Abstract
Men1 is a tumor suppressor gene mutated in endocrine neoplasms. Besides its endocrine role, the Men1 gene product menin interacts with the mixed lineage leukemia (MLL) protein, a histone H3 lysine 4 methyltransferase. Although menin and MLL fusion proteins cooperate to activate Homeobox (Hox) gene expression during transformation, little is known about the normal hematopoietic functions of menin. Here, we studied hematopoiesis after Men1 ablation. Menin loss modestly impaired blood neutrophil, lymphocyte, and platelet counts. Without hematopoietic stress, multilineage and myelo-erythroid bone marrow progenitor numbers were preserved, while B lymphoid progenitors were decreased. In contrast, competitive transplantation revealed a marked functional defect of long-term hematopoietic stem cells (HSC) in the absence of menin, despite normal initial homing of progenitors to the bone marrow. HoxA9 gene expression was only modestly decreased in menin-deficient HSCs. These observations reveal a novel and essential role for menin in HSC homeostasis that was most apparent during situations of hematopoietic recovery, suggesting that menin regulates molecular pathways that are essential during the adaptive HSC response to stress.
Introduction
Multiple endocrine neoplasia type I (MEN1) is a hereditary tumor syndrome caused by mutations in the MEN1 gene, which encodes the protein menin.1 MEN1 patients display a spectrum of benign and malignant endocrine lesions, including hyperparathyroidism as well as pituitary and enteropancreatic tumors. Heterozygous loss-of-function mutations are found in MEN1 patients, and additional somatic loss of heterozygosity in the MEN1 locus leads to mutation of both normal MEN1 alleles in tumors. Menin is predominantly a nuclear protein that has been reported to interact with a variety of partners, including nuclear factor kappa B (NF-κB), JunD, and SMADs, as well as with proteins involved in DNA repair, such as FANCD2, although it is unclear if these interactions are relevant to menin's activity as a tumor suppressor.1 Further, chromatin immunoprecipitation coupled with nucleotide array analysis has revealed binding of menin to a large number of genomic sites, suggesting a broad regulatory potential.2,3
A hematopoietic role for menin was first suggested through its identification in a complex containing the mixed lineage leukemia (MLL) gene product or its homologue MLL2.4,5 MLL is a homologue of the Drosophila Trithorax gene, an epigenetic regulator that antagonizes the activity of Polycomb family members and is essential for maintaining expression of target genes, such as members of the Homeobox (Hox) family.6 MLL is a large protein with multiple functional domains, including a C-terminal Su(var)3-9, enhancer-of-zeste, Trithorax (SET) domain that has histone methyltransferase activity, leading to histone H3 lysine 4 (H3K4) methylation, a hallmark of active genes.4,7 Translocations and rearrangements of the MLL gene arise in acute myeloid and lymphoid leukemias.8 These translocations generate fusion proteins consisting of the N-terminal portion of MLL and various partners, causing overexpression of Hox genes and other targets. Importantly, the menin/MLL interaction appears essential for the effects of MLL fusion proteins and the maintenance of Hox gene expression in leukemic cells.9-11
Despite its importance in leukemic transformation, the normal function of menin in hematopoiesis remains elusive. We have previously reported that Men1 inactivation decreases colony formation from bone marrow hematopoietic progenitors.10 In addition, menin's partner MLL is essential to establish normal hematopoiesis in the embryo.12-14 Furthermore, recent reports indicate that MLL controls the function of fetal and adult hematopoietic stem cells (HSCs) and normal Hox gene expression.15,16 These observations raise the possibility that menin might physiologically regulate hematopoiesis in association with MLL or through alternative mechanisms. Here, we investigated the impact of menin loss on hematopoietic progenitors. Menin had modest effects on hematopoiesis in steady-state conditions, but was absolutely essential for long-term HSCs in the setting of competitive transplantation, despite normal initial homing of the progenitors to the bone marrow. Menin loss resulted in only a modest decrease in HSC expression of HoxA9, which is induced in leukemia cells transformed by MLL fusion proteins. These results identify menin as an essential regulator of HSC homeostasis specifically in situations of hematopoietic stress.
Methods
Mice
Men1f/f mice were kindly provided by Francis Collins (National Institute for Human Genome Research, Bethesda, MD).17 These mice harbor loxP sites flanking exons 3 to 8 of the Men1 gene. Transgenic mice expressing a tamoxifen (TAM)–inducible Cre (Cre-ERTm) under the control of the ubiquitous UBC9 promoter were generated by lentitransgenesis and kindly provided by Dr Eric Brown (University of Pennsylvania, Philadelphia, PA).18 Cre-ERTm Men1f/f transgenic mice were maintained on a mixed 129SvEv-C57BL/6 background (CD45.2+). Cre-ERTm+Men1f/f and control Cre-ERTm+Men1+/+ mice were used in all experiments, unless indicated otherwise. Excision of the floxed allele was achieved by oral TAM gavage (MP Biomedicals, Solon, OH; 200 mg/kg daily for 2 days, followed by the same dose over 2 additional days after 1 day off). Control mice were treated with the same regimen. The efficiency of Men1 Cre-mediated excision was assessed by polymerase chain reaction (PCR) and agarose gel electrophoresis, using 3 primers in a single reaction: 5′-CCCACATCCAGTCCCTCTTCAGCT-3′; 5′-AAGGTACAGCAGAGGTCACAGAG-3′; 5′-GACAGGATTGGGAATTCTCTTTT-3′ (wild-type, 300 bp; floxed, 340 bp; Cre-excised allele, 500 bp). C57BL/6.SJL-Ptprca mice (B6.SJL, CD45.1+) were from the National Cancer Institute (Frederick, MD). Experimental protocols were approved by the University of Pennsylvania's Office of Regulatory Affairs or by the University of Michigan's Committee on Use and Care of Animals.
Antibodies
The following antibodies were obtained from BD Pharmingen (San Diego, CA): anti-CD45.2 (104), c-Kit (2B8), CD48 (HM48-1), CD34 (RAM34), CD43 (S7), B220 (RA3-6B2), CD19 (1D3), Gr1 (RB6-8C5), TER119, TCRβ (H57-597), CD4 (RM4-5), CD3 (145-2C11), CD8 (53-6.7), NK1.1 (PK136), CD11c (HL3); eBioscience (San Diego, CA): CD45.1 (A20), Sca-1 (E13-161.7), CD127/IL-7Rα (A7R34), CD135/Flt3 (A2F10), CD93 (AA4.1), CD16/32 (93), CD11b (M1/70), or BioLegend (San Diego, CA): CD150 (TC15-12F12.2). Fluorescein isothiocyanate (FITC)–labeled polyclonal anti-sIgM antibodies were from Jackson Immunoresearch (West Grove, PA). Biotinylated antibodies were revealed with streptavidin-Pacific Blue (Invitrogen, Carlsbad, CA; Molecular Probes, Eugene, OR) or phycoerythrin (PE)–Texas Red (Caltag, Burlingame, CA). Lineage+ cells were defined with anti-CD11b, Gr1, TER119, B220, CD19, CD11c, CD8, CD4, CD3, TCRβ and NK1.1, except for myelo-erythroid progenitors where CD11b was omitted.
Flow cytometry and cell sorting
After blocking unspecific binding with unlabeled rat plus mouse IgG (Sigma-Aldrich, St Louis, MO), cells were stained on ice in PBS plus 4% fetal calf serum (FCS) and sorted on FACSAria (BD Biosciences, San Jose, CA) or MoFlo (Cytomation, Fort Collins, CO). Analysis was performed on LSR II, FACSCanto, or FACSAria (BD Biosciences). Files were analyzed in FlowJo (TreeStar, San Carlos, CA).
Competitive transplantation assays
Bone marrow (BM) was harvested from Men1f/f Cre-ERTm+ and control Cre-ERTm+Men1+/+ or Cre-ERTm−Men1f/f mice at least 3 weeks after TAM administration. After red blood cell lysis using ACK lysis buffer (Cambrex, East Rutherford, NJ), nucleated BM cells were counted and resuspended in ice-cold phosphate-buffered saline (PBS). These cells (CD45.2+) were mixed at specific ratios with 106 CD45.1+ competitor BM cells from B6-SJL mice (1:1, 3:1, 9:1). This mixture was injected intravenously through the tail vein to cohorts of lethally irradiated (900 rads) B6-SJL mice. Recipient mice were maintained on antibiotics for 2 weeks after transplantation, and their peripheral blood was assessed by flow cytometry. In some experiments, TAM was administered only after reconstitution, as described in “Cell-autonomous hematopoietic defects after Men1 inactivation.”
Homing assays
For analysis of homing into nonirradiated hosts, 2.5 × 107 BM cells from TAM-treated CD45.2+Cre-ERTm+Men1f/f or control Cre-ERTm−Men1f/f mice were injected into the tail vein of CD45.1+ B6-SJL recipients. Three days later, BM from recipient mice was analyzed by flow cytometry for the presence of CD45.2+ donor cells within the Lin−Sca-1hic-Kithi (LSK) population. To assess homing into irradiated hosts, BM from TAM-treated Cre-ERTm+Men1f/f or Cre-ERTm−Men1f/f mice was labeled with CFSE (Invitrogen, Molecular Probes). After washing with PBS, 3 × 107 cells were injected into the tail vein of B6 recipient mice that had been lethally irradiated 8 hours previously (900 rads). Six hours later, the number of CFSE+ Lin− cells in the recipient bone marrow and spleen was determined by flow cytometry.
Peripheral blood count analysis
Blood samples were collected in EDTA (ethylenediaminetetraacetic acid)–containing Microtainer tubes (BD Biosciences) and analyzed on a Hemavet counter with a mouse-specific software (Drew Scientific, Oxford, CT).
Quantitative RT-PCR
RNA was isolated using the RNEasy Micro Kit (QIAGEN, Valencia, CA). cDNA was prepared with Superscript II (Invitrogen). Reverse transcriptase (RT)–PCR was performed with TaqMan PCR Master Mix and analyzed on ABI Prism 7900 (Applied Biosystems, Foster City, CA) or Mastercycler ep realplex (Eppendorf, Westbury, NY). Relative transcript abundance was calculated using the ΔΔCt method after normalization with Hprt1. The following Taqman primer/probe combinations were obtained from Applied Biosystems: Hprt1 (Mm03024075_m1), Men1 (Mm00484963_m1), HoxA9 (Mm00439364_m1), Meis1 (Mm00487664_m1), HoxA10 (Mm00433966_m1), and p18Ink4c (Mm00483243_m1).
Results
Abnormalities of peripheral blood cell counts in the absence of menin
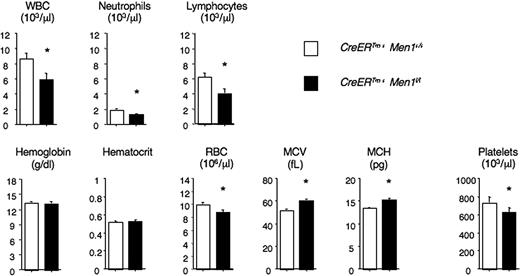
Homozygous Men1-deficient mice have multiple abnormalities leading to embryonic death (E11.5-13.5).19 To avoid this early lethality, we studied mice carrying a conditional Men1 allele.10,17,20 Men1f/f mice were crossed to transgenic mice ubiquitously expressing CreERTm. After TAM administration, CreERTm+Men1f/f multipotent BM progenitors in the LSK subset, containing HSCs, routinely had a greater than 90% to 95% reduction in Men1 mRNA (data not shown). Furthermore, PCR analysis showed effective Cre-mediated Men1 excision in total BM, as well as in purified short-lived BM myeloid cells (Figure S1; available on the Blood website; see the Supplemental Materials link at the top of the online article). To assess the impact of menin loss on adult hematopoiesis, we administered TAM to CreERTm+Men1f/f and control CreERTm+Men1+/+ mice. We previously reported that this led to decreased blood leukocyte numbers, but the effect on all lineages was not monitored.10 Thus, we measured complete blood counts in menin-deficient and control mice (Figure 1). The white blood cell count, including neutrophils and lymphocytes, significantly decreased after Men1 excision. In addition, red cell numbers were reduced, but average red cell size and hemoglobin content increased, accounting for normal hemoglobin levels despite reduced red cell numbers. We also noticed a modest but statistically significant decrease in the platelet count of menin-deficient mice (Figure 1). Altogether, menin loss led to abnormalities in all3 lineages, but without overt hematopoietic failure during steady-state hematopoiesis.
Modest impact of menin loss on peripheral blood counts during steady-state hematopoiesis. Blood cell counts were determined in the absence of menin. Tamoxifen (TAM) was administered to CreERTm+Men1f/f and control CreERTm+Men1+/+ mice. Results are shown as mean plus or minus SEM for a large cohort of mice at least 3 weeks after TAM (n = 46 and n = 65). *P < .05 (Student t test). WBC indicates white blood cells; RBC, red blood cells; MCV, mean corpuscular volume; and MCH, mean corpuscular hemoglobin.
Modest impact of menin loss on peripheral blood counts during steady-state hematopoiesis. Blood cell counts were determined in the absence of menin. Tamoxifen (TAM) was administered to CreERTm+Men1f/f and control CreERTm+Men1+/+ mice. Results are shown as mean plus or minus SEM for a large cohort of mice at least 3 weeks after TAM (n = 46 and n = 65). *P < .05 (Student t test). WBC indicates white blood cells; RBC, red blood cells; MCV, mean corpuscular volume; and MCH, mean corpuscular hemoglobin.
Preserved number of menin-deficient BM stem and progenitor cell subsets
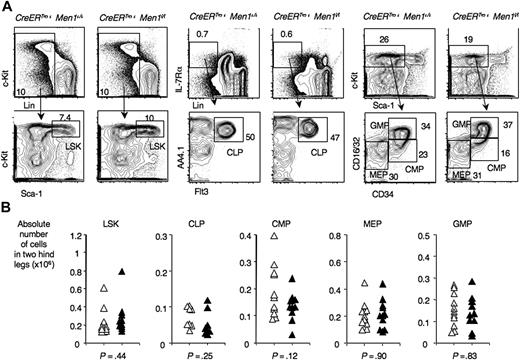
To investigate if normal numbers of BM progenitors could be maintained in the absence of menin, we studied primitive hematopoietic progenitors after TAM administration to CreERTm+Men1f/f and control CreERTm+Men1+/+ mice. The frequencies of LSK progenitors, containing HSCs, were not significantly different between these 2 cohorts of mice (Figure 2). The same was true for common lymphoid progenitors. Among myelo-erythroid progenitors,21 there was a nonsignificant trend for a decreased frequency of common myeloid progenitors. These results indicate that menin is not required in baseline conditions to maintain normal numbers of multipotent hematopoietic progenitors, common lymphoid progenitors, and myelo-erythroid progenitors, in contrast to observations made after Mll inactivation.15
Preserved numbers of multilineage, myelo-erythroid, and common lymphoid progenitors after Men1 inactivation. Flow cytometric analysis of progenitor populations in the BM of control CreERTm+Men1+/+ mice and menin-deficient CreERTm+Men1f/f mice. The analysis was performed at least 3 weeks after TAM administration. (A) Representative examples, with progenitor populations identified as follows: LSK progenitors (containing hematopoietic stem cells); CLP, common lymphoid progenitors (Lin−IL-7Rα+AA4.1+Flt3+); CMP, common myeloid progenitors (Lin−Sca-1–c-kithiCD34+CD16/32lo); MEP: megacaryocyte-erythroid progenitors (Lin−Sca-1–c-kithiCD34−CD16/32lo); GMP, granulocyte-macrophage progenitors (Lin−Sca-1–c-kithiCD34hiCD16/32hi). (B) Absolute cell numbers in calculated in 2 hind legs. ▵: control CreERTm+Men1+/+; ▴: CreERTm+Men1f/f mice. P values indicate the results of a Student t test.
Preserved numbers of multilineage, myelo-erythroid, and common lymphoid progenitors after Men1 inactivation. Flow cytometric analysis of progenitor populations in the BM of control CreERTm+Men1+/+ mice and menin-deficient CreERTm+Men1f/f mice. The analysis was performed at least 3 weeks after TAM administration. (A) Representative examples, with progenitor populations identified as follows: LSK progenitors (containing hematopoietic stem cells); CLP, common lymphoid progenitors (Lin−IL-7Rα+AA4.1+Flt3+); CMP, common myeloid progenitors (Lin−Sca-1–c-kithiCD34+CD16/32lo); MEP: megacaryocyte-erythroid progenitors (Lin−Sca-1–c-kithiCD34−CD16/32lo); GMP, granulocyte-macrophage progenitors (Lin−Sca-1–c-kithiCD34hiCD16/32hi). (B) Absolute cell numbers in calculated in 2 hind legs. ▵: control CreERTm+Men1+/+; ▴: CreERTm+Men1f/f mice. P values indicate the results of a Student t test.
Decreased number of BM B lymphoid progenitors after Men1 inactivation
Next, we studied the effect of menin loss on B lineage development. Developing BM B-cell progenitors were identified by flow cytometry in the following way: pro-B cells (Hardy fractions B,C) as B220+CD43+AA4.1+CD19+; pre-B cells (fraction D) as B220+CD43−AA4.1+sIgM−; and immature B cells (fraction E) as B220+CD43−AA4.1+sIgM+ (Figure 3A).22,23 We observed a significant decrease in pro-B, pre-B, and immature B cell numbers in menin-deficient mice (Figure 3B). Although there was variability between mice, the defect was profound in the majority of the animals studied. In contrast to other progenitors, these results reveal a physiologic requirement for menin to maintain normal BM B lineage progenitor numbers downstream of common lymphoid progenitors. This indicates that the lymphoid lineage is particularly sensitive to the absence of menin.
Decreased numbers of BM B lineage progenitors after Men1 inactivation. (A) BM B lineage progenitors were defined by flow cytometry. Pro-B cells: B220+CD43+AA4.1+CD19+; pre-B cells: B220+CD43−AA4.1+sIgM−; immature B: B220+CD43−AA4.1–sIgM+. (B) Absolute cell numbers calculated in 2 hind legs. ▵: control CreERTm+Men1+/+; ▴: CreERTm+Men1f/f mice. P values indicate the results of a Student t test (*, statistically significant, P < .05).
Decreased numbers of BM B lineage progenitors after Men1 inactivation. (A) BM B lineage progenitors were defined by flow cytometry. Pro-B cells: B220+CD43+AA4.1+CD19+; pre-B cells: B220+CD43−AA4.1+sIgM−; immature B: B220+CD43−AA4.1–sIgM+. (B) Absolute cell numbers calculated in 2 hind legs. ▵: control CreERTm+Men1+/+; ▴: CreERTm+Men1f/f mice. P values indicate the results of a Student t test (*, statistically significant, P < .05).
Impaired function of menin-deficient long-term HSCs
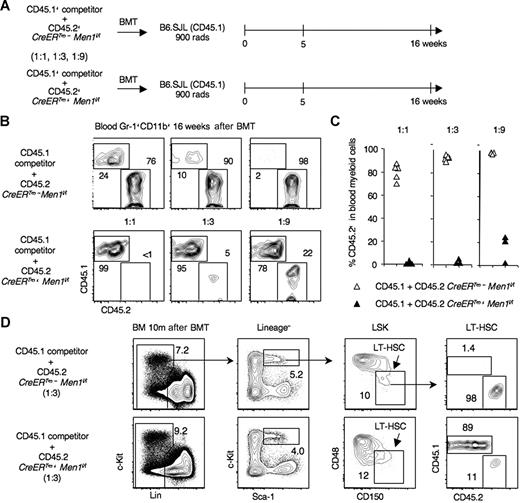
Because Men1 inactivation only caused modest abnormalities in steady-state hematopoiesis, we examined the impact of menin loss on HSC function during hematopoietic stress. Competitor CD45.1+ BM cells were combined with an increasing number of tester BM cells from TAM-treated CD45.2+CreERTm+Men1f/f or control CreERTm−Men1f/f mice (1:1, 1:3, and 1:9 ratios), and transplanted into lethally irradiated CD45.1+ B6.SJL mice (Figure 4A). Sixteen weeks after BM transplantation (BMT), control CD45.2+CreERTm−Men1f/f cells contributed to at least 60% to 70% of blood myeloid cells in recipients injected with a 1:1 competitor:tester ratio. In cohorts of recipients receiving 1:3 or 1:9 mixtures, more than 90% of the cells were CD45.2+ (Figure 4B,C), indicating excellent control long-term HSC (LT-HSC) function. In contrast, menin-deficient CD45.2+CreERTm+Men1f/f myeloid cells were barely detectable (< 1%) in 1:1 recipients. When the ratio of menin-deficient cells was raised 3-fold, chimerism remained less than 10%. Even when 9-fold more menin-deficient BM cells were injected, the contribution of CD45.2+ cells remained less than 25% (Figure 4B,C). The chimerism in B and T cells was similar, indicating a multilineage defect (data not shown). The poor performance of menin-deficient cells was already apparent 5 weeks after BMT (data not shown).
Markedly impaired function of menin-deficient hematopoietic stem cells after competitive transplantation. (A) Experimental design. BM was harvested from TAM-treated CreERTm+Men1f/f or CreERTm−Men1f/f mice (CD45.2+). CD45.1+ competitor B6.SJL BM cells were mixed with an increasing number of CD45.2+ tester cells (1:1, 1:3, 1:9 ratios) and used to reconstitute lethally irradiated B6.SJL mice. (B) Contribution of CD45.2+ and CD45.1+ cells to blood Gr1+CD11b+ myeloid cells 16 weeks after transplantation. Representative examples are shown. (C) Percentage of CD45.2+ cells in blood myeloid cells at 16 weeks for each recipient (triangles) in 1 representative experiment. Similar data were obtained in 4 independent experiments. (D) Impaired repopulation of the long-term HSC compartment by menin-deficient progenitors. BM was analyzed 10 months after transplantation. A representative example is shown for recipients of a 1:3 competitor:tester ratio. Long-term HSCs were identified among LSK progenitors using SLAM markers, as follows: CD150+CD48−Lin–Sca-1hic-kithi cells.24
Markedly impaired function of menin-deficient hematopoietic stem cells after competitive transplantation. (A) Experimental design. BM was harvested from TAM-treated CreERTm+Men1f/f or CreERTm−Men1f/f mice (CD45.2+). CD45.1+ competitor B6.SJL BM cells were mixed with an increasing number of CD45.2+ tester cells (1:1, 1:3, 1:9 ratios) and used to reconstitute lethally irradiated B6.SJL mice. (B) Contribution of CD45.2+ and CD45.1+ cells to blood Gr1+CD11b+ myeloid cells 16 weeks after transplantation. Representative examples are shown. (C) Percentage of CD45.2+ cells in blood myeloid cells at 16 weeks for each recipient (triangles) in 1 representative experiment. Similar data were obtained in 4 independent experiments. (D) Impaired repopulation of the long-term HSC compartment by menin-deficient progenitors. BM was analyzed 10 months after transplantation. A representative example is shown for recipients of a 1:3 competitor:tester ratio. Long-term HSCs were identified among LSK progenitors using SLAM markers, as follows: CD150+CD48−Lin–Sca-1hic-kithi cells.24
These findings suggested a profound defect of menin-deficient HSCs during hematopoietic stress. To investigate whether this defect involved long-term HSCs, we analyzed recipient mice 10 months after BMT and assessed CD45.1/CD45.2 chimerism among CD150+CD48−LSK cells, highly enriched for LT-HSC (Figure 4D).24 In control chimeras, more than 90% of cells in the LT-HSC fraction were derived from tester BM. In contrast, the contribution of CD45.2+ menin-deficient cells was markedly decreased (Figure 4D). Altogether, these results revealed a major defect of LT-HSC function in the absence of menin. Although it is possible that additional abnormalities were present at subsequent differentiation steps, the LT-HSC defect accounted for the main abnormalities observed after transplantation of menin-deficient progenitors. Thus, in a classical situation of hematopoietic recovery, menin loss led to a marked LT-HSC defect at the apex of the hematopoietic hierarchy.
Normal homing of menin-deficient progenitors to the BM
Because menin loss only led to modest hematopoietic abnormalities in steady-state conditions, with preserved numbers of HSCs, but to a profound LT-HSC engraftment defect after transplantation, we investigated if menin was required for homing of primitive hematopoietic progenitors to the BM. First, we transferred large numbers of control or menin-deficient BM cells into unirradiated CD45.1+ congenic recipients (Figure 5A). In this sytem, the HSC phenotype was well preserved, allowing us to track rare CD45.2+ donor cells within the primitive LSK compartment in the BM. We found that the number of donor LSK cells that had homed to host BM 3 days later was similar after transfer of menin-deficient as compared with control BM. To investigate if homing was preserved after lethal irradiation, we transplanted CFSE-labeled control or menin-deficient BM (Figure 5B). Six hours later, we detected similar numbers of CFSE+ Lin− progenitors in BM and spleen of recipient mice in both groups. This indicated that the homing of menin-deficient progenitors to hematopoietic organs was preserved, both in irradiated and unirradiated hosts.
Normal homing of menin-deficient hematopoietic progenitors. (A) Unirradiated hosts: BM from TAM-treated CD45.2+Cre-ERTm+Men1f/f or control Cre-ERTm−Men1f/f mice was injected into the tail vein of CD45.1+ B6-SJL recipients (2.5 × 107 cells/mouse). Three days later flow cytometry was used to analyze the homing efficiency of CD45.2+ donor LSK cells in the host bone marrow. Contour plots are representative of 3 individual recipients in each group. (B) Irradiated hosts: lethally irradiated (900 rads) B6 mice were transplanted with 3 × 107 CFSE-labeled cells from TAM-treated CD45.2+Cre-ERTm+Men1f/f or Cre-ERTm−Men1f/f mice. The host BM and spleen were analyzed by flow cytometry 6 hours after transfer. Graphs show the absolute number of CFSE+Lin− cells recovered from 2 hind legs (mean + SEM). Contour plots are representative of 4 individual recipients in each group. WT indicates recipients of control cells; and KO, recipients of menin-deficient cells.
Normal homing of menin-deficient hematopoietic progenitors. (A) Unirradiated hosts: BM from TAM-treated CD45.2+Cre-ERTm+Men1f/f or control Cre-ERTm−Men1f/f mice was injected into the tail vein of CD45.1+ B6-SJL recipients (2.5 × 107 cells/mouse). Three days later flow cytometry was used to analyze the homing efficiency of CD45.2+ donor LSK cells in the host bone marrow. Contour plots are representative of 3 individual recipients in each group. (B) Irradiated hosts: lethally irradiated (900 rads) B6 mice were transplanted with 3 × 107 CFSE-labeled cells from TAM-treated CD45.2+Cre-ERTm+Men1f/f or Cre-ERTm−Men1f/f mice. The host BM and spleen were analyzed by flow cytometry 6 hours after transfer. Graphs show the absolute number of CFSE+Lin− cells recovered from 2 hind legs (mean + SEM). Contour plots are representative of 4 individual recipients in each group. WT indicates recipients of control cells; and KO, recipients of menin-deficient cells.
Defect in BM recovery after chemoablation
As menin-deficient BM progenitors were sensitive to hematopoietic stress during BM transplantation, we studied menin-deficient BM after chemoablation with 5-fluorouracil (5-FU). BM was examined 6 days after 5-FU administration to TAM-treated CreERTm+Men1f/f or control CreERTm−Men1f/f mice. A decreased percentage of Gr1+CD11b+ cells was observed in the menin-deficient BM, showing impaired myeloid recovery (Figure S2A). Furthermore, we observed a decrease in the percentage of Lin−Sca-1−c-Kithi progenitors, a population containing committed myelo-erythroid progenitors (Figure S2B). These findings suggested that menin was functionally important for progenitor recovery after chemoablation.
Cell-autonomous hematopoietic defects after Men1 inactivation
As Men1 excision occurred in multiple cell types in CreERTm+Men1f/f mice, we investigated if loss of menin caused the hematopoietic defects through cell-autonomous mechanisms. We performed a mixed BMT of CD45.1+ competitor cells with CD45.2+CreERTm+Men1f/f or control CreERTm−Men1f/f BM into lethally irradiated CD45.1+ B6.SJL mice, without administering TAM before BMT (Figure 6A). Twelve weeks later, when mixed chimerism was established, the CD45.1/CD45.2 ratio was measured in blood myeloid cells, which gave a baseline level of chimerism of ca. 70% CD45.2+ blood myeloid cells (Gr1+CD11b+) in both groups (Figure S3). We then administered TAM and tracked blood populations over time (Figure 6B). When normalized to baseline chimerism, the contribution of control CD45.2+CreERTm−Men1f/f to myeloid, B and T cells remained stable up to 16 weeks after TAM administration. In contrast, the contribution of CD45.2+CreERTm+Men1f/f cells to all lineages progressively decreased (Figures 6B, S3). These findings showed that cell-autonomous effects accounted for the observed hematopoietic abnormalities. When chimerism was analyzed in LT-HSC and downstream populations, the contribution of menin-deficient cells to LT-HSCs was preserved, but dropped progressively along early myeloid differentiation (Figure 6C). The modest consequences of menin loss mostly downstream of LT-HSC in steady-state conditions contrasted with the profound LT-HSC defect during hematopoietic recovery after transplantation (Figure 4). However, when mice were subjected to an additional round of hematopoietic stress through injection of 5-FU, a significant decrease in the contribution of menin-deficient cells to the LT-HSC compartment was apparent (Figure 6D). These findings indicate that menin is required in LT-HSCs specifically in situations of hematopoietic stress, and not in steady-state conditions.
Cell-autonomous basis of multilineage hematopoietic defects after Men1 inactivation. (A) Experimental design. CD45.2+ BM was harvested from CreERTm+Men1f/f or control CreERTm−Men1f/f mice (CD45.2+) without prior TAM, mixed at a 1:1 ratio with competitor CD45.1+ BM and transplanted into lethally irradiated B6.SJL mice. After 12 weeks to establish mixed chimerism, TAM was administered. (B) The contribution of CD45.2+ cells to blood myeloid (Gr1+CD11b+), B (B220+CD19+), and T (Thy1.2+) cells was assessed before administration of TAM and 2, 6, 10, and 16 weeks thereafter. Results are shown as mean plus or minus SEM and normalized to the chimerism observed at baseline. ▵: CD45.1+ competitor and CD45.2+CreERTm−Men1f/f; ▴: CD45.1+ competitor and CD45.2+CreERTm+Men1f/f. (C) Contribution of CD45.2+ cells (% + SEM) to the BM LT-HSC compartment (CD150+CD48−LSK cells), other LSK progenitors containing short-term HSCs, CMPs, and GMPs 16 weeks after TAM administration. (D) Relative contribution of CD45.2+ cells to the CD150+CD48−LSK BM LT-HSC compartment in a group of mice transplanted as described in panel A, followed by challenge with 5-FU. Data were acquired 16 days after 5-FU injection. Results were normalized to the chimerism in the control group. * indicates statistical significance (P < .05).
Cell-autonomous basis of multilineage hematopoietic defects after Men1 inactivation. (A) Experimental design. CD45.2+ BM was harvested from CreERTm+Men1f/f or control CreERTm−Men1f/f mice (CD45.2+) without prior TAM, mixed at a 1:1 ratio with competitor CD45.1+ BM and transplanted into lethally irradiated B6.SJL mice. After 12 weeks to establish mixed chimerism, TAM was administered. (B) The contribution of CD45.2+ cells to blood myeloid (Gr1+CD11b+), B (B220+CD19+), and T (Thy1.2+) cells was assessed before administration of TAM and 2, 6, 10, and 16 weeks thereafter. Results are shown as mean plus or minus SEM and normalized to the chimerism observed at baseline. ▵: CD45.1+ competitor and CD45.2+CreERTm−Men1f/f; ▴: CD45.1+ competitor and CD45.2+CreERTm+Men1f/f. (C) Contribution of CD45.2+ cells (% + SEM) to the BM LT-HSC compartment (CD150+CD48−LSK cells), other LSK progenitors containing short-term HSCs, CMPs, and GMPs 16 weeks after TAM administration. (D) Relative contribution of CD45.2+ cells to the CD150+CD48−LSK BM LT-HSC compartment in a group of mice transplanted as described in panel A, followed by challenge with 5-FU. Data were acquired 16 days after 5-FU injection. Results were normalized to the chimerism in the control group. * indicates statistical significance (P < .05).
Gene expression analysis of menin-deficient hematopoietic progenitors
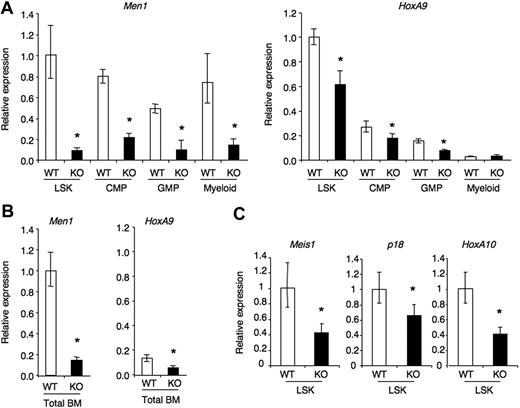
To investigate the potential mechanisms whereby menin regulates hematopoietic progenitors, we studied expression of a selected group of genes that are regulated by MLL and menin during leukemic transformation (Figure 7). Among these, HoxA9 was of particular interest because it is consistently induced by MLL fusion proteins and its overexpression can rescue the consequences of menin loss in leukemic cells.10,25 Furthermore, HoxA9−/− mice have impaired HSC function.26,27 To evaluate the impact of menin loss in HSCs and during differentiation, we harvested RNA from sorted LSK cells, common myeloid progenitors, granulocyte-macrophage progenitors, and BM myeloid cells 4 weeks after TAM administration to CreERTm+Men1f/f or control CreERTm−Men1f/f mice. Quantitative real-time RT-PCR showed a profound decrease in the amount of Men1 mRNA in all these cell subsets (Figure 7A), indicating efficient Men1 inactivation in progenitors that persisted throughout differentiation. HoxA9 expression was highest in the LSK compartment and sharply down-regulated in progressively more differentiated populations (Figure 7A). Menin loss only led to a modest (ca. 40%) decrease in HoxA9 expression in LSK cells as well as downstream progenitors. The effect of menin loss on HoxA9 expression was detectable in total BM cells, although the overall expression was lower (Figure 7B). Other putative targets of MLL and menin in leukemic cells include Meis1, HoxA10, and p18Ink4c. Expression of these genes was also modestly decreased in menin-deficient LSK cells, down approximately to levels that would be observed in mice with heterozygous loss of these genes. Furthermore, when a similar analysis was performed at later time points after TAM inactivation, decreased expression of these genes was less prominent (Meis1, p18Ink4c), or not detectable (HoxA9), despite persistent Men1 inactivation (data not shown). This suggests the existence of time-dependent compensatory mechanisms in HSCs in controlling the expression of Hox genes in the absence of menin. Altogether, menin loss led to a profound defect in HSC function in situations of hematopoietic stress, but only to a modest decrease in the expression of Hox and Hox-related genes that are regulated by menin in MLL fusion protein-transformed leukemic cells.
Gene expression analysis in menin-deficient hematopoietic progenitors. (A) The following cell populations were purified from the BM of control Cre-ERTm−Men1f/f (WT) or Cre-ERTm+Men1f/f (KO) 4 weeks after administration of tamoxifen: Lin−Sca-1hic-Kithi (LSK progenitors, containing HSCs); Lin−Sca-1–c-KithiCD16/32loCD34+ (CMP); Lin−Sca-1–c-KithiCD16/32hiCD34+ (GMP) and Gr1+CD11b+ myeloid cells. Quantitative RT-PCR was used to assess the abundance of Men1 and HoxA9 transcripts, relative to Hprt1. (B) Relative expression of HoxA9 and Men1 in total BM from TAM-treated Cre-ERTm−Men1f/f (WT) or Cre-ERTm+Men1f/f (KO) mice. Data are normalized to the WT LSK shown in panel A. (C) Relative expression of Meis1, p18Ink4c, and HoxA10 in control (WT) and menin-deficient (KO) LSK progenitors. Each sample was assayed in triplicate and expressed after normalization to 1 in WT LSK. Data are representative of 2 to 3 individual mice. Error bars represent the 95% confidence interval. * indicates statistical significance (P < .05).
Gene expression analysis in menin-deficient hematopoietic progenitors. (A) The following cell populations were purified from the BM of control Cre-ERTm−Men1f/f (WT) or Cre-ERTm+Men1f/f (KO) 4 weeks after administration of tamoxifen: Lin−Sca-1hic-Kithi (LSK progenitors, containing HSCs); Lin−Sca-1–c-KithiCD16/32loCD34+ (CMP); Lin−Sca-1–c-KithiCD16/32hiCD34+ (GMP) and Gr1+CD11b+ myeloid cells. Quantitative RT-PCR was used to assess the abundance of Men1 and HoxA9 transcripts, relative to Hprt1. (B) Relative expression of HoxA9 and Men1 in total BM from TAM-treated Cre-ERTm−Men1f/f (WT) or Cre-ERTm+Men1f/f (KO) mice. Data are normalized to the WT LSK shown in panel A. (C) Relative expression of Meis1, p18Ink4c, and HoxA10 in control (WT) and menin-deficient (KO) LSK progenitors. Each sample was assayed in triplicate and expressed after normalization to 1 in WT LSK. Data are representative of 2 to 3 individual mice. Error bars represent the 95% confidence interval. * indicates statistical significance (P < .05).
Discussion
Epigenetic changes stand as an important mechanism to control cellular memory and maintain the stem cell fate. In this regard, Bmi-1, a member of the Polycomb complex that regulates histone H3 lysine 27 methylation, plays a crucial role in regulating HSC function.28,29 In addition, recent progress has identified the biochemical activity of the Trithorax homologue MLL as a histone H3 lysine 4 methyltransferase. The tumor suppressor menin was identified as part of the MLL complex, and its presence appeared critical for epigenetic changes at the Hox locus, Hox gene expression, as well as proliferation of leukemic cells transformed by MLL fusion proteins. We have uncovered a major requirement for menin in supporting HSC function that was most apparent during hematopoietic recovery. Moreover, menin loss impaired the normal development of B lineage progenitors. These findings suggest that lymphoid progenitors are sensitive to the loss of menin even in steady-state conditions, while HSCs show a profound requirement for this protein specifically during hematopoietic stress.
Recent work has identified an important role for MLL in adult HSC function.15,16 Interestingly, loss of MLL led to rapid hematopoietic failure in one report, a more severe phenotype than observed after loss of menin.15 Thus, menin may be essential only for a subset of the physiologic functions of MLL. It remains to be determined if other proteins can substitute for menin to support MLL function in certain conditions, or if MLL can exert some of its effects totally independently of menin. An improved understanding of the precise role of menin in the MLL complex during transcriptional regulation will be important to answer this question. On the other hand, menin has also been isolated in association with MLL2, a MLL homologue with methyltransferase activity.7 Interestingly, a menin-containing MLL2 complex was recently reported to interact with the β-globin locus.30 Therefore, menin may regulate more than one methyltransferase complex in hematopoietic tissues. Finally, menin may act independently of MLL in some contexts. Large-scale mapping of menin binding to genomic sites in HeLa cells and endocrine tissues using chromatin immunoprecipation and microarray analysis has revealed menin binding to multiple sites, sometimes in the absence of MLL complex members.3 If such findings apply to the hematopoiesis, they would suggest that menin may regulate certain genes independently of MLL.
A striking feature of our observations was the severe impairment of LT-HSC function after BMT, as opposed to mild hematopoietic abnormalities without HSC loss in steady-state conditions. Several scenarios could explain these findings. First, menin might sustain epigenetic memory of the stem cell fate during the rapid proliferative burst of HSCs after transplantation. Second, menin may regulate pathways that are only used by HSCs during replicative stress, being essential, for example, to support HSC expansion or survival in these conditions. Third, it was possible that menin would be critical for normal HSC homing to the BM. However, this appears unlikely, as stem/progenitor abnormalities were apparent in menin-deficient mice during recovery from chemoablation, a situation that does not require HSC engraftment. Furthermore, we did not detect any defect in the homing ability of menin-deficient progenitors to the hematopoietic organs of unirradiated or irradiated hosts. This is similar to observations with MLL-deficient progenitors showing no obvious defect in progenitor homing to the BM.16 Future work to explore these possibilities will bring interesting insights, because the regulatory pathways used by HSCs during stress are poorly understood.
Previous reports indicate that, in leukemic cells driven by MLL fusion proteins, MLL and menin regulate the expression of Hox genes, including HoxA9.4,5,10 Up-regulated expression of Hox family members has been suggested to play an essential role in MLL fusion protein-mediated transformation,25,31 although transformation by some MLL fusion proteins in the absence of HoxA9 or HoxA7 alone remained possible.32 Furthermore, loss of MLL in adult hematopoiesis was shown to result in decreased HoxA9 expression, and HoxA9 deficiency caused defects in HSC and lymphoid progenitor function that shared characteristics with the defects observed after menin loss.15,26,27 Surprisingly, however, we only detected a modest impact of menin loss on HoxA9 expression in the HSC compartment. The magnitude of this effect was comparable at most to the decrease in HoxA9 transcripts that would be expected in HoxA9+/− mice. In contrast to HoxA9−/− mice that show impaired HSC function in competitive repopulation assays and leukopenia in steady-state conditions, normal complete blood counts have been observed in HoxA9+/− mice (although the HSC function was not reported).26,27 Thus, the modest decrease in HoxA9 expression observed after Men1 inactivation is unlikely to explain all the hematopoietic defects in these mice, including the abnormalities observed in the peripheral blood. However, it is possible that relatively small decreases in HoxA9 mRNA levels could have disproportionate functional effects when arising acutely, as seen in knockdown experiments. Besides HoxA9, we have observed modest decreases in the expression of other Hox and Hox-related genes after menin loss, such as HoxA10 and Meis1. Individually modest decreases in the expression of several Hox-related genes could have a synergistic effect and account for the HSC defect of menin-deficient progenitors. Alternatively, stress conditions may induce expression of menin-dependent MLL target genes and magnify the differences observed in steady-state conditions. Finally, menin may regulate other targets that are functionally relevant during hematopoietic recovery. In any case, studying menin-deficient HSCs appears as an attractive strategy to identify the Hox-dependent or Hox-independent molecular pathways that are specifically required for the HSC response(s) to hematopoietic stress.
Our studies have uncovered a novel physiologic function for the tumor suppressor menin in hematopoietic stem and progenitor cell homeostasis. Menin was particularly important during hematopoietic recovery after BMT or chemoablation. As menin cooperates with MLL fusion proteins to immortalize leukemia stem cells, yet menin has only modest effects on HSCs in steady-state conditions, our findings suggest the existence of a therapeutic window to target menin or the menin-MLL interaction in leukemia stem cells while sparing adjacent normal stem cells, at least in the absence of hematopoietic stress. This suggests that blocking menin or the menin-MLL interaction during postremission therapy may be an attractive therapeutic strategy to treat leukemias driven by MLL fusion proteins.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Dr Sean Morrison and Dr Jay Hess for advice and critical reading of the manuscript.
This work was supported by grants from the National Institutes of Health (Bethesda, MD) and a Leukemia & Lymphoma Society (White Plains, NY) Specialized Center of Research (SCOR) grant (R01 CA113962 and R01CA100912, X.H. and W.S.P.). I.M. was supported by a grant from the Damon Runyon Cancer Research Foundation (New York, NY; DRG-102-05) and by the University of Michigan's Biological Sciences Scholar Program (Ann Arbor, MI).
National Institutes of Health
Authorship
Contribution: I.M. designed and performed research, analyzed data, and wrote the manuscript; Y.-X.C. designed and performed research, participated in data analysis, and edited the manuscript; A.F., Y.Y., A.T.T., and O.S. performed research; W.S.P. assisted in research design and edited the manuscript; and X.H. designed research, participated in data analysis, and edited the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Ivan Maillard, Center for Stem Cell Biology, Life Sciences Institute, University of Michigan, Ann Arbor, MI 48109; e-mail: imaillar@umich.edu; or Xianxin Hua, Abramson Family Cancer Research Institute, Department of Cancer Biology, University of Pennsylvania, Philadelphia, PA 19104; e-mail: huax@mail.med.upenn.edu.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal