Abstract
The production of mature cells necessitates that lineage-committed progenitor cells be constantly generated from multipotential progenitors. In addition, the ability to respond rapidly to physiologic stresses requires that the signals that regulate the maintenance of progenitor populations be coordinated with the signals that promote differentiation of progenitors. Here we examine the signals that are necessary for the maintenance of the BMP4-dependent stress erythropoiesis pathway. Our previous work demonstrated that BMP4, stem cell factor, and hypoxia act in concert to promote the expansion of a specialized population of stress erythroid progenitors in the spleen during the recovery from acute anemia. Our analysis shows that acute anemia leads to an almost complete mobilization of BMP4-responsive stress erythroid burst-forming units; therefore, new stress progenitors must be recruited to the spleen to replenish this system. We show that bone marrow cells can home to the spleen and, in response to a signal in the spleen microenvironment, Hedgehog, they develop into BMP4-responsive stress progenitors. Hedgehog induces the expression of BMP4, and together these 2 signals are required for the development of BMP4-responsive stress progenitors. These data demonstrate that the interplay between these 2 signals is crucial for maintenance of this stress response pathway.
Introduction
Acute anemia induces a systemic response designed to increase the transport of oxygen to hypoxic tissues. One aspect of this response is the increased production of erythrocytes.1 Our previous analysis has demonstrated that acute anemia leads to the rapid expansion and differentiation of a specialized population of stress erythroid progenitors in the spleen.2 These progenitors are resident in the spleen. However, their differentiation is tightly regulated and only occurs at times of acute erythropoietic need. Part of this regulation stems from the fact that this response requires 3 signals (BMP4, stem cell factor [SCF], and hypoxia), and the expression BMP4 is in part regulated by hypoxia.2,3 In response to these 3 signals, stress erythroid burst-forming units (BFU-E) are rapidly expanded in the spleen, which in vivo translates into a 45-fold increase in stress BFU-E. These progenitors are ideally suited to respond to acute anemia in that they exhibit a greater potential to generate new erythrocytes than bone marrow steady-state progenitors.2,3
Stress response pathways by definition must be able to transiently mount a response to alleviate a physiologic stress, but once equilibrium is restored, the pathway is inactivated.1 In addition, stress response pathways must have a mechanism by which they are able to maintain readiness for subsequent challenges. In this work, we extend our analysis of the BMP4-dependent stress erythropoiesis pathway by investigating the mechanisms that regulate the maintenance of stress erythroid progenitors in the murine spleen. Here we find that acute anemia mobilizes essentially all progenitors that can respond to BMP4, which results in a loss of responsiveness to immediate rechallenge with acute anemia. We observe that, after recovery from anemia, the BMP4-responsive stress progenitors are replenished. Although our previous work showed that bone marrow cells do not respond to BMP4 like spleen stress erythroid progenitors,2 here we show that bone marrow cells can home to the spleen and differentiate into splenic BMP4-responsive stress erythroid progenitors. These data suggest that the spleen microenvironment contains a signal that promotes the development of stress erythroid progenitors. We have identified that signal as Hedgehog. Treatment of bone marrow cells with Hedgehog induces BMP4 expression, and these 2 signals act in concert to promote the development of BMP4-responsive stress erythroid progenitors. Furthermore, mutations that block Hedgehog signaling compromise the development of BMP4-responsive stress erythroid progenitors in the spleen after recovery from acute anemia and render the mice incapable of responding to subsequent anemic challenges. Taken together, our data show that BMP4 and Hedgehog are specific signals in the spleen that are required to maintain extramedullary stress erythroid progenitors.
Methods
Mice
C57BL/6 and B6.SJLPtprcaPep3b/BoyJ (CD45.1) mice were bred in our colony. The WBB6F1-KitW/Wv mice and the Smoothened (Smo) conditional allele Smotm2amc/J4 were purchased from JAX Mice and Services (Bar Harbor, ME). The conditional Patched allele (Ptcfx) was provided by Brandon Wainwright (Institute for Molecular Bioscience, University of Queensland, Brisbane, Queensland, Australia).5 Smo and Ptc mutant alleles were crossed onto the C57BL/6 background at least 5 generations. C57BL/6-Smotm2amc/J mice were crossed with the interferon-inducible Cre recombinase transgenic mouse line, B6.Cg-Tg(Mx1-cre)1Cgn/J mice6 to generate C57BL/6-Smotm2amc;Mx1cre. C57BL/6-Ptcfx mice were crossed with the tamoxifen-inducible Cre recombinase transgenic line, C57BL/6-CAGGCre-ER7 to generate C57BL/6-Ptcfloxed; CAGGCre-ER mice. Deletion of Smo using poly(I)poly(C) injection to induce MX1-cre expression and deletion of Ptc using 4-hydroxytamoxifan to activate CreER were done as previously described.8,9 The efficiency of deletion was measured by polymerase chain reaction (PCR) analysis as described.4,5 Induction of acute anemia by phenylhydrazine injection was done as previously described.2,3 All procedures were approved by the Institutional Animal Care and Use Committee of the Pennsylvania State University.
Analysis of stress BFU-E
Stress BFU-E and steady-state BFU-E were assayed as previously described.2,3,10 In some experiments, other factors were added to the methylcellulose BFU-E cultures. For experiments investigating the ability of Sonic Hedgehog (Shh) to induce stress BFU-E, bone marrow cells were preincubated in Iscove modified Dulbecco medium plus 5% fetal calf serum supplemented 200 ng/mL Shh (R&D Systems, Minneapolis, MN) for 24 hours before plating cells in methylcellulose media. For experiments using the BMP4 antagonist Noggin, 200 μg/mL Noggin (R&D Systems) was added.
Transplantation of bone marrow or spleen cells into WBB6F1 KitW/Wv mice
A total of 2 × 106 bone marrow or spleen cells were isolated from C57BL/6 and injected into the tail vein of WBB6F1 KitW/Wv mice. Bone marrow and spleen cells were isolated on the indicated days and the presence of stress BFU-E, BMP4-responsive stress progenitors, BMP4R cells, and total BFU-E were assayed. Additional transplantations were done as described using bone marrow cells from CD45.1, poly(I)poly(C)-treated C57BL/6-Smotm2amc/tm2amc; Mx1-Cre, C57BL/6-Smotm2amc/+; Mx1-Cre or control C57BL/6-Smotm2amc/+ mice.
Immunofluoresence analysis of BMP4 expression by donor bone marrow cells in the spleen of transplanted mice
Spleens were harvested 1 week after 2 × 106 CD45.1 spleen cells were transplanted into WBB6F1 KitW/Wv mice. The spleens were processed for paraffin sections as previously described.2,10 The sections were stained with anti-BMP4 antibodies (Novocastra, Newcastle, United Kingdom; and Vector Laboratories, Burlingame, CA) using an Alexa Fluor 660 (Invitrogen, Carlsbad, CA) secondary antibody as previously described2,10 and with fluorescein isothiocyanate–conjugated anti-CD45.1 (BD Biosciences Phar-Mingen, San Diego, CA) at a 1:100 dilution. Slides were analyzed by confocal microscopy using an Olympus FV300 confocal microscope (Tokyo, Japan).
Analysis of BMP4 and Hedgehog expression in the spleen
RNA was isolated from the indicated cells or tissues using TRIzol reagent (Invitrogen) according to the manufacturer's instructions. Expression of BMP4 mRNA was analyzed by real-time PCR using Taqman gene expression assay for murine BMP4 (Applied Biosystems, Foster City, CA). The analysis of Shh, Desert Hedgehog (Dhh), and Indian Hedgehog (Ihh) was analyzed by reverse-transcribed PCR (RT-PCR) using the following primers.11 Shh (570 bp): forward, 5′-TCC GAA TTT AAG GAA CTC ACC-3′; reverse, 5′-GGC TCC AGC GTC TCG ATC ACG TAG-3′. Dhh (791 bp): forward, 5′-GAC CTC GTA CCC AAC TAC AAC CCC G-3′; reverse, 5′-ACG TCG TTG ACC AGC AGC GTC C-3′. Ihh (668 bp): forward, 5′-CAA GCT CGT GCC TCT TGC CTA CAA G-3′; reverse, 5′-GCA CAT CAC TGA AGG TGG GGG TCC-3′. Expression of Smoothened in deleted floxed strains was done by RT-PCR using the following primers: forward, 5′-AAC TAT CGG TAC CGT GCT GG-3′; reverse, 5′-GCT GAA GGT GAT GAG CAC AA-3′.
Results
BMP4-dependent stress progenitors completely differentiate during the recovery from acute anemia
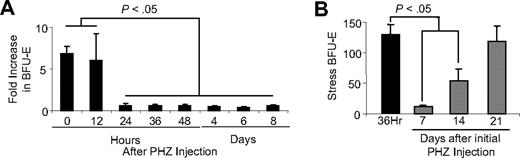
Our previous analysis of the recovery from phenylhydrazine (PHZ)–induced acute anemia showed that BMP4-responsive stress progenitors, BMP4R cells, which are resident in the spleen, rapidly proliferate in response to BMP4, SCF, and hypoxia-dependent signals to expand the population of stress BFU-E. By 36 hours after treatment, these progenitors expand 45-fold, which leads to recovery in approximately 6 to 7 days.2,3 We would predict that mice would be able to activate this stress erythropoiesis pathway multiple times in response to future anemic conditions. However, the mechanisms by which this pathway is maintained are not well understood. To address this question, we tested whether the number of BMP4R cells is maintained during the recovery period. C57BL/6 control mice were treated with PHZ, and the increase in stress BFU-E after treatment with BMP4 was used as a measure of BMP4R cells. The data in Figure 1A show that, in the first 12 hours after treatment, BMP4R cells were present in the spleen as demonstrated by the 5- to 7-fold increase in stress BFU-E with the inclusion of BMP4 in the media. Our previous work showed that BMP4 expression is induced in the spleen starting at 24 hours after PHZ treatment.2 At this point, we no longer observed BMP4R cells in the spleen, and it appeared that all BMP4R cells had differentiated into stress BFU-E. This lack of response was not simply the result of the high expression of BMP4 in the spleen causing the differentiation of all BMP4R cells because no response was also observed at later time points (days 4-8) when expression of BMP4 is no longer observed.2 These data indicate that excess BMP4R cells are not maintained in the spleen, and PHZ-induced acute anemia results in the complete activation of the BMP4-dependent stress erythropoiesis pathway.
Analysis of BMP4R stress erythroid progenitors during and after the recovery from acute anemia. (A) C57BL/6 mice were injected with PHZ to induce acute anemia. Spleen cells were isolated on the indicated days. BMP4R cells were measured by determining the fold increase in stress BFU-E when the number of stress BFU-E generated when cells were plated in Epo (3 U/mL) plus BMP4 (15 ng/mL) was compared with the number of stress BFU-E generated when cells were plated in Epo alone. (B) C57BL/6 mice were treated with PHZ to induce anemia and allowed to recover for 7 days. The mice were then challenged with a second dose of PHZ as indicated. At 36 hours after the second dose, stress BFU-E were measured by plating spleen cells in methylcellulose media containing Epo (3 U/mL) alone. The response of an untreated mouse 36 hours after the initial dose of PHZ is shown in black. For all assays, at least 3 mice were used at each time point.
Analysis of BMP4R stress erythroid progenitors during and after the recovery from acute anemia. (A) C57BL/6 mice were injected with PHZ to induce acute anemia. Spleen cells were isolated on the indicated days. BMP4R cells were measured by determining the fold increase in stress BFU-E when the number of stress BFU-E generated when cells were plated in Epo (3 U/mL) plus BMP4 (15 ng/mL) was compared with the number of stress BFU-E generated when cells were plated in Epo alone. (B) C57BL/6 mice were treated with PHZ to induce anemia and allowed to recover for 7 days. The mice were then challenged with a second dose of PHZ as indicated. At 36 hours after the second dose, stress BFU-E were measured by plating spleen cells in methylcellulose media containing Epo (3 U/mL) alone. The response of an untreated mouse 36 hours after the initial dose of PHZ is shown in black. For all assays, at least 3 mice were used at each time point.
The complete mobilization of BMP4R cells and stress BFU-E suggest that there must be mechanisms by which this response is replenished. We next tested how long a control mouse takes to recover after an initial treatment with PHZ before it can respond like an untreated mouse. C57BL/6 mice were treated with PHZ, and 7, 14, and 21 days after the first treatment, the mice were rechallenged with a second dose of PHZ. We measured stress BFU-E 36 hours after the second treatment with PHZ because our previous work demonstrated that at this time point the maximal expansion of stress BFU-E was observed.2 We observed that mice treated with a second dose of PHZ 7 days after the initial dose were unable to expand stress BFU-E in their spleens. However, by 21 days after the initial treatment, mice were able to expand stress BFU-E similarly to control untreated mice. Interestingly, at 14 days, there was an intermediate response suggesting that BMP4R cells gradually repopulate the spleen after recovery from acute anemia.
Bone marrow cells can home to the spleen and differentiate into BMP4R cells
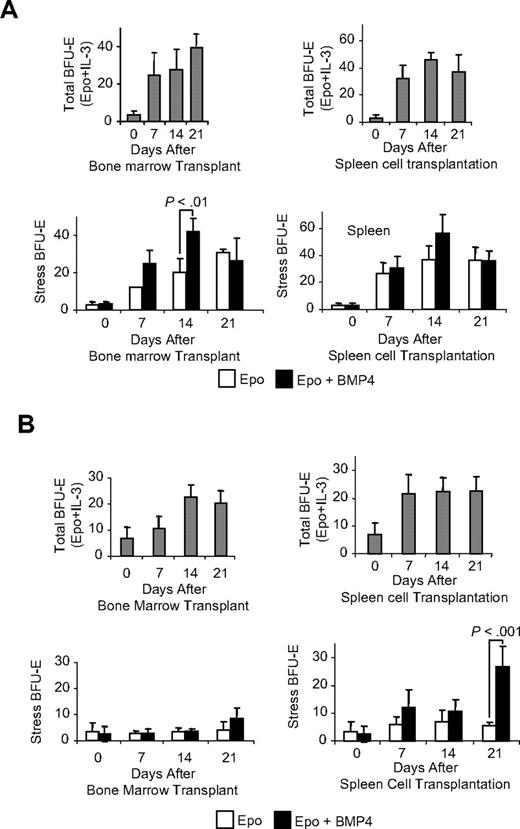
The complete differentiation of BMP4R cells during the recovery from acute anemia and their gradual replenishment during the 2 weeks after recovery suggest that immature progenitors, which are either present in the spleen or migrate to the spleen, give rise to new BMP4R cells. To distinguish between these possibilities, we established a transplantation assay that took advantage of our previous observation that mice mutant for the Kit receptor, WBB6F1 KitW/Wv (W/Wv), lacked BMP4R cells in their spleens.3 In addition, these mice have been used extensively in transplantation studies because transplantations into W/Wv mice do not require myeloablative preconditioning before transplantation.12 We transplanted bone marrow or spleen cells from C57BL/6 control mice into W/Wv mice. We then analyzed the spleens of recipient mice 7, 14, and 21 days after transplantation for BMP4R cells and stress BFU-E. Stress BFU-E are able to form colonies in media containing only erythropoietin (Epo), in contrast to steady-state BFU-E, which require a burst-promoting factor such as interleukin-3 (IL-3) in addition to Epo. BMP4R cells differentiate into stress BFU-E after exposure to BMP4. The increase in stress BFU-E when BMP4 is included in the media is indicative of BMP4R cells. Transplantation of spleen or bone marrow cells resulted in an increase in the total number of BFU-E (stress + steady-state BFU-E) as measured by plating cells in Epo plus IL-3 on each of the days analyzed. The number of stress BFU-E in the spleen also increased. In mice transplanted with bone marrow cells, the number of stress BFU-E steadily increased on days 7, 14, and 21 (Figure 2A). In contrast, when W/Wv mice were transplanted with spleen cells, we observed that the number of stress BFU-E did not significantly change from 7 to 21 days after transplantation (Figure 2A). The situation was quite different when we examined BMP4R cells by assaying the increase in stress BFU-E when BMP4 was added to the cultures. Only transplantations using bone marrow cells were able to give rise to BMP4R cells. We observed a significant increase in stress BFU-E when BMP4 was included in the media 14 days after transplanting bone marrow cells (Figure 2A). Fourteen days after transplantation is functionally equivalent to reappearance of BMP4R cells 21 days after the initial treatment of control mice with PHZ. BMP4R cells were not observed at 21 days after bone marrow cell transplantation. This difference may be caused by the mobilization of new donor-derived BMP4R cells to alleviate the anemia of the W/Wv mice. Spleen cell transplantations did not result in BMP4R cells in the recipient spleens at any of the time points. These data suggest that the bone marrow, but not the spleen, contains an immature progenitor that gives rise to BMP4R cells.
Transplanted bone marrow cells give rise to BMP4R cells in the recipient spleen. WBB6F1 KitW/Wv mice were transplanted with C57BL/6 donor bone marrow cells or spleen cells, and the development of stress BFU-E was assayed. (A) Analysis of stress BFU-E in the spleens of transplanted mice after transplantation with bone marrow cells (left) or spleen cells (right). (Top graphs) Total BFU-E observed after spleen cells were plated on indicated days in media containing Epo + IL-3. (Bottom graphs) Stress BFU-E observed on the indicated days when spleen cells were plated in media containing Epo or Epo + BMP4 as indicated. (B) Analysis of stress BFU-E in the bone marrow of transplanted mice after transplantation of bone marrow cells (left) or spleen cells (right). (Top graphs) Total BFU-E observed after spleen cells were plated on indicated days in media containing Epo + IL-3. (Bottom graphs) Stress BFU-E observed on the indicated days when spleen cells were plated in media containing Epo or Epo + BMP4 as indicated. For each time point, 3 recipient mice were analyzed. Significant differences are indicated in the figure.
Transplanted bone marrow cells give rise to BMP4R cells in the recipient spleen. WBB6F1 KitW/Wv mice were transplanted with C57BL/6 donor bone marrow cells or spleen cells, and the development of stress BFU-E was assayed. (A) Analysis of stress BFU-E in the spleens of transplanted mice after transplantation with bone marrow cells (left) or spleen cells (right). (Top graphs) Total BFU-E observed after spleen cells were plated on indicated days in media containing Epo + IL-3. (Bottom graphs) Stress BFU-E observed on the indicated days when spleen cells were plated in media containing Epo or Epo + BMP4 as indicated. (B) Analysis of stress BFU-E in the bone marrow of transplanted mice after transplantation of bone marrow cells (left) or spleen cells (right). (Top graphs) Total BFU-E observed after spleen cells were plated on indicated days in media containing Epo + IL-3. (Bottom graphs) Stress BFU-E observed on the indicated days when spleen cells were plated in media containing Epo or Epo + BMP4 as indicated. For each time point, 3 recipient mice were analyzed. Significant differences are indicated in the figure.
We also tested whether the transplanted cells could give rise to BMP4R cells and stress BFU-E in the bone marrow of recipient mice. Analysis of bone marrow cells from recipient mice showed that transplanted either bone marrow or spleen resulted in an increase in the total number of BFU-E (Figure 2B). However, when we examined stress BFU-E and BMP4R cells, the situation was different. As expected, transplanting bone marrow cells did not result in an increase in stress BFU-E or BMP4R cells in the bone marrow of recipients (Figure 2B). Spleen cell transplantations, however, did result in BMP4R cells in the bone marrow 21 days after transplantation (Figure 2B). These data suggest that the spleen contains BMP4R cells, which expand in the bone marrow microenvironment. It also suggests that, once a cell adopts the splenic BMP4R cell potential, it maintains that potential even if it finds itself in a different microenvironment.
Treatment of bone marrow cells with Hedgehog results in the development of BMP4R cells and stress BFU-E
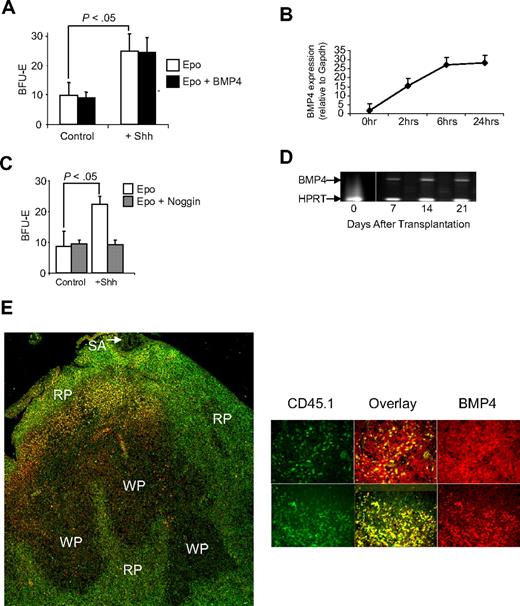
Our previous work showed that bone marrow cells do not respond to BMP4 like spleen BMP4R cells.2 However, when transplanted bone marrow cells migrate to the spleen, they acquire the ability to respond to BMP4. Taken together, these observations suggest that there is a signal in the spleen microenvironment that promotes the differentiation of bone marrow progenitors into BMP4R cells. To identify this signal, we relied on earlier observations about the role of BMP4 signaling in chondrocyte development. In this system, it was proposed that somatic mesoderm cells develop into chondrocytes in response to BMP4.13-15 However, when presomitic mesoderm cells were cultured in the presence of BMP4, they failed to differentiate into chondrocytes unless they were first treated with Shh.13 This analysis showed that Shh made the cells competent to respond to BMP4; together, these 2 factors promote the differentiation of chondrocytes. Using this paradigm, we proposed that hedgehog signals promote the differentiation of bone marrow progenitors into BMP4R cells. If Hedgehog is the signal in the spleen microenvironment that promotes the development of BMP4R cells, we would predict that culturing bone marrow cells with Hedgehog would result in the development of BMP4R cells in vitro. Treatment of bone marrow cells with Shh resulted in a significant increase in the number of stress BFU-E when cells were grown in methylcelluolose media containing only Epo (Figure 3A). However, when we added BMP4 to the media, no increase in stress BFU-E was observed. In the chondrocyte system, treatment of presomitic mesoderm cells with Shh induces the expression of BMP4.13 We tested whether bone marrow cells treated with Shh also expressed BMP4. The data in Figure 3B show that, similar to presomitic mesoderm, bone marrow cells express BMP4 in response to Shh treatment. Furthermore, when we sorted bone marrow hematopoietic cells from nonhematopoietic stromal cells, the Kit plus hematopoietic cells express BMP4 when cultured in media containing Shh (data not shown). Therefore, the expression of endogenous BMP4 by bone marrow progenitor cells would induce the expansion of stress BFU-E and mask the effect of exogenously added BMP4. To demonstrate whether this possibility were true, we treated bone marrow cells with Shh in the presence or absence of the BMP4 antagonist, Noggin.16-18 In Figure 3C, we show that inclusion of Noggin in the culture blocks the increase in stress BFU-E induced by Shh. These data show that hedgehog-dependent signaling induces BMP4 expression, and these 2 signals act in concert to promote the differentiation of bone marrow progenitor cells into stress BFU-E.
Treatment of bone marrow cells with Sonic Hedgehog induces stress BFU-E formation. (A) Bone marrow cells were preincubated overnight in Iscove modified Dulbecco medium plus 5% fetal calf serum supplemented with (+Shh) or without (Control) Sonic Hedgehog. Afterward, the cells were plated in methylcellulose media containing either Epo (3 U/mL) alone or Epo + BMP4 (15 ng/mL), and BFU-E were scored. (B) Real-time RT-PCR analysis of the expression of BMP4 by bone marrow cells cultured in the presence or absence of Shh. (C) Bone marrow cells were preincubated for 24 hours with Shh as described in panel A and then plated in methylcellulose media containing Epo or Epo + Noggin (200 μg/mL), and BFU-E were scored. (D) RT-PCR analysis of BMP4 expression in the spleen of WBB6F1 KitW/Wv recipient mice transplanted with CD45.1 donor bone marrow cells. The vertical white line between time 0 and 7 days has been inserted to indicate a repositioned gel lane. (E) Analysis of BMP4 expression by CD45.1+ donor cells in the spleen of KitW/Wv mice after bone marrow transplantation. (Left) BMP4 is shown in red and CD45.1 in green in a low power (original magnification ×20) analysis of BMP4 expression. Overlap between the 2 signals is shown in yellow. SA indicates splenic artery; RP, red pulp; and WP, white pulp. (Right) Spleen sections from additional mice at higher power (original magnification ×40). BMP4 is shown in red, CD45.1 in green, and the overlap in signals as yellow. All assays were done in triplicate and are representative of 2 independent experiments. Significant differences are indicated in the figure. Slides were viewed with an Olympus BX61Epi Fluorescence microscope (Olympus, Center Valley, PA) using a UPlanF1 lens at 40×/0.75 NA and Slow Fade Gold antifade agent (Invitrogen, Carlsbad, CA). Images were acquired using an Olympus DP71 camera (Olympus) and were processed with DP-BSW Basic software for the DP71 camera and Adobe Photoshop imaging software (Adobe, San Jose, CA).
Treatment of bone marrow cells with Sonic Hedgehog induces stress BFU-E formation. (A) Bone marrow cells were preincubated overnight in Iscove modified Dulbecco medium plus 5% fetal calf serum supplemented with (+Shh) or without (Control) Sonic Hedgehog. Afterward, the cells were plated in methylcellulose media containing either Epo (3 U/mL) alone or Epo + BMP4 (15 ng/mL), and BFU-E were scored. (B) Real-time RT-PCR analysis of the expression of BMP4 by bone marrow cells cultured in the presence or absence of Shh. (C) Bone marrow cells were preincubated for 24 hours with Shh as described in panel A and then plated in methylcellulose media containing Epo or Epo + Noggin (200 μg/mL), and BFU-E were scored. (D) RT-PCR analysis of BMP4 expression in the spleen of WBB6F1 KitW/Wv recipient mice transplanted with CD45.1 donor bone marrow cells. The vertical white line between time 0 and 7 days has been inserted to indicate a repositioned gel lane. (E) Analysis of BMP4 expression by CD45.1+ donor cells in the spleen of KitW/Wv mice after bone marrow transplantation. (Left) BMP4 is shown in red and CD45.1 in green in a low power (original magnification ×20) analysis of BMP4 expression. Overlap between the 2 signals is shown in yellow. SA indicates splenic artery; RP, red pulp; and WP, white pulp. (Right) Spleen sections from additional mice at higher power (original magnification ×40). BMP4 is shown in red, CD45.1 in green, and the overlap in signals as yellow. All assays were done in triplicate and are representative of 2 independent experiments. Significant differences are indicated in the figure. Slides were viewed with an Olympus BX61Epi Fluorescence microscope (Olympus, Center Valley, PA) using a UPlanF1 lens at 40×/0.75 NA and Slow Fade Gold antifade agent (Invitrogen, Carlsbad, CA). Images were acquired using an Olympus DP71 camera (Olympus) and were processed with DP-BSW Basic software for the DP71 camera and Adobe Photoshop imaging software (Adobe, San Jose, CA).
The ability of Shh to induce BMP4 expression in vitro suggested that transplanted bone marrow cells homing to the spleen would also express BMP4. We tested this hypothesis by examining BMP4 expression in W/Wv mice that had been transplanted with bone marrow cells. We used donor bone marrow cells from B6.SJL-Ptprca Pep3b/BoyJ, which carry the CD45.1 allele, which can be distinguished from recipient WBB6F1-W/Wv cells, which carry the CD45.2 allele. Our previous work showed that W/Wv mice do not express BMP4 in the spleen.3 However, when we examined the expression of BMP4 in the spleen by RT-PCR on days 7, 14, and 21 after transplantation, we observed BMP4 expression at all 3 time points (Figure 3D). This observation supports the idea that bone marrow cells have migrated into the spleens of the W/Wv recipient mice and now express BMP4. We tested whether donor bone marrow cells homing to the spleen would express BMP4. We stained spleen sections derived from W/Wv mice 7 days after transplantation with anti-BMP4 and anti-CD45.1 to distinguish donor-derived cells. The data in Figure 3E show that BMP4 is expressed by donor bone marrow cells. These data are consistent with the hypothesis that hedgehog signaling in the spleen induces the expression of BMP4 by bone marrow cells homing to the spleen. In addition, we also observed BMP4 expression by recipient spleen cells, which before transplantation do not express BMP4. These data suggest the possibility that donor-derived BMP4R cells may in part regulate BMP4 expression in the spleen and the expression of BMP4 by recipient cells may represent an attempt by donor cells to induce recovery from anemia in W/Wv mice. Further analysis will be needed to investigate this question.
Dhh and Ihh are expressed in the spleen
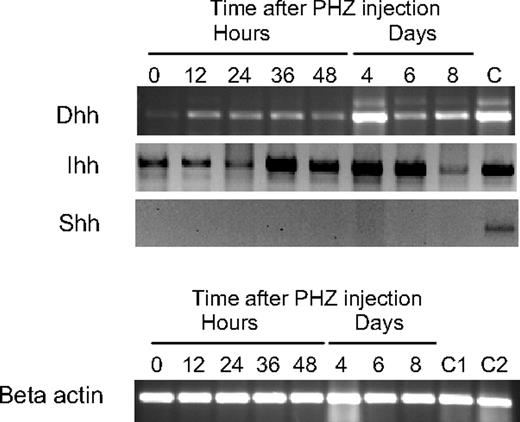
There are 3 vertebrate hedgehog genes, Shh, Dhh, and Ihh.19 Shh and Ihh have been implicated in hematopoiesis previously.20-25 We examined the expression of the 3 hedgehog family members in the spleen during the recovery from PHZ-induced anemia by RT-PCR. In Figure 4, we show that Dhh and Ihh are expressed in the spleen. Ihh is expressed in untreated animals and continuously throughout the recovery period. Dhh is expressed at low levels in untreated animals, but after PHZ treatment Dhh expression is up-regulated and maintained during the recovery from PHZ-induced acute anemia. Our previous work showed that, during the recovery from acute anemia, BMP4 expression is induced in nonhematopoietic stromal cells in the red pulp of the spleen. Similarly, nonhematopoietic cells in the fetal liver express BMP4 during embryogeneiss.2,10 We wanted to determine which cells express Dhh in the spleen, so we examined spleen sections for BMP4 and Dhh expression by immunofluorescence. We observed that Dhh and BMP4 are coexpressed, which suggests that nonhematopoietic stromal cells express both BMP4 and Dhh (Figure S1, available on the Blood website; see the Supplemental Materials link at the top of the online article). Further studies will need to be done to localize Ihh expression in the spleen during the recovery from acute anemia.
Expression of Hedgehog family members in the spleen. (Top) RT-PCR analysis of Ihh, Shh, and Dhh in the spleen at the indicated times after induction of acute anemia by PHZ injection. C indicates positive control RT-PCR analysis for Ihh, Dhh, and Shh. Dhh control used spleen RNA, and Ihh and Shh controls used thymus RNA. (Bottom) β-Actin control RT-PCR was used as a loading control. C1 indicates Dhh control RNA; C2, control RNA for Ihh and Shh.
Expression of Hedgehog family members in the spleen. (Top) RT-PCR analysis of Ihh, Shh, and Dhh in the spleen at the indicated times after induction of acute anemia by PHZ injection. C indicates positive control RT-PCR analysis for Ihh, Dhh, and Shh. Dhh control used spleen RNA, and Ihh and Shh controls used thymus RNA. (Bottom) β-Actin control RT-PCR was used as a loading control. C1 indicates Dhh control RNA; C2, control RNA for Ihh and Shh.
Mutation of the hedgehog-signaling pathway prevents development of BMP4R cells in the spleen
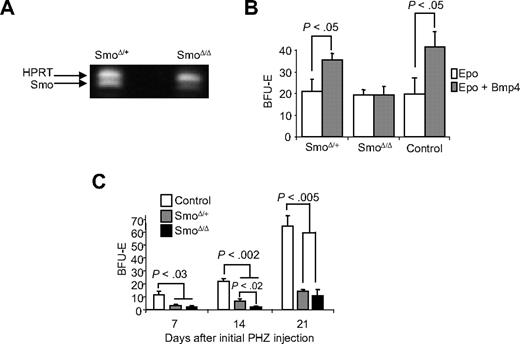
Our in vitro data show that Shh can induce the BMP4-dependent expansion of stress BFU-E, so we next tested whether donor bone marrow cells that were mutant in the hedgehog-signaling pathway could generate new BMP4R cells when transplanted into W/Wv mice. Hedgehog signaling uses 2 receptors, Ptc and Smo.26 Ptc is the negative receptor, which inhibits Smo signaling. On binding hedgehog, Ptc inhibition of Smo is released and Smo transmits the signal. Mutation of Smo blocks hedgehog signaling. Because Smo−/− mice are embryonic lethal, the role of Smo-dependent signaling in adult stress erythropoiesis has not been investigated.27 We used a conditional allele of Smo (Smotm2amc), which is a floxed allele of Smo and the interferon inducible Mx1-Cre.4,6 We deleted Smo in the bone marrow by repeated injections of poly(I)poly(C).9 Deleted bone marrow expressed very little Smo mRNA (Figure 5A). The deletion of Smo did not adversely affect hematopoiesis in the injected mice, which is consistent with previous work28 (data not shown). SmoΔ/Δ, SmoΔ/+, and control bone marrow cells were transplanted into W/Wv mice. The ability of mutant and control cell to home to the spleen was no different from wild-type control cells (data not shown). Fourteen days later, we examined the ability of spleen cells from the transplanted mice to respond to BMP4 in BFU-E colony assays. As shown in Figure 5B, SmoΔ/Δ cells were unable to respond to BMP4, whereas control and SmoΔ/+ cells did respond to BMP4. These data demonstrate that donor bone marrow cells require hedgehog signaling to develop into BMP4-responsive splenic stress BFU-E.
Mutation of the Hedgehog receptor Smoothened blocks the ability of bone marrow cells to generate new BMP4R cells in the spleen. (A) RT-PCR analysis of Smo expression in SmoΔ/+ and SmoΔ/Δ after poly(I)poly(C) treatment to delete the conditional allele. Hypoxanthine-guanine phosphoribosyl transferase expression is used as a loading control. (B) SmoΔ/+, SmoΔ/Δ, and control bone marrow cells were transplanted into WBB6F1 KitW/Wv recipient mice. Fourteen days after transplantation, cells were plated in methylcellulose media containing Epo or Epo + BMP4, and BFU-E were scored. Significant differences are indicated in the figure. At least 3 recipient mice were analyzed for each donor cell genotype. (C) SmoΔ/+, SmoΔ/Δ, and control mice were treated with PHZ to induce acute anemia and allowed to recover for 7 days. The mice were challenged with PHZ a second time on the indicated days after the initial treatment. At 36 hours after the second treatment, spleen cells were isolated and plated in methylcellulose media containing Epo alone, and BFU-E were scored. Significant differences are indicated in the figure. At least 3 mice per genotype were analyzed at each time point.
Mutation of the Hedgehog receptor Smoothened blocks the ability of bone marrow cells to generate new BMP4R cells in the spleen. (A) RT-PCR analysis of Smo expression in SmoΔ/+ and SmoΔ/Δ after poly(I)poly(C) treatment to delete the conditional allele. Hypoxanthine-guanine phosphoribosyl transferase expression is used as a loading control. (B) SmoΔ/+, SmoΔ/Δ, and control bone marrow cells were transplanted into WBB6F1 KitW/Wv recipient mice. Fourteen days after transplantation, cells were plated in methylcellulose media containing Epo or Epo + BMP4, and BFU-E were scored. Significant differences are indicated in the figure. At least 3 recipient mice were analyzed for each donor cell genotype. (C) SmoΔ/+, SmoΔ/Δ, and control mice were treated with PHZ to induce acute anemia and allowed to recover for 7 days. The mice were challenged with PHZ a second time on the indicated days after the initial treatment. At 36 hours after the second treatment, spleen cells were isolated and plated in methylcellulose media containing Epo alone, and BFU-E were scored. Significant differences are indicated in the figure. At least 3 mice per genotype were analyzed at each time point.
Using this transplantation model, we have established a role for hedgehog signaling in development of BMP4R cells in the spleen. In Figure 1B, we show that, after PHZ induced acute anemia, the recovery of the BMP4-dependent stress erythropoiesis pathway occurs 21 days after the initial PHZ treatment. We next investigated the ability of SmoΔ/Δ mice to recover BMP4R cells in the spleen after PHZ-induced anemia. Smotm2amc/tm2amc;Mx1-cre, Smotm2amc/+;Mx1-cre, and control Smotm2amc/+ mice were treated with poly(I)-poly(C) to induce interferon and delete the Smo gene. The mice were injected with PHZ to induce acute anemia. Seven, 14, and 21 days after the initial PHZ treatment, we examined the ability of these mice to expand stress BFU-E in response to a second PHZ treatment. All genotypes recovered from the initial PHZ treatment similarly (data not shown). In Figure 5C, we show that control mice exhibit an expansion of stress BFU-E at 21 days, which is similar to that observed in untreated mice (Figure 1B). In contrast, SmoΔ/Δ mice were unable to recover BMP4R cells in the spleen, even at 21 days after the initial anemia. Furthermore, none of these SmoΔ/Δ mice survived longer than 48 hours after the second PHZ treatment (data not shown). These data show that in vivo hedgehog signaling is required for the recovery and maintenance of the BMP4-dependent stress erythropoiesis pathway in the spleen; and in the absence of Hedgehog signaling, mice are unable to recover from repeated PHZ treatments.
Surprisingly, the SmoΔ/+ mice also exhibited a phenotype in this assay. At 14 days, the recovery of the heterozygous mice was significantly greater than the homozygous mutant mice, but less than control mice, which is consistent with the analysis of the transplantations of Smo+/− and Smo−/− transplantations into W/Wv mice in Figure 5B. However, by 21 days, the heterozygotes did not fully recover their ability to respond to a second dose of PHZ. Haplo-insufficient phenotypes for Smo have not been reported previously in mice, but in humans heterozygous mutations in the hedgehog-signaling pathway can cause pathology.29-32
Mutation of Patched circumvents the need for Hedgehog and leads to stress BFU-E in the bone marrow
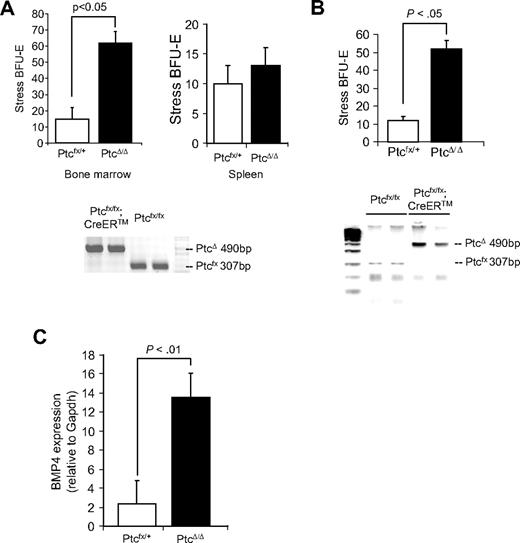
Hedgehog signaling is tightly regulated by the negative receptor Ptc, which inhibits the signaling activity of Smo.33 We hypothesized that mutation of Ptc in the bone marrow could lead to the expansion of stress BFU-E in the bone marrow where few stress BFU-E are normally observed. We tested this idea by crossing a conditional allele of Ptc (Ptcfx) with the tamoxifen-inducible Cre recombinase strain Cre-ERT. We treated Ptcfx/fx;CreERT and Ptcfx/+ control mice with repeated injections of 4-hydroxy tamoxifen (4OHT),8 after which bone marrow cells were isolated and plated in methylcellulose media containing only Epo to score stress BFU-E. In Figure 6A, we show that PtcΔ/Δ mice exhibited a 4-fold increase in the number of stress BFU-E in the bone marrow compared with control mice. Although these data suggest that deleting Ptc in bone marrow releases Smo-dependent signaling resulting in the development of stress BFU-E, it is possible that deleting Ptc could increase the number of stress BFU-E in the spleen, which could then migrate to the bone marrow. We addressed this possibility in 2 ways. When we examined the spleens of PtcΔ/Δ mice, we found that the number of stress BFU-E was unchanged, which is consistent with the constitutive expression of Ihh and Dhh in the spleen. Second, we isolated bone marrow cells from Ptcfx/fx;CreERT and Ptcfx/+ control mice and deleted Ptc with 4OHT in vitro. We plated the treated bone marrow cells in methylcellulose to assay for stress BFU-E. Similar to what we observed when Ptc was deleted in vivo, PtcΔ/Δ cells exhibited a 5-fold increase in the number of stress BFU-E (Figure 6B). Indeed, PtcΔ/Δ cells generated significantly larger colonies (data not shown). We also observed that deletion of Ptc induces the expression of BMP4, which mimics the effects of Shh treatment of bone marrow cells (Figure 6C). Taken together, these data suggest that hedgehog signaling promotes the development of stress BFU-E and hedgehog signaling is tightly regulated so that access to Hedgehog is compartmentalized, which promotes the development of BMP4-responsive stress BFU-E only in the spleen.
Mutation of Ptc induces stress BFU-E formation in the bone marrow in vivo and in vitro. (A, top) Bone marrow or spleen cells as indicated were isolated from PtcΔ/+ and PtcΔ/Δ mice and plated in methylcellulose media containing Epo alone, and BFU-E were scored. (A, bottom) PCR analysis of Ptc deletion by 4-hydroxytamoxifen. (B) Bone marrow cells were isolated from Ptcfloxed/+ and Ptcfloxed/floxed;CreER, and deletion of Ptc was induced in vitro by incubating the cells overnight with 4-hydroxytamoxifen. (Top) The cells were washed and plated in methylcellulose media containing Epo alone, and BFU-E were scored. (bottom) PCR analysis of Ptc deletion by 4-hydroxytamoxifen. (C) Real-time RT-PCR analysis of BMP4 expression by in vitro deleted PtcΔ/+ and PtcΔ/Δ bone marrow cells. Significant differences are indicated in the figure. All assays were done in triplicate and are representative of 2 independent experiments.
Mutation of Ptc induces stress BFU-E formation in the bone marrow in vivo and in vitro. (A, top) Bone marrow or spleen cells as indicated were isolated from PtcΔ/+ and PtcΔ/Δ mice and plated in methylcellulose media containing Epo alone, and BFU-E were scored. (A, bottom) PCR analysis of Ptc deletion by 4-hydroxytamoxifen. (B) Bone marrow cells were isolated from Ptcfloxed/+ and Ptcfloxed/floxed;CreER, and deletion of Ptc was induced in vitro by incubating the cells overnight with 4-hydroxytamoxifen. (Top) The cells were washed and plated in methylcellulose media containing Epo alone, and BFU-E were scored. (bottom) PCR analysis of Ptc deletion by 4-hydroxytamoxifen. (C) Real-time RT-PCR analysis of BMP4 expression by in vitro deleted PtcΔ/+ and PtcΔ/Δ bone marrow cells. Significant differences are indicated in the figure. All assays were done in triplicate and are representative of 2 independent experiments.
Discussion
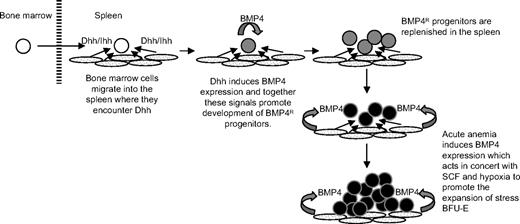
Here we show that Hedgehog-dependent signaling is required for the maintenance of the BMP4-dependent stress erythropoiesis pathway in the murine spleen. These data suggest a model (Figure 7) where bone marrow cells migrate to the spleen after recovery from acute anemia. Although our previous work demonstrated that bone marrow cells are unable to respond to BMP4 like spleen stress BFU-E2, once in the spleen, the bone marrow cells encounter Dhh or Ihh. Hedgehog signaling induces the expression of BMP4 in the bone marrow cells, and these 2 signals are both required to promote the differentiation of bone marrow progenitors into BMP4-responsive spleen stress erythroid progenitors. Our analysis of SmoΔ/Δ mice showed that deletion of Smo affected the recovery of spleen stress progenitors to such an extent that the mice were unable to recover from the anemia induced by a second dose of PHZ. These data demonstrate the key role played by Hedgehog signaling in the maintenance of the BMP4-dependent stress erythropoiesis pathway in the spleen. These data also suggest a wider role for BMP4-dependent signals. Our observation that Noggin can completely inhibit the ability of Shh to induce the differentiation of stress BFU-E (Figure 3C) shows that BMP4 plays an early role in the development of stress erythroid progenitors where it acts in concert with Hedgehog. This role is in addition to the role that we described previously where BMP4, SCF, and hypoxia are required for the rapid expansion of stress BFU-E during the recovery from acute anemia.2,3
Model for the role of Hedgehog in the recovery of the BMP4-dependent stress erythropoiesis pathway in the spleen.
Model for the role of Hedgehog in the recovery of the BMP4-dependent stress erythropoiesis pathway in the spleen.
Hedgehog- and BMP4-signaling pathways are required in a wide range of developmental systems34-37 and during hematopoiesis,20-25,27,28 they have been shown to play role in the expansion of stem cells.20,25 However, the situation that is most similar to stress erythropoiesis is somitic chondrocyte development. In this system, Shh initiates a chondrocyte development program, which allows somitic mesoderm cells to differentiate into chondrocytes in response to BMP4.13-15 Similar to what we observe in stress progenitors, Shh induces BMP4 in somitic mesoderm cells. The 2 signals cooperate to establish a chondrocyte gene expression pattern that is subsequently activated and extended by BMP4 signaling during differentiation. Based on our observations, we propose that hedgehog signaling induces a pro-stress erythropoiesis fate that allows bone marrow cells to respond to BMP4. We hypothesize that Hedgehog (Ihh or Dhh) and BMP4 in the spleen act to promote the development of BMP4-responsive stress erythroid progenitors, which in turn, at times of acute erythropoietic stress rapidly respond to BMP4, SCF, and hypoxia to generate new erythrocytes.
Our data also suggest that spleen BMP4-responsive stress erythroid progenitors represent a lineage committed progenitor, which does not rely on spleen-specific signals to maintain this cell fate. Our transplantation experiments showed that BMP4-responsive stress progenitors from the spleen maintained their potential to respond to BMP4, even though they had lodged in the bone marrow. Furthermore, the compartmentalization of the stress erythropoiesis pathway to the spleen appears to be maintained by regulating access to hedgehog. Although others have reported that Shh and Ihh are expressed in the bone marrow,20,38 progenitors destined to become BMP4 response stress progenitors do not appear to have access to this source of Hedgehog. However, when we deleted Ptc, BMP4-responsive cells were readily observed in the bone marrow. These data, coupled with observation that in vitro deletion of Ptc also leads to the development of stress BFU-E, suggests that activation of hedgehog-signaling pathways by Ptc mutation and the subsequent induction of BMP4 expression are sufficient to promote the differentiation of stress erythroid progenitors in the absence of hedgehog-secreting cells.
The role hedgehog and BMP4 in regulating the development of stress erythroid progenitors may not be limited to the adult spleen. Recent work from our laboratory has shown that the BMP4-dependent stress erythropoiesis pathway is required to rapidly generate new erythrocytes at a critical juncture in fetal development.10 BMP4 expression is first observed in the fetal liver at embryonic day 14.5 (E14.5). Stress BFU-E rapidly expand at E15.5 where they make up the majority of BFU-E in the fetal liver. Before the expansion of stress BFU-E at E15.5, we detected BMP4R cells in the fetal liver. Because hematopoietic progenitor cells do not develop de novo in the fetal liver,39-41 we proposed that definitive progenitors from the yolk sac may seed the fetal liver at earlier times and develop into BMP4-responsive stress progenitors. In support of this idea, we showed that yolk sac contains progenitor cells that are capable of differentiating into stress BFU-E when cocultured on AFT024 fetal liver stromal cells.42 This cell line expresses BMP4 at high levels, but BMP4 alone is unable to cause yolk sac progenitor cells to differentiate into stress BFU-E. It is tempting to speculate that, similar to the adult spleen, hedgehog signals may be involved in the development of BMP4-responsive stress progenitors in the fetal liver. New experiments will be needed to test this hypothesis.
In conclusion, we have shown that the BMP4-responsive stress erythropoiesis pathway is maintained by bone marrow cells that migrate to the spleen. Once in the spleen, they encounter Hedgehog, which induces BMP4 expression. These 2 signals together promote the differentiation of bone marrow progenitor cells into BMP4-responsive stress erythroid progenitors, which maintains the BMP4-dependent stress erythropoiesis pathway in the spleen.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the members of the Paulson Laboratory and the Center for Molecular Immunology and Infectious Disease for their comments on the paper, Brandon Wainwright and C. C. Hui for providing the Ptcfloxed mice, and Bennie Luscher for providing the CreER mice.
This work was supported by National Institutes of Health grant HL70720 (R.F.P.) and, in part, by a grant with the Pennsylvania Department of Health using Tobacco Settlement Funds.
The Pennsylvania Department of Health specifically disclaims responsibility for any analyses, interpretations, or conclusions.
National Institutes of Health
Authorship
Contribution: J.M.P., O.F.H., and P.P. performed experiments and analyzed data; S.H. and A.K.K. performed experiments; and R.F.P. designed experiments, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Robert F. Paulson, Center for Molecular Immunology and infectious Disease, Department of Veterinary and Biomedical Sciences, 115 Henning Building, Pennsylvania State University, University Park, PA 16802; e-mail rfp5@psu.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal