP) or washed mouse platelets were prepared as previously described.27
Static platelet adhesion assays
Static platelet adhesion assays were performed according to Yuan et al.28 Washed platelets derived from wild-type, ceacam1+/−, and ceacam1−/− mice were examined for binding to immobilized type I fibrillar collagen and fibrinogen in the absence of Mg2+ over time, fixed, and quantitated by differential interference contrast (DIC) microscopy (63× oil objective).
Platelet adhesion and thrombus formation under flow
Platelet adhesion and thrombus formation under flow was performed as previously described.1
Platelet aggregation assays
Platelet aggregation assays were monitored by measuring light transmission using a 4-channel platelet aggregometer (Chrono-log, Havertown, PA).28,29 PRP was diluted in RCD buffer, pH 7.4, (108 mM NaCl, 38 mM KCl, 1.7 mM NaHCO3, 21.2 mM sodium citrate, 27.8 mM glucose, and 1.1 mM MgCl2·6H2O) to generate a platelet count of 100 × 109/L. Reactions were performed in glass cuvettes in a 250 μL volume in the presence of 100 μg/mL fibrinogen and 1 mM CaCl2 at 37°C with constant stirring (1000 rpm).
Flow cytometry
The assessment of surface and intracellular expression of CEACAM1 was performed essentially as previously described.30 The only exception was that platelets were labeled with either preimmune rabbit serum (1:500), rabbit anti-mouse CEACAM1 2457 serum (1:500) or FITC-conjugated antibody to P-selectin (10 μg/mL), and where appropriate, then labeled with swine anti–rabbit F(ab′)2 FITC-conjugated antibody (1:200; Dako).
5-Hydroxytryptamine dense granule secretion
Dense granule secretion was assessed essentially as described with the inclusion of 2 mM imipramine hydrochloride (Sigma-Aldrich, St Louis, MO).1
Ferric chloride–induced model of thrombosis and intravital microscopy
Wild-type, ceacam1+/− or ceacam1−/− C57BL/6 mice (4-5 weeks old) were anaesthetized with a ketamine/xylazine (200:10 mg/kg) mixture and the mesentery was exteriorized through a midline abdominal incision. A catheter was inserted into the jugular vein to deliver rhodamine dye and anesthetic as required. Mesenteric arterioles (60-100 μm in diameter) were visualized with a Zeiss Axiovert 135 M1 microscope (Carl Zeiss, Gottingen, Germany), and z-stack slices of the vessel captured with a AxioCam MRm camera (Carl Zeiss). A strip of filter paper (4 mm) was dipped in a 462 mM (7.5% wt/vol) FeCl3 solution for 3 seconds, applied to a 2- to 5-mm length of arteriole for 4 minutes and then removed.30,31 Z-stack images of the vessel were taken over 5 2-minute cycles (determined as the time the vessel begins to occlude). The rendered z-stack images were deconvolved with AxioVision Rel 4.6 software (Carl Zeiss), to produce a 3-dimensional image, and the volume of the thrombi were determined from the area and height of the clot. To facilitate detection of quantitative differences, the evaluator of the platelet thrombi was blinded to the genotype of the mice: wild-type, ceacam1+/−, and ceacam1−/−. Stability was determined by calculating the percentage of the vessel that was occupied by the thrombus over a period of 2 minutes, and scored from 1 to 10, with 1 representing 0% to 10% occupancy and 10 representing 91% to 100% occupancy (ie, complete vessel occlusion).
Pulmonary thromboembolism mouse model
Mouse models of pulmonary thromboembolism were essentially performed as previously described.32 The only exceptions include that 350 μg/kg of type I fibrillar collagen and 0.200 μL/g tissue thromboplastin (Thromborel S) were used in respective models. The end point of the pulmonary thromboembolism was defined as the time to restrained breathing within 10-second intervals.
Statistical analysis
Data were checked for normal distribution using the Shapiro-Wilk normality test and statistical significance was determined using the unpaired Student t test. P values of .05 or less were taken to indicate significant differences.
Results
CEACAM1-deficient mice have normal hematopoiesis
Unlike other CEACAM1 family members, CEACAM1 is broadly expressed on a wide range of cells including platelets and immune, epithelial, and endothelial cells.13 To test whether deletion of CEACAM1 influenced hematopoiesis and particularly platelet production, we determined peripheral blood hematologic parameters for age- and sex-matched wild-type versus CEACAM1-deficient mice. As shown in Table 1, ceacam1−/− mice, compared with wild-type C57BL/6 mice, displayed normal white blood cell (WBC) counts, red blood cell (RBC) counts and other parameters including hemoglobin and platelet count, compared with parameters from wild-type C57BL/6 mice (P > .05, n = 18 for each type of mouse). Differential counting of WBCs revealed that ceacam1−/− mice had an increased percentage of neutrophils compared with wild-type mice (P < .05).
Summary of peripheral blood hematologic parameters for wild-type and ceacam1−/− mice
| Hematologic parameter . | ceacam1+/+ (n = 18) . | ceacam1−/− (n = 18) . |
|---|---|---|
| WBC, ×109/L | 1.63 ± 0.17 | 1.81 ± 0.14 |
| Hb, g/L | 107.8 ± 2.7 | 108.3 ± 3.0 |
| RBC, ×1012/L | 8.34 ± 0.148 | 8.66 ± 0.230 |
| PCV, L/L | 0.396 ± 0.007 | 0.412 ± 0.009 |
| MCV, fl | 474.8 ± 1.35 | 478.1 ± 2.87 |
| MCH, pg | 129.6 ± 3.37 | 125.5 ± 1.53 |
| MCHC, ×103 g/L | 2.73 ± 0.07 | 2.62 ± 0.03 |
| Plat, ×109/L | 776 ± 39 | 680 ± 65 |
| Neut/WBC, % | 9.71 ± 0.84 | 13.78 ± 1.88 |
| Lymph/WBC, % | 83.8 ± 1.7 | 78.2 ± 2.7 |
| Mono/WBC, % | 6.29 ± 1.21 | 7.56 ± 1.14 |
| Hematologic parameter . | ceacam1+/+ (n = 18) . | ceacam1−/− (n = 18) . |
|---|---|---|
| WBC, ×109/L | 1.63 ± 0.17 | 1.81 ± 0.14 |
| Hb, g/L | 107.8 ± 2.7 | 108.3 ± 3.0 |
| RBC, ×1012/L | 8.34 ± 0.148 | 8.66 ± 0.230 |
| PCV, L/L | 0.396 ± 0.007 | 0.412 ± 0.009 |
| MCV, fl | 474.8 ± 1.35 | 478.1 ± 2.87 |
| MCH, pg | 129.6 ± 3.37 | 125.5 ± 1.53 |
| MCHC, ×103 g/L | 2.73 ± 0.07 | 2.62 ± 0.03 |
| Plat, ×109/L | 776 ± 39 | 680 ± 65 |
| Neut/WBC, % | 9.71 ± 0.84 | 13.78 ± 1.88 |
| Lymph/WBC, % | 83.8 ± 1.7 | 78.2 ± 2.7 |
| Mono/WBC, % | 6.29 ± 1.21 | 7.56 ± 1.14 |
P > .05 for all parameters except neutrophils per WBC (P < .05).
WBC indicates white blood cell; Hb, hemoglobin; RBC, red blood cell; PCV, packed cell volume; MCV, mean cell volume; MCH, mean cell hemoglobin; MCHC, mean cell hemoglobin concentration; Plat, platelets; Neut, neutrophils; Lymph, lymphocytes; and Mono, monocytes.
CEACAM1 is expressed on the surface and in intracellular pools in murine and human platelets
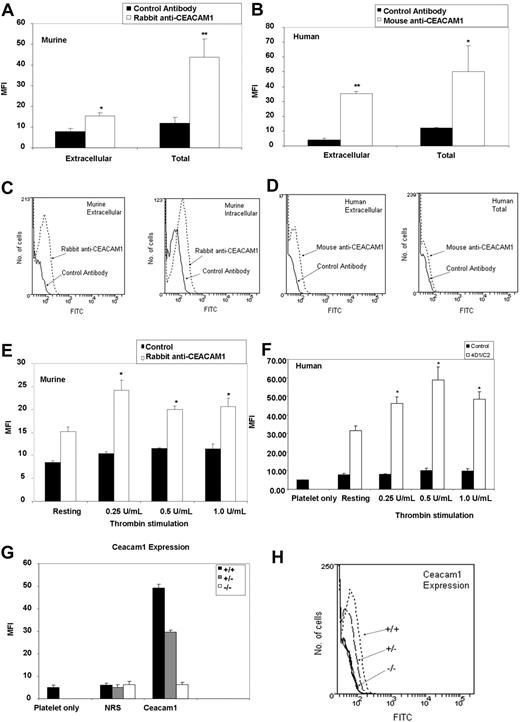
The only evidence in the literature in relation to CEACAM1 in platelets was the demonstration that C-CAM was present in the intracellular compartment of rat platelets.17 To determine the presence of CEACAM1 protein expressed on the surface and in intracellular pools of resting murine and human platelets, and whether this expression is up-regulated by intracellular granule release, we examined the binding of specific polyclonal anti-murine CEACAM1 antibody to resting and thrombin-stimulated wild-type murine and human platelets, monitored by flow cytometry. To characterize the surface expression of CEACAM1 in murine platelets, washed platelets were incubated with either normal rabbit pre-immune serum (1:500 dilution) or polyclonal anti–mouse CEACAM1 antibody (1:500 dilution), followed by FITC-labeled anti-rabbit conjugate (1:200 dilution), and then analyzed by flow cytometry. In addition, characterization of the total distribution of CEACAM1 in murine platelets was determined by permeabilization of the platelet membrane with 0.1% (wt/vol) saponin. As shown in Figure 1A and C, resting murine platelets treated with specific polyclonal anti-CEACAM1 antibody had a mean fluorescence intensity (MFI) that was twice that of platelets treated with normal rabbit pre-immune serum (1:500 dilution; 15.24 ± 1.61 vs 7.74 ± 1.71, respectively; P < .005; n = 3). This finding indicated the presence of CEACAM1 on the surface of murine platelets. In addition, the MFI of saponin-treated murine platelets was 4 times higher after treatment with a specific polyclonal anti-CEACAM1 antibody than with normal rabbit pre-immune serum (1:500 dilution; 43.64 ± 8.84 vs 11.99 ± 2.75; P < .05; n = 3). This finding indicated the presence of CEACAM1 in an intracellular distribution in murine platelets. As shown in Figure 1B and D, the MFI of resting human platelets was 7 times higher with a specific monoclonal anti-CEACAM1 antibody than with an isotype control antibody (35.3 ± 1.3 vs 4.0 ± 1.2; P < .005; n = 3). In addition, the MFI of saponin-treated human platelets was 4 times higher with a specific monoclonal anti-CEACAM1 antibody than with an isotype control antibody (50.25 ± 17.4 vs 12.05 ± 0.35; P < .05; n = 3). Cell surface expression of CEACAM1 was increased upon agonist stimulation with different doses of thrombin compared with resting murine platelets and normal pre-immune rabbit serum (24.08 ± 2.3 vs 15.25 ± 0.93; P < .05; n = 3; 8.41 ± 0.37; Figure 1E). A similar trend was observed with cell surface expression of CEACAM1 after agonist stimulation with different doses of thrombin compared with resting human platelets and negative isotype antibody (58.93 ± 7.11 vs 31.63 ± 2.59; P < .05, n = 3; 10.23 ± 1.15; Figure 1F). At higher concentrations of thrombin, a modest reduction in CEACAM1 surface expression was observed. The CEACAM1 expression of ceacam1+/− platelets was 60% of that of wild-type murine platelets (Figure 1G,H). These results indicate that CEACAM1 is expressed on the surface and in intracellular pools of resting murine and human platelets.
CEACAM1 is expressed on the surface and in intracellular pools in murine and human platelets. (A) Flow cytometric analysis of CEACAM1 surface and total expression on resting murine platelets. Platelets were stained with a polyclonal anti-murine CEACAM1 2457 antibody followed by a secondary FITC-conjugated anti-rabbit antibody. Normal rabbit serum and ceacam1−/− platelets were included as negative controls. Data were collected through a live platelet gate based on forward versus side scatter profiles on a FACSCanto flow cytometer (BD Biosciences). Results combine data derived from 3 independent experiments and are represented as mean fluorescence intensity (MFI) plus or minus SEM. (B) Flow cytometric analysis of CEACAM1 surface and total expression on resting human platelets. Platelets were stained with a monoclonal anti–human CEACAM1 4D1C2 antibody followed by a secondary FITC-conjugated anti–mouse antibody. Normal mouse IgG and ceacam1−/− platelets were included as negative controls. Data were collected through a live platelet gate based on forward- versus side-scatter profiles on a FACSCanto flow cytometer. Results combine data derived from 3 independent experiments and are represented as MFI plus or minus SEM. (C,D) Representative histogram profiles of CEACAM1 surface and total expression on resting murine and human platelets. CEACAM1 surface and total expression was determined as described in panels A and B. (E) Agonist stimulation of murine platelets using thrombin (0.25-1.00 U/mL) over a dose-dependent range. CEACAM1 surface expression was determined as described in panel A. (F) Agonist stimulation of human platelets using thrombin (0.25-1.00 U/mL) over a dose-dependent range. CEACAM1 surface expression was determined as described in panel B. (G) Flow cytometric analysis of CEACAM1 surface expression on resting murine platelets from wild-type versus ceacam1+/− and ceacam1−/− mice. CEACAM1 surface expression was determined as described in panel A. NRS indicates normal rabbit serum. (H) Representative histogram profiles of CEACAM1 surface expression on resting murine platelets from wild-type versus ceacam1+/− and ceacam1−/− mice. *P < .05; **P < .005.
CEACAM1 is expressed on the surface and in intracellular pools in murine and human platelets. (A) Flow cytometric analysis of CEACAM1 surface and total expression on resting murine platelets. Platelets were stained with a polyclonal anti-murine CEACAM1 2457 antibody followed by a secondary FITC-conjugated anti-rabbit antibody. Normal rabbit serum and ceacam1−/− platelets were included as negative controls. Data were collected through a live platelet gate based on forward versus side scatter profiles on a FACSCanto flow cytometer (BD Biosciences). Results combine data derived from 3 independent experiments and are represented as mean fluorescence intensity (MFI) plus or minus SEM. (B) Flow cytometric analysis of CEACAM1 surface and total expression on resting human platelets. Platelets were stained with a monoclonal anti–human CEACAM1 4D1C2 antibody followed by a secondary FITC-conjugated anti–mouse antibody. Normal mouse IgG and ceacam1−/− platelets were included as negative controls. Data were collected through a live platelet gate based on forward- versus side-scatter profiles on a FACSCanto flow cytometer. Results combine data derived from 3 independent experiments and are represented as MFI plus or minus SEM. (C,D) Representative histogram profiles of CEACAM1 surface and total expression on resting murine and human platelets. CEACAM1 surface and total expression was determined as described in panels A and B. (E) Agonist stimulation of murine platelets using thrombin (0.25-1.00 U/mL) over a dose-dependent range. CEACAM1 surface expression was determined as described in panel A. (F) Agonist stimulation of human platelets using thrombin (0.25-1.00 U/mL) over a dose-dependent range. CEACAM1 surface expression was determined as described in panel B. (G) Flow cytometric analysis of CEACAM1 surface expression on resting murine platelets from wild-type versus ceacam1+/− and ceacam1−/− mice. CEACAM1 surface expression was determined as described in panel A. NRS indicates normal rabbit serum. (H) Representative histogram profiles of CEACAM1 surface expression on resting murine platelets from wild-type versus ceacam1+/− and ceacam1−/− mice. *P < .05; **P < .005.
CEACAM1 serves as a negative regulator of collagen-GPVI–mediated platelet responses
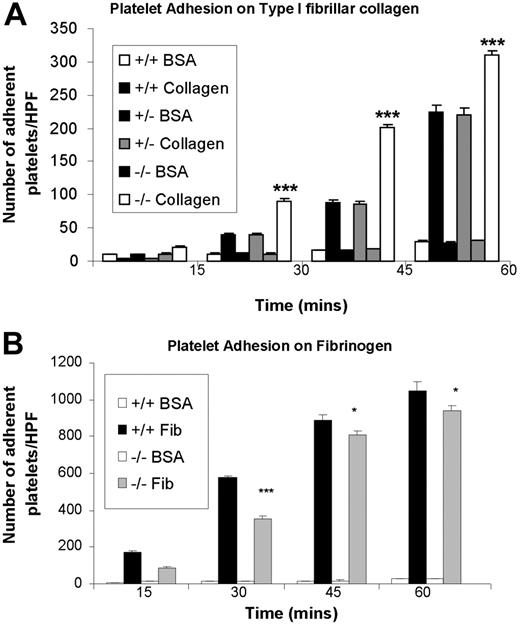
Recent studies in T cells have demonstrated that CEACAM1 acts as an inhibitory coreceptor via the interface of its intrinsic ITIMs with recruitment and activation of SHP-1 protein-tyrosine phosphatase.16 This finding supports the concept in immunologic systems that SHP-1 protein-tyrosine phosphatase acts as a negative regulator.33 However, in collagen-GPVI–mediated platelet responses, SHP-1 has been proposed to act as a positive regulator.12 Given this controversy, it is important to define the functional role of CEACAM1 negative regulatory signaling in platelets. To test the hypothesis that CEACAM1, like PECAM-1, normally functions to modulate collagen-GPVI–mediated platelet responses, we first examined the ability of platelets derived from wild-type versus CEACAM1-deficient mice to bind, over time, to immobilized type I fibrillar collagen and fibrinogen in the absence of Mg2+. As shown in Figure 2A, ceacam1−/− platelets bound significantly better at all time points to type I fibrillar collagen than did wild-type and ceacam1+/− platelets (P < .005; n = 3). This effect was specific as adhesion of bovine serum albumin over the same time points was insignificant for wild-type, ceacam1+/−, and ceacam1−/− platelets. This hyper-responsive platelet adhesion observed with ceacam1−/− platelets was selective for type I fibrillar collagen as platelet adhesion on fibrinogen did not show increased adhesion (Figure 2B). In fact, a mild reduction in platelet adhesion on fibrinogen was observed in ceacam1−/− platelets but not wild-type or ceacam1+/− platelets (P < .05; n = 3).
ceacam1−/− platelets demonstrate increased adhesion on immobilized type I fibrillar collagen. (A) Wild-type, ceacam1+/−, and ceacam1−/− platelets were allowed to adhere to type I fibrillar collagen (50 μg/mL) in the absence of magnesium, or to plates coated with bovine serum albumin (50 μg/mL) for 15, 30, 45, or 60 minutes at 37°C. After removal of nonadherent platelets, adherent platelets were measured as described in “Methods.” Each assay was performed in triplicate and is representative of 3 independent experiments. Each data point represents platelet adhesion per high-powered field and expressed as the mean plus or minus SEM. Note that wild-type, ceacam1+/−, and ceacam1−/− platelets bound to a similar extent on BSA-coated platelets, where at all time points, ceacam1−/− platelets showed higher levels of adhesion to type I fibrillar collagen than did wild-type and ceacam1+/− platelets (***P < .005; n = 3). (B) Platelet adhesion on fibrinogen (50 μg/mL) was assessed for wild-type, ceacam1+/−, and ceacam1−/− platelets at 15, 30, 45, and 60 minutes at 37°C, as described in panel A.
ceacam1−/− platelets demonstrate increased adhesion on immobilized type I fibrillar collagen. (A) Wild-type, ceacam1+/−, and ceacam1−/− platelets were allowed to adhere to type I fibrillar collagen (50 μg/mL) in the absence of magnesium, or to plates coated with bovine serum albumin (50 μg/mL) for 15, 30, 45, or 60 minutes at 37°C. After removal of nonadherent platelets, adherent platelets were measured as described in “Methods.” Each assay was performed in triplicate and is representative of 3 independent experiments. Each data point represents platelet adhesion per high-powered field and expressed as the mean plus or minus SEM. Note that wild-type, ceacam1+/−, and ceacam1−/− platelets bound to a similar extent on BSA-coated platelets, where at all time points, ceacam1−/− platelets showed higher levels of adhesion to type I fibrillar collagen than did wild-type and ceacam1+/− platelets (***P < .005; n = 3). (B) Platelet adhesion on fibrinogen (50 μg/mL) was assessed for wild-type, ceacam1+/−, and ceacam1−/− platelets at 15, 30, 45, and 60 minutes at 37°C, as described in panel A.
To examine the functional role of CEACAM1 negative regulatory signaling in platelets, we performed a time course (0-3 minutes) stimulation of wild-type and ceacam1−/− platelets with collagen-related peptide (CRP) and determined global tyrosine phosphorylation profiles of 30 μg of platelet lysates. As shown in Figure 3A, CRP stimulation of ceacam1−/− platelets showed an increased level of tyrosine phosphorylation over time of several proteins including Syk, with both quantitative and kinetic differences from the tyrosine phosphorylation observed in wild-type platelets. In addition, Erk-2 blot was included as a loading control (Figure 3A bottom panel). Measurement of tyrosine phosphorylation of PLCγ2 was performed by immunoprecipitation of resting versus CRP stimulated wild-type and ceacam1−/− platelets (time [T] = 90 seconds) followed by immunoblotting with an anti-phosphotyrosine antibody (Figure 3B). PLCγ2 antigen loading was confirmed by reprobing with a polyclonal anti-PLCγ2 antibody. As shown in Figure 3B, resting and CRP stimulated ceacam1−/− platelets showed increased tyrosine phosphorylation of PLCγ2 compared with wild-type platelets. These results support the concept that CEACAM1 acts as a negative regulator to modulate ITAM-coupled signaling pathways.
ceacam1−/− platelets display hyper-phosphorylated proteins including PLCγ2 after CRP stimulation over time. (A) Tyrosine phosphorylation in whole-cell platelet lysates from wild-type and ceacam1−/− mice were stimulated with CRP (10 μg/mL) over 3 minutes. Then 30 μg of each platelet lysate was loaded onto a 10% sodium dodecyl sulfate–polyacrylamide gel and tyrosine phosphorylation was detected on the western blot, using a horseradish peroxidase (HRP)–conjugated anti-phosphotyrosine RC20 antibody. Erk-2 blot (bottom panel) was included as a protein loading control. This is a representative blot of 3 experiments. (B) PLCγ2 was immunoprecipitated from lysates of wild-type or ceacam1−/− platelets stimulated by CRP (10 μg/mL) at time (T) 0 and 90 seconds and immunoblotted for phosphotyrosine using a HRP-conjugated anti-phosphotyrosine RC20 antibody. PLCγ2 antigen is included as a loading control of the immunoprecipitates (bottom panel). Relative intensity of tyrosine phosphorylated PLCγ2 to total PLCγ2 antigen was quantitated using ImageJ software version 1.40g (available from http://rsbweb.nih.gov/ij/) from 3 replicate experiments.
ceacam1−/− platelets display hyper-phosphorylated proteins including PLCγ2 after CRP stimulation over time. (A) Tyrosine phosphorylation in whole-cell platelet lysates from wild-type and ceacam1−/− mice were stimulated with CRP (10 μg/mL) over 3 minutes. Then 30 μg of each platelet lysate was loaded onto a 10% sodium dodecyl sulfate–polyacrylamide gel and tyrosine phosphorylation was detected on the western blot, using a horseradish peroxidase (HRP)–conjugated anti-phosphotyrosine RC20 antibody. Erk-2 blot (bottom panel) was included as a protein loading control. This is a representative blot of 3 experiments. (B) PLCγ2 was immunoprecipitated from lysates of wild-type or ceacam1−/− platelets stimulated by CRP (10 μg/mL) at time (T) 0 and 90 seconds and immunoblotted for phosphotyrosine using a HRP-conjugated anti-phosphotyrosine RC20 antibody. PLCγ2 antigen is included as a loading control of the immunoprecipitates (bottom panel). Relative intensity of tyrosine phosphorylated PLCγ2 to total PLCγ2 antigen was quantitated using ImageJ software version 1.40g (available from http://rsbweb.nih.gov/ij/) from 3 replicate experiments.
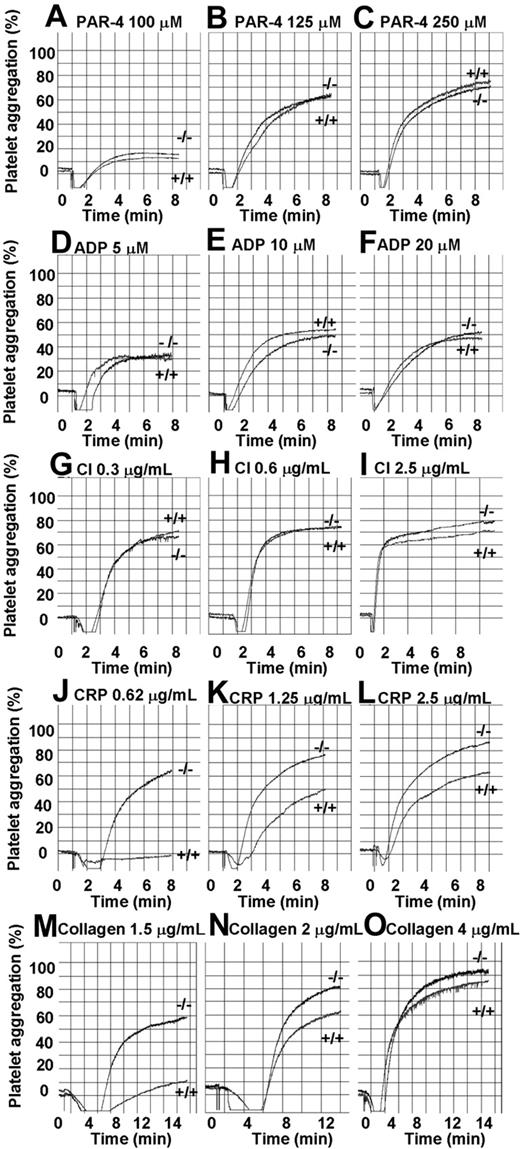
As CEACAM1 has been demonstrated to function as a regulator in T cells upon T-cell antigen receptor ligation, we wanted to test the hypothesis that CEACAM1 serves to negatively regulate ITAM-bearing collagen-GPVI–mediated platelet responses. To test the possibility in platelets, we compared the aggregation responses of platelets from wild-type and CEACAM1-deficient mice over a dose-dependent range of GPVI selective agonists, CRP (0.62-2.5 μg/mL; Figure 4J-L) and acid soluble type I collagen (1.5-4 μg/mL; Figure 4M-O) with G-protein–coupled agonists, PAR-4 (100-250 μM; Figure 4A-C), ADP (5-20 μM; Figure 4D-F) and calcium ionophore (CI; 0.3-2.5 μg/mL; Figure 4G-I). As shown in Figure 4, wild-type and ceacam1−/− platelets display equivalent platelet aggregation profiles over a dose-dependent range of PAR-4, ADP, and calcium ionophore. In contrast, ceacam1−/− platelets show enhanced amplitude and slope of platelet aggregation responses to CRP and collagen particularly at subthreshold doses. This hyperresponsive effect is independent of GPVI expression and other platelet glycoprotein expression (integrin α2β1, GPIb-IX-V complex, integrin αIIbβ3, CD9, CD44, PECAM-1), as ceacam1−/− platelets are comparable to wild-type platelets (Table 2). These results indicate that the absence of the Ig-ITIM superfamily member CEACAM1 leads to hyperresponsive GPVI-mediated platelet responses to collagen and CRP.
ceacam1−/− platelets show hyper-responsive aggregation in response to stimulation with GPVI-selective agonists. Aggregation responses of PRP (platelet count adjusted to 100 × 109/L) for wild-type and ceacam1−/− mice were determined after activation with different concentrations of various agonists: PAR-4 agonist peptide (100-250 μM), ADP (5-20 μM), calcium ionophore (0.3-2.5 μg/mL), collagen-related peptide (0.62-2.5 μg/mL) and collagen (1.5-4 μg/mL) respectively. Note that ceacam1−/− platelets are hyper-responsive in collagen and CRP-induced platelet aggregation. These results are representative of at least 3 independent experiments.
ceacam1−/− platelets show hyper-responsive aggregation in response to stimulation with GPVI-selective agonists. Aggregation responses of PRP (platelet count adjusted to 100 × 109/L) for wild-type and ceacam1−/− mice were determined after activation with different concentrations of various agonists: PAR-4 agonist peptide (100-250 μM), ADP (5-20 μM), calcium ionophore (0.3-2.5 μg/mL), collagen-related peptide (0.62-2.5 μg/mL) and collagen (1.5-4 μg/mL) respectively. Note that ceacam1−/− platelets are hyper-responsive in collagen and CRP-induced platelet aggregation. These results are representative of at least 3 independent experiments.
Glycoprotein expression in ceacam1−/− mice
| Glycoprotein . | MFI in wild-type mice (n = 3) . | MFI in ceacam1−/− mice (n = 3) . |
|---|---|---|
| GPVI | 87.9 ± 5.9 | 78.3 ± 9.8 |
| Integrin αbβ3 | 254.7 ± 15.6 | 245.0 ± 16.5 |
| GPIbα/IX/V complex | 242.6 ± 23.2 | 231.9 ± 29.4 |
| Integrin α2β1 | 60.7 ± 1.9 | 51.9 ± 2.7 |
| PECAM-1 | 39.9 ± 12.7 | 32.4 ± 10.1 |
| CD9 | 258.2 ± 1.0 | 256.3 ± 43.7 |
| CD44 | 134.7 ± 2.5 | 113.3 ± 10.8 |
| CEACAM1 | 39.7 ± 1.4 | 3.8 ± 0.04 |
| Glycoprotein . | MFI in wild-type mice (n = 3) . | MFI in ceacam1−/− mice (n = 3) . |
|---|---|---|
| GPVI | 87.9 ± 5.9 | 78.3 ± 9.8 |
| Integrin αbβ3 | 254.7 ± 15.6 | 245.0 ± 16.5 |
| GPIbα/IX/V complex | 242.6 ± 23.2 | 231.9 ± 29.4 |
| Integrin α2β1 | 60.7 ± 1.9 | 51.9 ± 2.7 |
| PECAM-1 | 39.9 ± 12.7 | 32.4 ± 10.1 |
| CD9 | 258.2 ± 1.0 | 256.3 ± 43.7 |
| CD44 | 134.7 ± 2.5 | 113.3 ± 10.8 |
| CEACAM1 | 39.7 ± 1.4 | 3.8 ± 0.04 |
Cell surface expression of platelet glycoprotein expression was monitored by flow cytometry using specific monoclonal antibodies for wild-type and ceacam1−/− mice. MFIs are reported as mean plus or minus SEM for three independent experiments.
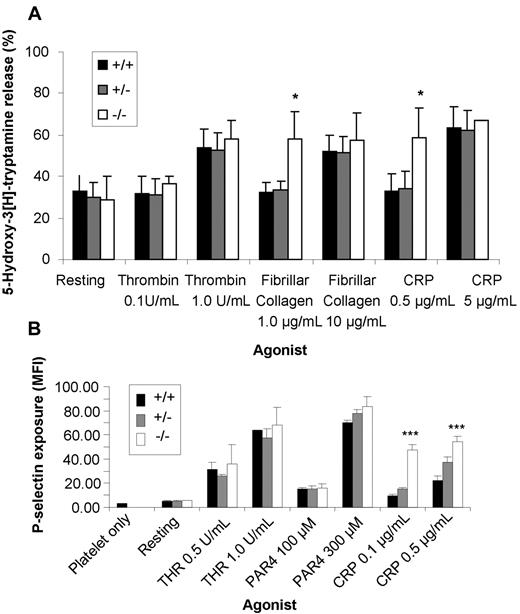
As ceacam1−/− platelets displayed enhanced adhesion of type I fibrillar collagen and hyperresponsive GPVI-mediated platelet aggregation responses, we wanted to determine whether the absence of CEACAM1 influenced the platelet-release reaction stimulated by GPVI-specific agonists restricted to GPVI. To test this possibility, we compared the ability of wild-type, ceacam1+/−, and ceacam1−/− platelets to secrete alpha (P-selectin) and dense granule serotonin upon stimulation of GPVI. As shown in Figure 5A,B, ceacam1−/− platelets displayed increased release of their alpha and dense granules at subthreshold doses of fibrillar collagen and CRP compared with low or high doses of thrombin-treated wild-type and ceacam1+/− platelets (*P < .05; ***P < .001; n = 3). These data are consistent with the concept that CEACAM1 normally acts as a negative regulator of platelet GPVI-mediated alpha and dense granule release.
ceacam1−/− platelets display a lower threshold in alpha and dense granule release in response to stimulation with GPVI-selective agonists. (A) Wild-type (■), ceacam1+/− ( ), and ceacam1−/− (□) platelets were stimulated with varying concentrations of GPVI-selective agonists including type I fibrillar collagen in the absence of magnesium and CRP, or with thrombin. The amount of 5-hydroxytryptamine released was measured as described in “Methods.” The assays were performed in triplicate, and the results expressed as the mean plus or minus SEM. These results are representative of 3 independent experiments. Note that the release of dense granular contents in response to stimulation at subthreshold levels of GPVI-selective agonists was greater in ceacam1−/− platelets than in wild-type and ceacam1+/− platelets. (B) Surface expression of P-selectin (alpha granule release) was determined for washed platelets stimulated by thrombin, PAR-4 agonist peptide, and CRP at different concentrations. The platelets were then stained with either a buffer control or FITC-P-selectin monoclonal antibody for both wild-type and ceacam1−/− platelets. FITC-labeled samples were analyzed on a FACSCanto analyzer. Results are representative of 3 independent experiments.
), and ceacam1−/− (□) platelets were stimulated with varying concentrations of GPVI-selective agonists including type I fibrillar collagen in the absence of magnesium and CRP, or with thrombin. The amount of 5-hydroxytryptamine released was measured as described in “Methods.” The assays were performed in triplicate, and the results expressed as the mean plus or minus SEM. These results are representative of 3 independent experiments. Note that the release of dense granular contents in response to stimulation at subthreshold levels of GPVI-selective agonists was greater in ceacam1−/− platelets than in wild-type and ceacam1+/− platelets. (B) Surface expression of P-selectin (alpha granule release) was determined for washed platelets stimulated by thrombin, PAR-4 agonist peptide, and CRP at different concentrations. The platelets were then stained with either a buffer control or FITC-P-selectin monoclonal antibody for both wild-type and ceacam1−/− platelets. FITC-labeled samples were analyzed on a FACSCanto analyzer. Results are representative of 3 independent experiments.
ceacam1−/− platelets display a lower threshold in alpha and dense granule release in response to stimulation with GPVI-selective agonists. (A) Wild-type (■), ceacam1+/− ( ), and ceacam1−/− (□) platelets were stimulated with varying concentrations of GPVI-selective agonists including type I fibrillar collagen in the absence of magnesium and CRP, or with thrombin. The amount of 5-hydroxytryptamine released was measured as described in “Methods.” The assays were performed in triplicate, and the results expressed as the mean plus or minus SEM. These results are representative of 3 independent experiments. Note that the release of dense granular contents in response to stimulation at subthreshold levels of GPVI-selective agonists was greater in ceacam1−/− platelets than in wild-type and ceacam1+/− platelets. (B) Surface expression of P-selectin (alpha granule release) was determined for washed platelets stimulated by thrombin, PAR-4 agonist peptide, and CRP at different concentrations. The platelets were then stained with either a buffer control or FITC-P-selectin monoclonal antibody for both wild-type and ceacam1−/− platelets. FITC-labeled samples were analyzed on a FACSCanto analyzer. Results are representative of 3 independent experiments.
), and ceacam1−/− (□) platelets were stimulated with varying concentrations of GPVI-selective agonists including type I fibrillar collagen in the absence of magnesium and CRP, or with thrombin. The amount of 5-hydroxytryptamine released was measured as described in “Methods.” The assays were performed in triplicate, and the results expressed as the mean plus or minus SEM. These results are representative of 3 independent experiments. Note that the release of dense granular contents in response to stimulation at subthreshold levels of GPVI-selective agonists was greater in ceacam1−/− platelets than in wild-type and ceacam1+/− platelets. (B) Surface expression of P-selectin (alpha granule release) was determined for washed platelets stimulated by thrombin, PAR-4 agonist peptide, and CRP at different concentrations. The platelets were then stained with either a buffer control or FITC-P-selectin monoclonal antibody for both wild-type and ceacam1−/− platelets. FITC-labeled samples were analyzed on a FACSCanto analyzer. Results are representative of 3 independent experiments.
CEACAM1-deficient mice display increased thrombus growth in vitro, in vivo, and enhanced susceptibility to type I collagen induced pulmonary thromboembolism
While platelets lack the prototypic Ig-ITIM superfamily member, FcγRIIb, other surrogate Ig-ITIM superfamily members, like PECAM-1, serve as potent negative regulators of platelet thrombus formation. It is however, not clearly defined which Ig-ITIM superfamily members are functionally important in regulating in vivo platelet thrombus formation and this issue warrants investigation. To explore the possibility that CEACAM1 is important in modulation of platelet thrombus formation in vivo, we compared age- and sex-matched wild-type versus ceacam1−/− mice in platelet adhesion and thrombus growth on the exposed extracellular matrix of the injured mesenteric arterioles (60- to 100-μm-sized vessels). We used a vascular injury model with ferric chloride-mediated denuding of endothelium, monitored by fluorescence intravital microscopy. The ferric chloride (FeCl3) oxidative injury model was used to induce formation of free radicals to disrupt the vascular endothelium in mesenteric arterioles.30 Platelet thrombus formation was monitored in real time by assessing the accumulation of fluorescently labeled platelets. After application of FeCl3 to the mesentery, platelets in both wild-type and ceacam1−/− mice rapidly commenced interacting with the injured vessel wall and within 10 to 15 minutes after the injury, vessel occlusion occurred. Thrombus characteristics were assessed in real time, measuring the area, height, and volume occupied by the thrombus, as well as a stability score for each thrombus over a 2-minute time frame. Typically, ceacam1−/− mice showed increased thrombus growth and more stable thrombi over time than did wild-type control mice (Figure 6A). Typically, ceacam1−/− arterioles showed a larger thrombus area (reflecting surface coverage of platelets) over a 2-minute time interval than did wild-type and ceacam1+/− arterioles (6212 ± 268.7 vs 3294 ± 223.4 vs 2859 ± 293.9 μm2, respectively; P < .001; n = 15; Figure 6B). In addition, ceacam1−/− mice developed thrombi that were significantly larger in volume than wild-type and ceacam1+/− mice (122 400 ± 6794 vs 49 430 ± 4602 vs 63 680 ± 5478 μm3, respectively; P < .001; n = 15; Figure 6C). Moreover, the thrombi formed in ceacam1−/− arterioles were typically more stable than those in wild-type and ceacam1+/− arterioles (4.067 ± 0.371 vs 2.400 ± 0.214 vs 1.050 ± 0.120; P < .001; n = 15; Figure 6D). In contrast, ceacam1−/− mice showed no abnormalities in time to the first thrombi larger than 20 μm2, number of thrombi greater than 20 μm2, increase in thrombus diameter over 1 minute, thrombus height, and time to vessel occlusion (P > .05; n = 15; data not shown). To directly test the contribution of the collagen GPVI receptor in this thrombus growth phenotype in ceacam1−/− arterioles, we deleted GPVI in vivo using JAQ1 treatment (100 μg/mouse) for 5 days before ferric chloride injury and intravital studies.23 A different cohort of ceacam1−/− mice were similarly treated with control IgG (100 μg/mouse). As shown in Figure 6E and F, JAQ1-treated ceacam1−/− mice showed a 3-fold reduction in thrombus volume and 2-fold reduction in stability score compared with control IgG treated and untreated ceacam1+/+ and ceacam1−/− arterioles (n = 10 arterioles from 3 mice/group; P < .001). Importantly, ceacam1−/− arterioles showed reversal of the more stable thrombus phenotype upon JAQ1 treatment and of the thrombus growth phenotype compared with ceacam1+/+ arterioles. We confirmed successful clearance of GPVI after JAQ1 treatment by western blotting of platelet lysates from ceacam1−/− platelets (data not shown). Overall, these data support the concept that the in vivo thrombus growth phenotype in ceacam1−/− arterioles is dependent on the presence of the collagen GPVI receptor.
Thrombi are larger and more stable in ceacam1−/− mice both in vitro and in vivo. (A) Images of thrombus formation in response to ferric chloride induced vascular injury was visualized in arterioles of wild-type versus ceacam1−/− mice over time. The different lengths of time after ferric chloride application are indicated. Note that ceacam1−/− arterioles formed larger thrombi over time than did wild-type control arterioles (n = 15). (B-D) Quantitative analysis of arterial thrombogenesis of wild-type (●), ceacam1+/− (■), and ceacam1−/− (▴) arterioles. Compared with wild-type and ceacam1+/−, arterioles, ceacam1−/− arterioles exhibited a significantly larger thrombus area at 2 minutes (3294 ± 223.4 vs 2859 ± 294 vs 6212 ± 268.7 μm2, respectively; ***P < .001; n = 15), greater stability in thrombi formed (2.40 ± 0.21 vs 1.05 ± 0.12 vs 4.07 ± 0.37, respectively; ***P < .001; n = 15), and greater thrombus volume (49 430 ± 4602 vs 63 680 ± 5478 vs 122 400 ± 6794 μm3, respectively; ***P < .001; n = 15). The primary stability of the thrombi was scored from 1 to 10, with 1 being 0% to 10% occupancy and 10 being 91% to 100% occupancy (ie, complete vessel occlusion) monitored over time. (E,F) Platelet thrombus formation after inhibition of GPVI using monoclonal antibody JAQ1 administration to ceacam1+/+ and ceacam1−/− mice compared with control IgG treated ceacam1+/+ and ceacam1−/− or untreated ceacam1+/+ and ceacam1−/− mice. Mice received either 100 μg control IgG or JAQ1 antibody and were left for 5 days before ferric chloride injury and intravital microscopy. Compared with untreated ceacam1−/− or control IgG treated ceacam1−/− arterioles, JAQ1 treated ceacam1−/− arterioles exhibited a 3-fold smaller thrombus volume at 2 minutes (135 500 ± 2137 vs 134 000 ± 1837 vs 40 400 ± 1127 μm3, respectively; ***P < .001; n = 10 arterioles from 3 mice/group) and a 2-fold lower stability score at 2 minutes (6.150 ± 0.212 vs 5.700 ± 0.300 vs 3.350 ± 0.212, respectively; ***P < .001; n = 10 arterioles from 3 mice/group). In contrast, compared with untreated ceacam1+/+ or control IgG treated ceacam1+/+ arterioles, JAQ1 treated ceacam1+/+ arterioles exhibited a 3-fold smaller thrombus volume at 2 minutes (107 800 ± 3526 vs 102 100 ± 2607 vs 32 050 ± 599 μm3, respectively; ***P < .001; n = 10 arterioles from 3 mice/group) and a moderately lower stability score at 2 minutes (2.750 ± 0.261 vs 3.000 ± 0.236 vs 2.150 ± 0.076, respectively; ***P < .001; n = 10 arterioles from 3 mice/group). (G) DiOC6-labeled whole blood of wild-type, ceacam1+/−, and ceacam1−/− mice was perfused over 100 μg/mL type I fibrillar collagen-coated microslides at a wall shear rate of 1800 seconds−1. Thrombi (1-μm sections) were imaged at 4 minutes with a Zeiss Axiovert 135 inverted microscope (Carl Zeiss) using a 60×/0.4 NA objective at 37°C and captured with an Axiocam MRm camera (Carl Zeiss), and thrombus volume was quantified using Slidebook software (Intelligent Imaging Innovations, Denver, CO). Each data point showed on the graph represents the thrombus volume for each individual mouse performed independently.
Thrombi are larger and more stable in ceacam1−/− mice both in vitro and in vivo. (A) Images of thrombus formation in response to ferric chloride induced vascular injury was visualized in arterioles of wild-type versus ceacam1−/− mice over time. The different lengths of time after ferric chloride application are indicated. Note that ceacam1−/− arterioles formed larger thrombi over time than did wild-type control arterioles (n = 15). (B-D) Quantitative analysis of arterial thrombogenesis of wild-type (●), ceacam1+/− (■), and ceacam1−/− (▴) arterioles. Compared with wild-type and ceacam1+/−, arterioles, ceacam1−/− arterioles exhibited a significantly larger thrombus area at 2 minutes (3294 ± 223.4 vs 2859 ± 294 vs 6212 ± 268.7 μm2, respectively; ***P < .001; n = 15), greater stability in thrombi formed (2.40 ± 0.21 vs 1.05 ± 0.12 vs 4.07 ± 0.37, respectively; ***P < .001; n = 15), and greater thrombus volume (49 430 ± 4602 vs 63 680 ± 5478 vs 122 400 ± 6794 μm3, respectively; ***P < .001; n = 15). The primary stability of the thrombi was scored from 1 to 10, with 1 being 0% to 10% occupancy and 10 being 91% to 100% occupancy (ie, complete vessel occlusion) monitored over time. (E,F) Platelet thrombus formation after inhibition of GPVI using monoclonal antibody JAQ1 administration to ceacam1+/+ and ceacam1−/− mice compared with control IgG treated ceacam1+/+ and ceacam1−/− or untreated ceacam1+/+ and ceacam1−/− mice. Mice received either 100 μg control IgG or JAQ1 antibody and were left for 5 days before ferric chloride injury and intravital microscopy. Compared with untreated ceacam1−/− or control IgG treated ceacam1−/− arterioles, JAQ1 treated ceacam1−/− arterioles exhibited a 3-fold smaller thrombus volume at 2 minutes (135 500 ± 2137 vs 134 000 ± 1837 vs 40 400 ± 1127 μm3, respectively; ***P < .001; n = 10 arterioles from 3 mice/group) and a 2-fold lower stability score at 2 minutes (6.150 ± 0.212 vs 5.700 ± 0.300 vs 3.350 ± 0.212, respectively; ***P < .001; n = 10 arterioles from 3 mice/group). In contrast, compared with untreated ceacam1+/+ or control IgG treated ceacam1+/+ arterioles, JAQ1 treated ceacam1+/+ arterioles exhibited a 3-fold smaller thrombus volume at 2 minutes (107 800 ± 3526 vs 102 100 ± 2607 vs 32 050 ± 599 μm3, respectively; ***P < .001; n = 10 arterioles from 3 mice/group) and a moderately lower stability score at 2 minutes (2.750 ± 0.261 vs 3.000 ± 0.236 vs 2.150 ± 0.076, respectively; ***P < .001; n = 10 arterioles from 3 mice/group). (G) DiOC6-labeled whole blood of wild-type, ceacam1+/−, and ceacam1−/− mice was perfused over 100 μg/mL type I fibrillar collagen-coated microslides at a wall shear rate of 1800 seconds−1. Thrombi (1-μm sections) were imaged at 4 minutes with a Zeiss Axiovert 135 inverted microscope (Carl Zeiss) using a 60×/0.4 NA objective at 37°C and captured with an Axiocam MRm camera (Carl Zeiss), and thrombus volume was quantified using Slidebook software (Intelligent Imaging Innovations, Denver, CO). Each data point showed on the graph represents the thrombus volume for each individual mouse performed independently.
To further investigate the role of CEACAM1 in modulating platelet thrombus formation on immobilized collagen, in vitro flow studies were performed with blood obtained from wild-type, ceacam1+/−, and ceacam1−/− mice. DiOC6-labeled whole blood was perfused through type I collagen-coated microcapillary tubes at a wall shear rate of 1800 seconds−1, and thrombi were imaged by confocal microscopy after 4 minutes of blood perfusion. ceacam1−/− platelets (136 200 ± 18 640 μm3) displayed greater thrombus volume than wild-type (83 870 ± 8065 μm3) and ceacam1+/− platelets (86 640 ± 15 330 μm3; P < .05; n = 9; Figure 6F). Collectively, these data demonstrate that CEACAM1 is required for modulation of platelet-collagen interactions in platelet thrombus growth in vitro and in vivo.
To further determine the consequences of CEACAM1 deficiency in vivo, we tested wild-type, ceacam1+/−, and ceacam1−/− mice in a model of pulmonary thromboembolism induced by infusion of type I fibrillar collagen or tissue thromboplastin. Typically, ceacam1−/− mice displayed greater susceptibility to type I collagen induced pulmonary thromboembolism than did wild-type and ceacam1+/− mice with a more than 70% reduction in circulating platelet counts within 3 minutes of challenge (Figure 7A,B). After challenge with type I fibrillar collagen, ceacam1−/− mice reached maximal clinical scoring of restrained breathing earlier than wild-type and ceacam1+/− mice (252.1 ± 15.4 vs 343.5 ± 23.7 vs 327.0 ± 28.3 seconds, respectively; P < .005; n = 20). In this system, wild-type, ceacam1+/−, and ceacam1−/− mice had comparable reductions in circulating platelet numbers over a 3-minute period after challenge with type I fibrillar collagen (73.49 ± 3.15% vs 77.24 ± 2.25% vs 74.21 ± 3.50%, respectively; P > .05; n = 20). It should be noted that early time points could not be tested for detection of kinetic differences in platelet consumption due to animal welfare considerations. Consistent with these observations, histologic sections of lung tissue and analysis of occlusive thrombi in the lungs at the end of the experiment revealed similar numbers of occluded vessels among wild-type, ceacam1+/−, and ceacam1−/− mice (data not shown). In contrast, wild-type and ceacam1−/− mice were equally susceptible and had comparable reductions in circulating platelets over time, after tissue thromboplastin-induced pulmonary thromboembolism (Figure 7C,D; P > .05; n = 10). Collectively, these data highlight that CEACAM1 regulates platelet-collagen interactions in controlling susceptibility to type I collagen–induced pulmonary thromboembolism in vivo.
ceacam1−/− mice are more susceptible to type I collagen–induced pulmonary thromboembolism. (A) The percent reduction in platelet count over 3 minutes for wild-type (●), ceacam1+/− (■), and ceacam1−/− (▴) mice after intravenous injection of 350 μg/kg type I fibrillar collagen was determined. Note that the mice of each genotype showed a similar reduction in platelet count over time (77.24 ± 2.25% vs 73.49 ± 3.16% vs 74.21 ± 3.51%, respectively; P > .05; n = 20 mice/group). (B) The time until restrained breathing, quantified by a breathing interval of 10 seconds, for wild-type (●), ceacam1+/− (■), and ceacam1−/− (▴) mice after intravenous injection of 350 μg/kg of type I fibrillar collagen. Compared with the wild-type and ceacam1+/− mice, the ceacam1−/− mice showed greater susceptibility to type I collagen-induced pulmonary thromboembolism (343.5 ± 23.7 vs 327.0 ± 28.3 vs 252.1 ± 15.4 seconds, respectively; **P < .001; n = 20 mice/group). (C) The percent reduction in platelet count over 3 minutes for wild-type (●) and ceacam1−/− (▴) mice after intravenous injection of 0.200 μL/g tissue thromboplastin Thromborel S was determined. Note that wild-type and ceacam1−/− mice showed a similar reduction in platelet count over time (94.30 ± 2.16% vs 93.22 ± 1.76%, respectively; P > .05; n = 10 mice/group). (D) The time until restrained breathing, quantified by a breathing interval of 10 seconds, for wild-type (●) and ceacam1−/− (▴) mice after intravenous injection of 0.200 μL/g tissue thromboplastin Thromborel S was determined. Note that wild-type control mice and ceacam1−/− mice showed similar susceptibility to tissue thromboplastin induced pulmonary thromboembolism (223.8 ± 8.0 vs 214.2 ± 4.1 seconds; P > .05; n = 10 mice/group).
ceacam1−/− mice are more susceptible to type I collagen–induced pulmonary thromboembolism. (A) The percent reduction in platelet count over 3 minutes for wild-type (●), ceacam1+/− (■), and ceacam1−/− (▴) mice after intravenous injection of 350 μg/kg type I fibrillar collagen was determined. Note that the mice of each genotype showed a similar reduction in platelet count over time (77.24 ± 2.25% vs 73.49 ± 3.16% vs 74.21 ± 3.51%, respectively; P > .05; n = 20 mice/group). (B) The time until restrained breathing, quantified by a breathing interval of 10 seconds, for wild-type (●), ceacam1+/− (■), and ceacam1−/− (▴) mice after intravenous injection of 350 μg/kg of type I fibrillar collagen. Compared with the wild-type and ceacam1+/− mice, the ceacam1−/− mice showed greater susceptibility to type I collagen-induced pulmonary thromboembolism (343.5 ± 23.7 vs 327.0 ± 28.3 vs 252.1 ± 15.4 seconds, respectively; **P < .001; n = 20 mice/group). (C) The percent reduction in platelet count over 3 minutes for wild-type (●) and ceacam1−/− (▴) mice after intravenous injection of 0.200 μL/g tissue thromboplastin Thromborel S was determined. Note that wild-type and ceacam1−/− mice showed a similar reduction in platelet count over time (94.30 ± 2.16% vs 93.22 ± 1.76%, respectively; P > .05; n = 10 mice/group). (D) The time until restrained breathing, quantified by a breathing interval of 10 seconds, for wild-type (●) and ceacam1−/− (▴) mice after intravenous injection of 0.200 μL/g tissue thromboplastin Thromborel S was determined. Note that wild-type control mice and ceacam1−/− mice showed similar susceptibility to tissue thromboplastin induced pulmonary thromboembolism (223.8 ± 8.0 vs 214.2 ± 4.1 seconds; P > .05; n = 10 mice/group).
Discussion
Using several approaches, we have defined a novel role of CEACAM1 as a new inhibitory coreceptor in murine platelets that serves to negatively modulate platelet-collagen interactions and regulate platelet thrombus formation in vitro and in vivo. This study suggests that CEACAM1 serves an autoregulatory role when platelets come into contact with each other when exposed to type I collagen. Firstly, CEACAM1 is expressed on the surface and in intracellular pools of murine and human platelets. Secondly, CEACAM1 serves as a negative regulator of collagen-GPVI–mediated platelet responses in vitro. Thirdly, CEACAM1 serves to negatively regulate platelet thrombus formation in vitro and in vivo under conditions where type I collagen is exposed in the injured arteriole and in the presence of the collagen GPVI receptor on platelets. Finally, CEACAM1 serves as a negative regulator of thrombosis after type I collagen–induced pulmonary thromboembolism in vivo.
There is little evidence in the literature in relation to the presence and functional role of CEACAM1 in platelets. The only exception is a report by Hansson et al, which suggested that C-CAM was present in the intracellular compartment of rat platelets.17 The authors only found significant amounts of C-CAM when 125I-labeled rat platelets were solubilized with detergent and immunoprecipitated with C-CAM antibodies. They suggested that almost no 125I-labeled C-CAM could be immunoprecipitated from intact rat platelets under resting or ADP-activated conditions. This study suggested that intracellularly expressed C-CAM may play a functional role under conditions of platelet activation. This concept is supported by studies of CEACAM1 in leukocytes. Traditionally, CEACAM1 (formerly known as CD66a) was defined as a neutrophil-specific “activation antigen” in that it is detected in low density on resting cells but its surface expression is up-regulated upon stimulation with the chemotactic peptide formyl-methionyl-leucyl-phenylalanine (FMLP), CI A23187, and 12-O-tetradeconoyl-phorbol-13-acetate.34 Subcellular localization studies revealed that CEACAM1 was present in the secondary granules of neutrophils suggesting that antigens could be recruited to the cell surface with activation.35 In contrast, in mouse spleen T lymphocytes, CEACAM1 was not present on the surface of resting cells but was rapidly up-regulated on CD4+ and CD8+ T cells after activation with either Con A or anti-CD3 stimulation.36 Overall, these studies in leukocytes are somewhat different from the CEACAM1 expression profile that we have observed in murine and human platelets with moderate basal expression on the surface of resting platelets with an intracellular pool evident (Figure 1A-D). This difference was further supported by agonist stimulation using thrombin that resulted in up-regulation of cell surface expression of CEACAM1 on murine and human platelets (Figure 1E,F).
There is now a consensus that platelet thrombus formation under physiologic conditions involves platelet adhesion to the subendothelial matrix component type I collagen, which becomes exposed by tissue trauma or diseases such as atherosclerotic plaque formation. A “2-site, 2-step” model for platelet-collagen interactions has been proposed that requires the initial engagement of collagen to be mediated by the high-affinity receptor integrin α2β1, followed by activation generated through the low-affinity collagen receptor GPVI.37,38 In this model, integrin α2β1 is now thought to have a supportive, rather than a primary role as the adhesive collagen receptor, while GPVI is a major platelet-collagen activating receptor that interacts with collagen fibrils via a triplicate GPO sequence (glycine, proline, hydroxyproline) and integrin α2β1 interacts with collagen via GFOFER (glycine, phenylalanine, hydroxyproline, glutamic acid and arginine).39-41 For in vitro studies, we used CRP as the GPVI-selective agonist because this peptide contains 10 GPO repeat sequences and serves as a potent activator of GPVI-mediated signaling and does not recognize integrin α2β1.42 In this way, we can differentiate GPVI-mediated platelet responses from integrin α2β1–mediated platelet responses. Signaling through GPVI/FcRγ-chain ITAM-dependent pathway is thought to be counterbalanced by proteins bearing ITIM motifs such as those contained in members of the Ig-ITIM superfamily. As mouse platelets lack the low-affinity ITAM containing FcγRIIa, but contain the GPVI/FcRγ-chain ITAM-dependent pathway, we wanted to test the hypothesis that CEACAM1 may serve as an inhibitory coreceptor in platelets to negatively regulate collagen-GPVI–mediated platelet responses. Consistent with this hypothesis, ceacam1−/− platelets displayed hyper-tyrosine phosphorylated proteins including PLCγ2 after GPVI stimulation compared with wild-type platelets, indicating modulation of ITAM-associated signaling events in murine platelets (Figure 3).
To test this hypothesis, platelet aggregation studies were conducted to examine the slope and amplitude of responses of wild-type versus CEACAM1-deficient platelets using a range of agonists and dose-dependent ranges, including collagen and CRP. In addition, to test specificity of platelet responses, different agonists were selected to stimulate different receptor signaling pathways including G-protein–coupled receptors, using PAR-4 and ADP; calcium ion channels, using CI; and collagen-coupled receptors, using acid soluble collagen and CRP. ceacam1−/− platelets displayed normal platelet aggregation responses over a dose-dependent range of PAR-4, ADP, and CI. In contrast, ceacam1−/− platelets displayed enhanced platelet aggregation responses over a dose-dependent range of collagen and CRP (Figure 4). This hyperresponsive feature was most evident at subthreshold concentrations of collagen and CRP, indicating that CEACAM1 serves to negatively regulate collagen-GPVI–mediated ITAM platelet responses. Importantly, the fact that the platelet aggregation responses to PAR-4, ADP, and CI were normal reveals that ceacam1−/− platelets do not have a global platelet defect and that the defect is restricted to GPVI-mediated platelet-collagen interactions.
This hyperresponsive feature of ceacam1−/− platelets with subthreshold doses of collagen and CRP is consistent with the phenotype observed by us and others with pecam-1−/− platelets.1,2 The subtle phenotype was most evident at concentrations of collagen at 1 μg/mL but was not so evident at higher concentrations of collagen 10 to 30 μg/mL. This was also the case using CRP as the GPVI-selective agonist. At 0.5 μg/mL CRP, wild-type platelets were not responsive while pecam-1−/− platelets yielded an approximately 70% CRP-mediated platelet aggregation response. At 5 to 10 μg/mL CRP, wild-type and pecam-1−/− platelets were less hyperresponsive.1,2
Platelets from ceacam1−/− mice exhibited increased adhesion on type I fibrillar collagen but not fibrinogen. This hyper-responsive adhesion phenotype of ceacam1−/− platelets on type I fibrillar collagen was evident at all time points. In addition, ceacam1−/− platelets displayed a lowered threshold for alpha and dense granule release relative to wild-type and ceacam1+/− platelets after stimulation with the GPVI-selective ligands CRP (particularly at 0.1-0.5 μg/mL) and type I collagen (at 1 μg/mL), but not the G-protein–coupled receptor agonist thrombin. Overall, these features suggest that in the absence of an inhibitory coreceptor, platelets are more responsive to stimulation by collagen agonists particularly involving collagen-GPVI receptor–mediated signaling. This trend is consistent with CEACAM1 serving as a negative regulator of platelet-collagen interactions.
To investigate the role of CEACAM1 in thrombus formation in vitro and in vivo, we used intravital microscopy to study thrombus formation in real time, ferric chloride–induced vascular injury of mesenteric arterioles, and in vitro flow studies on immobilized type I collagen. This ferric chloride–induced vascular injury model results in exposure of subendothelial collagens including type I collagen. This enables collagen-GPVI platelet interactions to occur with exposed type I collagen. Under these conditions, we demonstrated that thrombi formed in mesenteric arterioles after ferric chloride injury in CEACAM1 knockout mice were significantly larger in area and volume, reflecting increased thrombus growth than those formed in wild-type and ceacam1+/− mice (Figure 6B,C). These differences in thrombus size were consistent over time. In addition, thrombi formed in CEACAM1 knockout mice appeared to have greater stability than those in wild-type and ceacam1+/− mice (Figure 6D). In addition, this in vivo thrombus growth phenotype observed in ceacam1−/− mice was dependent on the presence of the collagen GPVI receptor on platelets (Figure 6E,F). These data are consistent with CEACAM1 playing a role in negative regulation of thrombus growth in vivo in mesenteric arterioles. In addition, our studies have shown an important role for CEACAM1 in regulating thrombus formation on immobilized collagen under in vitro flow conditions (Figure 6F). Because CEACAM1 is expressed on platelets, leukocytes, and endothelium, further studies will be required to dissect out the relative importance of platelet CEACAM1 versus endothelial CEACAM1 in regulating thrombus growth in vivo. Importantly, CEACAM1 appears to be a second example of an Ig-ITIM superfamily member that plays an important functional role in negatively regulating stationary adhesion of platelet-collagen interactions and thrombus growth in vivo, but not tethering and rolling platelets on the thrombogenic collagen surface.
Previous studies with PECAM-1–deficient mice in the ferric chloride induced vascular injury model of carotid arteries revealed a subtle defect in the time to 75% vessel occlusion.43 The mean occlusion rate in pecam-1−/− mice was shorter than in wild-type control mice. However, the magnitude of difference was only very modest. A more noticeable difference was observed in the kinetics of platelet thrombus formation using the laser-induced injury model that requires tissue factor generated platelet/fibrin thrombus and not type I collagen exposure.43 Therefore, it appears that PECAM-1 and CEACAM1 both play a role in negatively regulating platelet-collagen interactions in vivo.
To examine the consequences of CEACAM1 deficiency in vivo in platelet-collagen interactions, we used a second model of type I collagen–induced pulmonary thromboembolism. In this model, type I fibrillar collagen is infused intravenously and platelet thrombi are deposited in the lung architecture leading to development of pulmonary thromboemboli and restrained breathing that in wild-type mice ultimately leads to morbidity. Using this model, monitoring of the restrained breathing rate of the mice revealed that, compared with wild-type and ceacam1+/− mice, ceacam1−/− mice have enhanced susceptibility to type I collagen–induced pulmonary thromboembolism but not tissue thromboplastin–induced pulmonary thromboembolism (Figure 7B,D). ceacam1−/− mice showed a reduction in time for restrained breathing after challenge with type I fibrillar collagen compared with wild-type and ceacam1+/− mice (Figure 7B) despite having comparable consumption of platelets (Figure 7A). These data further implicate CEACAM1 as a physiologic regulator of platelet-collagen interactions in vivo.
In conclusion, this study has defined a new inhibitory coreceptor, CEACAM1, present in murine and human platelets and expressed on the surface and in intracellular compartments. CEACAM1 serves as a negative physiologic regulator of platelet-collagen interactions as shown by its hyperresponsive features in collagen- and CRP-mediated platelet aggregation, increased adhesion on type I collagen, and enhanced alpha and dense granule release using GPVI-selective ligands. In vitro and in vivo, CEACAM1 serves to negatively regulate the arteriolar thrombus growth and thrombosis associated with type I collagen–induced pulmonary thromboembolism. These negative regulatory properties of CEACAM1 may prove to be of physiologic benefit in diseased vessels such as those with ruptured atherosclerotic plaques where type I collagen is exposed.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by grants from the National Heart Foundation of Australia and the National Health and Medical Research Council (NHMRC) of Australia (D.E.J.), and the Canadian Institutes of Health Research (N.B.). D.E.J. is a recipient of an NHMRC Senior Research Fellowship.
Authorship
Contribution: C.W., Y.L., J.Y., R.C., J.W., A.R., and L.O. performed research and analyzed data; N.B. supplied the CEACAM1-deficient mice; B.N. supplied the JAQ1 antibody and advice for the GPVI depletion experiment; H.N. directed the in vitro flow experiments; and D.E.J. directed the research and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Assoc Prof Denise E. Jackson, FAIMS, PhD, NHMRC Senior Research Fellow, Kronheimer Building, Burnet Institute incorporating the Austin Research Institute, Studley Road, Heidelberg, Victoria, 3084, Australia; e-mail: djackson@burnet.edu.au.
References
Author notes
*C.W. and Y.L. contributed equally to this manuscript.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal