Abstract
The high iron demand associated with enhanced erythropoiesis during high-altitude hypoxia leads to skeletal muscle iron mobilization and decrease in myoglobin protein levels. To investigate the effect of enhanced erythropoiesis on systemic and muscle iron metabolism under nonhypoxic conditions, 8 healthy volunteers were treated with recombinant erythropoietin (rhEpo) for 1 month. As expected, the treatment efficiently increased erythropoiesis and stimulated bone marrow iron use. It was also associated with a prompt and considerable decrease in urinary hepcidin and a slight transient increase in GDF-15. The increased iron use and reduced hepcidin levels suggested increased iron mobilization, but the treatment was associated with increased muscle iron and L ferritin levels. The muscle expression of transferrin receptor and ferroportin was up-regulated by rhEpo administration, whereas no appreciable change in myoglobin levels was observed, which suggests unaltered muscle oxygen homeostasis. In conclusion, under rhEpo stimulation, the changes in the expression of muscle iron proteins indicate the occurrence of skeletal muscle iron accumulation despite the remarkable hepcidin suppression that may be mediated by several factors, such as rhEpo or decreased transferrin saturation or both.
Introduction
The glycoprotein hormone erythropoietin (Epo) acts as the main regulator of erythropoiesis by promoting the differentiation of erythroid cell precursors, thus ultimately leading to an increase in red blood cell production and hemoglobin levels. Recombinant human Epo (rhEpo), which mimics the action of endogenous Epo and was first introduced into clinical practice 20 years ago, is extremely useful in correcting certain types of anemia, is widely used, and is considered to be a major advance in therapy. The presence of Epo receptors (EpoRs) in various nonerythropoietic tissues1 indicates that Epo also has nonhematopoietic activity, but its effects outside the erythron have not been completely characterized.
The erythroid cell proliferation stimulated by rhEpo is associated with a dramatic alteration in iron metabolism to satisfy the high demand for iron to synthesize hemoglobin.2 Iron homeostasis is primarily modulated by hepcidin, a liver peptide hormone that inhibits cellular iron release by triggering the internalization and degradation of the cellular iron exporter ferroportin, thus inhibiting iron absorption by the intestine and iron release by reticuloendothelial cells.2-5 Hepatic hepcidin expression is down-regulated in response to iron deficiency, anemia, or hypoxia,6 which increases iron release and availability, and is up-regulated in response to iron overload or inflammation.7
It has been established that, whether associated with anemia in patients with β-thalassemia8-11 or occurring in the absence of anemia or tissue hypoxia in experimental animals,12-16 a high degree of erythropoietic activity suppresses hepcidin production, but the signals involved in this regulation are poorly understood. In this regard, an interesting clue has been given by showing that, as a result of its interactions with liver Epo receptors, Epo plays a direct role in regulating hepcidin expression in hepatoma cell lines,17,18 and by noting the emerging role of GDF-15, a stress-responsive member of the TGF-β cytokine superfamily, in blunting hepcidin expression.11
The hepcidin-ferroportin interaction provides the mechanistic link between the iron supply by absorptive enterocytes and reticuloendothelial cells, and the iron demand of erythroid cells. However, the effects of hepcidin on ferroportin in other cells have not been fully characterized, thus raising the question of the exact role of hepcidin in iron mobilization from storage sites.
Skeletal muscle is important in iron metabolism because it represents approximately 40% of human body mass and contains 10% to 15% of body iron, mainly located in myoglobin, a hemoprotein involved in oxygen storage, and its diffusion from capillaries to mitochondria.19 Disrupted iron balance in skeletal muscle may therefore interact with local oxygen homeostasis. We have recently reported that, in humans exposed to high altitude, the iron requirement associated with hypoxia-induced erythropoiesis leads to muscle iron loss and decreased myoglobin levels, which suggests the priority of systemic oxygen homeostasis over muscle oxygen homeostasis.20 However, the coexistence of hypoxia and functional iron deficiency secondary to erythropoiesis during high-altitude hypoxia prevented us from identifying the relative importance of these 2 stimuli in triggering iron redistribution.
Because it is possible to dissociate increased erythropoietic iron requirements from hypoxia in humans by administering rhEpo under normoxic conditions, the aim of this study was to examine the effect of accelerated erythropoiesis (and the consequent high iron need of the bone marrow) on iron metabolism in healthy humans injected with low doses of rhEpo for 1 month by simultaneously evaluating the expression of iron and hemoproteins in 2 compartments “competing” for iron: blood (the central erythropoietic compartment), and skeletal muscle.
The results of this study provide new information about the effects of rhEpo administration on systemic iron traffic and insights into the molecular mechanisms involved in iron and oxygen homeostasis in human skeletal muscle.
Methods
Participants
The study involved 8 healthy male volunteers, age (± SD) 23 (± 3) years, height 181 (± 7) cm, body weight 77 (± 5) kg, and maximal oxygen uptake 51 (± 7) mL/minute per kg, all of whom gave written informed consent in accordance with the Declaration of Helsinki. The study was approved by the Ethics Committee of Copenhagen and Frederiksberg counties, Denmark.
Procedures
Treatment with rhEpo.
The treatment protocol is shown in Figure 1A. After baseline measurements were made, 4 weeks of rhEpo treatment (epoetin β; NeoRecormon; Roche, Mannheim, Germany) was started with 5000 IU (∼ 65 IU/kg body weight) in 0.3 mL saline being injected subcutaneously once every other day for 2 weeks, and then once a week in the third and fourth weeks. This protocol replicates the initial part of our study in which subjects were administered the same rhEpo dose for 1 month, followed by once-weekly injections for a further 3 months.21 The rhEpo dose and schedule were chosen on the basis of clinical evidence showing the efficacy of low-dose rhEpo in renal anemia.22,23 The duration of rhEpo treatment was reduced in this study because we have previously observed that the main hematologic variables (total red blood cell volume, arterial oxygen content, and hemoglobin concentration) reach a plateau during the first month,21 thus indicating that this was the period of greatest erythropoietic activity and therefore the optimal “time window” in which to investigate alterations in iron metabolism. All of the injections were given between 8:00 and 10:00 am and were preceded by 30 minutes of supine rest. The subjects did not receive any iron supplementation.
Complementary experiment.
To gain additional insights into the response to rhEpo, we examined skeletal muscle and blood from healthy subjects undergoing different rhEpo treatments as reported in detail elsewhere.24 Briefly, study A evaluated the effects of long-term (14 weeks) low-dose rhEpo administration, and the biopsy and blood samples were collected from 8 healthy subjects before and after treatment; study B evaluated the effect of a single dose of rhEpo (15000 IU), and the biopsy and blood samples were obtained from 4 healthy subjects before and 10 hours after the injection.
Experimental setup.
The experimental design of the study is summarized in Figure 1A. The measurements consisted of venous blood and urine samples taken at baseline and on days 2, 4, 6, 8, and 28 of rhEpo treatment; a biopsy sample from the vastus lateralis muscle was taken at baseline and on days 8 and 28 of treatment; and the total red blood cell volume was determined at baseline and on days 2, 4, 6, 10, 14, and 28. On days 2, 4, 6, 8, 10, and 14, the samples were taken between 8:00 and 10:00 am from fasting subjects after 20 minutes of supine rest, just before the next rhEpo injection (ie, 48 hours after the previous injection); the day-28 samples were taken 7 days after the previous injection. The dual aims of the sampling on day 8 were to evaluate the muscle response to high erythropoietic activity and high Epo levels and to compare this response with data previously obtained after 7 to 9 days of exposure to high-altitude hypoxia20 (even though rhEpo stimulation could not replicate exactly the Epo kinetics observed during the high-altitude condition). The aim of the sampling on day 28 was to evaluate the effect of prolonged rhEpo treatment on skeletal muscle after Epo levels had returned to normal values.
Blood samples.
Blood samples taken from a forearm vein during supine rest just before the muscle biopsy were divided into aliquots, which were either immediately centrifuged and plasma stored at −20°C or allowed to clot at 4°C, centrifuged, and the serum stored at −20°C. The baseline serum and plasma values correspond to the mean of 2 independent samples drawn on separate days.
Urine samples.
Freshly collected, early morning urine was preserved with 0.01% sodium azide in polypropylene tubes, divided into aliquots, and then stored at −80°C for later analysis.
Muscle biopsies.
Under local anesthesia (lidocaine), a resting muscle biopsy was obtained from the middle portion of the vastus lateralis muscle with the use of the percutaneous needle biopsy technique with suction. The muscle specimen (an average of 90 mg) was immediately frozen in liquid nitrogen and stored at −80°C for subsequent analysis.
Total red blood cell volume.
Total red blood cell volume (RBCV) was measured using a carbon-monoxide (CO) rebreathing technique, as previously described.21,25 The CO-rebreathing method (1) has error of measurement similar to that of the radioactive chromium technique, which is the reference method, and (2) does not need to be corrected for loss of CO to myoglobin (≤ 1% of the CO dose).26 The administered volume of CO was approximately 1.3 mL/kg body mass. Baseline RBCV corresponds to the mean of 2 determinations made on separate days. Here, the mean error of measurement (coefficient of variation) is 2.5% for RBCV.
Blood analyses
Hemoglobin (Hb) and hematocrit (Hct) levels were determined by a blood gas analyser (ABL700; Radiometer, Copenhagen, Denmark). Plasma Epo was assessed with the use of an enzyme-linked immunoabsorbent assay (ELISA) kit (R&D Systems, Minneapolis, MN), and the serum concentrations of soluble transferrin receptor (sTfR), ferritin, transferrin, and ceruloplasmin were assessed by nephelometry (Behring Nephelemeter Analyser II; Dade Behring GmbH, Marburg, Germany) with the use of N latex sTfR, N latex ferritin, human transferrin, and human ceruloplasmin reagents (Dade Behring). Serum GDF-15 levels were determined by immunoradiometric assay, as previously described.27 Serum iron concentrations were assessed by ferrozin colorimetry with the use of an iron reagent (Advia1650; Bayer Diagnostic, Dublin, Ireland).
Urinary hepcidin assay
Hepcidin was measured by surface/enhanced laser desorption/ionization time-of-flight mass spectrometry (SELDI-TOF-MS) assay, as previously described,28 with synthetic 25-hepcidin (Peptides International, Louisville, KY) being used for calibration purposes. The relative concentrations of urinary hepcidin were expressed as mega intensity units per millimole of creatinine, as previously reported.28
Muscle biopsy analyses
Protein analyses.
Muscle tissue was homogenized in 20 mM Tris, pH 8, 200 mM LiCl, 1 mM EDTA, 0.5% Nonidet P40, and protease inhibitors (Sigma Chemical, Milan, Italy). The lysates were incubated on ice for 30 minutes, and the debris was pelleted at 14500g at 4°C. The supernatant was divided into aliquots and stored at −80°C. Protein concentration was determined with the use of the Dc protein assay (BioRad, Segrate, Italy).
For the immunoblot analyses, equal amounts of protein were resolved by SDS–polyacrylamide gel electrophoresis and transferred onto PVDF membranes (Amersham Biosciences, Milan, Italy). After assessing transfer by Ponceau S staining, the membranes were incubated with monoclonal anti-TfR1 (Zymed, San Francisco, CA) or affinity-purified IgG raised against a 19-aa peptide of ferroportin (Alpha Diagnostic, San Antonio, TX). In preliminary experiments the specificity of the anti–ferroportin antibody was checked by observing the disappearance of the band corresponding to the predicted molecular mass of ferroportin after preincubation with a 50-fold molar excess of the peptide used as antigen (Alpha Diagnostic). The primary antibodies were revealed by HRP-conjugated secondary antibodies (Amersham Biosciences) and a chemiluminescence kit (Amersham Biosciences), after which the filters were stained with amido black to assess equal protein loading. The antigens were quantitated by densitometry, with the values being calculated after normalization to the amount of amido black–stained proteins.
Ferritin concentrations were determined in cell lysates by an ELISA based on monoclonal antibodies specific for the human H and L subunits, as previously described.29
Myoglobin analysis was performed by 2-dimensional difference gel electrophoresis (2D-DIGE). Muscle tissue was ground in lysis buffer (pH 8.5) containing 7 M urea, 2 M thiourea, 4% CHAPS, 65 mM DTT, 0.5% IPG buffer, 30 mM Tris, and 1 mM PMSF and was solubilized by sonication on ice. Protein extracts were labeled with Cy5 dye (GE Healthcare, Milano, Italy), and the internal standard, generated by pooling together an equal amount of protein extract from each sample, was labeled with Cy3 dye (GE Healthcare). Samples were separated on immobilized pH gradient (IPG) strips (3-10 NL pH gradient), by a 200- to 8000-voltage gradient with an IPG-Phor unit (GE Healthcare). The second dimension separation was performed as previously described.20 The gels were visualized by a Typhoon 9200 laser scanner at 532 and 633 nm and emission filters of 520 and 670 nm (GE Healthcare), and the images were analyzed by a DeCyder DIA module V. 6.5 (GE Healthcare). The spots abundance, provided by the software, is the sum of the pixel values within each spot boundary normalized against the internal standard.
RNA isolation, reverse transcription, and polymerase chain reaction.
Total RNA was extracted and reverse transcribed with the Superscript II RNase H− system and Oligo dT (Invitrogen, Carlsbad, CA), as previously described.20
The mRNA content of TfR1 and ferroportin was determined by fluorescence-based real-time polymerase chain reaction (ABI PRISM 7900 Sequence Detection System; Applied Biosystems, Foster City, CA) with the use of previously described primers and probes designed from human-specific sequences available elsewhere.20 Probes labeling, amplification (in triplicates), and normalization to the cDNA content to give an mRNA expression ratio were performed as previously described.20 The amount of RNA/cDNA hybrids was determined as described,20 with the exception that the OliGreen reagent (Invitrogen) was used instead of the PicoGreen reagent.
Total muscle iron content.
The total iron content in muscle tissue was determined by atomic absorption spectrometry, as previously described.30
Statistics
The effect of rhEpo on the different variables parameters (3 or more repeated measures) was evaluated with the use of Friedman nonparametric test and, when the P value was significant, planned pair-wise specific comparisons (rhEpo versus baseline) were made with Wilcoxon test. The mRNA and urinary hepcidin data were log transformed before making the statistical analysis. The statistical calculations were made with the use of Statview software (version 5.0; SAS Institute, Cary, NC). A P value less than .05 was considered statistically significant.
Results
rhEpo stimulates iron incorporation in erythropoietic tissue
Circulating Epo levels before and during rhEpo treatment are shown in Figure 1B and are also partially reported elsewhere.31 On starting rhEpo administration, plasma Epo increased 2 to 3 times over baseline levels, remained high for as long as the participants were treated with rhEpo every other day, and then returned to pretreatment levels when rhEpo was administered once a week.
Experimental design and plasma Epo levels in response to rhEpo treatment. (A) Summary of the experimental procedures. The participants were subcutaneously injected with rhEpo 5000 IU on each occasion: every other day for 14 days and weekly during the last 2 weeks (large arrows). RBCV indicates total red blood cell volume. All of the measurements were made just before the next rhEpo injection. (B) The time course of plasma Epo in response to rhEpo treatment. Additional blood sampling on day 21 indicates the level of plasma Epo before the rhEpo injection on day 21. However, the absence of a measurement on day 23 prevented us from addressing the response of plasma Epo 48 hours after the previous rhEpo injection on day 21. Values are mean plus or minus SD for 8 participants. The statistical differences from pre-rhEpo values were calculated with the Wilcoxon test; *P < .05.
Experimental design and plasma Epo levels in response to rhEpo treatment. (A) Summary of the experimental procedures. The participants were subcutaneously injected with rhEpo 5000 IU on each occasion: every other day for 14 days and weekly during the last 2 weeks (large arrows). RBCV indicates total red blood cell volume. All of the measurements were made just before the next rhEpo injection. (B) The time course of plasma Epo in response to rhEpo treatment. Additional blood sampling on day 21 indicates the level of plasma Epo before the rhEpo injection on day 21. However, the absence of a measurement on day 23 prevented us from addressing the response of plasma Epo 48 hours after the previous rhEpo injection on day 21. Values are mean plus or minus SD for 8 participants. The statistical differences from pre-rhEpo values were calculated with the Wilcoxon test; *P < .05.
The response of erythroid tissue was evaluated by analyzing blood variables and total red blood cell volume. The hematologic response to rhEpo, indicating a progressive rise in Hct and Hb levels, and total red blood cell volume are summarized in Table 1. As expected, rhEpo favored iron entry into erythropoietic tissue, as shown by the progressive increase in soluble TfR (Table 2). This acquisition of iron by erythroid cells led to a decrease in body iron storage, as indicated by the progressive reduction in serum iron and ferritin levels, as well as transferrin saturation; however, ceruloplasmin and transferrin levels were not significantly altered (Table 2). These results indicate that the expected higher acquisition of iron by the erythropoietic compartment occurs in subjects treated with rhEpo.
Hemoglobin, hematocrit and total red blood cell volume
| . | Pre . | Days of rhEpo treatment . | |||||
|---|---|---|---|---|---|---|---|
| 0 . | 2 . | 4 . | 6 . | 10 . | 14 . | 28 . | |
| Hemoglobin (g.L−1) | 143 ± 6 | 142 ± 6 | 144 ± 4 | 145 ± 7 | 150 ± 8* | 150 ± 7* | 153 ± 11* |
| Hematocrit (%) | 43.9 ± 1.8 | 43.5 ± 1.8 | 44.3 ± 1.4 | 44.5 ± 2.2 | 45.9 ± 2.3* | 46.1 ± 2.2* | 46.8 ± 3.5* |
| RBCV (mL) | 2825 ± 295 | 2854 ± 316 | 2861 ± 366 | 2908 ± 362 | 2917 ± 407 | 3072 ± 343* | 3109 ± 441* |
| . | Pre . | Days of rhEpo treatment . | |||||
|---|---|---|---|---|---|---|---|
| 0 . | 2 . | 4 . | 6 . | 10 . | 14 . | 28 . | |
| Hemoglobin (g.L−1) | 143 ± 6 | 142 ± 6 | 144 ± 4 | 145 ± 7 | 150 ± 8* | 150 ± 7* | 153 ± 11* |
| Hematocrit (%) | 43.9 ± 1.8 | 43.5 ± 1.8 | 44.3 ± 1.4 | 44.5 ± 2.2 | 45.9 ± 2.3* | 46.1 ± 2.2* | 46.8 ± 3.5* |
| RBCV (mL) | 2825 ± 295 | 2854 ± 316 | 2861 ± 366 | 2908 ± 362 | 2917 ± 407 | 3072 ± 343* | 3109 ± 441* |
Mean values (± SD) for 8 participants. Venous blood samples and total red blood cell volume (RBCV; determined by means of a carbon monoxide rebreathing technique) were obtained before (Pre) and on several occasions during treatment with recombinant human Epo (rhEpo). The statistical differences from pre-rhEpo values were calculated using the Wilcoxon test.
P < .05.
Serum iron parameters
| . | Pre . | Days of rhEpo treatment . | ||||
|---|---|---|---|---|---|---|
| 0 . | 2 . | 4 . | 6 . | 8 . | 28 . | |
| sTfR (nmol.L−1) | 16.6 ± 4.0 | 17.9 ± 5.8 | 19.3 ± 7.2* | 22.7 ± 7.8* | 25.7 ± 6.5* | 30.1 ± 9.0* |
| Ferritin (μg.L−1) | 74.9 ± 65.4 | 74.7 ± 76.6 | 60.1 ± 64.7* | 46.4 ± 50.0* | 36.6 ± 36.2* | 36.5 ± 29.4* |
| Transferrin (g.L−1) | 2.7 ± 0.1 | 2.7 ± 0.2 | 2.7 ± 0.3 | 2.8 ± 0.3 | 2.9 ± 0.3 | 2.9 ± 0.3 |
| Serum iron (μmol.L−1) | 18.1 ± 5.0 | 12.4 ± 3.7* | 9.4 ± 5.7* | 8.3 ± 3.1* | 7.2 ± 3.3* | 10.5 ± 4.5* |
| Iron saturation of transferrin (%) | 27 ± 8 | 19 ± 6 | 14 ± 9 | 12 ± 5* | 10 ± 5* | 15 ± 6* |
| Ceruloplasmin (g.L−1) | 0.26 ± 0.03 | 0.27 ± 0.06 | 0.28 ± 0.06 | 0.29 ± 0.06 | 0.29 ± 0.06 | 0.25 ± 0.03 |
| . | Pre . | Days of rhEpo treatment . | ||||
|---|---|---|---|---|---|---|
| 0 . | 2 . | 4 . | 6 . | 8 . | 28 . | |
| sTfR (nmol.L−1) | 16.6 ± 4.0 | 17.9 ± 5.8 | 19.3 ± 7.2* | 22.7 ± 7.8* | 25.7 ± 6.5* | 30.1 ± 9.0* |
| Ferritin (μg.L−1) | 74.9 ± 65.4 | 74.7 ± 76.6 | 60.1 ± 64.7* | 46.4 ± 50.0* | 36.6 ± 36.2* | 36.5 ± 29.4* |
| Transferrin (g.L−1) | 2.7 ± 0.1 | 2.7 ± 0.2 | 2.7 ± 0.3 | 2.8 ± 0.3 | 2.9 ± 0.3 | 2.9 ± 0.3 |
| Serum iron (μmol.L−1) | 18.1 ± 5.0 | 12.4 ± 3.7* | 9.4 ± 5.7* | 8.3 ± 3.1* | 7.2 ± 3.3* | 10.5 ± 4.5* |
| Iron saturation of transferrin (%) | 27 ± 8 | 19 ± 6 | 14 ± 9 | 12 ± 5* | 10 ± 5* | 15 ± 6* |
| Ceruloplasmin (g.L−1) | 0.26 ± 0.03 | 0.27 ± 0.06 | 0.28 ± 0.06 | 0.29 ± 0.06 | 0.29 ± 0.06 | 0.25 ± 0.03 |
Mean values (± SD) for 8 participants. Venous blood samples were obtained before (Pre) and on several occasions during treatment with recombinant human Epo (rhEpo). The statistical differences from pre-rhEpo values were calculated using the Wilcoxon test.
sTfR indicates soluble transferrin receptor.
P < .05.
Treatment with rhEpo is associated with a decrease in hepcidin levels
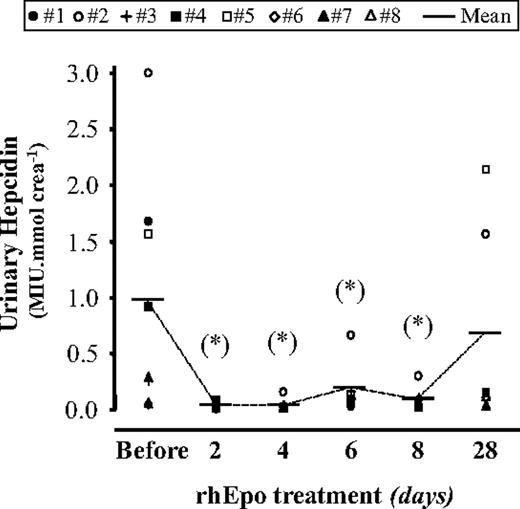
To investigate the role of hepcidin, the principal regulator of systemic iron homeostasis,2-4 we measured urinary hepcidin levels, which were promptly (within 48 hours) and dramatically down-regulated by rhEpo treatment (Figure 2). The levels remained low for as long as the rhEpo concentration was high (Figure 1B) and returned to near baseline values at the end of treatment.
Treatment with rhEpo induces a strong and early decrease in urinary hepcidin. Urinary hepcidin levels were determined by SELDI-TOF-MS and are expressed in mega intensity units (MIU) per millimole of creatinine. Individual data for 8 participants, as well as mean values, are shown. The statistical differences from pre-rhEpo values were calculated with the Wilcoxon test; *P < .05.
Treatment with rhEpo induces a strong and early decrease in urinary hepcidin. Urinary hepcidin levels were determined by SELDI-TOF-MS and are expressed in mega intensity units (MIU) per millimole of creatinine. Individual data for 8 participants, as well as mean values, are shown. The statistical differences from pre-rhEpo values were calculated with the Wilcoxon test; *P < .05.
Transferrin saturation, which is thought to be a factor controlling hepcidin expression,32-34 did not decline as strongly and reached statistical significance only at day 6 (Table 2). We also measured serum GDF-15, which has been related to hepcidin inhibition,11 and found that GDF-15 levels were only marginally increased by rhEpo (Figure 3). Furthermore, the small transient increase in GDF-15 occurred later than the decrease in hepcidin, thus suggesting that other factors are involved in the early fall in hepcidin levels.
Serum GDF-15 marginally increases during rhEpo treatment. The serum levels of GDF-15 were determined by immunoradiometry. Values are mean plus or minus SE for 8 participants. The statistical differences from pre-rhEpo values were calculated with the Wilcoxon test; *P < .05.
Serum GDF-15 marginally increases during rhEpo treatment. The serum levels of GDF-15 were determined by immunoradiometry. Values are mean plus or minus SE for 8 participants. The statistical differences from pre-rhEpo values were calculated with the Wilcoxon test; *P < .05.
rhEpo administration is associated with increased iron storage in skeletal muscle
The increased iron incorporation in erythropoietic tissue secondary to rhEpo treatment, together with the reduced hepcidin levels, suggested increased iron mobilization from the storage compartment. To investigate the underlying mechanisms, we explored the expression of the main proteins of iron metabolism in the biopsy specimens of skeletal muscle. First of all, concomitantly with high plasma Epo levels, we found a 10-fold increase in the L ferritin subunit on day 8 of rhEpo treatment, which returned to baseline levels at the end of treatment (Figure 4A). The amount of the H ferritin subunit did not significantly change throughout the treatment (Figure 4A).
Short-term rhEpo treatment increases L ferritin and total iron content in skeletal muscle; long-term rhEpo treatment decreases muscle L ferritin. The figure shows the changes in the ferritin and iron content of muscle biopsy samples obtained from the vastus lateralis before and after 8 and 28 days of rhEpo treatment. The ferritin heavy (H) and light (L) subunit contents (A) were determined by ELISA with the use of monoclonal antibodies against the corresponding recombinant human ferritin subunits and are expressed in relation to their pretreatment values, arbitrarily defined as 100. The values are all representative of 3 independent determinations. Total iron content (B) was determined by atomic absorption spectrometry, measured in μg/100 mg dry weight and expressed in relation to its pretreatment value, arbitrarily defined as 100. Values are mean plus or minus SE for 8 participants. (C,D) Complementary experiments involving independent groups of participants (see “Methods”). (C) Values are mean muscle ferritin levels plus or minus SE measured in 4 participants before and 10 hours after the injection of a single rhEpo dose of 15000 IU (complementary experiment B). (D) Values are mean muscle ferritin levels plus or minus SE measured in 8 participants before and after 14 weeks of rhEpo treatment, expressed in relation to the pretreatment value, arbitrarily defined as 100 (complementary experiment A). The statistical differences from pre-rhEpo values were calculated with the Wilcoxon test; *P < .05; ‡P = .05.
Short-term rhEpo treatment increases L ferritin and total iron content in skeletal muscle; long-term rhEpo treatment decreases muscle L ferritin. The figure shows the changes in the ferritin and iron content of muscle biopsy samples obtained from the vastus lateralis before and after 8 and 28 days of rhEpo treatment. The ferritin heavy (H) and light (L) subunit contents (A) were determined by ELISA with the use of monoclonal antibodies against the corresponding recombinant human ferritin subunits and are expressed in relation to their pretreatment values, arbitrarily defined as 100. The values are all representative of 3 independent determinations. Total iron content (B) was determined by atomic absorption spectrometry, measured in μg/100 mg dry weight and expressed in relation to its pretreatment value, arbitrarily defined as 100. Values are mean plus or minus SE for 8 participants. (C,D) Complementary experiments involving independent groups of participants (see “Methods”). (C) Values are mean muscle ferritin levels plus or minus SE measured in 4 participants before and 10 hours after the injection of a single rhEpo dose of 15000 IU (complementary experiment B). (D) Values are mean muscle ferritin levels plus or minus SE measured in 8 participants before and after 14 weeks of rhEpo treatment, expressed in relation to the pretreatment value, arbitrarily defined as 100 (complementary experiment A). The statistical differences from pre-rhEpo values were calculated with the Wilcoxon test; *P < .05; ‡P = .05.
In line with the higher level of L ferritin, which is related to intracellular iron deposits,35 muscle total iron content was also increased on day 8 (Figure 4B); it showed a tendency to decrease by day 28 but remained significantly higher than pretreatment levels.
The dramatic increase in muscle L ferritin observed on day 8 of rhEpo treatment prompted us to examine additional samples obtained during our complementary studies.24 Four subjects who had received a single rhEpo dose (study B) showed a doubling in muscle L ferritin levels 10 hours after the injection (Figure 4C), at a time when their serum iron variables showed no change (results not shown). On the contrary, in the second study (study A), we found that muscle L ferritin levels decreased after long-term (14 weeks) rhEpo administration (Figure 4D), that is, long after total red blood cell volume had stabilized21 and at a time when serum iron variables had returned to normal levels (results not shown).
We next examined the expression of TfR1, which mediates iron entry into cells (reviewed in Andrews2 ). As shown in Figure 5, both TfR1 mRNA and protein levels increased markedly and progressively in the skeletal muscle tissue. Similarly, the mRNA levels of ferroportin (the only iron export protein),2,3 doubled on day 8 of rhEpo treatment and peaked on day 28 (Figure 6A). To evaluate ferroportin protein levels by immunoblot analysis, we first characterized the immunoreactivity of the antibody. Figure 6B shows that an excess of a blocking peptide prevented the immunodetection of a band migrating at 62 kDa, which corresponds to the predicted mass of ferroportin.2,3 Therefore, we focused the analysis of ferroportin expression in the muscle samples on this band. Ferroportin increased on day 8 and then tended to return toward baseline values but remained significantly higher than pretreatment levels (Figure 6C,D).
Treatment with rhEpo increases transferrin receptor (TfR1) expression in skeletal muscle. The figure shows the changes in TfR1 expression in muscle biopsy samples obtained from the vastus lateralis before and after 8 and 28 days of rhEpo treatment. (A) TfR1 mRNA levels were normalized to total cDNA content and are expressed in relation to the pretreatment value, arbitrarily defined as 100. (B) Equal amounts of proteins (as assessed by amido black staining) from the muscle biopsy extracts were loaded onto SDS polyacrylamide gels, immunoblotted with antibodies against TfR1, and visualized by chemiluminescence. The result shown is representative of 3 independent experiments obtained with extracts from 2 participants (#1 and #2). (C) The densitometric quantification of the immunoblot analysis of TfR1 protein content before and after 8 and 28 days of rhEpo treatment. Values are mean ± SE for 8 participants. The statistical differences from pre-rhEpo values were calculated with the Wilcoxon test; *P < .05.
Treatment with rhEpo increases transferrin receptor (TfR1) expression in skeletal muscle. The figure shows the changes in TfR1 expression in muscle biopsy samples obtained from the vastus lateralis before and after 8 and 28 days of rhEpo treatment. (A) TfR1 mRNA levels were normalized to total cDNA content and are expressed in relation to the pretreatment value, arbitrarily defined as 100. (B) Equal amounts of proteins (as assessed by amido black staining) from the muscle biopsy extracts were loaded onto SDS polyacrylamide gels, immunoblotted with antibodies against TfR1, and visualized by chemiluminescence. The result shown is representative of 3 independent experiments obtained with extracts from 2 participants (#1 and #2). (C) The densitometric quantification of the immunoblot analysis of TfR1 protein content before and after 8 and 28 days of rhEpo treatment. Values are mean ± SE for 8 participants. The statistical differences from pre-rhEpo values were calculated with the Wilcoxon test; *P < .05.
Treatment with rhEpo increases ferroportin expression in skeletal muscle. The figure shows the changes in ferroportin (fpn) expression in muscle biopsy samples obtained from the vastus lateralis before and after 8 and 28 days of rhEpo treatment. (A) Ferroportin mRNA levels were normalized to total cDNA content and are expressed in relation to the pretreatment value, arbitrarily defined as 100. (B) Experiments confirm antibody specificity by peptide competition: muscle extract from the same biopsy was blotted with anti–ferroportin antibody preincubated in the presence or absence of a molar excess of ferroportin peptide. The similar intensity of the band above 110 kDa indicates equal protein loading. (C) Equal amounts of proteins (as assessed by amido black staining) from muscle biopsy extracts were loaded onto SDS polyacrylamide gels, immunoblotted with antibodies against ferroportin, and visualized by chemiluminescence. The result shown is representative of 3 independent experiments obtained with extracts from 2 participants (#1 and #2). (D) The densitometric quantification of the immunoblot analysis of ferroportin protein content before and after 8 and 28 days of rhEpo treatment. Values are mean ± SE for 8 participants. The statistical differences from pre-rhEpo values were calculated with the Wilcoxon test; *P < .05.
Treatment with rhEpo increases ferroportin expression in skeletal muscle. The figure shows the changes in ferroportin (fpn) expression in muscle biopsy samples obtained from the vastus lateralis before and after 8 and 28 days of rhEpo treatment. (A) Ferroportin mRNA levels were normalized to total cDNA content and are expressed in relation to the pretreatment value, arbitrarily defined as 100. (B) Experiments confirm antibody specificity by peptide competition: muscle extract from the same biopsy was blotted with anti–ferroportin antibody preincubated in the presence or absence of a molar excess of ferroportin peptide. The similar intensity of the band above 110 kDa indicates equal protein loading. (C) Equal amounts of proteins (as assessed by amido black staining) from muscle biopsy extracts were loaded onto SDS polyacrylamide gels, immunoblotted with antibodies against ferroportin, and visualized by chemiluminescence. The result shown is representative of 3 independent experiments obtained with extracts from 2 participants (#1 and #2). (D) The densitometric quantification of the immunoblot analysis of ferroportin protein content before and after 8 and 28 days of rhEpo treatment. Values are mean ± SE for 8 participants. The statistical differences from pre-rhEpo values were calculated with the Wilcoxon test; *P < .05.
In brief, although we cannot prove that the alterations in the expression of the major iron proteins in response to rhEpo treatment are accompanied by corresponding changes in protein expression on the cell surface, the present data suggest that iron incorporation into muscle is greater than iron exportation from muscle, and so muscle iron accumulates.
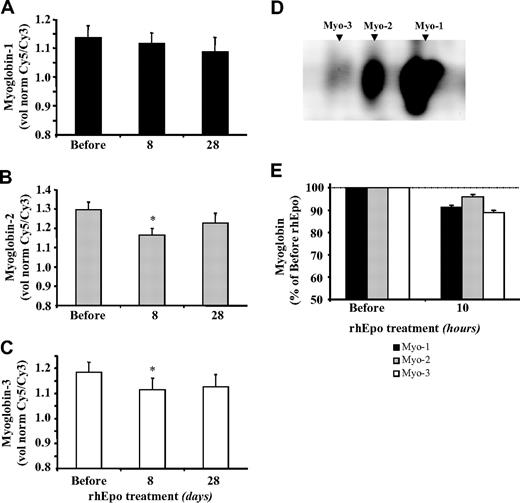
Myoglobin content remains unchanged after rhEpo administration
To obtain further insights into the interactions between iron and oxygen regulation in skeletal muscle tissue, we analyzed myoglobin protein expression in muscle biopsy specimens by 2D-DIGE gel electrophoresis followed by fluorescent staining (Figure 7D). No significant changes in the amount of the major myoglobin isoform 1 were detected in the various samples at the different time points (Figure 7A), but there was a transient small decrease in isoforms 2 and 3 on day 8 (Figure 7B,C). Because myoglobin isoform 1 accounts for 75% to 80% of the total under control conditions,36 our results indicate that no major alterations in myoglobin expression occurred during rhEpo-induced erythropoiesis, thus suggesting no change in muscle oxygen homeostasis. The results of our complementary study (study B) support this finding, because they showed no change in myoglobin 10 hours after a single rhEpo injection (Figure 7E).
Treatment with rhEpo does not alter myoglobin protein expression in skeletal muscle. The figure shows the changes in myoglobin levels in muscle biopsy samples obtained from the vastus lateralis before and after 8 and 28 days of rhEpo treatment. The myoglobin content of the muscle biopsy extracts was analyzed by 2D-DIGE. The quantification of the separated isoforms was performed with a DeCyder DIA module V. 6.5. The values are given as volumes normalized against the internal standard and are mean plus or minus SE for 8 participants. (A) The quantitative changes in relation to myoglobin isoform 1 (main isoform, 75%-80% of the total),36 (B) the quantitative changes in relation to myoglobin isoform 2 (15%-20% of the total), and (C) the quantitative changes in relation to myoglobin isoform 3 (5% of the total). Values are mean plus or minus SE for 8 participants. (D) The 3 myoglobin isoforms were separated by 2D gel electrophoresis in a 3 to 10 NL pH gradient as first dimension. (E) Refers to complementary experiment B: muscle myoglobin protein expression was measured before and 10 hours after the injection of a single rhEpo dose of 15000 IU. Values are mean plus or minus SE for 4 participants. The statistical differences from pre-rhEpo values were calculated with the Wilcoxon test; *P < .05.
Treatment with rhEpo does not alter myoglobin protein expression in skeletal muscle. The figure shows the changes in myoglobin levels in muscle biopsy samples obtained from the vastus lateralis before and after 8 and 28 days of rhEpo treatment. The myoglobin content of the muscle biopsy extracts was analyzed by 2D-DIGE. The quantification of the separated isoforms was performed with a DeCyder DIA module V. 6.5. The values are given as volumes normalized against the internal standard and are mean plus or minus SE for 8 participants. (A) The quantitative changes in relation to myoglobin isoform 1 (main isoform, 75%-80% of the total),36 (B) the quantitative changes in relation to myoglobin isoform 2 (15%-20% of the total), and (C) the quantitative changes in relation to myoglobin isoform 3 (5% of the total). Values are mean plus or minus SE for 8 participants. (D) The 3 myoglobin isoforms were separated by 2D gel electrophoresis in a 3 to 10 NL pH gradient as first dimension. (E) Refers to complementary experiment B: muscle myoglobin protein expression was measured before and 10 hours after the injection of a single rhEpo dose of 15000 IU. Values are mean plus or minus SE for 4 participants. The statistical differences from pre-rhEpo values were calculated with the Wilcoxon test; *P < .05.
Discussion
The increased red cell production that occurs in physiologic and clinical situations threatening tissue oxygen availability is mediated by Epo and requires an increased iron supply to erythropoietic tissue. Greater duodenal iron absorption and faster iron release from macrophages, as well as mobilization from storage organs such as the liver, are recognized mechanisms ensuring that iron supply matches the demands of stimulated erythropoiesis (reviewed in Andrews2 and De Domenico3 ). We have recently demonstrated in healthy humans that skeletal muscle tissue can be another significant source of iron during high altitude–induced erythropoiesis.20
Our findings quite surprisingly showed that skeletal muscle does not lose its iron in healthy participants treated with low-dose rhEpo for 4 weeks. On the contrary, skeletal muscle tissue accumulates iron despite systemic iron deficiency secondary to rhEpo-induced erythropoiesis and hepcidin suppression promoting iron store mobilization. The following discussion considers the potential mechanisms by which this occurs, and we also discuss the new insights into iron-oxygen interactions in humans shown by our finding that muscle iron responds differently to accelerated erythropoiesis when hypoxia is superimposed20 or not (present study).
Hepcidin suppression after rhEpo administration
One major finding of this study is the rapid and marked reduction in hepcidin levels that takes place after rhEpo administration. This is consistent with the idea that erythropoietic activity has a dominant suppressive effect on hepcidin, as indicated by several studies on rhEpo-treated animals12-16 and patients with hereditary8-10 or acquired37 anemias with ineffective erythropoiesis. Nevertheless, to the best of our knowledge, this is the first study that directly shows a marked suppression of hepcidin after pharmacologic stimulation of erythropoietic activity with clinically relevant doses of rhEpo in humans in the absence of anemia or hypoxia. Remarkably, the suppression occurred rapidly, that is, by the second day of rhEpo administration. On the basis of our data, it seems unlikely that this effect is mediated by an increase in GDF-15 levels.11 Early hepcidin down-regulation was concomitant with the decrease of serum iron (and to some extent of transferrin saturation), thus suggesting a possible role for circulating iron as a signaling factor. Nevertheless, we noted that hepcidin returned to normal values at the end of treatment, when serum iron was still low, but there was plenty of iron in the erythroid compartment, pointing out other regulatory mechanisms. Indeed, our in vivo data suggest a possible direct involvement of Epo in hepcidin regulation and are in line with in vitro experiments showing that the interaction of Epo with EpoR inhibits hepcidin transcription in hepatic cellular models.17,18
Whatever the molecular mechanism(s) involved in hepcidin down-regulation, our findings may help to clarify the complex pathophysiologic changes that occur in patients treated with pharmacologic doses of rhEpo, such as those with advanced chronic renal failure, and ultimately lead to a more rational use of erythropoietic-stimulating agents or iron therapy or both in these clinical settings.38 Epo could directly stimulate erythropoiesis by 2 mechanisms: (1) enhancement of erythrocyte survival and differentiation and (2) down-regulation of hepcidin, hence increasing the availability of iron, a key component of functional hemoglobin. These mechanisms may also be at the basis of the positive effect of therapy with erythropoietic agents on the correction of anemia in a large proportion of patients with inflammatory disease39 (albeit not in all because inflammation may contribute to anemia by hepcidin-independent mechanisms40 ).
Moreover, our finding that Epo-treated subjects accumulate skeletal muscle iron despite hepcidin suppression suggests that hepcidin is not the only factor controlling muscle iron metabolism in this condition and supports the idea that the response to hepcidin may be cell type and tissue specific.41
Alterations in muscle iron metabolism after rhEpo administration: potential mechanisms
In the skeletal muscle of our rhEpo-treated subjects, increased iron content was associated with the induction of L ferritin and an increased expression of TfR1 and ferroportin. What triggers lead to these unexpected changes in iron homeostasis?
A direct role of Epo may be suggested. Two recent studies have shown that STAT5, a target of Epo,1 is involved in the transcriptional up-regulation of TfR1 in erythroid cells.42,43 EpoRs have been identified in human muscle cells,24 and this mechanism may also function in skeletal muscle, thus explaining the increased TfR1 expression found in the muscles of rhEpo-treated subjects. Higher TfR1 levels may increase the uptake of transferrin-bound iron, which is then stored within L subunit-rich ferritin. Because increased L ferritin levels in the absence of hematologic changes were also found in subjects acutely treated with rhEpo (see Figure 4C), a direct effect of rhEpo cannot be excluded, although we have not found any effect on ferritin levels in rat c2c12 cells exposed to rhEpo before (myoblasts) or after serum deprivation-induced differentiation (myotubes; G.C., S.R., unpublished observation, May 2008). We therefore favor the interpretation that L ferritin induction is secondary to iron accumulation and not to a direct effect of rhEpo. Notably, on day 28 L ferritin returned to baseline, whereas cellular iron remained higher than pretreatment levels, but the available data do not allow us to identify the localization of this iron.
Increased ferroportin expression may also be iron-mediated. It has been shown that iron loading increases ferroportin mRNA and protein levels in macrophages44,45 and other cells,3,46-49 by acting at transcriptional45,49 and posttranscriptional47 levels. In addition to iron, ferroportin expression is posttranslationally mediated by hepcidin.5,50 The extremely low levels of hepcidin in our rhEpo-treated subjects may cooperate with intracellular muscle iron loading to favor ferroportin expression at the cell membrane. Nevertheless, because muscle ultimately gains iron after rhEpo suggests that ferroportin-induced iron exportation does not operate properly or is overridden by TfR1-mediated iron uptake in this tissue.
In summary, we propose that under acute rhEpo stimulation (up to 8 days), muscle iron might contribute to erythropoiesis through ferroportin-induced iron export (as suggested by Figure 6); however, iron traffic alterations within skeletal muscle result in iron accumulation (Figure 4A,B and also Figure 4C). Under chronic rhEpo stimulation, the muscle begins to lose some of its iron, which yet remains at a level higher than baseline (Figure 4A,B after 1 month). However, the decrease in L ferritin content (Figure 4D) suggests that in the long term (14 weeks) muscle would eventually lose iron.
rhEpo versus prolonged hypoxia: why does muscle iron respond differently?
One of the aims of the present study was to dissociate the effect of accelerated erythropoiesis from that of hypoxia, but we acknowledge some limitations. First, circulating Epo levels differed between the 2 conditions: although at high altitude serum Epo levels increased dramatically (450%) during the first days of acclimatization51 and then progressively declined (40% above normal after 7-9 days20 ), rhEpo provoked a sustained increase in serum Epo over the first week (164% on day 8). Second, it may be difficult to completely divorce hypoxic effects from rhEpo effects. Indeed, both conditions are known to stimulate cytokine production, which, in turn, may alter iron metabolism. Furthermore, rhEpo depletes iron store; thus, it is not possible to exclude an effect of the hypoxia-inducible factor (HIF-1) also in this condition. Finally, rhEpo, alike high-altitude exposure, promotes plasma volume contraction.21 Nevertheless, the analysis of the present results in view of our high-altitude study20 gives additional insights into the interactions between erythropoiesis and iron metabolism.
The observation that rhEpo-induced erythropoiesis increases muscle iron content (this study), whereas it is decreased by erythropoiesis associated with high-altitude hypoxia,20 raises the question as to which molecular pathways are involved and highlights the potential role of hypoxia in triggering muscle iron mobilization. One possible mechanism involves an increase in TfR1 expression after rhEpo treatment that is directly mediated by Epo,42 rather than the previously observed TfR1 down-regulation.20 However, although ferroportin induction was observed both at high altitude and after rhEpo treatment, only hypoxia resulted in effective muscle iron mobilization, whereas rhEpo unexpectedly led to muscle iron accumulation. The mechanism underlying such dissociation cannot be evidenced from our data; however, we speculate that increased TfR1 may counteract muscle iron export during normoxic rhEpo stimulation, whereas decreased TfR1 would cooperate with iron export during sustained hypoxia. Moreover, our rhEpo-treated subjects did not show the increase in serum ceruloplasmin that is induced by exposure to high altitude20 and is possibly a direct effect of hypoxia (given that ceruloplasmin expression is up-regulated by HIF-152 ). Because the ferroxidase activity of ceruloplasmin is necessary for facilitating ferroportin-mediated iron export activity,53 in the absence of increased ceruloplasmin, the enhanced ferroportin expression in skeletal muscle may not be sufficient to match the increased uptake of transferrin-bound iron, and this may facilitate iron storage.
The finding that rhEpo did not alter the levels of myoglobin isoform 1 (ie, the main, biologically relevant myoglobin isoform36 ) despite muscle iron accumulation differs from our previous data indicating a large (35%) decrease in myoglobin isoform 1 during prolonged hypoxia, along with muscle iron loss.20
We propose that in the absence of hypoxia, rhEpo treatment promotes iron accumulation in iron-replete muscle cells, which respond by storing iron into ferritin (at least temporarily). By contrast, under more severe conditions such as hypoxia or blood loss, muscle cells may contribute some of their iron from myoglobin to help restoring oxygen transport capacity. Accordingly, the skeletal muscle should not be regarded as a “real” iron storage compartment like macrophages or liver, except under massive stress.
In conclusion, our results provide new insights into the interactions between erythropoiesis and iron metabolism in humans by showing that rhEpo administration induces a strong and early suppression of hepcidin. Second, the demonstration of iron accumulation in skeletal muscle tissue despite hepcidin suppression highlights the role of hepcidin-independent mechanisms in regulating iron traffic in this tissue.
By raising the possibility of a direct effect of rhEpo on hepcidin suppression in humans, our data may have clinical implications for optimizing the use of erythropoiesis-stimulating agents. Another clinically important finding is that rhEpo administration may favor iron loading in skeletal muscle. Whether this response is biologically relevant to muscle function cannot be established from our data, but this should be considered during rhEpo therapy especially when high doses are administered.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Paolo Arosio for critically reading the manuscript.
This work was supported by research funding from Anti-Doping Denmark (C.L.); from the Secrétariat d'Etat aux Sports, à la Jeunesse et à la Vie Associative (Direction des Sports; P.R.); from Telethon Italy (GGP06213) and the Cariverona Foundation (Verona, Italy; D.G.); from the Associazione Everest-K2-CNR and Fondo per gli Investimenti della Ricerca di Base RBRN07BMCT (C.G.); and from the Italian University and Scientific Research Ministry (MIUR; G.C.).
Authorship
Contribution: P.R. designed and performed the study, collected and analyzed data, and wrote the paper; S.R. and D.G. contributed vital new reagents or analytical tools, collected and analyzed data, and wrote the paper; N.J.A.-A., J.J.T., and A.M.N. performed the research; C.G., A.A., N.C., A.C., A.V., P.S., T.K., K.C.W., and S.M. contributed vital new reagents or analytical tools and collected data; C.L. designed and performed the study, contributed vital new reagents or analytical tools, collected and analyzed data, and wrote the paper; and G.C. designed the study, contributed vital new reagents or analytical tools, collected and analyzed data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Gaetano Cairo, University of Milan School of Medicine, Department of Human Morphology and Biomedical Sciences Città Studi, Via Mangiagalli 31, 20133 Milano, Italy; e-mail: gaetano.cairo@unimi.it.
References
Author notes
*C.L. and G.C. contributed equally to this study.






This feature is available to Subscribers Only
Sign In or Create an Account Close Modal