Abstract
Phase 1 testing of ezatiostat, a glutathione S-transferase P1-1 inhibitor, for the treatment of myelodysplastic syndrome was conducted in a multidose-escalation study. Patients received 10 dose levels (200, 400, 1000, 1400, 2000, 2400, 3000, 4000, 5000, and 6000 mg) of ezatiostat tablets in divided doses on days 1 to 7 of a 21-day cycle for a maximum of 8 cycles. The safety and pharmacokinetics of ezatiostat were evaluated. Forty-five patients with low to intermediate-2 International Prognostic Scoring System risk myelodysplastic syndrome were enrolled. No dose-limiting toxicities were observed. The most common grade 1 or 2, respectively, treatment-related adverse events were nonhematologic: nausea (56%, 9%), diarrhea (36%, 7%), vomiting (24%, 7%), abdominal pain (9%, 0%), constipation (4%, 9%), anorexia (3%, 7%), and dyspepsia (3%, 7%). Concentration of the primary active metabolite, TLK236, increased proportionate to ezatiostat dosage. Seventeen hematologic improvement (HI) responses by International Working Group criteria were observed at dose levels of 200 to 6000 mg/day with 11 HI responses at doses of 4000 to 6000 mg/day. HI responses occurred in all lineages including 3 bilineage and 1 complete cytogenetic response. Decreased number of red blood cell and platelet transfusions and in some cases transfusion independence were attained. Extended dose schedules of ezatiostat tablets are under investigation. This study was registered at http://www.clinicaltrials.gov as NCT00280631.
Introduction
Myelodysplastic syndrome (MDS) is a heterogeneous group of clonal hematopoietic stem cell disorders characterized by dysplasia in 1 or more myeloid, erythroid, and megakaryocytic lineages, leading to ineffective blood cell production and a variable risk of transformation to acute myelogenous leukemia (AML).1-4 The treatment options available to patients with MDS are largely based upon the patient's age and prognosis as determined by the International Prognostic Scoring System (IPSS).5 For patients in the low to intermediate 1 (INT-1) IPSS risk categories, the goal of treatment is to improve ineffective hematopoiesis while providing the appropriate supportive care. In higher risk patients, the goal is to extend survival and delay transformation to AML.
Current therapeutic strategies for patients with low or INT-1 risk include supportive care6 and low-intensity therapy such as chemotherapy with low-dose cytarabine, azacitidine, decitabine, or biologic response modifiers (eg, amifostine, pentoxifylline, lisofylline, interferon alpha, anti-thymocyte globulin [ATG], cyclosporine, retinoids, and vitamin D analogs), or nonchemotherapeutic agents such as thalidomide or thalidomide analogs.7,8
The National Comprehensive Cancer Network (NCCN) Practice Guidelines recommend the use of erythropoietin (EPO) and other stem cell factors for anemia.7 For patients with refractory anemia (RA) and a serum EPO level of less than 500 mU/mL, EPO may be given subcutaneously for 2 to 3 months. Concomitant oral iron may be beneficial even without iron deficiency in the absence of iron overload. In RA with ringed sideroblasts (RARS), EPO can be combined synergistically with granulocyte colony-stimulating factor (G-CSF) for 3 months. G-CSF or granulocyte-macrophage colony-stimulating factor (GM-CSF) should not be administered routinely for infection prophylaxis, but should be considered for recurrent or resistant infections in neutropenic patients.
To date, only a few treatments have had an impact on the natural history of MDS. Recent results from a clinical trial with azacitidine in MDS patients have shown that the drug improves survival and quality of life for patients with MDS compared with best supportive care.9,10 Allogeneic bone marrow transplantation is a potentially curative procedure with a high treatment-related mortality (40% to 50%), but is applicable to only a minority of patients of younger age (those < 60 years old) with good performance status and a suitable donor.11,12 Otherwise, management is largely supportive, consisting of treatment of infections, transfusions of red cells for anemia, platelet transfusions to reduce bleeding, and use of hematopoietic growth factors.
Recently, azacitidine was approved by the Food and Drug Administration (FDA) based upon a 16% response rate in azacitidine-treated patients compared with 0 percent receiving best supportive care in a 191-patient study.9,10 In 2006, Revlimid (lenalidomide), a thalidomide analog, was approved by the FDA for treatment of patients with transfusion-dependent anemia due to low or INT-1 risk MDS associated with a deletion 5q cytogenetic abnormality with or without additional cytogenetic abnormalities. Dacogen (decitabine), a hypomethylating agent, was also approved by the FDA in 2006 for treatment of patients with MDS including previously treated and untreated, de novo and secondary MDS of all French-American-British (FAB) subtypes and INT-1, INT-2, and high-risk IPSS groups. Attempts to use differentiating agents such as all-trans retinoic acid or vitamin D analogs have been disappointing,13,14 although one study reported a beneficial effect of vitamin D in the progression to acute leukemia.15 Trials of low-dose cytarabine have demonstrated hematologic response rates of 10% to 25%.16
Growth factors may improve cytopenias but do not induce complete remissions and may not prolong survival. Recently, weekly dosing of recombinant human erythropoietin alpha therapy resulted in improvement in erythropoiesis in a subset of MDS patients who were unresponsive to conventional dosing.17,18 Intensive chemotherapy induces complete remissions in 40% to 50% of patients but is associated with serious morbidity and mortality, with repeated and prolonged periods of hospitalization required and consequent impaired quality of life.
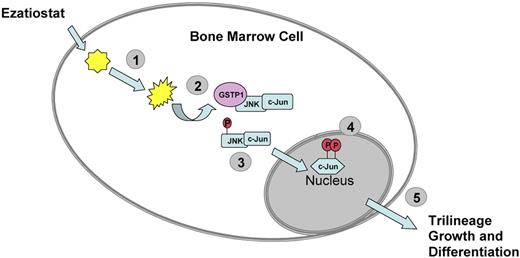
In conclusion, the need for new drugs with novel mechanisms of action remains. Ezatiostat hydrochloride (TLK199 tablets), a glutathione analog prodrug, is currently being developed for the treatment of cytopenias associated with MDS or chemotherapy. Ezatiostat is a synthetic tripeptide analog of glutathione that has been shown to stimulate the proliferation of myeloid precursors.19 Ezatiostat is metabolized to TLK117. TLK117 selectively binds to and inhibits glutathione S-transferase P1-1 (GST P1-1), an enzyme that is overexpressed in many human cancers. GST P1-1 is known to bind to and inhibit Jun-N-terminal kinase (JNK), a key regulator of cellular proliferation, differentiation, and apoptosis20 (Figure 1). TLK117 facilitates dissociation of GST P1-1 from JNK, leading to activation of JNK and the subsequent promotion of growth and maturation of hematopoietic progenitors in preclinical models, while promoting apoptosis in human leukemia cell lines. Ezatiostat has been shown to stimulate the multilineage differentiation of hematopoietic progenitors in vitro and overcomes the block in myeloblast differentiation (ineffective myelopoiesis) in leukemia cell lines.19,21,22
Ezatiostat HCl (TLK199). (1) Ezatiostat enters the cell and is hydrolyzed to its corresponding diacid. (2) The diacid form of ezatiostat binds to GST P1-1 and disrupts the interaction of GST P1-1 with JNK. (3) JNK is activated by phosphorylation and in turn activates c-Jun. (4) c-Jun and other regulators promote transcription of genes that lead to proliferation and differentiation of normal hematopoietic cells or apoptosis of malignant cells. (5) Signaling pathways are activated to promote growth and differentiation of hematologic progenitors that form neutrophils, erythrocytes, and platelets.
Ezatiostat HCl (TLK199). (1) Ezatiostat enters the cell and is hydrolyzed to its corresponding diacid. (2) The diacid form of ezatiostat binds to GST P1-1 and disrupts the interaction of GST P1-1 with JNK. (3) JNK is activated by phosphorylation and in turn activates c-Jun. (4) c-Jun and other regulators promote transcription of genes that lead to proliferation and differentiation of normal hematopoietic cells or apoptosis of malignant cells. (5) Signaling pathways are activated to promote growth and differentiation of hematologic progenitors that form neutrophils, erythrocytes, and platelets.
The absorption, distribution, metabolism, and excretion (ADME) properties of TLK199 were characterized in the rat and dog. The primary metabolite of TLK199 is to the phenylglycine monoester (TLK236). Unchanged TLK199 was not detected in blood, although the metabolites TLK117 and TLK236 were detected, indicating that the systemic clearance of the parent compound was both rapid and extensive. In a 14-day multiple dose study, up to 10 mg/kg TLK199 twice daily was administered to dogs orally. TLK199 was absorbed but was quickly biotransformed primarily to TLK236. Concentrations of TLK199 and TLK235, glutamate monomer, were either very low or below the detection limit. TLK117 is the predominant metabolite in the rat.
Toxicology studies included a 14-day repeat dose study in rats and dogs. Rats exhibited no significant toxicity following daily oral administration of TLK199 at doses up to 1000 mg/kg per day for 14 days. Dogs exhibited no significant toxicity following daily oral administration of TLK199 at doses as high as 20 mg/kg for 14 days.
On the basis of safety and promising hematologic activity of studies with the intravenous formulation of ezatiostat,23,24 we initiated a phase 1 study with an oral formulation. The goal of this study was to determine the maximum tolerated dose (MTD) or optimal biologic dose (OBD), the pharmacokinetics, safety profile, and preliminary evidence of hematologic improvement.
Methods
Patients
This study was conducted in accordance with the International Conference on Harmonization and Good Clinical Practice standards. Institutional review board (IRB) approval was obtained from all participating institutions: University of Massachusetts Medical School, Loyola University Medical School, and University of South Florida. (Note: J.G. moved to Southern Illinois University Simmons Cooper Cancer Institute, Springfield, IL; however, this institution did not participate in this study.) All patients provided written informed consent before study participation in accordance with the Declaration of Helsinki.
Eligible patients were 18 years or older, with histologically confirmed diagnosis of primary MDS, either newly diagnosed or for whom prior treatment was ineffective, and with an Eastern Cooperative Oncology Group (ECOG) performance status of 0 or 1. Patients were required to have adequate hepatic and renal function. No prior treatment with hematopoietic growth factors within 3 weeks of study entry was permitted. Other inclusion criteria were as follows: documented significant cytopenia for more than 2 months and ineligibility for stem cell bone marrow transplantation (BMT). Patients were excluded for prior allogeneic BMT, cytogenetic abnormalities consistent with de novo AML (such as t(15,17), t(9,11), t(8,21), t(9,22), t(8,16), and inv16),25,26 proliferative chronic myelomonocytic leukemia (CMML), prior leukemia, rapidly progressive AML, chronic myelogenous leukemia (CML) blast crisis, use of oral corticosteroids (> 10 mg), history of hepatitis B/C, HIV, or an active infection requiring intravenous antibiotics.
Study design
This phase 1 study of ezatiostat was conducted in patients with all World Health Organization (WHO) classification types of MDS.27 The objectives of the study were to evaluate safety, pharmacokinetics, and the hematologic improvement (HI) response rate. A cohort of patients was evaluated under fed/fast conditions to determine the effect of food on the pharmacokinetics of ezatiostat. Ezatiostat tablets were administered at 10 dose-escalation levels of 200, 400, 1000, 1400, 2000, 2400, 3000, 4000, 5000, and 6000 mg divided into 2 oral doses twice daily on days 1 to 7 of a 21-day treatment cycle. The dose schedule for ezatiostat tablets was chosen to mimic the schedule that led to hematologic improvement in the clinical phase 1-2a study of the intravenous liposomal formulation of TLK199 product in MDS patients.24 In addition, the dose schedule for TLK199 tablets mimics the schedule that led to steady-state pharmacokinetics after 3 to 5 days with the oral formulation nonclinical pharmacokinetic studies in animals. A minimum of 3 patients were treated at each dose level. If no more than none of 3 or 1 of 6 patients experienced a dose-limiting toxicity (DLT), 3 subsequent patients were enrolled at the next higher dose level. Dose-escalation continued until 2 or more patients in a cohort experienced a treatment-related DLT or dose escalation was completed based on the determination of the OBD. The MTD was defined as the highest dose at which none of 3 patients experienced a DLT or 1 full dose level below the level where a DLT was observed. If biologic activity (correction of neutropenia; improvement in anemia, platelet count, or blast count; reduction in red blood cells [RBCs] or platelet transfusion requirements) occurred before the MTD was established, the OBD was selected for future phase 2 studies. At the MTD or a dose level below the MTD, at least 6 additional patients were treated to further evaluate toxicity.
Patients were allowed to receive up to a maximum of 8 cycles of treatment unless unacceptable toxicity occurred. Treatment cycles continued until the patient experienced a lack of MDS response, disease progression, or unacceptable toxicity. The morning dose of ezatiostat tablets was administered after an overnight fast and the evening dose, approximately 12 hours later on an empty stomach (at least 3 hours after an evening meal). Twelve patients were enrolled to evaluate the pharmacokinetics of ezatiostat tablets under fed and fasted conditions. Under fed conditions, patients received the morning dose 30 minutes after a standard high-calorie meal followed by pharmacokinetic evaluation. Adverse events (AEs) were graded in accordance with the National Cancer Institute–Common Toxicity Criteria for Adverse Events, version 3.0 (NCI-CTCAE, version 3.0; Bethesda, MD). A hematologic DLT was defined as a grade 4 hematologic AE complicated by infection, severe hemorrhage, or marrow aplasia persisting more than 4 weeks. A nonhematologic DLT was defined as any treatment-related grade 3 or 4 nonhematologic AE occurring during the first treatment cycle.
Drug formulation and administration
Ezatiostat tablets are formulated as 100-mg and 500-mg tablets. Each tablet contains ezatiostat hydrochloride in a formulation containing the following excipients: mannitol, croscarmellose sodium, hypromellose, magnesium stearate, and Opadry Clear (Opadry Clear is a mixture of hypromellose and polyethylene glycol 400). Divided daily doses were administered orally approximately every 12 hours, 1 tablet at a time with adequate fluids.
Baseline and follow-up assessments
All patients underwent a screening evaluation including a complete medical history, a physical examination with vital signs, an assessment of ECOG performance status, an electrocardiogram, and a chest x-ray. Pretreatment laboratory evaluation included complete blood count (CBC) with differential and platelet count, serum erythropoietin, ferritin and transferrin levels, serum chemistry profile, urinalysis, and pregnancy test. On day 1 of each subsequent treatment cycle, a physical examination including vital signs and laboratory assessments (CBC with differential, platelet count, and chemistry profile) was obtained, use of concomitant medication(s) was documented, and AEs were assessed. Complete blood count with differential and platelet count was repeated weekly.
Dose modifications
Patients who experienced a treatment-related nonhematologic grade 3 or higher toxicity had treatment delayed by up to a maximum of 3 weeks until recovery to grade 1 or baseline and continued treatment at a reduced dose. Patients who did not meet the minimum retreatment criteria on day 21 of a treatment cycle had the subsequent cycle delayed and the AE re-evaluated. If recovery did not occur after a delay of 21 days, treatment was discontinued and patients were followed until resolution of the AE. Each dose of ezatiostat was reduced by the amount of 1 tablet for each dose reduction required.
Efficacy
Hematologic improvement (HI) response assessment was performed every 2 treatment cycles and was based upon the laboratory values indicating the best response during the previous 2 cycles. Although HI response assessments were performed every 2 cycles, treatment was to continue for at least 4 cycles before a decision to withdraw the patient from study treatment due to lack of MDS response was made.
The objective HI response was based on the standardized criteria for assessing MDS response as proposed by the International Working Group (IWG; 2000) for MDS.28,29 In addition to the peripheral cytopenia assessmentof HI, bone marrow assessments were reviewed beyond 4 months.
Patients with HI in the erythroid (E), neutrophil (N), and platelet (P) cell lines were summarized by cell lineage as HI-E, HI-N, and HI-P, respectively, based on the number of cytopenic peripheral blood cell lineages at baseline. The primary analysis was conducted with the IWG 2000 criteria and an exploratory analysis using IWG 2006 criteria was also performed.
Ezatiostat tablets pharmacokinetic assessment
Blood and urine samples were collected at specific time intervals to assess the pharmacokinetics of ezatiostat. Blood samples were collected on days 1 and 7 of the first treatment cycle. On day 1, urine samples were collected for 6 hours after ingestion of ezatiostat tablets. Plasma and urinary concentrations of ezatiostat and its metabolites (TLK235, TLK236, and TLK117) were analyzed from 400 μL whole blood supernatant or urine supernatant (after protein precipitation with 0.05 M acetic acid in acetonitrile). After evaporation to dryness and reconstitution, the extracts were analyzed by liquid chromatography–atmospheric pressure ionization mass spectrometry/mass spectrometry (LC-API/MS/MS). Run times were approximately 10 minutes. The lower limit of quantitation (LLQ) for ezatiostat and metabolites is 10 μg/mL. Twelve patients had pharmacokinetic evaluation under fed and fasted conditions.
Statistical analysis
Demographic and baseline MDS disease characteristics of all treated patients were summarized descriptively. The sample size, total number of cycles administered, median, and range of cycles per patient were summarized overall and by the following total daily dose levels of ezatiostat tablets: 200, 400, 1000, 1400, 2000, 2400, 3000, 4000, 5000, and 6000 mg.
The safety of ezatiostat was evaluated by the frequency, severity, and relationship to ezatiostat tablets of treatment-emergent AEs graded according to the NCI-CTCAE version 3.0 that occurred during the study treatment and follow-up period of 30 days after the last study drug treatment. The incidence and percentage of AEs related to study treatment judged by investigators were summarized overall and by dose level.
Results
Patient demographic characteristics
Forty-five patients (33 males and 12 females; Table 1) with histologically confirmed MDS were enrolled in the trial and treated at 3 centers in the United States between February 15, 2006, and October 15, 2007. The majority of patients were white (98%) with an ECOG performance status of 1 (56%). Ages ranged from 53 to 84 years (median, 71 years). Twenty-two patients (49%) had normal cytogenetics, 16 (36%) had abnormal cytogenetics, and 7 (26%) had unknown cytogenetics. The majority of patients, 28 (62%), had an INT-1 risk IPSS; 14 (31%) were low risk, and a minority of patients, 3 (7%), had an INT-2 risk MDS disease. The WHO subtypes included 9 patients (20%) with RA, 11 (24%) RARS, 3 (7%) refractory anemia with excess blasts (RAEB-1), 1 (2%) RAEB-II, 8 (18%) refractory cytopenias with multilineage dysplasia (RCMD), 2 (4%) RCMD-with ringed sideroblasts (RS), 5 (11%) MDS-unclassified (U), and 2 (4%) CMML. The majority of patients, 27 (60%), were red blood cell (RBC) transfusion dependent (defined as patients who required at least 4 units RBC transfusion over an 8-week time period before treatment), and only 5 patients (11%) were platelet transfusion dependent. Thirty-five (78%) patients had baseline anemia, 21 patients (47%) had baseline neutropenia, and 25 patients (56%) reported thrombocytopenia at baseline. Overall, 16 patients (26%) had a single lineage cytopenia; 22 (49%), bilineage cytopenia; and 7 (16%), trilineage cytopenia.
Patient demographics and MDS disease characteristics
| Demographics/characteristics . | n (%) . |
|---|---|
| Age, y* | |
| < 65 | 8 (18) |
| ≥ 65 | 37 (82) |
| Sex | |
| Male | 33 (73) |
| Female | 12 (27) |
| ECOG performance status | |
| 0 | 20 (44) |
| 1 | 25 (56) |
| Baseline cytogenetics | |
| Normal | 22 (49) |
| Abnormal | 16 (36) |
| Unknown | 7 (16) |
| IPSS classification | |
| Low risk | 14 (31) |
| INT-1 risk | 28 (62) |
| INT-2 risk | 3 (7) |
| High risk | 0 |
| FAB/WHO diagnosis classification | |
| RA† | 9 (20) |
| RARS† | 11 (24) |
| RAEB/RAEB-1† | 3 (7) |
| RAEB-II‡ | 1 (2) |
| RCMD‡ | 8 (18) |
| RCMD-RS‡ | 2 (4) |
| MDS-U‡ | 5 (11) |
| CMML§ | 2 (4) |
| Unknown | 4 (9) |
| Transfusion dependency | |
| RBC transfusion dependent | 27 (60) |
| Platelet transfusion dependent | 5 (11) |
| Cell-lineage cytopenia | |
| Anemia | 35 (78) |
| Neutropenia | 21 (47) |
| Thrombocytopenia | 25 (56) |
| Bilineage cytopenia | 22 (49) |
| Unilineage cytopenia | 16 (36) |
| Trilineage cytopenia | 7 (16) |
| Demographics/characteristics . | n (%) . |
|---|---|
| Age, y* | |
| < 65 | 8 (18) |
| ≥ 65 | 37 (82) |
| Sex | |
| Male | 33 (73) |
| Female | 12 (27) |
| ECOG performance status | |
| 0 | 20 (44) |
| 1 | 25 (56) |
| Baseline cytogenetics | |
| Normal | 22 (49) |
| Abnormal | 16 (36) |
| Unknown | 7 (16) |
| IPSS classification | |
| Low risk | 14 (31) |
| INT-1 risk | 28 (62) |
| INT-2 risk | 3 (7) |
| High risk | 0 |
| FAB/WHO diagnosis classification | |
| RA† | 9 (20) |
| RARS† | 11 (24) |
| RAEB/RAEB-1† | 3 (7) |
| RAEB-II‡ | 1 (2) |
| RCMD‡ | 8 (18) |
| RCMD-RS‡ | 2 (4) |
| MDS-U‡ | 5 (11) |
| CMML§ | 2 (4) |
| Unknown | 4 (9) |
| Transfusion dependency | |
| RBC transfusion dependent | 27 (60) |
| Platelet transfusion dependent | 5 (11) |
| Cell-lineage cytopenia | |
| Anemia | 35 (78) |
| Neutropenia | 21 (47) |
| Thrombocytopenia | 25 (56) |
| Bilineage cytopenia | 22 (49) |
| Unilineage cytopenia | 16 (36) |
| Trilineage cytopenia | 7 (16) |
ECOG indicates Eastern Cooperative Oncology Group; IPSS, International Prognostic Scoring System; WHO, World Health Organization; RA, refractory anemia; RARS, refractory anemia with ringed sideroblasts; RAEB, refractory anemia with excess blasts; RCMD, refractory cytopenias with multilineage dysplasia; RCMD-RS, refractory cytopenias with multilineage dysplasia with ringed sideroblasts; MDS-U, myelodysplastic syndrome-unclassified; CMML, chronic myelomonocytic leukemia; and RBC, red blood cells.
Age in years, median (range): 71 (53-84).
FAB/WHO.
WHO.
FAB.
Patients received a median of 1 prior therapy (range, 0-5 prior therapies): 20 (44%) received prior recombinant erythropoietin; 16 (36%), immunotherapy; 15 (33%), chemotherapy; 8 (18%), prior G-CSF; 7 (16%), corticosteroids; and 5 (11%), investigational agents.
Ezatiostat tablets study treatment administration
Ten dose levels ranging from 200 to 6000 mg were evaluated (Table 2). Forty-five patients received a total of 228 cycles of ezatiostat tablets. The median number of cycles per patient was 5 (range, 1-12 cycles). No DLTs were observed. Dose reductions due to AEs were infrequent with a total of 4 patients (9%) receiving a dose reduction (2 patients at the 3000 mg/day dose level, 1 patient at the 5000 mg/day dose level, and 1 patient at the 6000 mg/day dose level) for nausea.
Ezatiostat tablets: treatment administration
| TLK199 tablets: total daily dose, mg . | No. of patients . | Total no. of cycles administered . | Median no. of cycles per patient (range) . |
|---|---|---|---|
| 200 | 3 | 22 | 8 (5-9) |
| 400 | 3 | 15 | 5 (2-8) |
| 1000 | 3 | 19 | 6 (5-8) |
| 1400 | 3 | 18 | 8 (2-8) |
| 2000 | 3 | 19 | 8 (3-8) |
| 2400 | 3 | 14 | 5 (1-8) |
| 3000 | 4 | 27 | 6 (3-12) |
| 4000 | 5 | 22 | 4 (3-6) |
| 5000 | 6 | 34 | 5 (4-8) |
| 6000 | 12 | 38 | 3 (1-8) |
| Total | 45 | 228 | 5 (1-12) |
| TLK199 tablets: total daily dose, mg . | No. of patients . | Total no. of cycles administered . | Median no. of cycles per patient (range) . |
|---|---|---|---|
| 200 | 3 | 22 | 8 (5-9) |
| 400 | 3 | 15 | 5 (2-8) |
| 1000 | 3 | 19 | 6 (5-8) |
| 1400 | 3 | 18 | 8 (2-8) |
| 2000 | 3 | 19 | 8 (3-8) |
| 2400 | 3 | 14 | 5 (1-8) |
| 3000 | 4 | 27 | 6 (3-12) |
| 4000 | 5 | 22 | 4 (3-6) |
| 5000 | 6 | 34 | 5 (4-8) |
| 6000 | 12 | 38 | 3 (1-8) |
| Total | 45 | 228 | 5 (1-12) |
A total of 5 patients had a dose delay (1 each occurring at the 1000, 2400, 3000, 4000, and 6000 mg/day dose level). Two of the dose delays were due to AEs with the remaining 3 due to scheduling difficulties.
Safety
Ezatiostat tablets were well tolerated with no treatment-related deaths reported. The most common treatment-related AEs were nonhematologic (Tables 3,4). A total of 3 grade 4 hematologic AEs (neutropenia) occurred at 3000 mg, 4000 mg, and 6000 mg (n = 1 at each dose level, respectively); however, there were no grade 1, grade 2, or grade 3 ezatiostat tablet treatment-related hematologic AEs reported at any dose level. There was 1 grade 3 (pyrexia) and no grade 4 nonhematologic AEs. Nausea, diarrhea, vomiting, abdominal pain, constipation, anorexia, dyspepsia, and abnormal odor were the most frequently observed nonhematologic AEs with ezatiostat tablets. The majority of patients were instructed to take the tablets without food, until the fed/fast cohort enrolled at the end of the study. There were a total of 22 serious adverse events (SAEs) reported, 18 were unrelated to ezatiostat and 4 were ezatiostat tablet treatment related (esophagitis [n = 2], reflux esophagitis [n = 1], and fever [n = 1]). Three patients discontinued treatment with ezatiostat tablets due to AEs. One patient experienced a grade 3 esophageal ulceratius with hematochezia. One patient experienced an AE, mild elevation of hepatic enzymes, that the investigator considered to be possibly related to ezatiostat tablets and discontinued study treatment after 4 cycles. Because only 6 patients each were in the fed/fast 6000-mg cohort, a meaningful analysis of adverse events with and without food ingestion could not be conducted due to the small sample size. Further study of ingestion of ezatiostat tablets with food will be conducted.
Hematologic and nonhematologic adverse events related to ezatiostat tablets in 5% or more of patients: by grade
| Adverse event (preferred term) . | For all dose groups combined: maximum toxicity grade (N = 45), n (%) . | ||||
|---|---|---|---|---|---|
| Grade 1 . | Grade 2 . | Grade 3 . | Grade 4 . | Grade 5 . | |
| Hematologic | |||||
| Neutropenia | 0 | 0 | 0 | 3 (7) | 0 |
| Nonhematologic | |||||
| Nausea | 25 (56) | 4 (9) | 0 | 0 | 0 |
| Diarrhea | 16 (36) | 3 (7) | 0 | 0 | 0 |
| Vomiting | 11 (24) | 3 (7) | 0 | 0 | 0 |
| Anorexia | 3 (7) | 2 (4) | 0 | 0 | 0 |
| Abdominal pain | 4 (9) | 0 | 0 | 0 | 0 |
| Constipation | 4 (9) | 0 | 0 | 0 | 0 |
| Dyspepsia | 3 (7) | 1 (2) | 0 | 0 | 0 |
| Skin odor abnormal | 3 (7) | 1 (2) | 0 | 0 | 0 |
| Adverse event (preferred term) . | For all dose groups combined: maximum toxicity grade (N = 45), n (%) . | ||||
|---|---|---|---|---|---|
| Grade 1 . | Grade 2 . | Grade 3 . | Grade 4 . | Grade 5 . | |
| Hematologic | |||||
| Neutropenia | 0 | 0 | 0 | 3 (7) | 0 |
| Nonhematologic | |||||
| Nausea | 25 (56) | 4 (9) | 0 | 0 | 0 |
| Diarrhea | 16 (36) | 3 (7) | 0 | 0 | 0 |
| Vomiting | 11 (24) | 3 (7) | 0 | 0 | 0 |
| Anorexia | 3 (7) | 2 (4) | 0 | 0 | 0 |
| Abdominal pain | 4 (9) | 0 | 0 | 0 | 0 |
| Constipation | 4 (9) | 0 | 0 | 0 | 0 |
| Dyspepsia | 3 (7) | 1 (2) | 0 | 0 | 0 |
| Skin odor abnormal | 3 (7) | 1 (2) | 0 | 0 | 0 |
Hematologic and nonhematologic adverse events related to ezatiostat tablets in 5% or more of patients: by dose level
| Adverse event (preferred term) . | Dose level, mg, n (%) . | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| N = 3 . | N = 4 . | N = 5 . | N = 6 . | N = 12 . | ||||||
| 200 . | 400 . | 1000 . | 1400 . | 2000 . | 2400 . | 3000 . | 4000 . | 5000 . | 6000 . | |
| Hematologic | ||||||||||
| Neutropenia | 0 | 0 | 0 | 0 | 0 | 0 | 1 (25) | 1 (20) | 0 | 1 (8) |
| Nonhematologic | ||||||||||
| Nausea | 1 (33) | 0 | 0 | 3 (100) | 2 (67) | 2 (67) | 2 (50) | 3 (60) | 5 (83) | 11 (92) |
| Diarrhea | 0 | 0 | 1 (33) | 1 (33) | 1 (33) | 0 | 2 (50) | 3 (60) | 3 (50) | 8 (67) |
| Vomiting | 0 | 0 | 1 (33) | 0 | 0 | 1 (33) | 1 (25) | 3 (60) | 2 (33) | 6 (50) |
| Anorexia | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 (33) | 3 (25) |
| Abdominal pain | 0 | 0 | 0 | 0 | 1 (33) | 0 | 1 (25) | 1 (20) | 0 | 1 (8) |
| Constipation | 1 (33) | 0 | 1 (33) | 0 | 0 | 0 | 0 | 1 (20) | 0 | 1 (8) |
| Dyspepsia | 0 | 0 | 0 | 1 (33) | 0 | 0 | 1 (25) | 0 | 1 (17) | 1 (8) |
| Skin odor abnormal | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 4 (33) |
| Adverse event (preferred term) . | Dose level, mg, n (%) . | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| N = 3 . | N = 4 . | N = 5 . | N = 6 . | N = 12 . | ||||||
| 200 . | 400 . | 1000 . | 1400 . | 2000 . | 2400 . | 3000 . | 4000 . | 5000 . | 6000 . | |
| Hematologic | ||||||||||
| Neutropenia | 0 | 0 | 0 | 0 | 0 | 0 | 1 (25) | 1 (20) | 0 | 1 (8) |
| Nonhematologic | ||||||||||
| Nausea | 1 (33) | 0 | 0 | 3 (100) | 2 (67) | 2 (67) | 2 (50) | 3 (60) | 5 (83) | 11 (92) |
| Diarrhea | 0 | 0 | 1 (33) | 1 (33) | 1 (33) | 0 | 2 (50) | 3 (60) | 3 (50) | 8 (67) |
| Vomiting | 0 | 0 | 1 (33) | 0 | 0 | 1 (33) | 1 (25) | 3 (60) | 2 (33) | 6 (50) |
| Anorexia | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 (33) | 3 (25) |
| Abdominal pain | 0 | 0 | 0 | 0 | 1 (33) | 0 | 1 (25) | 1 (20) | 0 | 1 (8) |
| Constipation | 1 (33) | 0 | 1 (33) | 0 | 0 | 0 | 0 | 1 (20) | 0 | 1 (8) |
| Dyspepsia | 0 | 0 | 0 | 1 (33) | 0 | 0 | 1 (25) | 0 | 1 (17) | 1 (8) |
| Skin odor abnormal | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 4 (33) |
Pharmacokinetic analysis
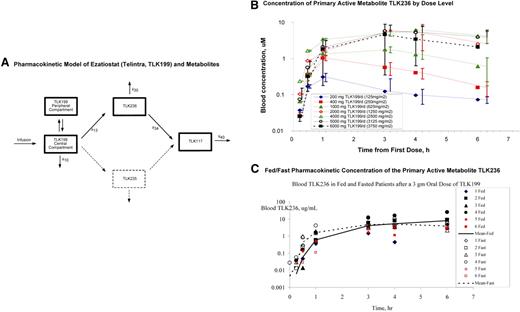
Concentrations of TLK199, TLK235, TLK236, and TLK117 were determined in whole blood by a high-performance liquid chromatography (HPLC) assay. The limit of quantification was 10 μg/mL for all 4 entities. The pharmacokinetic model for ezatiostat and its metabolites (Figure 2A) was derived from the concentrations of ezatiostat and metabolites in blood of patients administered ezatiostat intravenous formulation. The proposed pharmacokinetic model using nonlinear mixed effects was modeled by NONMEM (ICON Development Solutions, Ellicott City, MD). Ezatiostat undergoes de-esterification to both TLK235 and TLK236. Both TLK236 and TLK235 undergo further de-esterification to TLK117. This model is being further tested with the patient data collected in this and future studies.
Ezatiostat (TLK199) pharmacokinetics. (A) Pharmacokinetic model of ezatiostat and metabolites. Formation of metabolites is assumed to be unidirectional. TLK199 undergoes de-esterification to both TLK235 and TLK236; however, because the quantity of TLK235 measured in this study is consistently less than the level of quantification, this pathway is ignored (dashed lines). TLK236 undergoes further de-esterification to TLK117. Both TLK199 and TLK236 can be eliminated via more than one pathway. However, this study provides no insight into the fraction of each entity eliminated by each pathway. (B) Concentration of primary active metabolite TLK236 by dose level. Error bars represent SD of the mean at each sampling time point. (C) Fed/fast pharmacokinetic concentration of the primary active metabolite TLK236.
Ezatiostat (TLK199) pharmacokinetics. (A) Pharmacokinetic model of ezatiostat and metabolites. Formation of metabolites is assumed to be unidirectional. TLK199 undergoes de-esterification to both TLK235 and TLK236; however, because the quantity of TLK235 measured in this study is consistently less than the level of quantification, this pathway is ignored (dashed lines). TLK236 undergoes further de-esterification to TLK117. Both TLK199 and TLK236 can be eliminated via more than one pathway. However, this study provides no insight into the fraction of each entity eliminated by each pathway. (B) Concentration of primary active metabolite TLK236 by dose level. Error bars represent SD of the mean at each sampling time point. (C) Fed/fast pharmacokinetic concentration of the primary active metabolite TLK236.
Exposure to the primary active metabolite, TLK236, increased with ezatiostat tablet dose (Figure 2B). Ezatiostat tablets achieved a comparable exposure to ezatiostat administered intravenously with a liposomal formulation. Comparative fed/fast ezatiostat pharmacokinetics demonstrated similar results (Figure 2C). Food did not inhibit or delay absorption of ezatiostat tablets, thus patients will be allowed to ingest food on future studies. In summary, the exposure of the primary metabolite, TLK236, increases proportionate to ezatiostat dose. Fed/fast analysis revealed similar results. It was determined in this study that ezatiostat tablets may be ingested either 1 hour before or 1 hour after eating without affecting bioavailability.
Efficacy analysis
Forty-five patients were in the intent-to-treat population. Hematologic improvement responses were observed in all 3 cell lines at dose levels of 200 to 6000 mg/day in the efficacy evaluable population (Table 5). The IWG criteria were revised during the course of the study; therefore, HI-E analyses were performed using the original IWG 2000 criteria as the primary analysis and with the new IWG 2006 criteria as an exploratory analysis (Table 5). Eleven of the 17 HI responses were observed in the 4000 to 6000 mg/day dose range, reflecting a dose response. One bilineage response was reported at 5000 mg/day and 2 bilineage responses were reported at the 6000 mg/day dose level. One bilineage response was correlated with a complete cytogenetic response.
Efficacy
| . | Dose level . | |||
|---|---|---|---|---|
| 200-1400 mg/d . | 2000-4000 mg/d . | 5000-6000 mg/d . | All doses combined . | |
| HI response rates (efficacy evaluable population) | ||||
| IWG 2000 | ||||
| HI-E | 0/5 (0) | 3/11 (27) | 3/13 (23) | 6/29 (21) |
| HI-N | 0/5 (0) | 0/7 (0) | 4/7 (57) | 4/19 (21) |
| HI-P | 2/7 (29) | 2/7 (29) | 3/8 (38) | 7/21 (33) |
| Unilineage | 1/6 (17) | 1/5 (20) | 1/3 (33) | 3/14 (21) |
| Bilineage | 0/5 (0) | 0/9 (0) | 3/11 (27) | 3/25 (12) |
| Comparison of HI-E response rates | ||||
| IWG 2000 | 0/5 (0) | 3/11 (27) | 3/13 (23) | 6/29 (21) |
| IWG 2006 | 3/5 (60) | 5/11 (45) | 6/13 (45) | 14/29 (48) |
| Comparison of RBC transfusion independence and reduction | ||||
| IWG 2006 | ||||
| RBC transfusion independence | 3/5 (60) | 2/10 (20) | 3/8 (38) | 8/23 (35) |
| RBC transfusions reduced by 4 units/8 weeks | 3/5 (60) | 5/10 (50) | 6/8 (75) | 14/23 (61) |
| IWG 2000 | ||||
| RBC transfusions reduced by 50% | 2/5 (40) | 2/10 (20) | 5/8 (63) | 12/23 (52) |
| . | Dose level . | |||
|---|---|---|---|---|
| 200-1400 mg/d . | 2000-4000 mg/d . | 5000-6000 mg/d . | All doses combined . | |
| HI response rates (efficacy evaluable population) | ||||
| IWG 2000 | ||||
| HI-E | 0/5 (0) | 3/11 (27) | 3/13 (23) | 6/29 (21) |
| HI-N | 0/5 (0) | 0/7 (0) | 4/7 (57) | 4/19 (21) |
| HI-P | 2/7 (29) | 2/7 (29) | 3/8 (38) | 7/21 (33) |
| Unilineage | 1/6 (17) | 1/5 (20) | 1/3 (33) | 3/14 (21) |
| Bilineage | 0/5 (0) | 0/9 (0) | 3/11 (27) | 3/25 (12) |
| Comparison of HI-E response rates | ||||
| IWG 2000 | 0/5 (0) | 3/11 (27) | 3/13 (23) | 6/29 (21) |
| IWG 2006 | 3/5 (60) | 5/11 (45) | 6/13 (45) | 14/29 (48) |
| Comparison of RBC transfusion independence and reduction | ||||
| IWG 2006 | ||||
| RBC transfusion independence | 3/5 (60) | 2/10 (20) | 3/8 (38) | 8/23 (35) |
| RBC transfusions reduced by 4 units/8 weeks | 3/5 (60) | 5/10 (50) | 6/8 (75) | 14/23 (61) |
| IWG 2000 | ||||
| RBC transfusions reduced by 50% | 2/5 (40) | 2/10 (20) | 5/8 (63) | 12/23 (52) |
HI indicates hematologic improvement; HI-E, HI-erythroid; HI-N, HI-neutrophils; HI-P, HI-platelets; and RBC, red blood cells.
Of the 23 patients who were RBC transfusion dependent, 8 (35%) achieved transfusion independence and 14 (61%) had a reduction in the RBC transfusion requirements of 4 units/8 weeks (Table 5). One of 5 platelet transfusion–dependent patients achieved independence from platelet transfusions. Since this was a phase 1 study, a formal quality of life validated questionnaire was not incorporated; however, clinical benefit was assessed by key MDS-related symptoms in an informal questionnaire. These patient-reported outcomes were considered exploratory analyses; patients with HI responses in this study did report improvement in clinical symptoms (Table 6).
Patient reported outcomes
| Clinical benefit . | n (%) . |
|---|---|
| Increased energy | 13 (34) |
| Improved well-being | 9 (24) |
| Improved daily activities | 5 (13) |
| Increased mobility | 2 (5) |
| Decreased transfusion requirements | 8 (21) |
| Decreased bleeding events | 2 (5) |
| Decreased infections | 1 (3) |
| Decreased antibiotic requirements | 1 (3) |
| Decreased pain | 1 (3) |
| Decreased hospitalizations for fever/sepsis | 1 (3) |
| Other | 3 (8) |
| Clinical benefit . | n (%) . |
|---|---|
| Increased energy | 13 (34) |
| Improved well-being | 9 (24) |
| Improved daily activities | 5 (13) |
| Increased mobility | 2 (5) |
| Decreased transfusion requirements | 8 (21) |
| Decreased bleeding events | 2 (5) |
| Decreased infections | 1 (3) |
| Decreased antibiotic requirements | 1 (3) |
| Decreased pain | 1 (3) |
| Decreased hospitalizations for fever/sepsis | 1 (3) |
| Other | 3 (8) |
Discussion
This phase 1 study was the first clinical study of ezatiostat tablets in patients with MDS. The 7-day dose schedule of tablet administration represents one half the number of days of the maximum dose schedule evaluated in rats and dogs (ie, 14 days). Ezatiostat doses up to 6000 mg/day were evaluated without reaching a DLT. Adverse events were primarily mild or moderate in grade, severe events were relatively few, and an MTD was not defined.
Ezatiostat has shown significant stimulatory activity in vitro in human bone marrow progenitor cultures, as well as in several in vivo preclinical models of myelopoiesis. GST P1-1–null mice consistently demonstrate higher than normal neutrophil levels, in addition to a significant increase in the growth rate of their embryonal-derived fibroblast cells compared with the wild type.30 These findings are consistent with reports that GST P1-1 is a negative regulator of cellular growth and differentiation exerting its effect by binding to JNK.
In this oral study of ezatiostat tablets, levels of the major metabolite, TLK236, were comparable with the TLK236 exposure achieved with the intravenous formulation of ezatiostat. Comparable pharmacokinetic parameters were seen between the fed and fasted states in this clinical study.
Seventeen HI responses (IWG 2000 criteria) were observed that included lineages at all dose levels evaluated with 11 of the HI responses observed in the 4000 to 6000 mg/day dose range, suggesting a possible relationship between dose and HI response. Three bilineage responses were observed with 1 associated with a complete cytogenetic response. Hematologic improvement in all 3 lineages was observed in several MDS subtypes in this dose-escalation study. The MDS subtypes of responding patients included: RA, RARS, RCMD, and RSMDS-U. Due to the small sample size and different dose cohort assignments across subtypes, conclusions regarding which subtypes might most benefit from ezatiostat hydrochloride therapy cannot be made in this phase 1 study. Further study will be needed to identify the MDS subtypes that respond at ezatiostat hydrochloride's optimal dose and dose schedule.
An exploratory analysis suggests that the HI responses were also accompanied by improvement in clinical symptoms. Red blood cell (RBC) transfusion independence was achieved by IWG 2006 criteria in 8 patients (35%). Fourteen patients (61%) with RBC transfusion dependence achieved a 4-unit/8-week reduction in transfusion requirements. Twelve patients (52%) had transfusion requirements reduced by 50% (IWG 2000 criteria). One patient achieved platelet transfusion independence. A follow-up bone marrow evaluation was supposed to have been obtained at 4 months. Due to the insufficient number of follow-up bone marrows obtained, a meaningful data analysis of pre– and post–ezatiostat treatment effects on bone marrow was not performed. In a previous study of ezatiostat administered intravenously, an increase in bone marrow cellularity and maturation was reported.23,24 It is clear that early indications of efficacy on this 1-week dosing schedule are promising; however, to further enhance the efficacy observed with ezatiostat tablets, phase 2 testing will evaluate extended dosing schedules in MDS.
In conclusion, the tolerability and HI responses seen with ezatiostat tablets across all 3 cell lineages, including independence or reduction of RBC and platelet transfusion requirements, indicate clinical safety, tolerability, and early indications of hematologic improvement in patients with MDS. These findings support the further development of extended schedules of ezatiostat tablets in MDS and other hematologic malignancies.
Presented in abstract form at the 49th Annual Meeting of the American Society of Hematology, Atlanta, GA, December 8, 2007.31
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgment
This work was supported by Telik.
Authorship
Contribution: A.L., A.R., J.G., M.J., and G.L.B. designed the research protocol; A.R., N.G., S.S., J.G., J.L., M.M., and A.L. were involved in treating patients and collecting data; L.M. conducted the statistical analysis; and A.R., N.G., A.L., M.J., J.G.K., and G.L.B. wrote the paper with contributions from the other authors. All authors approved the final version of the paper.
Conflict-of-interest disclosure: G.L.B., M.J., J.G.K., and L.M. are employed by Telik, whose investigational drug candidate was studied in the present work. J.G. is a consultant to Telik. The remaining authors declare no competing financial interests.
Correspondence: Azra Raza, New York Medical College, Director, MDS Program, St Vincent's Comprehensive Cancer Center, 325 West 15th St, New York, NY 10011; e-mail: araza@aptiumoncology.com.