Abstract
Understanding the pathways that regulate the human T-cell acute lymphoblastic leukemia (T-ALL) initiating cells (T-LiC) activity has been hampered by the lack of biologic assays in which this human disease can be studied. Here we show that coculture of primary human T-ALL with a mouse stromal cell line expressing the NOTCH ligand delta-like-1 (DL1) reproducibly allowed maintenance of T-LiC and long-term growth of blast cells. Human T-ALL mutated or not on the NOTCH receptor required sustained activation of the NOTCH pathway via receptor/ligand interaction for growth and T-LiC activity. On the reverse, inhibition of the NOTCH pathway during primary cultures abolished in vitro cell growth and in vivo T-LiC activity. Altogether, these results demonstrate the major role of the NOTCH pathway activation in human T-ALL development and in the maintenance of leukemia-initiating cells.
Introduction
T-cell acute lymphoblastic leukemia (T-ALL) is a hematologic disorder resulting from the accumulation of cells blocked at specific stages of T-cell differentiation and is a heterogeneous pathology.1,2 Actual treatments of T-ALL leave uncured 10% to 20% of patients3 with a better efficiency obtained using treatments designed for children.4 However, these treatments have numerous side effects that led to recent attempts to reduce chemotherapy dosage in certain patients.5 The development of resistance to chemotherapy requires the finding of complementary therapeutic approaches. The recent description of rare cells, capable of initiating leukemia, called leukemia-initiating cells (LiC)6-8 that could be insensitive to actual treatments, points at the importance of targeting these cells to ensure durable remission.9 Thus, an important challenge is to elucidate the molecular mechanisms that drive LiC activity
Transgenic mouse models of T-ALL have greatly contributed to our knowledge of T-ALL development. In humans, a great amount of information came from recent microarray analysis of T-ALL samples and from surface expression of markers implicated in normal T-cell development that allowed their appropriate classification.1,10 However, the relevance of such molecular and cellular approaches is still missing for primary patient samples. Indeed, from a functional viewpoint, human T-ALL biology has been performed so far with long-term maintained patient-derived cell lines, mainly because experimental conditions that allow the survival and proliferation of blast cells from patient samples are very limited. Animal models of human T-ALL have been developed by transplanting patient cells into immune deficient mice, but they required particular protocols, such as preconditioning mice with cord blood cells 1 week before transplanting T-ALL cells.11 A simpler and straightforward protocol has recently been described. This study showed high levels of human T-ALL engraftment generated from a rare CD34+/CD4−CD7− cell population enriched in its ability to initiate leukemia in serial transplanted nonobese diabetic/severe combined immunodeficiency (NOD-Scid) mice, a characteristic of human T-ALL initiating cell (T-LiC) activity.8
To gain access into T-ALL biology, we developed an in vivo assay that supports human T-ALL growth from primary patients. Our data suggest that T-ALL development in NOD-Scid mice is initiated by a minority of cells, and further analysis suggests that the T-ALL development capacity does not strictly correlate with CD34 surface expression. Importantly, we report that NOTCH pathway regulates human T-LiC activity. NOTCH receptor pathway is implicated in normal T-cell development.12 NOTCH receptor activation is also one of the molecular pathways involved in T-ALL.2,10,13,14 Using a newly developed long-term coculture assay, we show that sustained levels of NOTCH activation in primary T-ALL blasts lead to in vitro cell proliferation. Serial transplantation of cultured blasts into NOD-Scid mice indicated that the T-LiC activity is maintained selectively on NOTCH activation. Importantly, the T-LiC activity is abolished after inhibition of NOTCH using γ-secretase inhibitors for all T-ALL samples. This study demonstrates, for the first time, the importance of the NOTCH pathway in the self-renewal of human LiC from primary patient samples. The functional in vitro and in vivo assays we describe here provide new and major approaches to study the molecular pathways that regulate T-ALL development and T-LiC properties starting with patient samples.
Methods
The use of mice and human primary samples in this study was reviewed and approved by scientific experts at the Ligue National contre le Cancer and Inserm.
Animals
NOD/Ltsz-scid/scid (NOD-Scid) mice were housed in the pathogen-free facility of the Institut André Lwoff (Villejuif, France). NOD-Scid mice were irradiated at 3.25 Gy before transplantation. Mice were anesthetized with ketamin and xylazin before intrabone injection (IBI) and with isoflurane before intravenous injection (IVI). Mice were killed at indicated time points or when showing signs of sickness (ruffled hair, hunched back). Hematopoietic organs were analyzed separately for human cell engraftment, including the injected bone when IBI was used. When secondary and tertiary transplantations were performed, recipient mice received either the same number of human blasts as primary mice or the total cells contained in the injected bone marrow (IBM) of primary mice when no human cells could be detected in primary mice, following the same injection route as primary recipients. For cells injected after culture, all transplantations were performed directly into the bone of recipient mice to avoid any cell loss, especially for conditions where no proliferation was observed. All experimental procedures were done in compliance with French Ministry of Agriculture regulations for animal experimentation.
T-ALL samples
Primary samples were collected after informed consent was obtained from all patients' parents in accordance with the Declaration of Helsinki and national ethics rules. Blood cells were Ficolled, immunophenotyped, and either transplanted and cultured directly or frozen in fetal calf serum, 10% dimethyl sulfoxide (DMSO) containing 25 ng/mL interleukin-7 (IL-7) for further use.
MS5 cells
Mouse stromal MS5 cells were originally obtained from Dr K. Mori (Nagata University, Japan)15 and maintained as described previously.16 MS5 cells were transduced with lentiviral TRIP-ΔU3-EF1α-huDelta-like1(DL1)–IRES-GFP or TRIP-ΔU3-EF1α-GFP vectors,17 such that they were 100% GFP+ by fluorescence-activated cell sorter (FACS). The human dl1 cDNA was originally kindly provided by Dr E. Parreira (Gulbenkian Instituto, Lisboa, Portugal).
Culture conditions
T-ALL cells (100-250 × 103/well) were cultured in reconstituted alpha-minimum essential medium supplemented with 10% fetal calf serum (06450; StemCell Technologies, Vancouver, BC) and 10% human AB serum (J. Boy, Reims, France), in the presence of recombinant human stem cell factor (50 ng/mL; Amgen, Thousand Oaks, CA), Flt3-L (20 ng/mL; Diaclone, Besançon, France), insulin (20 nM; Sigma-Aldrich, St Louis, MO), and IL-7 (10 ng/mL; R&D Systems, Minneapolis, MN). In specific experiments, GSI (N-[N-(3,5-difluorophenacetyl)L-alanyl]-S-phenylglycine t-butyl ester (DAPT); Calbiochem, San Diego, CA; 10 μM) was added in the culture medium either during the overall culture period or during the last 7 days. GSI was diluted into DMSO and control cultures included DMSO in medium. Medium was half changed twice a week, and every stromal layer was renewed once a week. At passage time point, cells were counted and phenotyped when enough cells were available for FACS analysis.
Flow cytometry
Immunophenotyping of T-ALL blasts was performed on a FACSCalibur flow cytometer (BD Biosciences, San Jose, CA) using phycoerythrin (PE)–, PE-Cyanin 5 (PC5), allophycocyanin-, and fluorescein isothiocyanate–conjugated mouse anti–human specific monoclonal antibodies CD45 (J.33), CD34 (581), CD15 (80H5), CD19 (J4.119), CD4 (13B8.2), CD3 (UCHT1), CD8 (B9.11), and CD7 (8H8.1). All antibodies were from Beckman Coulter (Fullerton, CA), including isotype controls. Briefly, cells positive for the human CD45 marker were gated according to isotype controls, and expression of lineage markers was done on gated human CD45+ cells from engrafted mice or from cultures as previously reported.18
Western blotting
DL1 expression was analyzed using 10 μg of proteins obtained from MS5-DL1 or MS5-GFP cells in Western blot analysis as previously described.19 Antibodies were against human DL1 (C20; Santa Cruz Biotechnology, Santa Cruz, CA) and β-actin (AC-15; Sigma-Aldrich).
SCL/TAL1 expression was measured in 5 × 105 T-ALL blasts before or after transplantation into NOD-Scid mice, using mouse monoclonal BTL73 antibody (kindly provided by Dr Danièle Mathieu, IGM, Montpellier, France). eEF2k protein was used as internal control (3692; Cell Signaling Technology, Danvers, MA).
Gene expression analysis
Quantitative polymerase chain reaction (Q-PCR) analysis was performed as previously described.18 Sequences of primers are: β2-microglobulin (b2m) forward (F): CACAGCCCAAGATAGTTAAGT; reverse (R): CCAGCCCTCCTAGAGC; hes1 F: CAACACGACACCGGATAAACC; R: CCAGAATGTCCGCCTTC; pTα F: GTGTCCAGCCCTACCCA; R: ATCCACCAGCAGCATGATTG; deltex F: TTCTGACTTCAGGAGCGAAAG; R: TGCCCACTCCCAACGA. Four measurements were performed for each sample, and data were normalized over b2m values.
γTCR gene rearrangements
Rearrangements of γ-TCR were assayed by PCR analysis using a kit named TCR-γ gene clonality assay according to the manufacturer's guidelines (InVivoScribe, San Diego, CA). PCR products were sequenced to confirm the clonal origin of the cells. Water replaced DNA in the negative control.
Analysis of notch1 mutations in the studied T-ALL
Sequencing was performed according to Weng et al.14
Statistical analysis
Statistical analysis was done using a Mann-Whitney nonparametric test (mouse data) and using the Student test (Q-PCR analysis).
Results
Human T-ALL development in NOD-Scid mice is generated from a minority of cells
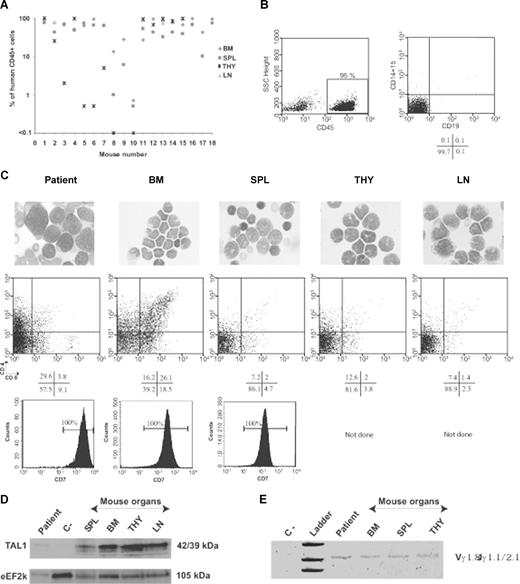
Previous models of myeloid, B-lymphoid, or T-lymphoid leukemia have shown that the NOD-Scid mouse transplantation assay is the only way to characterize the leukemia-initiating cell compartment in vivo.20 Thus, total mononuclear cells from 10 patients with newly diagnosed T-ALL were transplanted into NOD-Scid mice. All samples engrafted NOD-Scid recipients, resulting in severe morbidity 2 to 3 months after injection of the leukemic cells and coincident with high levels of human CD45+ cells in hematopoietic organs (Table 1; Figure 1A). Only human cells with blastic T-cell phenotype could be detected (Figure 1B,C, and Figure S1, available on the Blood website; see the Supplemental Materials link at the top of the online article). These cells originated from leukemic samples because we could detect the TAL1 protein in engrafted blasts from a T-ALL harboring an interstitial sil-tal deletion (Figure 1D). Moreover, assessment of γ-TCR gene rearrangements showed a clonal behavior of the engrafted human T-ALL blasts as in the original sample (Figure 1E). CD4, CD8, and CD34 expression of the engrafted cells differed from that of patient samples and sometimes from one organ to the other (Figures 1C, S1), indicating that the expression of these cell surface markers might be sensitive to the microenvironment.
T-ALL engraft NOD-Scid mice and grow in contact with MS5 stromal cells
| T-ALL samples . | NOD-Scid recipients . | Growth in culture . | |||||
|---|---|---|---|---|---|---|---|
| MS5-DL1 . | MS5-GFP . | ||||||
| Primary . | Secondary . | Tertiary . | −GSI . | +GSI . | −GSI . | +GSI . | |
| M18μ | 18/18* | 7/10 | nd | +++ | − | − | − |
| 65%-99%† | 40%-80% | ||||||
| 10-12 wk‡ | 10-12 wk | ||||||
| M22nμ | 8/10 | 2/2 | 2/2 | +++ | − | + | − |
| 50%-99% | 4%; 5% | 70%; 90% | |||||
| 9-12 wk | 12 wk | 12 wk | |||||
| M29μ | 3/3 | nd | nd | +++ | nd | + | nd |
| 14%-30% | |||||||
| 20 wk | |||||||
| M30μ | 20/20 | 2/2 | 7/7 | +++ | − | +++ | − |
| 85%-99% | 55%; 70% | 80%-99% | |||||
| 6-10 wk | 6-8 wk | 8 wk | |||||
| M34μ | 7/7 | nd | nd | +++ | − | + | − |
| 68%-99% | |||||||
| 10-12 wk | |||||||
| M40μ | 1/1 | 2/2 | nd | +++ | − | + | − |
| 95% | 0.5%; 85% | ||||||
| 10 wk | 11 wk | ||||||
| M52nd | 1/3 | 2/2 | nd | +++ | − | + | nd |
| 87% | 87%; 84% | ||||||
| 8 wk | 8 wk | ||||||
| M53μ | 2/3 | nd | nd | +++ | − | − | nd |
| 97% | |||||||
| 10 wk | |||||||
| M61nμ | 3/3 | 2/2 | nd | +++ | − | + | − |
| 95%-97% | 7%; 97% | ||||||
| 10 wk | 8-10 wk | ||||||
| M66nμ | 2/2 | nd | nd | +++ | − | + | − |
| 81%; 97% | |||||||
| 8 wk | |||||||
| T-ALL samples . | NOD-Scid recipients . | Growth in culture . | |||||
|---|---|---|---|---|---|---|---|
| MS5-DL1 . | MS5-GFP . | ||||||
| Primary . | Secondary . | Tertiary . | −GSI . | +GSI . | −GSI . | +GSI . | |
| M18μ | 18/18* | 7/10 | nd | +++ | − | − | − |
| 65%-99%† | 40%-80% | ||||||
| 10-12 wk‡ | 10-12 wk | ||||||
| M22nμ | 8/10 | 2/2 | 2/2 | +++ | − | + | − |
| 50%-99% | 4%; 5% | 70%; 90% | |||||
| 9-12 wk | 12 wk | 12 wk | |||||
| M29μ | 3/3 | nd | nd | +++ | nd | + | nd |
| 14%-30% | |||||||
| 20 wk | |||||||
| M30μ | 20/20 | 2/2 | 7/7 | +++ | − | +++ | − |
| 85%-99% | 55%; 70% | 80%-99% | |||||
| 6-10 wk | 6-8 wk | 8 wk | |||||
| M34μ | 7/7 | nd | nd | +++ | − | + | − |
| 68%-99% | |||||||
| 10-12 wk | |||||||
| M40μ | 1/1 | 2/2 | nd | +++ | − | + | − |
| 95% | 0.5%; 85% | ||||||
| 10 wk | 11 wk | ||||||
| M52nd | 1/3 | 2/2 | nd | +++ | − | + | nd |
| 87% | 87%; 84% | ||||||
| 8 wk | 8 wk | ||||||
| M53μ | 2/3 | nd | nd | +++ | − | − | nd |
| 97% | |||||||
| 10 wk | |||||||
| M61nμ | 3/3 | 2/2 | nd | +++ | − | + | − |
| 95%-97% | 7%; 97% | ||||||
| 10 wk | 8-10 wk | ||||||
| M66nμ | 2/2 | nd | nd | +++ | − | + | − |
| 81%; 97% | |||||||
| 8 wk | |||||||
Fresh or thawed newly diagnosed T-ALL blasts were transplanted into NOD-Scid mice. Three routes of transplantation were used: IVI (107 cells), intraperitoneum (107 cells), or IBI (1.5-2.5 × 105 cells) for M18, M22, and M30, whereas the other samples were injected either IVI (M61, M66) or IBI (M29, M34, M40, M52, M53). Analysis of engraftment was performed when mice showed signs of sickness. Secondary and tertiary transplantations were done using total cells from the spleen, bone marrow (BM), or lymph nodes of engrafted mice using the same route of transplantation as primary mice. A mouse was considered positive when ≥ 0.1% CD45+ human cells were detected. In the most engrafted and sick mice, we observed a severe BM aplasia and few human cells, whereas spleen was massively infiltrated with blasts. Shown are engraftment levels in the mouse BM. T-ALL samples were cocultured with MS5-GFP or MS5-DL1 cells, with or without γ -secretase inhibitor (GSI). Proliferation and phenotype analysis were done weekly.
− indicates no cell recovered from the culture; +, no amplification of cell number; +++, amplification (≥ 4 times) of cell number; nd, not done; μ, T-ALL samples with a notch1 mutation detected; and nd, no mutation detected.
Positive/total mouse.
Percentage of CD45+ cells.
Time of analysis.
T-ALL development in NOD-Scid mice. Human T-ALL blasts were identified using CD45-specific antibodies and further characterized with myeloid and lymphoid subset-specific antibodies. Shown are representative results from a group of mice transplanted with the M18 T-ALL sample that carries a sil-tal interstitial deletion (Table 1, additional data). (A) Engraftment levels in the mouse hematopoietic organs. BM indicates bone marrow; SPL, spleen; THY, thymus; LN, lymph nodes. (B) Human B lymphoid and myeloid cells are absent from the BM of T-ALL–transplanted mice. Human CD45+ cells were gated according to isotype controls. Expression of CD19 B-cell marker or of CD15 myeloid cell marker was analyzed in the gated human CD45+ cell population. (C) May-Grünwald-Giemsa staining of cytospins (top panels). Slides were viewed with a laborlux 5 Leitz microscope (Leitz France, Rueil Malmaison, France) using Leitz microscope lens (oil immersion, ×25). Pictures were acquired using a Sony-CCD iris color video camera (Sony France, Paris, France) and images were processed using Adobe Photoshop software. CD4/CD8 (middle panels), and CD7 (bottom panels) expression analysis of gated human CD45+ cells engrafting different hematopoietic sites. Positivity bars were set according to isotype controls. Indicated are percentage of positive cells. ND indicates not done. (D) SCL/TAL1 protein levels in human cells engrafting different hematopoietic organs were determined using Western blot. eEF2k protein levels were used for loading normalization. Negative control (ie, C−) are proteins from cells (mouse + human) present in the spleen of a mouse transplanted with M22 T-ALL that does not express SCL/TAL1. (E) PCR analysis of γ-TCR gene rearrangements in the BM, THY, and SPL of a representative mouse transplanted with M18 T-ALL. Cells from patient were used in parallel. Negative (C−) control contained water.
T-ALL development in NOD-Scid mice. Human T-ALL blasts were identified using CD45-specific antibodies and further characterized with myeloid and lymphoid subset-specific antibodies. Shown are representative results from a group of mice transplanted with the M18 T-ALL sample that carries a sil-tal interstitial deletion (Table 1, additional data). (A) Engraftment levels in the mouse hematopoietic organs. BM indicates bone marrow; SPL, spleen; THY, thymus; LN, lymph nodes. (B) Human B lymphoid and myeloid cells are absent from the BM of T-ALL–transplanted mice. Human CD45+ cells were gated according to isotype controls. Expression of CD19 B-cell marker or of CD15 myeloid cell marker was analyzed in the gated human CD45+ cell population. (C) May-Grünwald-Giemsa staining of cytospins (top panels). Slides were viewed with a laborlux 5 Leitz microscope (Leitz France, Rueil Malmaison, France) using Leitz microscope lens (oil immersion, ×25). Pictures were acquired using a Sony-CCD iris color video camera (Sony France, Paris, France) and images were processed using Adobe Photoshop software. CD4/CD8 (middle panels), and CD7 (bottom panels) expression analysis of gated human CD45+ cells engrafting different hematopoietic sites. Positivity bars were set according to isotype controls. Indicated are percentage of positive cells. ND indicates not done. (D) SCL/TAL1 protein levels in human cells engrafting different hematopoietic organs were determined using Western blot. eEF2k protein levels were used for loading normalization. Negative control (ie, C−) are proteins from cells (mouse + human) present in the spleen of a mouse transplanted with M22 T-ALL that does not express SCL/TAL1. (E) PCR analysis of γ-TCR gene rearrangements in the BM, THY, and SPL of a representative mouse transplanted with M18 T-ALL. Cells from patient were used in parallel. Negative (C−) control contained water.
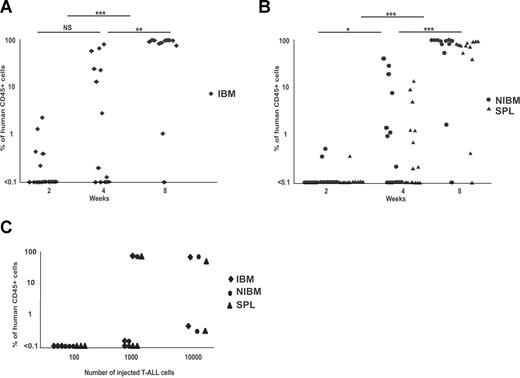
High levels of engraftment were obtained in 85% and 100% of, respectively, secondary and tertiary mice after transplantation of cells from primary mice (Table 1; Figure S1), indicating a self-renewing activity of human T-ALL samples. To understand the development of leukemia in NOD-Scid mice, kinetic analyses of engraftment levels were performed using the M30 T-ALL sample transplanted via an IBI. Two weeks after secondary or tertiary transplantations, human cells could be detected only in 29% (5 of 17) of the transplanted mice that had low levels (0.7% ± 0.7%; range, 0.2%-2.1%) of human CD45+ cells, mostly restricted to the IBM (Figure 2A). On the contrary, when analyses were performed later after IBI, most of the mice (73% at 4 weeks; 91% at 8 weeks) were engrafted and the levels of human CD45+/T-ALL blast cells were higher (up to 100% for 9 of 10 engrafted mice at 8 weeks) in their IBM and also in other hematopoietic sites, such as the spleen and the other noninjected bones (Figure 2B). In parallel, we performed a limiting dilution experiment using M30 T-ALL. Analysis of engraftment levels 2 months after injection revealed that 2 of 2 mice injected with 10 000 cells were positive for human cells (> 0.1% CD45+) in the 3 (IBM, non-IBM (NIBM), and spleen) analyzed hematopoietic sites (Figure 2C). When lower cell numbers were injected (1000 cells/mouse and 100 cells/mouse), the proportion of positive mice decreased (respectively, 2 of 3 and 0 of 3 positive mice). These results were supported by the fact that, of 10 mice injected with 10 000 M18 T-ALL cells, only 1 contained human cells after 2 months (data not shown). Altogether, these results suggest that human T-ALL in NOD-Scid mice is initiated by a fraction of the injected leukemic cells, which frequency varies in different patient samples and progressively spread the mouse hematopoietic organs after serial transplantations, in accordance with the recent description of T-LiC.8
Kinetic analysis of T-ALL engraftment after serial transplants. Blast cells from the spleen or bone marrow of secondary and tertiary engrafted mice were transplanted (250 000 cells/mouse) in the femur of recipient mice. CD45+ engraftment levels were measured over time in (A) the IBM and in (B) the spleen and noninjected bones (NIBM, right panel) of NOD-Scid recipients. *P < .05; **P < .01; ***P < .001; NS, not significant. (C) Limiting dilution experiment performed with M30 T-ALL. Cells were transplanted into mice and engraftment levels were tested in IBM, NIBM, and spleen 2 months after transplantation. Positivity was established when more than or equal to 0.1% human CD45+ cells were detected.
Kinetic analysis of T-ALL engraftment after serial transplants. Blast cells from the spleen or bone marrow of secondary and tertiary engrafted mice were transplanted (250 000 cells/mouse) in the femur of recipient mice. CD45+ engraftment levels were measured over time in (A) the IBM and in (B) the spleen and noninjected bones (NIBM, right panel) of NOD-Scid recipients. *P < .05; **P < .01; ***P < .001; NS, not significant. (C) Limiting dilution experiment performed with M30 T-ALL. Cells were transplanted into mice and engraftment levels were tested in IBM, NIBM, and spleen 2 months after transplantation. Positivity was established when more than or equal to 0.1% human CD45+ cells were detected.
In vitro growth of human T-ALL depends on NOTCH activation
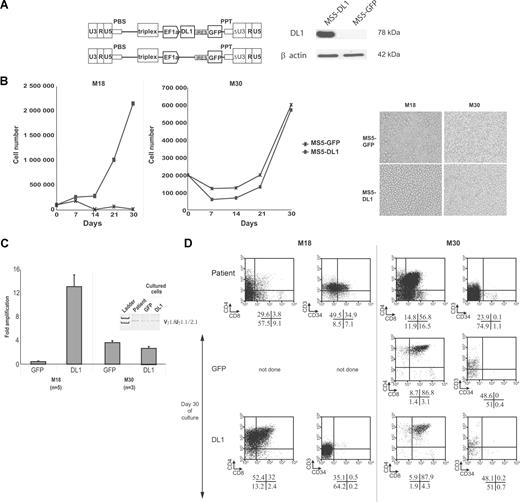
Understanding the pathways that regulate the T-LiC compartment represents a priority to elucidate the molecular mechanisms of T-ALL pathogenesis and requires the development of culture conditions, allowing the direct study of specific populations. Proliferation of primary T-ALL requires specific signals as in vitro growth or maintenance of T-ALL blasts has been very difficult to achieve.8,21,22 Because the NOTCH signaling pathway is crucial for T-cell development,12 we hypothesized that activation of NOTCH in T-ALL is also essential for in vitro growth. We genetically modified the mouse stromal cell line, MS5, for constitutive expression of high levels of human NOTCH ligand DL1 (MS5-DL1; Figure 3A), and we cocultured T-ALL samples, newly diagnosed during the course of the study, with MS5-DL1 cells or with MS5-GFP control cells. Nine of 10 T-ALL samples expanded only on MS5-DL1, with up to 12 times more cells recovered at day 30 compared with the input number of cells. Only a few cells were found after culture with MS5-GFP cells that express very weak endogenous DL1 levels (Table 1; Figure 3B,C left panels).23 One sample (M30) displayed similar blast growth on MS5-DL1 and on MS5-GFP cells (Figure 3B,C right panels). Compared with original T-ALL primary samples, cells recovered from cocultures harbored the same clonal γ-TCR rearrangement, indicating growth of leukemic T-ALL cells (Figure 3C), and expressed higher levels of CD4 and CD8 and decreased levels of CD34 expression (Figure 3D), suggesting blast cell differentiation.
In vitro T-ALL proliferation requires the activation of the NOTCH-signaling pathway. (A) Structure of the bicistronic TRIP-ΔU3-EF1α-IRES-GFP lentiviral vectors encoding or not human DL1 (left panel). Western blot analysis of DL1 expression in MS5 cells transduced with either of the 2 TRIP-ΔU3-EF1α vectors shown in the left panel. (B) Left panels: Representative growth curves observed with M18 and M30 T-ALL samples in coculture with MS5-GFP or MS5-DL1 cells. Right panels: Pictures of the leukemic cell cultures after 16 (M18) and 21 (M30) days of coculture with MS5 cells. Images were obtained using a Nikon Eclipse TE 2000-S (×200; Nikon France SAS, Champigny-Sur-Marne, France) microscope and captured using Eclipse-Net software. Images were processed using Adobe Photoshop software. (C) Amplification rate after 30 days of culture of M18 and M30. Fold amplification is referred to the initial number of plated cells. Shown are mean plus or minus SD of 6 (M18) and 3 (M30) experiments. (Inset) γ-TCR gene rearrangements of M30 at diagnosis and after 30 days of coculture with MS5 cells. (D) Phenotype of T-ALL leukemic cells before and after 30 days of culture with GFP- and DL1-expressing MS5 cells. Percentage of cells is indicated in each quadrant.
In vitro T-ALL proliferation requires the activation of the NOTCH-signaling pathway. (A) Structure of the bicistronic TRIP-ΔU3-EF1α-IRES-GFP lentiviral vectors encoding or not human DL1 (left panel). Western blot analysis of DL1 expression in MS5 cells transduced with either of the 2 TRIP-ΔU3-EF1α vectors shown in the left panel. (B) Left panels: Representative growth curves observed with M18 and M30 T-ALL samples in coculture with MS5-GFP or MS5-DL1 cells. Right panels: Pictures of the leukemic cell cultures after 16 (M18) and 21 (M30) days of coculture with MS5 cells. Images were obtained using a Nikon Eclipse TE 2000-S (×200; Nikon France SAS, Champigny-Sur-Marne, France) microscope and captured using Eclipse-Net software. Images were processed using Adobe Photoshop software. (C) Amplification rate after 30 days of culture of M18 and M30. Fold amplification is referred to the initial number of plated cells. Shown are mean plus or minus SD of 6 (M18) and 3 (M30) experiments. (Inset) γ-TCR gene rearrangements of M30 at diagnosis and after 30 days of coculture with MS5 cells. (D) Phenotype of T-ALL leukemic cells before and after 30 days of culture with GFP- and DL1-expressing MS5 cells. Percentage of cells is indicated in each quadrant.
Because activating notch1 mutations have been described in half T-ALL samples,14 we analyzed 9 of 10 T-ALL samples for such mutations. Six T-ALL samples displayed notch1 mutations, with 5 of them in sequences coding for the NOTCH1 PEST domain (exon 34/PEST domain for M18, M29, M30, M34, and M53; Table S1). Among the mutated T-ALL, only M30 cells could grow on MS5-GFP, whereas the other mutated cell samples were dependent on high DL1 expression for cell growth similar to the 3 nonmutated samples (Table 1). These results could indicate that mutations in the PEST domain are not equivalent as for DL1 dependence or that other genetic abnormalities are implicated in the behaviors of the different T-ALL. In M30, the mutation only deleted the last 37 C-terminal amino acids of the PEST domain, maintaining the FBW7 phosphodegron,24 whereas it is absent in M34 and M53. We thus also looked for mutations of the fbw7 gene in M30 and found a mutation that did not modify the amino acid sequence. These results indicated that M30 is a mutant of NOTCH1 but not of the FBW7 protein. In conclusion, NOTCH activation via ligand interaction is required for the proliferation of most T-ALL leukemic cells, and notch1 mutations cannot be used to predict NOTCH ligand-independent blast cell proliferation, explaining the weak oncogenic impact of notch1 mutations in transgenic animals.25
Decreased in vitro growth of primary T-ALL patient samples using γsecretase inhibitors
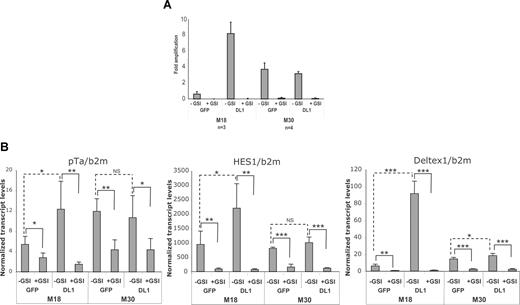
As the multienzymatic γ-secretase (GS) complex regulates the NOTCH signaling pathway,26 we studied the effect of inhibiting GS activity on the in vitro growth of T-ALL leukemic cells using DAPT, a well-known GS inhibitor (GSI). All studied T-ALL samples including M30 did not proliferate in the presence of 10 μM GSI (Table 1; Figure 4A), although lower doses of DAPT, such as 5 μM, were also efficient and could avoid potential toxicity resulting from high dosage (data not shown). To ensure that GSI targets the NOTCH pathway in the studied T-ALL samples, expression levels of NOTCH transcriptional target genes, hes1, pTα, deltex, and c-myc were quantified in T-ALL cells cultured on MS5-DL1 and MS5-GFP with or without GSI. Expression of genes, such as numb and spen, was also measured as genes not regulated by GS. Expression levels were quantified early after the initiation of culture and before GSI induces apoptosis27 (data not shown). M18 (Figure 4B) and M34 and M66 (data not shown) T-ALL leukemic cells cocultured on MS5-DL1 showed increased mRNA levels of deltex, hes1, and pTα compared with blasts cocultured on MS5-GFP, whereas M30 T-ALL had similar levels of these transcripts when cultured on either MS5-GFP or MS5-DL1 cell lines underlining differences among notch1-mutated T-ALL in terms of levels of NOTCH activation (Figure 4B). On the contrary, decreased deltex, hes1, and pTα transcript levels were found in all GSI-treated T-ALL cultures, independently of notch1 mutations (Figure 4B). These notch target gene expression levels correlated with intracellular NOTCH protein levels (data not shown). c-myc mRNA levels were similar in cells recovered from MS5-DL1 and MS5-GFP cocultures and decreased when GSI was added to the cultures, although not significantly (Figure S2). Finally, mRNA levels of numb and spen did not change in the different culture conditions, indicating the specific targeting of the NOTCH pathway (Figure S2). Altogether, these results showed that in vitro growth of all T-ALL leukemic cells requires the activation of the NOTCH signaling pathway and that the threshold of activation of the NOTCH signaling pathway is not equivalent in T-ALL bearing different mutations of the notch1 gene.
In vitro T-ALL growth is inhibited by γ-secretase inhibitors. (A) Day 30 amplification rate of M18 and M30 T-ALL leukemic cells cocultured with MS5-GFP and MS5-DL1 in presence (+GSI) or in absence (−GSI) of γ-secretase inhibitor. Shown are mean plus or minus SD of 3 (M18) and 4 (M30) experiments. (B) mRNA levels of pTα, HES1, and deltex measured on day 3 of culture of M18 and M30 cells. Shown are mean values plus or minus SD of 4 measurements normalized using β2-microglobulin mRNA levels. Data are expressed as percentage of b2m values. *P < .05; **P < .01; ***P < .001; NS, not significant.
In vitro T-ALL growth is inhibited by γ-secretase inhibitors. (A) Day 30 amplification rate of M18 and M30 T-ALL leukemic cells cocultured with MS5-GFP and MS5-DL1 in presence (+GSI) or in absence (−GSI) of γ-secretase inhibitor. Shown are mean plus or minus SD of 3 (M18) and 4 (M30) experiments. (B) mRNA levels of pTα, HES1, and deltex measured on day 3 of culture of M18 and M30 cells. Shown are mean values plus or minus SD of 4 measurements normalized using β2-microglobulin mRNA levels. Data are expressed as percentage of b2m values. *P < .05; **P < .01; ***P < .001; NS, not significant.
Maintenance of T-LiC activity relies on NOTCH activation
We next studied the role of the NOTCH signaling pathway in the T-LiC activity of T-ALL using in vivo transplantation that is the assay of T-LiC activity.8 Cells from 3 (M30, M18, and M22) T-ALL samples cultured on MS5-DL1 or MS5-GFP were recovered after 30 days of culture and transplanted into femurs of NOD-Scid mice (Figure 5A). Of 39 mice transplanted with T-ALL cells cocultured with MS5-DL1 cells, 100% contained human CD45+ T-ALL blasts in all hematopoietic organs, including NIBM, spleen, thymus, and lymph nodes (Figure 5B-D). Two types of engraftment were observed when leukemic blasts had been cocultured with MS5-GFP. T-ALL that did not proliferate on MS5-GFP did not engraft recipients, whereas the MS5-GFP growing M30 T-ALL engrafted 91% of transplanted mice (Figure 5B,C). This differential engraftment was not entirely related to the number of transplanted cells as M22 after coculture with MS5-GFP and MS5-DL1 generated similar cell numbers that engrafted differently (Table S2; Figure 5B). Altogether, these results show the importance of activating the NOTCH signaling pathway for efficient transplantation of T-ALL into NOD/SCID mice.
NOTCH regulates T-LiC activity in vivo. (A) Design of the experiments. (B,C) Analysis of human CD45+ cell engraftment in the IBM of mice transplanted 3 months before with M18, M22 (B), and M30 (C) T-ALL cultured during 30 days in contact with MS5-GFP, MS5-DL1 in presence (+GSI: 1 or 4 weeks), or in absence (−GSI/DMSO: 1 or 4 weeks) of GSI. Each mouse received all the cells obtained from one culture well directly into one femur. At the time of death, IBM was analyzed separately from the rest of the BM. (D) Phenotype of human CD45+ cells engrafting different mouse hematopoietic organs after transplantation of MS5-DL1/cocultured M18 (left panels) and M30 (right panels) T-ALL leukemic cells. SPL indicates spleen. (E) γ-TCR gene rearrangement of M30 T-ALL at diagnosis and after coculture with MS5-GFP (GFP) or MS5-DL1 (DL1) cells. Results obtained with human cells recovered from the SPL of mice transplanted with cells cocultured with MS5-DL1 (Mice/DL1) or MS5-GFP (Mice/GFP) are also shown. Controls are as in Figure 1.
NOTCH regulates T-LiC activity in vivo. (A) Design of the experiments. (B,C) Analysis of human CD45+ cell engraftment in the IBM of mice transplanted 3 months before with M18, M22 (B), and M30 (C) T-ALL cultured during 30 days in contact with MS5-GFP, MS5-DL1 in presence (+GSI: 1 or 4 weeks), or in absence (−GSI/DMSO: 1 or 4 weeks) of GSI. Each mouse received all the cells obtained from one culture well directly into one femur. At the time of death, IBM was analyzed separately from the rest of the BM. (D) Phenotype of human CD45+ cells engrafting different mouse hematopoietic organs after transplantation of MS5-DL1/cocultured M18 (left panels) and M30 (right panels) T-ALL leukemic cells. SPL indicates spleen. (E) γ-TCR gene rearrangement of M30 T-ALL at diagnosis and after coculture with MS5-GFP (GFP) or MS5-DL1 (DL1) cells. Results obtained with human cells recovered from the SPL of mice transplanted with cells cocultured with MS5-DL1 (Mice/DL1) or MS5-GFP (Mice/GFP) are also shown. Controls are as in Figure 1.
Engrafted cells taken from different transplanted mice exhibited the initial clonal γ-TCR rearrangement, suggesting the maintenance of T-LiC during coculture with MS5-DL1 cells (Figure 5E). To assay the T-LiC self-renewing activity, secondary and tertiary transplantations were performed with cells engrafting the IBM of primary mice. T-ALL blasts were detected in 100% (21 of 21) secondary and (9 of 9) tertiary mice when T-ALL leukemic cells had been previously cocultured with MS5-DL1 cells as in mice transplanted with uncultured patient cells (Table 2). Of all the T-ALLs cocultured with MS5-GFP, only secondary mice transplanted with cells from mice engrafted with M30 T-ALL contained human cells (Table 2).
Summary of T-ALL engraftment before and after coculture with MS5 cells
| . | NOD-Scid recipients (positive/total) . | ||
|---|---|---|---|
| I mice . | II mice . | III mice . | |
| Patients | 15/15 | 10/10 | ND |
| MS5-DL1/−GSI | 30/30 | 21/21 | 9/9 |
| MS5-DL1/+GSI | 2/16 | 0/2 | — |
| MS5-GFP/−GSI | 1/12* | 0/1* | — |
| 11/13† | 5/8† | ND | |
| . | NOD-Scid recipients (positive/total) . | ||
|---|---|---|---|
| I mice . | II mice . | III mice . | |
| Patients | 15/15 | 10/10 | ND |
| MS5-DL1/−GSI | 30/30 | 21/21 | 9/9 |
| MS5-DL1/+GSI | 2/16 | 0/2 | — |
| MS5-GFP/−GSI | 1/12* | 0/1* | — |
| 11/13† | 5/8† | ND | |
A total of 2.5 × 105 T-ALL blasts were either injected (Patients) using IBI or cultured with MS5-GFP and MS5-DL1 (± GSI) during 30 days. Wells were then harvested and cells recovered from individual wells were transplanted using IBI into a single mouse.
— indicates not applicable; and ND, not done.
Results from M18 and M22 T-ALL.
Results from M30 T-ALL. Results from primary/I mice are shown also in Figure 5B,C. II mice and III mice are secondary and tertiary transplanted mice, respectively.
To ensure that T-ALL development observed in mice serially transplanted with cultured cells was generated as with uncultured cells, a kinetic experiment was performed and human cells engrafting secondary mice were counted and phenotyped during 6 weeks. One to 2 weeks after transplantation, all the mice were positive for human CD45+ blast cells. Comparison with experiments performed with uncultured cells was not possible because more cells had been injected in the case of the cultured cells. However, the levels of engraftment were low (mean ± SD, 3% ± 2.9%; CD45+ cells, range, 0.1%-7.9%; n = 9 mice) and taken into consideration the absolute cell number of the mouse BM 1 week after irradiation (BM being then in aplasia), the human T-ALL cells represented only 0.9% plus or minus 0.6% (range, 1720-16 600 CD45+ cells/106 injected T-ALL blasts) of the injected cells. Phenotypic analysis of the CD45+ cells recovered from the injected BM at 1 week indicated that the cells were mostly CD7+/CD4+/CD8+/−/TCRab+/−/CD44+/CD34− (Figure S3). At 6 weeks, the remaining 2 mice analyzed were engrafted with high levels of leukemic blasts. These results indicated that T-ALL development from cultured cells arises progressively as observed for uncultured T-ALL cells.
Finally, T-ALL leukemic cells were cultured with GSI during the 30-day culture period or during the last 10 days and then transplanted into the BM of NOD-Scid recipients. Only 8.3% (1 of 12) of mice showed T-ALL development compared with 100% (30 of 30) of mice transplanted with untreated cells (Figure 5B,C). This result was obtained for the 3 (M18, M22, and M30) T-ALL tested, regardless of their notch1 mutation status. It showed that the few cells remaining after GSI treatment have no T-ALL engraftment ability. To investigate whether shorter in vitro exposure to GSI would effectively inhibit T-ALL development in vivo, in 2 independent experiments, we transplanted NOD/SCID mice with M22 and M30 cells that had been cultured for 4 weeks with MS5-DL1 or MS5-GFP cells but treated the last week only with GSI. In these experiments, we could not detect any engraftment in 7 of 9 mice transplanted with 1-week-treated GSI cells (Figure 5B; Table 3), and secondary recipient mice receiving leukemic cells from M22 engrafted primary recipient showed no human CD45+ leukemic cells (Table 2). As cell numbers of short-term GSI-treated cells were reduced compared with untreated cells (Table 3), the engraftment data suggested that a threshold of approximately 50 000 cells is required to get T-ALL development into NOD-Scid mice. However, previous experiments performed with M30 T-ALL showed that injection of 104 cells led to engraftment into transplanted mice (Figure 2C), suggesting that the cell number is an important but not the only relevant parameter to ensure engraftment. These results indicate that the inhibition of the NOTCH pathway severely impairs T-LiC activity, implying that sustained NOTCH activation is required during culture for maintenance and self-renewal of T-LiC activities.
Relation between cell numbers and engraftment levels of cultured T-ALL cells
| T-ALL sampleMouse . | −GSI . | +GSI . | ||
|---|---|---|---|---|
| Injected cells . | CD45+ cells, % . | Injected cells . | CD45+ cells, % . | |
| M22 | ||||
| 1 | 197 000 | 84 | 25 000 | 1 |
| 2 | 225 000 | 0.5 | 54 000 | 0 |
| 3 | 230 000 | 60 | 11 000 | 0 |
| 4 | 230 000 | 50 | 52 000 | 50 |
| 5 | 83 000 | 0.5 | 35 000 | 0 |
| M30 | ||||
| 1 | 81 000 | 0.3 | 29 500 | 0 |
| 2 | 61 000 | 0.8 | 31 500 | 0 |
| 3 | 56 000 | 74 | 35 000 | 0 |
| 4 | 74 000 | 90 | 21 000 | 0 |
| T-ALL sampleMouse . | −GSI . | +GSI . | ||
|---|---|---|---|---|
| Injected cells . | CD45+ cells, % . | Injected cells . | CD45+ cells, % . | |
| M22 | ||||
| 1 | 197 000 | 84 | 25 000 | 1 |
| 2 | 225 000 | 0.5 | 54 000 | 0 |
| 3 | 230 000 | 60 | 11 000 | 0 |
| 4 | 230 000 | 50 | 52 000 | 50 |
| 5 | 83 000 | 0.5 | 35 000 | 0 |
| M30 | ||||
| 1 | 81 000 | 0.3 | 29 500 | 0 |
| 2 | 61 000 | 0.8 | 31 500 | 0 |
| 3 | 56 000 | 74 | 35 000 | 0 |
| 4 | 74 000 | 90 | 21 000 | 0 |
T-ALL blasts (105 cells/well) were cultured during 4 weeks in contact with MS5-DL1 (M22) and MS5-GFP (M30) cells. One week before the termination of culture, 10 μM/mL GSI was added in medium refreshments. At the end of the culture period, every well was recovered, cells were counted, and each sample was individually transplanted into a single NOD-Scid mouse. Human cell engraftment levels (measured by FACS, using mouse antihuman CD45 antibodies) in the injected bone marrow were analyzed 2 months after transplantation.
Discussion
In the first part of this work, we show that T-ALL is initiated by a minority of blast cells after serial transplantation into NOD-Scid mice, as recently described.8 We subsequently describe, for the first time in primary human samples, that the NOTCH pathway regulates this T-ALL leukemic stem cell activity. NOTCH receptors has been long thought to belong to a group of pathways, such as Wnt, Sonic Hedge Hog, Bmi1 that are implicated in the regulation of normal stem cells and in cancer.28,29 NOTCH is implicated in rare translocations detected in T-ALL30 ; however, its role in normal hematopoietic stem cell maintenance is a matter of debate.31-33 Transplantation experiments of ICN1 gene-modified BM cells in mice leads to inevitable progenitor transformation.34 Moreover, Aster's group showed that NOTCH is frequently mutated in T-ALL and that inhibiting the NOTCH pathway in T-ALL-derived cell lines completely inhibited their growth.14 These findings are evidence that NOTCH pathway is a major player of leukemogenesis in animals or in long-term maintained cell lines. However, the role of NOTCH in patient samples, and particularly in preserving a leukemia stem cell activity in this pathology, has never been documented. Indeed, T-LiC have been described only very recently in humans,8 and so far no reproducible in vitro assay has been developed that could maintain such population and allow proliferation of the blastic population. In this report, we document such an assay and the idea of using genetically engineered stromal cells to provide a “bone marrow/thymus niche” was supported by the fact that such a system had previously been described to provide ideal conditions for normal T-cell development in culture.35,36 Whether the NOTCH stimulation itself is sufficient to allow T-LiC survival or whether the stromal MS5 support provides additional signals is an interesting and unresolved question. In addition, whether other ligands present on MS5 cells, such as Jagged1-2,37 may lead to qualitative or quantitative differences in the activation of NOTCH receptors is largely unknown. It has been previously shown that MS5 cells express DL1 transcripts and protein,23,37 although at much lower levels compared with transduced MS5-DL1 cell line. However, T-ALL could never be grown without stromal support, even when recovered after NOD-Scid transplantation and when notch1 gene was mutated (also in the case of M30 T-ALL), and even in presence of cytokines, such as rhu-IL7, Flt3-L, or SCF, suggesting that either the low levels of NOTCH ligands or other signals in MS5 cells are necessary to allow cell survival during the culture period. The fact that NOTCH activation, revealed by sustained deltex, hes1, and pTα expression, is present in the notch1-mutated M30 T-ALL cultured on MS5-GFP cells and decreases in the presence of GSI suggests the functionality of this pathway during culture. This result indicates that the sensitivity of certain T-ALL samples to low NOTCH stimulation is modified; and in the case of M30, this is possibly related, but not proven yet, to the fact that M30 exhibits a short PEST truncation, notably deleting the WSSSP site that, when mutated, can enforce the aggressiveness of leukemic progression,25 whereas the fbw7 gene contains a conservative mutation that does not affect the protein amino acid content. Such increase in NOTCH sensitivity could be further studied using MS5 cells expressing lower DL1 levels or graded quantities of immobilized NOTCH ligands.23,38 This last approach would then question whether NOTCH is the only pathway important in MS5 cells. In the majority of tested T-ALL, deltex/HES1/pTα expression was low during culture with MS5-GFP cells and their levels increased in contact with MS5-DL1, correlating fairly well with proliferation rate. It is well documented that DL1 has a dose-dependent effect on normal hematopoietic differentiation, including on apoptosis of normal progenitors.38 Whether this is also true for T-ALL is certainly an important question. Moreover, testing whether activation of NOTCH pathway via DL1 ligand can induce some levels of differentiation in blast cells is also an exiting feature. Indeed, if an increase in CD4 and CD8 expression in parallel to a marked decrease in CD34 expression is a sign of blast cell differentiation, then enhanced DL1/NOTCH interaction induces such a mechanism in our culture system. It has been previously shown that blast differentiation could be obtained in promyelocytic leukemia treated with retinoic acid39 as well as in acute myeloid leukemia treated with anti-CD44 antibodies.40 Thus high NOTCH activation could be another pathway that induces differentiation of leukemic blasts. Whether this idea could partly explain previous work showing that notch1 mutated T-ALL have an improved early treatment response and long-term outcome compared with T-ALL without such mutations3 will require further experiments.
Our study extends the list of hematologic disorders in which a LiC activity is documented.9 Although we did not address this point directly here, ie, by showing that indeed T-ALL are heterogeneous in terms of proliferation and NOD-Scid mice T-ALL initiation, a recent work indicated that such a population could be characterized in T-ALL under the CD34+/CD4−CD7− phenotype.8 However, when going through the phenotype of our T-ALL samples, the NOD-Scid–engrafted cells that reproducibly allow secondary and tertiary transplants and the cells recovered after culture with MS5 cells, we observed a variable phenotype evolution that questioned the use of cell surface markers for the identification of T-LiC. Moreover, we could not confirm a strict correlation between the transplantation potential and the reproducible presence of CD34+/CD4−CD7− cells even early after cell transplantation, suggesting that CD34 might not be a reliable and reproducible marker for T-LiC in all situations. Whether this discrepancy is explained by variability between T-ALL samples or between microenvironment interactions (mouse vs human) is being tested by sorting different cell populations from patients at diagnosis and assay their leukemic potential in vivo or after culture. Finally, clonal tracking of T-LiC through γ- and β-TCR rearrangements showed that leukemic cells, recovered from in vitro or in vivo experiments, exhibited always the same clonal rearrangement as the original patient. Although this approach did not allow dissection of the hierarchy of T-LiC as was done for acute myeloid leukemia using retroviral insertions, this result indicated that the T-LiC population probably resides in the T cell–committed progenitor and not in the hematopoietic stem cell compartment.
New therapeutic strategies of T-ALL now rely on the cellular and molecular characterization of T-LiC. Our study opens a new and major area of investigation in this field as we show that it is feasible to study and manipulate human T-LiC. Indeed, in vivo and in vitro assays we have developed can easily be used to test new drugs interfering with pathways that regulate the T-LiC activity. T-ALLs are characterized by chromosomal rearrangement or interstitial deletions that most often activate an oncogene. In this study, we show that the NOTCH signaling pathway regulates the engraftment and self-renewing properties of T-LiC in T-ALL with different activated oncogenes pinpointing the NOTCH signaling as a major target of future T-ALL therapies and challenging the notion that recurrent chromosomal abnormalities indicate therapeutic targets.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We acknowledge S. Gisselbrecht, S. Fichelson, A. Dubart-Kupperschmitt, and J. Dick for critical reading of the manuscript; A. Weng for helpful comments on notch1 mutations and on the writing of the paper; the staff of Inserm U602 animal facilities (Villejuif, France) for excellent support in mouse studies; M. Girault and M. Pauchard from Institut Cochin SCEA; C. Desouart for performing fbw7 sequencing on patients samples; Dr M. Adam and E. Zink for taking pictures of sample slides; and C. Kerdudo for helping with human leukemic samples. Most of the sequencing analyses were performed in the common facilities of Institut Cochin.
This work was supported by grants from Ligue Nationale contre le Cancer (LNCC; RAB05013KKA), Inserm (ASE04144/AMA03019), and Agence Nationale pour la Recherche (ANR05-R05105KK). F.A. was supported by a fellowship from LNCC. P.B.d.l.G. is a fellow of Fondation de France and of ANR (NT05-2-44232). B.G. is a fellow of LNCC.
Authorship
Contribution: F.A., P.-H.R., and F.P. designed and wrote the manuscript; F.A., P.B.d.l.G., B.G., M.-C.R., J.C., P.B., and F.P. performed experiments and analyzed data; M.F. helped design the experiments; and N.B., H.D., A.B., J.L.-P., and P.B. provided crucial biologic materials.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Françoise Pflumio, Laboratoire des cellules souches hématopoïétiques et leucémiques, Institut de Radiobiologie Cellulaire et Moléculaire, Commissariat à l'nergie Atomique, 18 Route du Panorama, 92260, Fontenay-aux-Roses, France; e-mail: francoise.pflumio@cea.fr.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal