Abstract
A noninferiority study was performed comparing low-dose and standard-dose prophylactic platelet transfusions. A double-blind randomized controlled trial (RCT) was performed in 6 sites in 3 countries. Thrombocytopenic adults requiring prophylactic platelet transfusion were randomly allocated to standard-dose (300-600 × 109 platelets/product) or low-dose (150- < 300 × 109 platelets/product) platelets. The primary outcome (World Health Organization [WHO] bleeding ≥ grade 2) was assessed daily through clinical examination, patient interview, and chart review. A WHO grade was assigned through adjudication. The Data Safety Monitoring Board stopped the study because the difference in the grade 4 bleeding reached the prespecified threshold of 5%. At this time, 129 patients had been randomized and 119 patients were included in the analysis (58 low dose; 61 standard dose). Three patients in the low-dose arm (5.2%) had grade 4 bleeds compared with none in the standard-dose arm. WHO bleeding grade 2 or higher was 49.2% (30/61) in the standard-dose arm and 51.7% (30/58) in the low-dose group (relative risk [RR], 1.052; 95% confidence interval [CI], 0.737-1.502). A higher rate of grade 4 bleeding in patients receiving low-dose prophylactic platelet transfusions resulted in this RCT being stopped. Whether this finding was due to chance or represents a real difference requires further investigation. These clinical studies are registered on http://www.clinicaltrials.gov as NCT00420914.
Introduction
Prophylactic platelet transfusions for patients with chemotherapy-induced thrombocytopenia became the standard of practice following the publication of several small randomized controlled trials in the late 1970s and early 1980s.1-3 The dose of the platelet product transfused was based upon the perceived need to raise the patient's platelet count to a safe level, combined with the feasibility of how many platelets could be recovered from one whole blood donation or collected using apheresis technology then available. For adult patients, this usually resulted in a single apheresis platelet or a pool of 4 to 6 whole blood–derived platelets, with the absolute number of platelets in the range of 300 to 600 × 109.4
Over the years, our understanding of bleeding in these thrombocytopenic patients has advanced and there is now evidence to suggest that patients require only approximately 7100 platelets/μL per day to maintain hemostasis.5 This raises the question as to whether the same degree of efficacy and safety can be achieved by transfusing a low-dose product.6,7 Such a strategy has potential economic and resource advantages as fewer platelet transfusions might be required and donor exposures might be reduced.6 Over the past 10 years, there have been 5 randomized controlled trials that have examined the efficacy of either low-dose or high-dose platelets compared with current standard practice.8-12 These studies suggested that there are higher posttransfusion increments and longer intervals between transfusions when higher doses of platelets are transfused. However, only 1 of the studies actually tested a low-dose strategy,11 and only 2 of these studies evaluated bleeding as an outcome with both studies having low power.11,12 Hence, the question of the optimal platelet dose remains unresolved.13 The BEST Collaborative has undertaken an international multicenter study to investigate 2 different dosing strategies for transfusion of platelets (SToP). The randomized controlled trial (RCT) reported in this paper was designed to demonstrate noninferiority of low-dose compared with standard-dose (current practice) prophylactic platelet transfusions with respect to the frequency of World Health Organization (WHO) grade 2 or higher bleeding in patients with chemotherapy-induced thrombocytopenia.
Methods
This RCT was a multicenter international study undertaken by the BEST Collaborative (http://www.bestcollaborative.org). Six academic teaching hospitals participated in the study: 3 Canadian sites, 1 Norwegian site, and 2 sites in the United States. The protocol for the study was approved by the research ethics board (REB) at all participating sites (Cedars-Sinai Institutional Review Board, Los Angeles; Committee for the Protection of Human Subjects, Dartmouth; Health Sciences/Faculty of Health Sciences, Research Ethics Board, Hamilton; Regional Komité for Medisinsk og Helsefaglig Forskningsetikk, Vest-Norge, Bergen; Hospital Research Ethics Board, Ottawa; and University Health Network Research Ethics Board, Toronto). The study was registered at http://www.clinicaltrials.gov, no. NCT00420914.
Patients were eligible for the study if they met the following inclusion criteria: hypoproliferative thrombocytopenia where the platelet count was expected to be less than 10 × 109/L for a minimum of 10 days; receiving treatment as an inpatient; and weight between 40 and 100 kg. Patients who met the inclusion criteria were excluded if any of the following characteristics were present: diagnosis of acute promyelocytic leukemia; a history or current diagnosis of immune thrombocytopenia, thrombotic thrombocytopenic purpura, or hemolytic uremic syndrome; evidence of WHO grade 2 bleeding or greater at the time of study assessment; indication for bedside leukoreduced platelet components; and pregnancy. Patients who met the eligibility criteria were invited to participate in the study. The rationale and objectives of the study were explained to patients by health care professionals not directly involved in the patients' care. Informed consent was required from all participants in accordance with the Declaration of Helsinki. Patients were allowed to withdraw from the study at any time or at the request of their treating physician. Patients were studied during only one hospital admission.
Two diagnostic groups were included: patients who underwent bone marrow/stem cell transplantation and patients who did not undergo transplantation. Patients who underwent were defined as inpatients undergoing a bone marrow or peripheral blood stem cell transplantation. All other patients were assigned to the nontransplantation strata. The treatment allocation scheme was computer generated, stratified by center and diagnostic group, and allocated through a secure central web-based randomization system. Block randomization was used with variable block sizes within strata to help conceal treatment allocation.
Eligible patients were to be randomized when they required their first study prophylactic platelet transfusion; however, in some cases patients were randomized on a Friday if it was anticipated that their first transfusion would occur on the weekend. Prophylactic platelet transfusions were indicated when the patient's platelet count fell below the trigger routinely used at the participating centers. Although most centers used a trigger of 10 × 109/L, higher triggers were used in special clinical circumstances (eg, sepsis) at the discretion of the treating physician. When the first platelet transfusion was not related to routine prophylaxis (eg, insertion of a Hickman catheter), the patient remained eligible for randomization when the count fell to the trigger threshold.
Interventions
The intervention under investigation was the use of a low-dose platelet component (1.5 to 3.0 × 1011 platelets/product) for prophylactic transfusions. Patients randomized to the standard-dose platelet arm were expected to receive products containing 3.0 to 6.0 × 1011 platelets per transfusion. Prior to study start-up, each center using whole blood–derived platelets used their quality control data to determine the number of platelets required in a pool to ensure that the platelet dose was within the prespecified ranges of the 2 treatment arms. Apheresis platelet collections were targeted so that a low-dose platelet product could be prepared by splitting a standard-dose apheresis platelet unit into 2. The Canadian sites used apheresis platelets (Cobe Spectra; CaridianBCT, Lakewood, CO), and whole blood–derived platelets prepared using the platelet-rich plasma (PRP) method (Nutricell AS3; Pall Medical, Glen Cove, NY), and all platelet units were prestorage leukoreduced. In Norway, the platelets were collected by apheresis (Amicus, version 2.51; Fenwal, Lake Zurich, IL) or made using a buffy coat technique: whole blood collected into a R6492 bag (Baxter Healthcare, Deerfield, IL); 5 buffy coats pooled using the Orbisca method (Gambro BCT, Karlskoga, Sweden); and storage in 65% Intersol (Baxter Healthcare) and 35% plasma. Both US sites used prestorage leukoreduced apheresis platelets (Trima Accel; CaridianBCT).
Duration of follow-up.
Data on the indication for platelet transfusions and signs and symptoms of bleeding were collected daily during each patient's period of thrombocytopenia. Day 0 of the period of thrombocytopenia was the first day that a prophylactic platelet transfusion was required (ie, patient's platelet count below the trigger level), and the last day was defined as the day of one or more of the following: 30 days of follow-up; patient withdrawal; death; or the day of the last platelet transfusion before marrow recovery (defined by a spontaneous recovery of the patient's platelet count to 50 × 109/L or higher).
Outcome measure (primary).
The primary outcome measure was the occurrence of a WHO grade 2 or higher bleed. Information on bleeding was captured from 2 sources. A daily bleeding assessment was performed each morning during the period of thrombocytopenia by personnel who were blinded to the platelet dose assigned to the patient. The assessment consisted of a physical examination for signs of petechiae, purpura, bruising; inspection of intravenous and central line sites for evidence of bleeding; and questioning the patient to determine whether any bleeding had occurred during the previous 24 hours. The patient's chart was also reviewed to capture any documented information related to bleeding in the 24-hour time period prior to the clinical assessment
Grading bleeds.
Bleeding was categorized using the WHO scale grades 1 to 4.14 Specific descriptors for each grade of bleeding were developed a priori (Table 1).
Classification and descriptions of the grades of bleeding
| WHO bleeding grade and characteristics . |
|---|
| Grade 1* |
| Mucocutaneous hemorrhage (oral blood blisters) |
| Petechiae (lesions < 2 mm in size) |
| Purpura < 2.54 cm (1-inch) diameter |
| Ecchymosis (lesions < 10 cm in size) |
| Oropharyngeal bleeding |
| Conjunctival bleeding |
| Epistaxis < 1 hour in duration and not requiring intervention |
| Abnormal vaginal bleeding (nonmenstrual) with spotting (< 2 pads per day) |
| Grade 2* |
| Ecchymosis (lesions > 10 cm in size) |
| Hematoma |
| Epistaxis > 1 hour in duration or packing required |
| Retinal hemorrhage without visual impairment |
| Abnormal vaginal bleeding (not normal menses) using > 2 pads/day |
| Melena, hematemesis, hemoptysis, hematuria, hematochezia |
| Bleeding from invasive sites, musculoskeletal bleeding |
| Grade 3† |
| Melena |
| Hematemesis |
| Hemoptysis |
| Hematuria—including intermittent gross bleeding without clots |
| Abnormal vaginal bleeding |
| Hematochezia |
| Epistaxis |
| Oropharyngeal |
| Bleeding from invasive sites, musculoskeletal bleeding, or soft tissue bleeding |
| Grade 4 |
| Debilitating bleeding including retinal bleeding with visual impairment (defined as a field deficit and there must be a consult note documenting visual impairment) |
| Nonfatal CNS bleeding with neurologic signs and symptoms |
| Fatal bleeding from any source |
| WHO bleeding grade and characteristics . |
|---|
| Grade 1* |
| Mucocutaneous hemorrhage (oral blood blisters) |
| Petechiae (lesions < 2 mm in size) |
| Purpura < 2.54 cm (1-inch) diameter |
| Ecchymosis (lesions < 10 cm in size) |
| Oropharyngeal bleeding |
| Conjunctival bleeding |
| Epistaxis < 1 hour in duration and not requiring intervention |
| Abnormal vaginal bleeding (nonmenstrual) with spotting (< 2 pads per day) |
| Grade 2* |
| Ecchymosis (lesions > 10 cm in size) |
| Hematoma |
| Epistaxis > 1 hour in duration or packing required |
| Retinal hemorrhage without visual impairment |
| Abnormal vaginal bleeding (not normal menses) using > 2 pads/day |
| Melena, hematemesis, hemoptysis, hematuria, hematochezia |
| Bleeding from invasive sites, musculoskeletal bleeding |
| Grade 3† |
| Melena |
| Hematemesis |
| Hemoptysis |
| Hematuria—including intermittent gross bleeding without clots |
| Abnormal vaginal bleeding |
| Hematochezia |
| Epistaxis |
| Oropharyngeal |
| Bleeding from invasive sites, musculoskeletal bleeding, or soft tissue bleeding |
| Grade 4 |
| Debilitating bleeding including retinal bleeding with visual impairment (defined as a field deficit and there must be a consult note documenting visual impairment) |
| Nonfatal CNS bleeding with neurologic signs and symptoms |
| Fatal bleeding from any source |
Does not require red cell transfusion.
Requiring red cell transfusion specifically for support of bleeding within 24 hours of onset.
Adjudication of bleeding.
Data from all bleeding assessments, daily transfusion requirements, and daily blood counts were initially reviewed independently by 2 trained adjudicators who were asked to grade bleeding (WHO grades 1-4), and to identify the site of bleeding (mucocutaneous; gastrointestinal; genitourinary; bronchopulmonary; musculoskeletal and soft tissue; body cavity; central nervous system; invasive site; ocular/eye; and other). If the 2 adjudicators disagreed in their assessments, the data were sent to another adjudicator. If agreement was not reached after 5 adjudicators, a meeting of adjudicators was held to achieve consensus. The adjudicated bleeding data were used in the analysis and for decisions made by the Data Safety Monitoring Board (DSMB).
Secondary outcomes
Additional prespecified secondary outcomes and analyses included the following: the frequency of individual grades of bleeding (grades 1-4); time to first bleed; recurrent event analysis to determine the mean number of bleeding days over time per 100 patients; duration of thrombocytopenia; platelet transfusions requirements; red cell transfusion requirements; the interval between platelet transfusions; and modeling the association between the platelet dose transfused and risk of bleeding over the next 24 hours.
Other data collection
The following baseline information was collected: ethnic origin, weight, height, age, sex, diagnosis, prior pregnancies and/or transfusions, ABO type, HLA antibody screen (if performed), platelet count, hematocrit, hemoglobin, PT, PTT, and fibrinogen. Laboratory test results captured daily included platelet count, hematocrit, and hemoglobin. The following results were recorded only if the tests were performed: prothrombin time (PT), partial thromboplastin time (PTT), fibrinogen, urine, hemoglobin. With each platelet transfusion given the laboratory recorded the product type, age, ABO group, and weight, and performed a platelet count on a sample from the product. The actual platelet dose per transfusion was calculated by correcting the platelet count by the weight × 1.03 (correction factor for viscosity). Red cell transfusion data were also recorded.
Statistical analysis
The frequency of the primary outcome (≥ grade 2 bleeding) was reported for each group.15 The relative risk (RR) of grade 2 or higher bleeding for the low-dose compared with the standard-dose groups was computed with an associated 95% confidence interval. The proportion of patients with at least one bleed of grade 2 or higher was estimated based on the cumulative incidence function and a Cox regression model was fit to yield a relative risk.15-17 Robust methods were used to estimate the rates of platelet transfusions, red blood cell (RBC) transfusions, and bleeding by group, and the groups were compared using robust log-rank test statistics that account for the association in multiple event times from the same patient over time.18,19 Kaplan-Meier estimates were also computed for the duration of thrombocytopenia for each group and these were compared using a Wald test from a Cox model.20,21 The dose-response relationship between the platelet dose transfused and the occurrence of bleeding on the day following the transfusion was examined using generalized estimating equations.22 This exploratory analysis was performed using platelet dose as a continuous variable, using the median as a cut point, and using the data categorized by quartiles and quintiles. These analyses were repeated using the dose adjusted for body weight. All analyses were carried out using SAS version 9.1 (SAS Institute, Cary, NC) and R version 2.6.0 (http://www.R-project.org, University of Auckland).
Sample size
The sample size was calculated based on a hypothesis of noninferiority. The frequency of bleeding in the standard-dose arm was estimated at 30% and the margin of noninferiority was set at an absolute difference of 15%. Therefore, noninferiority would be claimed if the one-sided upper 95% confidence interval for the absolute difference in bleeding risk in the low-dose and the standard-dose arms (low dose minus standard dose) was less than 0.15. Although this margin of noninferiority may seem wide, the international members of the BEST Collaborative felt it was a reasonable value as most bleeding in the composite outcome would be grade 2, and it allowed for a feasible size for the trial. Using a one-sided test with 80% power, it was determined that we would require 268 patients per treatment arm. If the lower limit of a 95% confidence interval excludes zero, then inferiority would be claimed.
Data Safety Monitoring Board
The independent DSMB was composed of an acute care physician, 2 hematologists, and a biostatistician. DSMB members were provided with monthly accrual tables and the cumulative frequency of bleeding events per treatment group by bleeding grade. The DSMB also reviewed all fatal and serious adverse events reported to the coordinating center. A prespecified safety stopping guideline suggested that the study be stopped if the difference in the proportion of patients with grade 4 bleeding between the 2 treatment arms exceeded 5% at any time after 50 patients had been enrolled per arm. The DSMB was blinded to the treatment groups when reviewing data and for the purpose of making decisions about stopping the study.
Results
Six centers participated in the study and recruited patients between October 2003 and June 2007, at which point the DSMB requested that enrollment be temporarily stopped until all bleeds had been adjudicated. This was accomplished by March 2008 at which time the DSMB reviewed the safety data and recommended that the study be terminated.
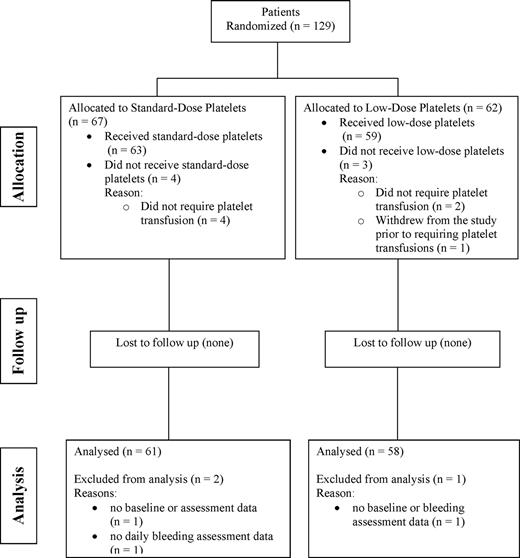
Figure 1 summarizes patient flow through the study. There were 129 patients who met the eligibility criteria and were randomized; however, 7 patients were not included in the analysis as 6 did not require platelet transfusions and 1 patient withdrew prior to receiving platelet transfusions. Hence, 122 patients were eligible for the analysis (59 in the low-dose arm and 63 receiving standard-dose products). Of these patients, 3 could not be included in the analysis due to missing data (standard-dose group n = 2; low-dose group n = 1); hence, 119 patients contributed data to the analyses (58 in the low-dose arm and 61 in the standard-dose arm).
Summary of the number and flow of patients through the various stages of the study.
Summary of the number and flow of patients through the various stages of the study.
The diagnostic categorization of the patients is summarized in Table 2. Most patients had a primary diagnosis of acute leukemia. Overall, 20 patients (16.9%) had received a hematopoietic stem cell/bone marrow transplant (9 patients in the standard-dose arm; 11 patients in the low-dose arm) with 12 (60.0%) of these being allogeneic and 8 (40.0%) being autologous.
Summary of the primary diagnosis on the 119 study patients included in the analyses
| Variable . | Standard-dose platelet arm, no. (%) . | Low-dose platelet arm, no. (%) . | Overall, no. (%) . |
|---|---|---|---|
| Primary diagnosis | |||
| Acute leukemia | 52 (85.2) | 51 (87.9) | 103 (86.6) |
| Chronic leukemia | 2 (3.3) | 0 (0.0) | 2 (1.7) |
| Lymphoma | 3 (4.9) | 4 (6.9) | 7 (5.9) |
| Myelodysplasia | 2 (3.3) | 1 (1.7) | 3 (2.5) |
| Plasma cell dyscrasia | 1 (1.6) | 1 (1.7) | 2 (1.7) |
| Nonhematopoietic solid tumors | 0 (0.0) | 1 (1.7) | 1 (0.8) |
| Other | 1 (1.6) | 0 (0.0) | 1 (0.8) |
| Overall | 61 | 58 | 119 |
| Acute leukemia | |||
| ALL | 5 (9.6) | 4 (7.8) | 9 (8.7) |
| AML | 47 (90.4) | 47 (92.2) | 94 (91.3) |
| Overall | 52 | 51 | 103 |
| Chronic leukemia/hairy cell leukemia | |||
| CML | 2 (100.0) | 0 (0.0) | 2 (0.0) |
| Overall | 2 | 0 | 2 |
| Lymphoma | |||
| Non-Hodgkin lymphoma | 2 (66.7) | 4 (100.0) | 6 (85.7) |
| Hodgkin lymphoma | 1 (33.3) | 0 (0.0) | 1 (14.3) |
| Overall | 3 | 4 | 7 |
| Nonhematopoietic solid tumor | |||
| Carcinoma | 0 (0.0) | 1 (100.0) | 1 (100.0) |
| Overall | 0 | 1 | 1 |
| Variable . | Standard-dose platelet arm, no. (%) . | Low-dose platelet arm, no. (%) . | Overall, no. (%) . |
|---|---|---|---|
| Primary diagnosis | |||
| Acute leukemia | 52 (85.2) | 51 (87.9) | 103 (86.6) |
| Chronic leukemia | 2 (3.3) | 0 (0.0) | 2 (1.7) |
| Lymphoma | 3 (4.9) | 4 (6.9) | 7 (5.9) |
| Myelodysplasia | 2 (3.3) | 1 (1.7) | 3 (2.5) |
| Plasma cell dyscrasia | 1 (1.6) | 1 (1.7) | 2 (1.7) |
| Nonhematopoietic solid tumors | 0 (0.0) | 1 (1.7) | 1 (0.8) |
| Other | 1 (1.6) | 0 (0.0) | 1 (0.8) |
| Overall | 61 | 58 | 119 |
| Acute leukemia | |||
| ALL | 5 (9.6) | 4 (7.8) | 9 (8.7) |
| AML | 47 (90.4) | 47 (92.2) | 94 (91.3) |
| Overall | 52 | 51 | 103 |
| Chronic leukemia/hairy cell leukemia | |||
| CML | 2 (100.0) | 0 (0.0) | 2 (0.0) |
| Overall | 2 | 0 | 2 |
| Lymphoma | |||
| Non-Hodgkin lymphoma | 2 (66.7) | 4 (100.0) | 6 (85.7) |
| Hodgkin lymphoma | 1 (33.3) | 0 (0.0) | 1 (14.3) |
| Overall | 3 | 4 | 7 |
| Nonhematopoietic solid tumor | |||
| Carcinoma | 0 (0.0) | 1 (100.0) | 1 (100.0) |
| Overall | 0 | 1 | 1 |
ALL indicates acute lymphoblastic leukemia; and CML, chronic myelogenous leukemia.
The demographic and baseline characteristics of the patients are summarized in Table 3. In each treatment group there was a similar distribution of characteristics with the exception of the mean (SD) platelet count at baseline, which was lower in the low-dose arm compared with the standard-dose group (31 × 109/L [SD26] vs 46 × 109/L [SD62]). This finding may be due to chance and the clinical relevance of this finding is unknown. Most patients (109/119; 91.6%) remained in the study until at least one of the prespecified criteria for the end of the period of thrombocytopenia; however, there were 10 patients who withdrew from the study early for the following reasons: patient decision to withdraw (n = 3: 2 standard dose; 1 low dose); and physician decision to withdraw (n = 7: 1 standard dose; 6 low dose). Data from these patients were included in the analysis up until the time of withdrawal.
Summary of the demographic and baseline characteristics of the 2 treatment groups
| Variable . | Treatment group . | Overall . | |
|---|---|---|---|
| Standard dose . | Low dose . | ||
| Sex, no. (%) | |||
| Male | 40 (65.6) | 37 (63.8) | 77 (64.7) |
| Female | 21 (34.4) | 21 (36.2) | 42 (35.3) |
| Overall no. | 61 | 58 | 119 |
| Ethnic origin, no. (%) | |||
| White | 56 (91.8) | 55 (94.8) | 111 (93.3) |
| African American or black | 1 (1.6) | 0 (0.0) | 1 (0.8) |
| American Indian/Alaska native | 1 (1.6) | 0 (0.0) | 1 (0.8) |
| Asian | 1 (1.6) | 0 (0.0) | 1 (0.8) |
| Other | 2 (3.3) | 3 (5.2) | 5 (4.2) |
| Overall no. | 61 | 58 | 119 |
| Previous transfusion, proportion (%) | |||
| Platelets | 33/61 (54.1) | 35/56 (62.5) | 68/117 (58.1) |
| Red cells | 45/60 (75.0) | 46/56 (82.1) | 91/116 (78.4) |
| Previous pregnancies, proportion (%) | |||
| Yes | 17/61 (27.9) | 15/57 (26.3) | 32/118 (27.1) |
| Blood group, no. (%) | |||
| O | 22 (36.1) | 18 (31.0) | 40 (33.6) |
| A | 35 (57.4) | 28 (48.3) | 63 (52.9) |
| B | 4 (6.6) | 9 (15.5) | 13 (10.9) |
| AB | 0 (0.0) | 3 (5.2) | 3 (2.5) |
| Overall no. | 61 | 58 | 119 |
| Rh type, no. (%) | |||
| Positive | 47 (77.0) | 48 (82.8) | 95 (79.8) |
| Negative | 14 (23.0) | 10 (17.2) | 24 (20.2) |
| Overall no. | 61 | 56 | 119 |
| Mean height, cm (SD) | 169 (10) | 171 (9) | 170 (10) |
| Mean weight, kg (SD) | 75.2 (15.6) | 78.7 (14.0) | 76.9 (14.9) |
| Mean baseline platelet count, ×109/L (SD) | 46 (62) | 31 (26) | 38 (48) |
| Variable . | Treatment group . | Overall . | |
|---|---|---|---|
| Standard dose . | Low dose . | ||
| Sex, no. (%) | |||
| Male | 40 (65.6) | 37 (63.8) | 77 (64.7) |
| Female | 21 (34.4) | 21 (36.2) | 42 (35.3) |
| Overall no. | 61 | 58 | 119 |
| Ethnic origin, no. (%) | |||
| White | 56 (91.8) | 55 (94.8) | 111 (93.3) |
| African American or black | 1 (1.6) | 0 (0.0) | 1 (0.8) |
| American Indian/Alaska native | 1 (1.6) | 0 (0.0) | 1 (0.8) |
| Asian | 1 (1.6) | 0 (0.0) | 1 (0.8) |
| Other | 2 (3.3) | 3 (5.2) | 5 (4.2) |
| Overall no. | 61 | 58 | 119 |
| Previous transfusion, proportion (%) | |||
| Platelets | 33/61 (54.1) | 35/56 (62.5) | 68/117 (58.1) |
| Red cells | 45/60 (75.0) | 46/56 (82.1) | 91/116 (78.4) |
| Previous pregnancies, proportion (%) | |||
| Yes | 17/61 (27.9) | 15/57 (26.3) | 32/118 (27.1) |
| Blood group, no. (%) | |||
| O | 22 (36.1) | 18 (31.0) | 40 (33.6) |
| A | 35 (57.4) | 28 (48.3) | 63 (52.9) |
| B | 4 (6.6) | 9 (15.5) | 13 (10.9) |
| AB | 0 (0.0) | 3 (5.2) | 3 (2.5) |
| Overall no. | 61 | 58 | 119 |
| Rh type, no. (%) | |||
| Positive | 47 (77.0) | 48 (82.8) | 95 (79.8) |
| Negative | 14 (23.0) | 10 (17.2) | 24 (20.2) |
| Overall no. | 61 | 56 | 119 |
| Mean height, cm (SD) | 169 (10) | 171 (9) | 170 (10) |
| Mean weight, kg (SD) | 75.2 (15.6) | 78.7 (14.0) | 76.9 (14.9) |
| Mean baseline platelet count, ×109/L (SD) | 46 (62) | 31 (26) | 38 (48) |
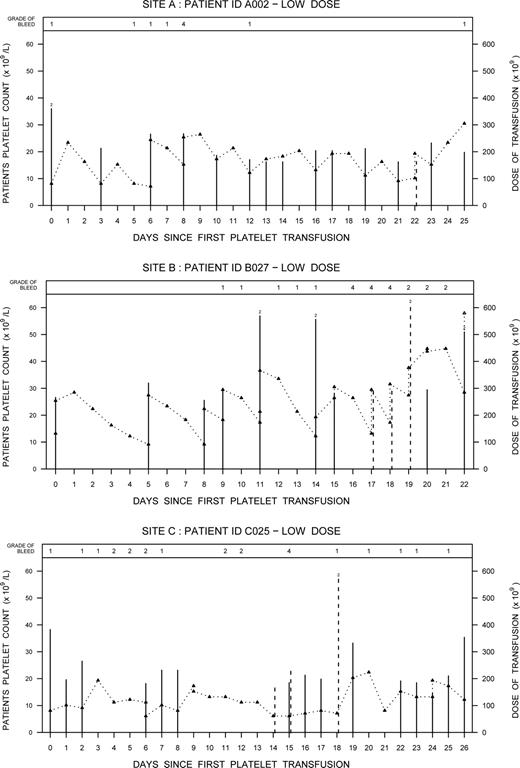
The frequency of grade 4 bleeding was 5.2% (3/58) in the low-dose arm (95% confidence interval [CI], 0.011-0.144) and 0% (0/61) in the standard-dose arm (95% CI, 0.000-0.059), which satisfied the condition in the informal stopping rule. Two of the 3 cases of grade 4 bleeding involved retinal bleeds with visual impairment confirmed through an ophthalmologic assessment. The third case involved a cerebral bleed. All 3 cases occurred at different participating sites: one site used only apheresis platelets, one site used buffy coat platelets, and the third site used both whole blood–derived PRP and apheresis platelets. Figure 2 summarizes the platelet counts, platelet dose transfused, and the occurrence of bleeds for the 3 patients. Relevant clinical information for each case is also summarized.
Event graphs for the 3 patients with grade 4 bleeds showing the patients' daily platelet counts (Δ) and the dose of platelets transfused during their thrombocytopenia periods. Solid lines represent prophylactic platelet transfusions and dashed lines indicate therapeutic platelet transfusions. When 2 platelet transfusions were given on the same day, the additive dose is plotted and a “2” is depicted at the top of the line.
Event graphs for the 3 patients with grade 4 bleeds showing the patients' daily platelet counts (Δ) and the dose of platelets transfused during their thrombocytopenia periods. Solid lines represent prophylactic platelet transfusions and dashed lines indicate therapeutic platelet transfusions. When 2 platelet transfusions were given on the same day, the additive dose is plotted and a “2” is depicted at the top of the line.
Case A002
A 69-year-old man with relapsed acute myeloid leukemia (AML) (weight, 99.5 kg) developed bilateral retinal hemorrhage with vision impairment on day 8 of the study. Concurrent clinical conditions included tracheal bronchitis and pneumonia. The patient remained on the study and over the next 2 weeks the hemorrhage and vision impairment resolved.
Case B027
A 36-year-old man with AML (weight, 76 kg) developed a right retinal hemorrhage with vision impairment on day 16 of the study. Concurrent clinical conditions included pneumonia and infection at the site of the Hickman catheter. On days 11 and 14 (prior to the bleed) the patient had received 2 platelet transfusions per day representing a total daily dose more than 550 × 109. The patient remained on the study after the grade 4 bleed but was transfused at a higher trigger. The patient's vision improved over time.
Case C025
A 52-year-old woman with AML (weight, 78.2 kg) developed a left temporal subdural hemorrhage presenting with slurred speech and right arm weakness on day 15 of the study. Concurrent clinical conditions included febrile neutropenia, insulin-dependent diabetes mellitus, peripheral vascular disease, and hypertension. The platelet dose and trigger threshold were increased as needed and the patient remained on the study.
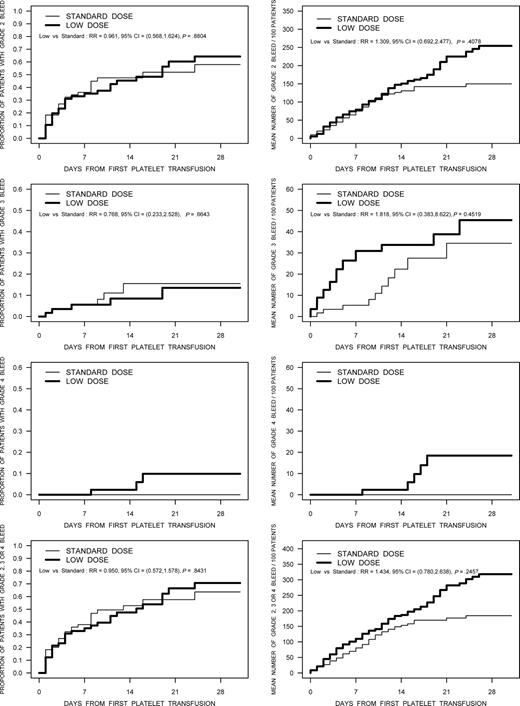
The proportion of patients with bleeding WHO grade 2 or higher was 51.7% (30/58) in the low-dose treatment arm, and 49.2% (30/61) in the standard-dose group (RR, 1.052; 95% CI, 0.737-1.502). The frequencies of bleeding by WHO grade in the 2 treatment arms are summarized in Table 4. The frequency of bleeding by grade for the individual country participants was similar, with the only exception being a higher frequency of grade 1 bleeds documented by the Canadian sites (96.1% of patients), compared with Norway (59.1%) and the United States (71.4%). In addition to the dichotomous description of bleeding, the distribution of time-to-first bleed and recurrent event analyses of the expected number of bleeds per 100 patients were analyzed for various grades of bleeding (Figure 3). None of these comparisons were statistically significant; however, in the recurrent event analyses there were trends toward a higher bleeding rate in the low-dose platelet arm for bleeding of grades 2 through 4.
Frequency of bleeding (WHO grades 1-4) in the 2 treatment groups
| Type of bleeding . | Treatment group . | |
|---|---|---|
| Standard dose, n = 61 . | Low dose, n = 58 . | |
| No. of patients with bleeding (%) | ||
| Grade 1 | 48 (78.7) | 53 (91.4) |
| Grade 2 | 28 (45.9) | 28 (48.3) |
| Grade 3 | 6 (9.8) | 5 (8.6) |
| Grade 4 | 0 (0.0) | 3 (5.2) |
| Grade 2 or higher | 30 (49.2) | 30 (51.7) |
| Percentage of days with bleeding (proportion)* | ||
| Grade 2 or higher | 8.5 (73/854) | 12.1 (111/918) |
| Grade 4 | 0.0 (0/854) | 0.5 (5/918) |
| Type of bleeding . | Treatment group . | |
|---|---|---|
| Standard dose, n = 61 . | Low dose, n = 58 . | |
| No. of patients with bleeding (%) | ||
| Grade 1 | 48 (78.7) | 53 (91.4) |
| Grade 2 | 28 (45.9) | 28 (48.3) |
| Grade 3 | 6 (9.8) | 5 (8.6) |
| Grade 4 | 0 (0.0) | 3 (5.2) |
| Grade 2 or higher | 30 (49.2) | 30 (51.7) |
| Percentage of days with bleeding (proportion)* | ||
| Grade 2 or higher | 8.5 (73/854) | 12.1 (111/918) |
| Grade 4 | 0.0 (0/854) | 0.5 (5/918) |
Expressed as a percentage of thrombocytopenic days.
The results of analyses using time to first bleed and the recurrent event analysis for bleeding designated as grade 2, grade 3, grade 4, and grade 2 or higher bleeding are shown in these figures. The figures in the left column are the time-to-first bleed analysis. The figures in the right column are the results of the recurrent event analysis.
The results of analyses using time to first bleed and the recurrent event analysis for bleeding designated as grade 2, grade 3, grade 4, and grade 2 or higher bleeding are shown in these figures. The figures in the left column are the time-to-first bleed analysis. The figures in the right column are the results of the recurrent event analysis.
Adjudication of bleeding presented challenges as discrepancies between the first 2 adjudicators occurred in 39% (433/1150) of the bleeding days adjudicated. However, through consensus, agreement could be reached. Most of the discrepancies occurred between the grade 1 and grade 2 classifications. Agreement was reached after 3 adjudicators for each of the 3 grade 4 bleeding events. The discrepancies between the adjudicators were due to a technical problem whereby the first adjudicator received incomplete information.
The platelet transfusions in each treatment group are summarized in Table 5. The triggers used for prophylactic platelet transfusions tended to be higher in the low-dose treatment group with 35.9% of transfusions (158/440) given at a trigger of 16 × 109/L or more compared with 24.7% (66/267) in the standard-dose group. The mean age of the platelets transfused was similar in the standard- and low-dose arms (3.4 ± 0.8 [SD] days and 3.6 ± 0.7 [SD] days, respectively). The total number of platelet transfusion episodes was 553 in the low-dose arm and 325 in the standard-dose group. The mean number of platelet transfusion episodes during the period of thrombocytopenia was significantly higher in the low-dose treatment group compared with standard (9.5 ± 7.8 [SD] vs 5.3 ± 3.3 [SD]; P < .001). The total number of platelet donor exposures was 1354 in the standard-dose arm and 1524 in the low-dose arm (P = .335). Most of the difference observed was due to more therapeutic platelet transfusions in the low-dose arm (low dose, 267; standard dose, 150). Total red cell transfusion requirements were not significantly different between the 2 treatment groups: standard dose, 176 red cells transfused; low dose, 208. The mean difference in red cell transfusions per thrombocytopenic day (low-standard) was −0.1 (95% CI, −0.06 to 0.04; P = .723).
Summary information of platelet transfusions per treatment group
| Characteristic . | Standard-dose platelet arm, n = 61 . | Low-dose platelet arm, n = 58 . | Mean difference, low dose minus standard dose (95% CI) . | P . |
|---|---|---|---|---|
| Mean (SD) duration of thrombocytopenia, d | 14.0 (9.1) | 15.8 (9.3) | 1.8 (−1.5-5.2) | .281 |
| Platelet transfusion episodes | ||||
| Prophylactic and therapeutic | ||||
| Total no. | 325 | 553 | ||
| Mean (SD) | 5.3 (3.2) | 9.5 (7.8) | 4.2 (2.0-6.4) | .001 |
| Prophylactic only | ||||
| Total no. | 274 | 447 | ||
| Mean (SD) | 4.5 (2.6) | 7.7 (7.1) | 3.2 (1.2-5.2) | .002 |
| Mean no. of platelet transfusions per thrombocytopenic day (SD) | ||||
| Prophylactic and therapeutic | 0.5 (0.3) | 0.6 (0.3) | 0.1 (0.1-0.3) | < .002 |
| Prophylactic only | 0.4 (0.2) | 0.5 (0.3) | 0.1 (0.01-0.2) | .039 |
| Donor exposures | ||||
| All transfusions, prophylactic and therapeutic | ||||
| Total no. | 1354 | 1524 | ||
| Mean (SD) | 22.2 (15.6) | 26.3 (28.2) | 4.1 (−4.3-12.4) | .335 |
| Prophylactic platelet transfusions only | ||||
| Total no. | 1203 | 1254 | ||
| Mean (SD) | 20.1 (15.0) | 22.8 (25.5) | 2.8 (−5.1-10.6) | .487 |
| Mean (SD) interval between platelet transfusions, d | 2.8 (1.8) | 1.8 (1.1) | −1.0 (−1.2, −0.7) | < .001 |
| Characteristic . | Standard-dose platelet arm, n = 61 . | Low-dose platelet arm, n = 58 . | Mean difference, low dose minus standard dose (95% CI) . | P . |
|---|---|---|---|---|
| Mean (SD) duration of thrombocytopenia, d | 14.0 (9.1) | 15.8 (9.3) | 1.8 (−1.5-5.2) | .281 |
| Platelet transfusion episodes | ||||
| Prophylactic and therapeutic | ||||
| Total no. | 325 | 553 | ||
| Mean (SD) | 5.3 (3.2) | 9.5 (7.8) | 4.2 (2.0-6.4) | .001 |
| Prophylactic only | ||||
| Total no. | 274 | 447 | ||
| Mean (SD) | 4.5 (2.6) | 7.7 (7.1) | 3.2 (1.2-5.2) | .002 |
| Mean no. of platelet transfusions per thrombocytopenic day (SD) | ||||
| Prophylactic and therapeutic | 0.5 (0.3) | 0.6 (0.3) | 0.1 (0.1-0.3) | < .002 |
| Prophylactic only | 0.4 (0.2) | 0.5 (0.3) | 0.1 (0.01-0.2) | .039 |
| Donor exposures | ||||
| All transfusions, prophylactic and therapeutic | ||||
| Total no. | 1354 | 1524 | ||
| Mean (SD) | 22.2 (15.6) | 26.3 (28.2) | 4.1 (−4.3-12.4) | .335 |
| Prophylactic platelet transfusions only | ||||
| Total no. | 1203 | 1254 | ||
| Mean (SD) | 20.1 (15.0) | 22.8 (25.5) | 2.8 (−5.1-10.6) | .487 |
| Mean (SD) interval between platelet transfusions, d | 2.8 (1.8) | 1.8 (1.1) | −1.0 (−1.2, −0.7) | < .001 |
The mean (SD) duration of thrombocytopenia was 15.8 (9.3) days and 14.0 (9.1) days for the low- and standard-dose groups, respectively (mean difference, 1.8; 95% CI, −1.5 to 5.2).
It was difficult for centers to consistently achieve a platelet dose within the prespecified range for each treatment arm. Overlap in dose distributions was seen in all 6 centers, but was highest in the centers preparing platelets from PRP. Overall, in the low-dose treatment group, 27.4% (101/368) of the prophylactic platelet transfusions were outside the predesignated range: 2.7% below 150 × 109 platelets/product (n = 10) and 24.7% above 300 × 109 platelets/product (n = 91). In the standard-dose group, 20.0% (51/255) of the prophylactic platelet transfusions were outside the predefined range: 6.7% below 300 × 109 platelets/product (n = 17) and 13.3% above the upper threshold of 600 × 109 platelet/product (n = 34).
Data from both treatment groups were combined to assess the relationship between prophylactic platelet dose and bleeding status on the following day. When platelet dose was treated as a continuous variable (odds ratio [OR], 0.98; 95% CI, 0.94-1.03) or as a categoric variable (OR, 0.98; 95% CI, 0.29-3.32), there was no significant relationship detected between dose and bleeding status on the following day.
Discussion
Current practice when providing prophylactic platelet transfusion support to patients with chemotherapy-induced thrombocytopenia is to transfuse a dose in the range of 300 to 600 × 109 platelets with each transfusion.4 Because this study was designed to explore the safety of shifting to a lower dose strategy, it was necessary to establish a DSMB and to establish a stopping rule that the DSMB could use for making decisions around safety. We selected a stopping rule that was based on grade 4 bleeding alone as this type of bleeding is clinically relevant and can result in severe (often permanent) morbidity or mortality. The stopping rule developed for this study was selected somewhat arbitrarily and designed to err on the side of patients' safety. It was not based on the need for statistical significance as grade 4 bleeds are rare,23,24 requiring many patients for a stopping rule based on statistical significance. To avoid the impact of high percentages resulting from a few events in a small number of patients, the 5% rule was initiated only after 50 patients had been enrolled in each arm. The investigators also felt that a 5% difference was meaningful as the literature estimates grade 4 bleeding to occur in 1% to 2% of this population.23,24 All grade 4 bleeds (n = 3) occurred in the low-dose treatment arm. This trending of severe bleeds to occur only in one treatment group and starting early in the study raised concerns for the DSMB, and when the 5% threshold was observed the study was stopped. Whether this difference is real or occurred by chance cannot be determined from this study. We looked at the association between actual platelet dose transfused and risk of bleeding within the next 24 hours and found no evidence of an association, which is consistent with the possibility that the grade 4 bleeding observed in the low-dose arm was in fact due to chance. However, supporting the suggestion that this difference may be real are the observations that the frequency of other types of bleeding (grade 1 and grade 2) were also higher with low-dose platelets and the recurrent event analysis, which captures the total burden of bleeding, trended in favor of a higher event rate in the low-dose arm.
The ethical rationale for performing a noninferiority study requires some hypothesis of benefit. Prior to initiating the study, we hypothesized 2 areas of potential benefit. First, we hypothesized that a low-dose platelet strategy would result in fewer platelets transfused and fewer donor exposures. Our findings indicate that a low-dose transfusion strategy resulted in significantly more platelet transfusion episodes, which is an observation consistent with reports from several other studies.8,9 From an operational perspective additional resources would be required for both the laboratory and the clinical areas to prepare, infuse, and monitor these additional transfusion events. The number of donor exposures from platelet transfusions was not significantly different between the 2 dosing strategies, but there was a trend for the low-dose group to require more therapeutic platelet transfusions. Second, it has been suggested that the period of thrombocytopenia may be shorter for patients who receive fewer platelets and/or who are maintained at a lower mean platelet count.25 The rationale for this hypothesis is that thrombopoietin can bind to circulating platelets, decreasing the systemic concentration of this growth factor available to stimulate the marrow for platelet production.26 Hence, patients receiving a higher dose of platelets through transfusion might be expected to have lower levels of thrombopoietin and longer periods of thrombocytopenia. To our knowledge, there are no human clinical data to validate this hypothesis and indeed the results from this study do not support this concept. The periods of thrombocytopenia were similar in both treatment groups. Hence, the hypothesized benefits from a low-dose platelet transfusion strategy are not supported by the results from our study.
The dosing strategy that was used in this study differed from the study that is being conducted by the Transfusion Medicine/Hemostasis Clinical Trials Network in the United States.25 In the US study, both children and adults were included so a decision was made to dose each transfusion based on the patient's body surface area. This study evaluated 3 dosing strategies for prophylactic platelet transfusions: low dose at 1.1 × 1011 platelets/m2 body surface area (BSA); standard dose at 2.2 × 1011 platelets/m2 BSA; and high dose at 4.4 × 1011 platelets/m2 BSA. In our study, we used a dosing strategy where each participating laboratory selected the number of whole blood–derived platelets to include in the pool to aim for a targeted low or standard dose, or split an apheresis platelet to give 2 low-dose products. Our approach was selected for several reasons: first, it has been shown that counting platelets is variable depending on the automated instrument being used compromising a reliable and precise measurement of dose,27-29 and second, we wanted to investigate a strategy that would be easily adaptable for implementation by the Transfusion Service Laboratory. The results clearly showed that this approach does not allow one to consistently target a dose within a specific range. There was overlap between the actual platelet dose transfused in the 2 treatment arms with 152 (24.4%) of 623 transfusions falling outside of the targeted dose. There are several reasons to explain this variation: the natural variability in donor platelet counts results in whole blood–derived products with a wide range of concentrations,30 and, the well-documented differences that can be obtained depending on the counting instrument used.27-29 These findings suggest that dosing platelets based on quality control data is not a reliable strategy for ensuring a standardized dose. Indeed, had this study not been stopped early for safety reasons, the ability to demonstrate noninferiority would have been compromised by the overlap in actual dose between the 2 groups.
It is also important to understand the rational for using WHO bleeding grade 2 or higher as the primary end point for this study. This end point was selected for several reasons. First, it has been the outcome used in recent platelet studies that has been accepted as the current gold standard by the transfusion community and regulatory bodies.11,25,31 Second, from a biologic perspective grade 2 bleeding is felt to be related to the patient's platelet count; hence, it has been used as a surrogate measure for more severe bleeding.32 Finally, it allowed for a feasible sample size as the baseline rate of grade 2 or higher bleeding was estimated a priori to be approximately 30% rather than the low frequency of 1% to 2% reported with grade 4 bleeding. However, one could question the actual clinical relevance of grade 2 bleeding especially when it is combined into a composite outcome with more severe bleeding grades. An analysis of the bleeding data from the transfusion trigger study published by Rebulla et al23 did not demonstrate that the presence of grade 2 bleeding increased the risk of grades 3 or 4 bleeding on the following day, suggesting that it may not be appropriate to combine grades 2, 3, and 4 bleeding into a single outcome.33 The adjudication of bleeding events was also challenging with initially minimal agreement between adjudicators; however, the discrepancies involved predominately grade 1 and grade 2 designations and could be resolved through consensus (detailed information to be presented in a separate report). We also acknowledge that the clinical relevance of the WHO bleeding scale is potentially questionable. Retinal bleeding with vision impairment (observed in 2 of the 3 patients with grade 4 bleeds) is categorized as a grade 4 bleed, although in both of these patients their vision impairment eventually resolved; hence, it was not catastrophic. Recently it has been suggested that mortality could be used as a more clinically relevant outcome for platelet transfusion studies.34 However, assigning a cause for mortality in patients with hematologic malignancies is complicated. Whether this becomes the gold standard for future platelet transfusion studies remains to be seen.
There are several limitations with this study. The generalizability of the study results is limited by the fact that centers were not able to collect demographic information on all patients who were screened for eligibility. Without this information there is no way to assess the potential of a selection bias related to study recruitment. Efforts were made to maintain a blinded study design; however, the fact that 7 patients were prematurely removed from the study at the request of the treating physician (6 in the low-dose arm) raises the possibility that blinding of health care providers may not have always been achieved. Recruitment at many of the sites was slow and sometimes interrupted due to limited resources; however, we cannot eliminate a potential recruitment bias. The issue of power due to the study being stopped prematurely also limits any conclusions about noninferiority. The clinical relevance of the primary outcome, the ability to grade bleeding, and the difficulties in providing a targeted dose are all potential limitations that have been discussed; however, despite these limitations the study provides useful information to inform future studies and allow for more effective clinical trial designs.
In summary, based on the study results we cannot make any conclusion about the noninferiority of low-dose platelets related to bleeding. Although 3 patients in the low-dose treatment arm had bleeds categorized as grade 4, all 3 patients remained on the study and had no long-term morbidity related to these bleeding events. However, even if a low-dose platelet transfusion strategy is safe (not inferior), the proposed benefits (fewer products being a transfused and a shorter duration of thrombocytopenia) were not found using the dosing strategy that was applied in this study.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors acknowledge Dr Sherrill Slichter, who conceived of the idea for this study and actively participated in the protocol development, as well as the late Dr Scott Murphy for his constant support and encouragement throughout the design and implementation phases of this study. Special thanks to the study personnel at each site who participated in recruitment and data collection; the individuals who participated in the adjudication of bleeding events; and the members of the Data Safety Monitoring Board (all groups listed in the Supplemental Appendix, available on the Blood website; see the Supplemental Materials link at the top of the online article).
Canadian Blood Services is acknowledged for the funding that supported coordination for the study and participation by 3 Canadian sites. R.J.C. holds a Canada Research Chair in Statistical Methods for Health Research.
Authorship
Contribution: N.M.H. designed the research proposal, performed research, and wrote the paper; R.J.C. designed the research proposal and data analysis, and contributed to writing the paper; A.T., C.T.K., E.K., J.M.B., and Z.M.S. performed research (site investigator) and provided feedback on the paper; T.H. designed the research proposal, performed research (site investigator), and provided feedback on the paper; J.P.A. provided input into study design, performed research (site investigator), and provided feedback on the paper; R.L.B. coordinated the study, managed the database, and provided feedback on the paper; and K.-A.L. performed analysis and provided feedback on the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
A complete list of the participating SToP Study Investigators of the BEST Collaborative appears in the Supplemental Appendix.
Correspondence: Nancy Heddle, Department of Medicine, McMaster University, 1200 Main Street West, HSC-3N43, Hamilton, ON L8N 3Z5; e-mail: heddlen@mcmaster.ca.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal