Abstract
The intracellular Ca2+ concentration of many nonexcitable cells is regulated by calcium store release and store-operated calcium entry (SOCE). In platelets, STIM1 was recently identified as the main calcium sensor expressed in the endoplasmic reticulum. To evaluate the role of the SOC channel moiety, Orai1, in platelet SOCE, we generated mice expressing a mutated, inactive form of Orai1 in blood cells only (Orai1R93W). Platelets expressing Orai1R93W were characterized by markedly reduced SOCE and impaired agonist-induced increases in [Ca2+]i. Orai1R93W platelets showed reduced integrin activation and impaired degranulation when stimulated with low agonist concentrations under static conditions. This defect, however, did not significantly affect the ability of Orai1R93W platelets to aggregate or to adhere to collagen under arterial flow conditions ex vivo. In contrast, these adherent Orai1R93W platelets were defective in surface phosphatidylserine exposure, suggesting that Orai1 is crucial for the platelets' procoagulant response rather than for other Ca2+-dependent cellular responses.
Introduction
In electrically nonexcitable cells such as lymphocytes and platelets, the major mechanism for Ca2+ entry is store-operated calcium entry (SOCE), a process controlled by the Ca2+ concentration in the endoplasmic reticulum (ER). Depletion of Ca2+ stores triggers activation of SOC channels in the plasma membrane.1 Two major players in lymphocyte SOCE have recently been identified2-6 : the 4-transmembrane–spanning calcium release-activated (CRAC) channel moiety Orai1 (CRACM1), and STIM1, a Ca2+ sensor expressed predominantly in the ER.
Orai1 deficiency in mice results in impaired Ca2+ influx and grossly defective degranulation in mast cells,7 and impaired SOCE and cytokine production in T cells.8 A naturally occurring mutation in Orai1 (R91W), found in patients with severe combined immunodeficiency (SCID), led to a marked reduction in SOCE in lymphocytes.4 In addition to Orai1, Orai2 and Orai3 are widely expressed in mammalian cells, and both molecules, when overexpressed together with STIM1, can form ion channels with properties similar to those of CRAC channels.9,10
In platelets, STIM1 was recently identified as the major Ca2+ sensor expressed in the ER.11 The nature of the SOC channel regulated by STIM1, however, remains elusive. Among the candidate proteins are several members of the canonical transient potential channel (TRPC) family,12,13 as well as Orai1.14 In this study, we provide evidence for Orai1 as the major SOC channel expressed in platelets.
Methods
Mice and generation of fetal liver chimeras
Gene targeting of the Orai1 gene is described in detail in Document S1 (available on the Blood website; see the Supplemental Materials link at the top of the online article). Fetal liver cells were obtained from E14.5 mouse embryos derived from matings of Orai1+/R93W mice on the C57BL/6 background. Orai1R93W/R93W and littermate wild-type control cells (CD45.2+) were injected intravenously into irradiated (4.5 + 4.5 Gy) Rag2−/−, γc−/− mice (Taconic Farms, Hudson, NY) or CD45.1+ wild-type C57BL/6 mice (Taconic Farms). Blood cell analyses were performed 5 to 6 weeks after transplantation. All mice were maintained in specific pathogen-free barrier facilities at Harvard Medical School and New York University School of Medicine and were used in accordance with protocols approved by the Institutional Animal Care and Use Committee (IACUC) at both institutions.
Aggregometry
Platelet-rich plasma (PRP) was obtained from heparinized whole blood by centrifugation at 100g for 10 minutes. Light transmission was measured by using PRP adjusted to 3 × 108 platelets per milliliter with modified Tyrode buffer. Agonists were added at the indicated concentrations and transmission was recorded over 10 minutes on a Chrono-log 4-channel optical aggregation system (Chrono-log, Havertown, PA).
Flow cytometry
Calcium flux measurement.
Washed platelets were incubated with 5 μM Fluo-3 for 15 minutes, activated with the indicated concentrations of PAR4p or Cvx, and immediately analyzed on a FACSCalibur (BD Biosciences, San Jose, CA).
Platelet activation.
Platelets were diluted in Tyrode buffer containing 1 mM CaCl2, activated with PAR4p and/or Cvx for 10 minutes, and stained for αIIbβ3 activation (JON/A-PE), P-selectin expression (α-Pselectin-FITC), or PS exposure (annexin V–Alexa 488).
Flow chamber studies.
Whole blood was perfused over a collagen-coated surface (100 μg/mL collagen Horm) at a wall shear rate of 1000 s−1 for 2 minutes, followed by perfusion with Tyrode buffer containing Alexa 594–coupled antibodies to GPIbα and annexin V–Alexa 488. Fluorescence signals were visualized with an Axiovert 135 inverted microscope (Carl Zeiss, Thornwood, NY) equipped with a silicon-intensified tube camera (C 2400; Hamamatsu, Middlesex, NJ). Images were analyzed using NIH Image 1.61 software (National Institutes of Health, Bethesda, MD).
More detailed information on reagents and methods used for this study is provided in Document S1.
Results and discussion
Generation of Orai1R93W chimeric mice
To study a potential role of Orai1 in platelet SOCE, we generated knockin mice expressing Orai1R93W instead of Orai1 (Figure 1A, Document S1). The equivalent R91W mutation in human SCID patients abrogates SOCE and CRAC channel currents (ICRAC) in T cells.15 Homozygous expression of Orai1R93W in mice was perinatally lethal with mice dying during the first day after birth. To circumvent this problem, we generated chimeric mice that express Orai1R93W in blood cells only. Fetal liver cells from Orai1+/+ and Orai1R93W/R93W embryos were transplanted into irradiated wild-type C57BL/6 or Rag2−/−, γc−/− mice. The CD45.1/CD45.2 screening system was used to confirm chimerism in some of the mice (Figure S1). All the screened chimeras expressed Orai1R93W in more than 98% of circulating blood cells. Examination of mice 6 weeks after fetal liver cell transplant demonstrated normal platelet volume (not shown) and surface expression of key platelet adhesion receptors (Figure S2) in Orai1R93W mice (ie, mice that received Orai1R93W/R93W fetal liver cells).
Impaired calcium influx in Orai1R93W platelets. (A) Schematic diagram showing the targeting strategy for Orai1R93W knockin mice. A detailed description of the targeting strategy is given in Document S1. (B) Calcium flux in Orai1R93W platelets. Fluo-3–labeled WT (gray line) or Orai1R93W platelets (black line) were stimulated with the indicated agonists in the presence of 0.5 mM CaCl2. In the thapsigargin (TG) experiment, cells were first treated with TG in the absence of extracellular Ca2+ followed by addition of 0.5 mM CaCl2 (arrows). Mean fluorescence intensity (MFI) was recorded over time on a FACSCalibur. Results are representative of 5 independent experiments. Cvx, convulxin; PAR4p, PAR4 receptor activating peptide.
Impaired calcium influx in Orai1R93W platelets. (A) Schematic diagram showing the targeting strategy for Orai1R93W knockin mice. A detailed description of the targeting strategy is given in Document S1. (B) Calcium flux in Orai1R93W platelets. Fluo-3–labeled WT (gray line) or Orai1R93W platelets (black line) were stimulated with the indicated agonists in the presence of 0.5 mM CaCl2. In the thapsigargin (TG) experiment, cells were first treated with TG in the absence of extracellular Ca2+ followed by addition of 0.5 mM CaCl2 (arrows). Mean fluorescence intensity (MFI) was recorded over time on a FACSCalibur. Results are representative of 5 independent experiments. Cvx, convulxin; PAR4p, PAR4 receptor activating peptide.
Impaired SOCE in Orai1R93W platelets
We next determined whether Orai1 is required for calcium flux in platelets (Figure 1B). Specific activation of PAR4 or GPVI with high doses of PAR4p or convulxin (Cvx), respectively, led to a rapid increase in [Ca2+]i that was sustained in Orai1+/+ platelets. In contrast, [Ca2+]i levels rapidly dropped back to baseline in activated Orai1R93W platelets, presumably due to impaired Ca2+ entry into the cell. A more prominent defect in Ca2+ mobilization was observed at low doses of the agonists, suggesting that Orai1-mediated SOCE is crucial for calcium flux under conditions of weak platelet activation. To more directly address whether Orai1 is required for SOCE, platelets were stimulated with the SR/ER Ca2+ ATPase (SERCA) pump inhibitor thapsigargin (TG). In the absence of extracellular calcium, TG induced weak Ca2+ store release in both Orai1+/+ and Orai1R93W platelets. Upon addition of extracellular calcium, Orai1+/+ but not Orai1R93W platelets responded with a marked and rapid influx of extracellular calcium. In summary, these results identify Orai1 as a major SOC channel expressed in platelets.
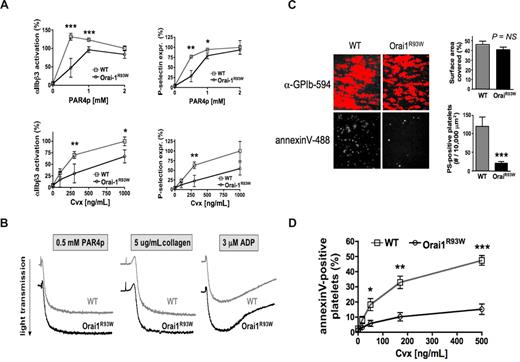
Orai1 regulates Ca2+-dependent platelet activation but has no apparent effect on platelet aggregation and thrombus formation in vitro
Platelet responses that are directly dependent on an increase in [Ca2+]i include integrin activation, granule release, and procoagulant activity.16,17 Orai1R93W platelets activated with PAR4p or the GPVI agonist Cvx showed a significant reduction in αIIbβ3 activation and P-selectin expression (a measure for alpha granule release) at low but not high doses of the agonist (Figure 2A). These data differ from the results obtained with STIM1-deficient platelets, which showed that a loss of STIM1-dependent SOCE predominantly affects GPVI- but not GPCR-dependent platelet activation.11 In contrast to STIM1−/− platelets, Orai1R93W platelets also showed a normal aggregation response toward all agonists tested, including the GPVI agonists convulxin (not shown) and collagen (Figure 2B). Furthermore, platelet adhesion to fibrillar collagen at a shear rate of 1000 s−1 was similar in blood from Orai1+/+ and Orai1R93W mice (Figure 2C). A likely explanation for the difference in platelet aggregation and adhesion to collagen observed in Orai1R93W and STIM1−/− platelets is based on our observation that Ca2+ release from intracellular stores is normal in Orai1R93W platelets, whereas it was shown to be impaired in STIM1−/− platelets.11 Similar observations have been reported for mast cells, where deficiency in STIM1, but not Orai1, resulted in reduced filling of intracellular calcium stores.7,18 Thus, while STIM1−/− platelets have defects in both Ca2+ release and influx, Orai1R93W platelets can mount a normal Ca2+ release response, which in turn facilitates residual granule release and integrin activation that seems sufficient to support platelet aggregation and adhesion to collagen in vitro. It is currently not clear why STIM1 is crucial for store refilling while Orai1 is not. One possibility is that STIM1 regulates this process independent of its role in SOC channel regulation. Alternatively, given a more pronounced defect in SOCE in Stim1-deficient than Orai1R93W platelets, one could speculate that other Ca2+ channels functionally compensate for a defect in Orai1. The Orai1 paralogues Orai2 and Orai3 can conduct Ca2+ when coexpressed with STIM19,10 and are expressed in platelets (Figure S3). It remains unclear at present, however, what the function of native Orai2 and Orai3 is. TRP channels have been implicated in SOC channel function but their role in platelet SOCE is controversial19-22 and needs further investigation. Theoretically, the R93W mutation may not completely abolish Orai1 function. This possibility, however, is not supported by our studies in T cells, which showed similar defects in SOCE and CRAC current in Orai1−/−7 and Orai1R93W mice (S.F., unpublished observations, April 2007).
Orai1 regulates Ca2+-dependent platelet responses. (A) Integrin activation and granule release. WT and Orai1R93W platelets were stimulated for 10 minutes with the indicated agonists, stained for expression of activated αIIbβ3 or P-selectin, and immediately analyzed (n = 5-6). (B) αIIbβ3-dependent platelet aggregation (results are representative of 3 independent experiments). (C) PS exposure on platelets adherent to collagen under flow. WT and Orai1R93W whole blood was perfused over collagen at a shear rate of 1000 s−1 for 2 minutes, and stained with annexin V–Alexa 488 and anti-GPIbα-Alexa 594. The surface area covered by platelets and the number of PS-positive cells was evaluated in 6 visual fields from 3 independent experiments. (D) PS exposure on platelets activated under static conditions. Platelets were stimulated with the combination of PAR4p (1 mM) and Cvx (indicated doses) for 10 minutes under static conditions, stained with annexin V–Alexa 488, and immediately analyzed. Results are expressed as mean plus or minus the standard error of the mean (SEM) (n = 7-8). *P < .05, **P < .001, ***P < .0001.
Orai1 regulates Ca2+-dependent platelet responses. (A) Integrin activation and granule release. WT and Orai1R93W platelets were stimulated for 10 minutes with the indicated agonists, stained for expression of activated αIIbβ3 or P-selectin, and immediately analyzed (n = 5-6). (B) αIIbβ3-dependent platelet aggregation (results are representative of 3 independent experiments). (C) PS exposure on platelets adherent to collagen under flow. WT and Orai1R93W whole blood was perfused over collagen at a shear rate of 1000 s−1 for 2 minutes, and stained with annexin V–Alexa 488 and anti-GPIbα-Alexa 594. The surface area covered by platelets and the number of PS-positive cells was evaluated in 6 visual fields from 3 independent experiments. (D) PS exposure on platelets activated under static conditions. Platelets were stimulated with the combination of PAR4p (1 mM) and Cvx (indicated doses) for 10 minutes under static conditions, stained with annexin V–Alexa 488, and immediately analyzed. Results are expressed as mean plus or minus the standard error of the mean (SEM) (n = 7-8). *P < .05, **P < .001, ***P < .0001.
Arguably the most prominent defect observed in Orai1R93W platelets was their inability to express PS on the cell surface. In flow chamber studies, the number of platelets expressing PS at the end of the perfusion period was reduced by more than 80% (Figure 2C). A similar defect in surface PS exposure was observed in platelets costimulated with PAR4p and Cvx under static conditions in vitro (Figure 2D). These findings are in agreement with previous studies, which demonstrated that PS exposure on platelets requires [Ca2+]i to be maintained at elevated levels over a prolonged period of time.23-25 Orai1R93W platelets, however, due to their defect in SOCE, are not able to mount a sustained calcium signal (Figure 1B). Interestingly, a small fraction of Orai1R93W platelets was able to express surface PS. It will be interesting to see if STIM1−/− platelets show an even greater defect in PS exposure, a finding that would suggest that there is some functional redundancy between Orai paralogues. Future in vivo studies will have to show how impaired procoagulant activity in Orai1R93W platelets affects thrombosis and hemostasis.
No obvious bleeding or clotting disorder was observed in SCID patients homozygous for the corresponding Orai1 R91W mutation. Neither did we detect any spontaneous bleeding complications in Orai1R93W mice, a finding that may not be surprising given the mild aggregation defect observed in vitro. Furthermore, studies in Scott patients, a familial platelet disorder with unknown etiology, demonstrated that an impaired PS exposure/procoagulant response in platelets is associated with a very mild risk of spontaneous bleeding.26 Since Orai1R93W platelets showed markedly impaired PS exposure similar to that described for platelets from Scott patients, we investigated if mutations in STIM1 or ORAI1 may be responsible for the clinical phenotype of the original patient with Scott syndrome.27 No mutations in STIM1 or ORAI1 genes of this patient were found (not shown).
In summary, our data identify Orai1 as a major SOC channel in platelets. Platelets expressing nonfunctional Orai1 show defects in agonist-induced calcium influx as well as Ca2+-regulated platelet functions, such as integrin activation, granule release, and PS exposure.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Note added in proof:
During the review of this paper a study by Braun et al (Blood. Prepublished on October 2, 2008, as DOI 10.1182/blood-2008-07-171611) was published showing impaired SOCE in platelets from Orai1−/− mice.
Acknowledgments
We thank Ying Jin for help with the RT-PCR assay and Peter Sims for providing DNA samples from a healthy volunteer and a Scott patient. We also thank Denisa Wagner and Anjana Rao for their support throughout the study and for use of their laboratory space.
This work was supported by National Institutes of Health (NIH) grants AI066128 (S.F.), GM075256 (Anjana Rao), and R37-HL41002 (Denisa Wagner), a Scientist Development Grant 0630044N from the American Heart Association (W.B.), a March of Dimes Foundation grant (S.F.), and a Uehara Memorial Foundation research fellowship (M.O.).
National Institutes of Health
Authorship
Contribution: W.B. designed the study, performed many of the experiments, analyzed the results, and wrote the paper; M.O. generated Orai1R93W/R93W mice; C.-A.M. helped generate and maintain Orai1R93W chimeric mice; R.C.R. sequenced human DNA samples; P.F.B. helped with the detection of Orai1/2/3 RNA in ultrapurified platelet preparations and megakaryocytic cells; and S.F. designed the study, generated Orai1R93W/R93W mice, analyzed the results, and wrote the paper.
Conflict-of-interest disclosure: S.F. is a scientific cofounder of Calcimedica, a company whose research on immune therapies includes a focus on inhibitors of the STIM-ORAI pathway. The remaining authors declare no competing financial interests.
Correspondence: Wolfgang Bergmeier, Cardeza Foundation and Department of Medicine, Thomas Jefferson University, 803 Curtis, 1015 Walnut Street, Philadelphia, PA 19007, e-mail: wolfgang.bergmeier@jefferson.edu; or Stefan Feske, Department of Pathology, New York University School of Medicine, 550 First Avenue, New York, NY 10016, e-mail: feskes01@nyumc.org.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal