Abstract
Protein 4.1R (4.1R) was first identified in red cells where it plays an important role in maintaining mechanical stability of red cell membrane. 4.1R has also been shown to be expressed in T cells, but its function has been unclear. In the present study, we use 4.1R-deficient mice to explore the role of 4.1R in T cells. We show that 4.1R is recruited to the immunologic synapse after T cell–antigen receptor (TCR) stimulation. We show further that CD4+ T cells of 4.1R−/− mice are hyperactivated and that they displayed hyperproliferation and increased production of interleukin-2 (IL-2) and interferon γ (IFNγ). The hyperactivation results from enhanced phosphorylation of LAT and its downstream signaling molecule ERK. The 4.1R exerts its effect by binding directly to LAT, and thereby inhibiting its phosphorylation by ZAP-70. Moreover, mice deficient in 4.1R display an elevated humoral response to immunization with T cell–dependent antigen. Thus, we have defined a hitherto unrecognized role for 4.1R in negatively regulating T-cell activation by modulating intracellular signal transduction.
Introduction
T cell–antigen receptor (TCR)–mediated signal transduction is a key event in the regulation of T-cell function. Signal transduction is initiated by the formation of an immunologic synapse which brings together a set of molecules involved in the transduction of multiple intracellular signaling pathways.1 The earliest biochemical event that follows the clustering of TCR complex and coreceptors is the activation of 2 members of the Src family of tyrosine kinases, Lck and Fyn.2 The activation of these kinases results in phosphorylation of immunoreceptor tyrosine-based motifs (ITAMs), which serve as a docking site for ZAP-70.3 On binding to ITAM motifs, ZAP-70 is phosphorylated and activated. The activated ZAP-70 phosphorylates several downstream substrates. T cells deficient in ZAP-70 have substantially decreased TCR-induced tyrosine phosphorylation of downstream signaling molecules.4 One of the most important of these substrates is linker for activation of T cells (LAT), an hematopoietic-specific transmembrane adaptor protein with no apparent enzymatic activity.5,6 It is known that tyrosine phosphorylation of LAT is required for it to function as an adapter molecule, because phosphorylated LAT serves as a docking site for several signaling molecules, such as Grb2, PLC-γ1, and the p85 subunit of phosphoinositide 3-kinase (PI3K)7-10 ; these together form the LAT signalosome that is responsible for initiating critical downstream events such as ERK activation. However, how the phosphorylation of LAT is regulated in T cells has been unclear
4.1R is the prototypal member of the 4.1 family of proteins that comprises 4.1R,11 4.1B,12 4.G,13 and 4.1N.14 These proteins serve as a bridge between transmembrane proteins and the actin cytoskeleton. The 4.1 family is characterized by the presence of 3 highly conserved domains: an N-terminal membrane binding domain (MBD), an internal spectrin-actin-binding domain (SABD), and a C-terminal domain (CTD). The membrane-binding domains of the 4.1 proteins are closely related, both in sequence and in structure, to the N-terminal domains of ezrin, radixin, and moesin (the ERM proteins), and are therefore commonly referred to as the FERM domains.15-17 Both 4.1 and ERM proteins bind to various transmembrane proteins through this domain. For example, it has been shown that the membrane-binding domain of 4.1R binds to the cytoplasmic tails of glycophorin C,18 to the anion exchanger band 3,19 and to CD44,20 and that the membrane-binding domains of ERM bind to intercellular adhesion molecules (ICAMs) CD43 and CD44.21 These membrane-binding activities are modulated by both phosphorylation and by the phospholipid PIP2.22-24
The functions of ERM proteins in different tissues in vivo and cell types in vitro have been relatively well studied.25-27 Several studies have implicated a role for ERM proteins in T-cell function,28-30 but the physiologic role of the 4.1 proteins in nonerythroid cells has remained essentially unknown. In the present study, we explore the function of 4.1R in T cells both in vitro and in vivo, with the aid of 4.1R−/− mice. Our results bring to light an unsuspected role for 4.1R in suppressing T-cell activation and show that it acts by negatively regulating TCR-mediated signal transduction through inhibition of LAT phosphorylation.
Methods
Generation and use of 4.1R knockout mice
The generation of 4.1R knockout mice has been described previously.31 The mice were backcrossed onto C57BL/6 background and were inbred for more than 20 generations. All the mice were maintained at the animal facility of New York Blood Center under pathogen-free conditions according to institutional guidelines. Animal protocols were reviewed and approved by the Institutional Animal Care and Use Committee. Unless otherwise stated, all the experiments were done on 8- to 10-week-old mice.
Flow cytometry
Single-cell suspensions from lymph node, spleen, bone marrow, thymus, or peritoneal wash were depleted of red blood cells, incubated with Fc-Block (CD16/32; BD PharMingen, San Diego, CA) for 10 minutes and stained for 30 minutes with combinations of the following antibodies (obtained from BD PharMingen or eBioscience, San Diego, CA): fluorescein isothiocyanate–conjugated (FITC) anti-IgM (II/41), anti-CD4 (RM 4-5), anti-CD5 (53-7.3), anti-CD8 (53-6.7), anti-CD40 (HM40-3), anti-CD43 (S7), anti-CD102 (mIC2/4), anti-Mac1 (M1/70), PE-conjugated (PE) anti-B220 (RA3-6B2), anti-CD3 (17A2), anti-CD4 (GK1.5), anti-CD8 (53-6.7), anti-CD54 (YN1/1.7.4), anti-CD62L (MEL-14), anti-CD69 (H1.2F3), anti-IgD (217-170), anti-GR1(RB6-8C5), anti-NK1.1(PK136), PERCP-conjugated anti-B220 (RA3-6B2), anti-CD3 (145-2C11), allophycocyanin-conjugated (APC) anti-CD4 (RM4-5), anti-CD11c (HL3), anti-CD19 (1D3), anti-CD25 (PC61), anti-CD44 (IM7), and anti-Ter119 (Ter119). Appropriate isotype controls were included in all cases. Data were acquired on a FACS (fluorescence-activated cell sorting)–CANTO flow cytometer (BD Biosciences, San Jose, CA) and analyzed using Diva software (BD Biosciences). Live cells were gated based on forward and side scatter. A gate characteristic of lymphoid cells on this basis was used for acquisition of peritoneal cells.
Cloning of 4.1R from CD4+ T cells
Total RNA was isolated from CD4+ T cells by RNeasy mini kit (QIAGEN, Valencia, CA). RNA (1 μg) was reverse transcribed into cDNA with random nonamers and M-MuLV reverse transcriptase (New England Biolabs, Ipswich, MA) for 50 minutes at 42°C. An equivalent of 5 ng cDNA was used for polymerase chain reaction (PCR). PCR was performed with the use of Accuprime Platinum Pfx DNA polymerase (Invitrogen, Carlsbad, CA). Transcripts of 4.1R can initiate at 2 separate start sites; therefore, the following set of PCR primers were used: ATG1 forward, ATGACAACAGAGAAGAGTTTAGTGGCTGAAGC; ATG2 forward, ATGCACTGTAAGGTCTCCTTGTTGGATGACACG; epb41 reverse, CTCCTCAGAGATCTCTGTCTCCTGGTGGA. The PCR products were cloned into pCR2.1-TOPO vector (Invitrogen), and 6 clones of each kind were sequenced.
Immunoblot analysis
CD4+ T cells from lymph nodes were purified by negative selection, using MARS beads (Miltenyi Biotec, Auburn, CA) according to the manufacturer's instructions. The whole-cell lysates of CD4+ T cells or thymocytes (106 cells) were separated by 10% sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to polyvinylidene difluoride (PVDF) membrane (Millipore, Billerica, MA). The membrane was probed with antibodies against 4.1R exon 13, 4.1B headpiece, 4.1G headpiece, and 4.1N headpiece,32 followed by horseradish peroxidase (HRP)–conjugated goat anti–rabbit IgG (Jackson ImmunoResearch Laboratories, West Grove, PA). The film was developed with the use of the Renaissance chemiluminescence detection kit (Pierce, Rockford, IL).
Immunofluorescence microscopy
Lymph nodes from 4.1R+/+ mice or 4.1R−/− mice were mechanically disrupted to create a single-cell suspension by passing the tissue through a 70-μm cell strainer (BD Biosciences) into RPMI media. For T-cell activation, cells were incubated with 10 μg/mL biotin–anti-CD3/anti-CD28 (BD PharMingen) on ice for 30 minutes. After washing out excess antibody, the bound antibodies were cross-linked by the addition of streptavidin-coated M-280 Dynabeads (Dynal Biotech, Lake Success, NY) at a bead-to-cell ratio of 1:5. The samples were then incubated at 37°C in a water bath for exactly 10 minutes, with occasional agitation. For an unstimulated control sample, the anti-CD3/CD28 antibodies were omitted. Cell/bead conjugates were seeded (2 × 106 cells/well) onto a poly-d-lysine–coated 8-well culture slide (BD Biosciences) and left to adhere on ice for 10 minutes. Samples were fixed in cold methanol at −20°C for 10 minutes. Fixed cells were permeabilized with phosphate-buffered saline (PBS)/0.1% Triton X-100 for 5 minutes at room temperature. The cells were double-stained with anti-4.1R exon 13 and anti-LAT antibodies. Samples were mounted in Vectashield hard-set mounting media (Vector Labs), and fluorescence images were obtained with a LSM 510 META confocal microscope (Carl Zeiss, Thornwood, NY) equipped with a Plan Neofluar 100×/1.3 oil-immersion objective. The software used for taking images was LSM 510 Meta 3.2. The images were further processed using Adobe Photoshop 7.0. To examine whether 4.1R is also present at the cell-cell contact between T cells and antigen-presenting cells, the conjugate assay between primary T-cell blasts from 5c.c7 T-cell receptor–transgenic mice and CH27 B cell lymphoma (IEk-positive) cells was performed as previously described.33 Cells were stained with rabbit polyclonal anti-4.1R exon 13 antibody followed by Alexa-488 anti–rabbit secondary antibody. Samples were processed and analyzed as described in this paragraph.
Activation assay, proliferation assay, and T-cell cytokine production
CD4+ T cells from lymph nodes were purified as described in “Immunoblot analysis.” Flow cytometric analysis confirmed that purity of the isolated cells was consistently greater than 90%. For activation assays, purified CD4+ T cells (106 in 1 mL) were stimulated with plate-bound monoclonal anti–mouse CD3ϵ antibody (clone 145-2C11; BD PharMingen) for 16 hours at 37°C, and cells were stained with FITC-conjugated anti-CD4, PE-conjugated anti-CD69, and 7AAD. Appropriate isotype controls were included in all cases. Data were acquired on a FACSCanto flow cytometer (BD Biosciences) and analyzed using Diva software (BD Biosciences). For T-cell proliferation, purified CD4+ T cells (105 in 100 μL) were activated with plate-bound monoclonal anti–mouse CD3ϵ antibody. 3H-thymidine (1 μCi [0.037 MBq]/well; Sigma-Aldrich, St Louis, MO) was added after 48 hours of stimulation, and its incorporation was measured 16 hours later. For IL-2 and IFN-γ assay, 105 cells (100 μL) were cultured in a 96-well plate, precoated with anti-CD3ϵ antibody. The supernatant was collected 48 hours later, and IL-2 or IFN-γ in the culture medium was measured with enzyme-linked immunoabsorbent assay (ELISA) kits (BD PharMingen). The results were averaged from triplicate samples.
In vitro T-cell expansion using carboxyfluorescein succinimidyl ester
Purified CD4+ T cells were suspended in PBS/0.1% BSA at a final concentration of 5 × 107 cells/mL. A volume of 1 μL of 10 mM carboxyfluorescein succinimidyl ester (CFSE; Invitrogen) was added, and the cells were labeled for 10 minutes at 37°C. The unbound CFSE was quenched by adding 5 vol ice-cold RPMI 1640/10% FBS and incubated for 5 minutes on ice. Labeled cells were washed 3 times with the culture medium. Labeled cells were suspended at a concentration of 106 cells/mL in the culture medium and plated to anti-CD3–coated a 96-well plate at 105 cells per well. After 48 hours of culture at 37°C, cells were harvested and washed with 3 mL ice-cold PBS/0.1% BSA. Cells were resuspended in 100 μL PBS/0.1% BSA, stained with APC-conjugated rat anti–mouse CD4+ antibody and with 7-AAD to exclude dead cells. Flow cytometry was done on a FACSCanto cytometer (BD PharMingen) with Diva software (BD Biosciences).
Cell signaling
T cells were stimulated with hamster anti–mouse CD3ϵ (clone 145-2C11) at 37°C for various times. Cells were lysed in ice-cold lysis buffer (10 mM Tris-HCl, pH 7.5, 1 mM MgCl2, 0.5% CHAPS, 1 mM EDTA, 100 μM Na3VO4, 5 mM NaF, 20 μg/mL leupeptin, and 1 mM benzamidine). Cell lysates were separated on SDS-PAGE, and proteins were transferred to PVDF membranes. The expression of the signal molecules were determined by immunoblotting with the cognate antibodies (anti-Lck, anti–ZAP-70, anti-LAT, anti-PLC γ1, anti-Akt, and anti-ERK), and their phosphorylation levels were determined by Western blotting with phosphoantibodies. Anti-LAT pY132 was from Abcom (Cambridge, MA), pY171 from Cell Signaling Technology (Danvers, MA), pY191 from Upstate (Charlottesville, VA), and pY226 from BD PharMingen. Other phosphoantibodies are: pLck (Tyr394) from Upstate; pAkt (Ser473), pERK (Thr202/Tyr204), and pPLC-γ1(Tyr783) from Cell Signaling; pZAP70 (Tyr292) from Santa Cruz Biotechnology (Santa Cruz, CA). Blots were developed with enhanced chemiluminescence detection reagents (Pierce).
Intracellular staining
For intracellular staining of phospho-ERK, CD4+ T cells were purified as in “Immunoblot analysis.” Cells were stimulated with hamster anti-CD3ϵ (clone 145-2C11) at 37°C for 15 minutes. Cells were fixed in 1.5% PFA for 10 minutes at 37°C and then permeabilized with 100% methanol for 10 minutes. Cells were washed in BD PharMingen Stain Buffer twice and stained with PE-conjugated phosphor-ERK antibody (clone 20a; BD Biosciences). For intracellular staining of IL-2, 1 × 105 CD4+ T cells were cultured in a 96-well plate precoated with 2.5 μg/mL anti-CD3e (clone 145-2C11; BD PharMingen) and 2.5 μg/mL anti-CD28 (BD PharMingen) at 37°C for 16 hours. T cells were stimulated with PMA (50 ng/mL) and Ionomycin(500 nM) in the presence of Brefeldin A for 6 hours, then fixed and stained with the use of the BD PharMingen intracellular cytokine staining kit, following the manufacturer's instructions. Appropriate isotype controls were included in all cases. Data were acquired on a FACSCanto flow cytometer (BD Biosciences) and analyzed with Diva software (BD Biosciences).
Coimmunoprecipitation of LAT with 4.1R
Lymph nodes from 4.1R+/+ or 4.1R−/− mice were homogenized in 1.5 mL ice-cold RIPA buffer (50 mM Tris-HCl, pH 7.4, 150 mM NaCl, 1% NP-40, 1 mM EDTA, 1 mM PMSF, 1 mM NaF, 1 mM Na3VO4, 1 μg/mL of aprotinin, leupeptin, and pepstatin). Supernatant was collected after centrifugation at 16 000g at 4°C for 10 minutes, and the concentration of the supernatant was determined by the Bradford method with the use of BSA as standard. Extract (500 μg) was incubated with 5 μg rabbit anti-4.1R polyclonal antibody or preimmune IgG in 500 μL RIPA buffer (Active Motif, Carlsbad, CA) at 4°C overnight with rotation. The immunoprecipitates were isolated with Protein A beads and separated by 10% SDS-PAGE followed by transferring onto PVDF membrane. The membrane was probed with rabbit anti-4.1R exon 13 polyclonal antibody and with monoclonal anti-LAT antibody (Santa Cruz Biotechnology).
Construction, expression, and purification of recombinant 4.1R fragments and cytoplasmic domain of LAT
Subcloning, expression, and purification of 80-kDa 4.1R and its 4 domains (30, 16, 10, and 22/24 kDa) were described previously.24,32 Lobe A, lobe B, and lobe C of the 30-kDa membrane binding domain were subcloned into pGEX-4T-2 vector (GE Healthcare, Little Chalfont, United Kingdom) with EcoRI and SalI upstream and downstream, respectively. The cytoplasmic domain of LAT was cloned into pMAL-p2x vector (New England BioLabs) with BamHI and SalI or into pET28b(+) vector (Novagen, Madison, WI) with the use of NcoI and NotI upstream and downstream, respectively. Restriction enzymes were from New England BioLabs. The template used to amplify the cytoplasmic region of LAT with PCR was from ATCC (Manassas, VA). The GST-tagged recombinant proteins were purified using glutathione-Sepharose 4B affinity column (GE Healthcare), and the MBP-tagged cytoplasmic domain of LAT was purified on an amylose resin affinity column (New England BioLabs). Protein concentration was determined spectrophotometrically, using extinction coefficients calculated from the tryptophan and tyrosine contents.34 Proteins were dialyzed against binding buffer (10 mM Tris-HCl, pH 7.4, 150 mM NaCl, 0.1% Tween-20).
Pull-down assays and surface plasmon resonance assay
Pull-down assays were performed as previously described.35 To examine the binding of 4.1R to the cytoplasmic domain of LAT, MBP or MBP-tagged LAT cytoplasmic domain was coupled to amylose beads (New England BioLabs). The binding of 4.1R was detected by Western blotting, using anti-4.1R antibody. To examine the binding of cytoplasmic domain of LAT to 4.1 domains and subdomains of 30 kDa, GST-tagged recombinant 4.1R polypeptides were coupled to glutathione-Sepharose 4B beads (GE Healthcare), and the binding of His-tagged LAT cytoplasmic domain was detected by anti-His antibody (generated and affinity-purified by our laboratory). Surface plasmon resonance assay was also performed as previously described.35 GST-tagged lobe C was covalently coupled to a CM-5 biosensor chip with the use of an amino coupling kit according to the manufacturer's instruction. Increasing concentrations of His-tagged cytoplasmic domain of LAT were injected onto the lobe C–coated surface. Sensograms (plots of changes in RU on the surface as a function of time) derived were analyzed with the software BIAeval 3.0. (Biacore, Piscataway, NJ). Binding affinity was estimated by curve fitting with the use of a 1:1 binding model.
In vitro phosphorylation of cytoplasmic domain of LAT
His-tagged cytoplasmic domain of LAT (at a concentration of 1 μM) was incubated with 100 ng ZAP-70 (Upstate), in the absence or presence of 4.1R or recombinant 4.1R fragments (1 μM). The reaction mixture (final volume, 25 μL) contained 50 mM Tris-HCl, pH 7.5, 1 mM ATP, 0.1 mM EGTA, 0.01% β-mercaptoethanol, and 0.1 mM sodium orthovanadate. The reactions were performed for 10 minutes at 30°C. The reaction was terminated by the addition of 25 μL sample buffer followed by heating for 5 minutes at 95°C. Phosphorylation of LAT was detected by Western blotting with anti-phosphotyrosine antibody 4G10 (Upstate).
T cell–dependent antibody response in vivo
Eight- to 10-week-old 4.1R+/+ (n = 8) or 4.1R−/− (n = 10) mice were immunized with 50 μg NP-KLH (Sigma-Aldrich) by intraperitoneal injection. Serum was collected at day 10 after primary immunization for IgM measurement. A booster injection was given at day 13, and at day 23 serum was collected for IgG1 measurement. The antibody concentrations were determined by ELISA with mouse immunoglobulin kits (Southern Biotechnology Associates, Birmingham, AL).
Results
4.1R knockout mice
The erythroid phenotype of 4.1R knockout mice, characterized by a moderately severe anemia, has been previously described.31 The mice were backcrossed onto C57BL/6 background and inbred for more than 20 generations. Breeding experiments with homozygous mice showed that, although 4.1R−/− mice were fertile and viable, the litter size was smaller than that of wild-type mice (4-6 for knockout mice compared with 8-10 for wild-type mice). Furthermore, breeding of heterozygous mice showed that both 4.1R−/− and 4.1R+/− mice were born in sub-Mendelian ratios (1.0:1.4:0.55 for WT/Het/KO).
Expression of 4.1R in T cells
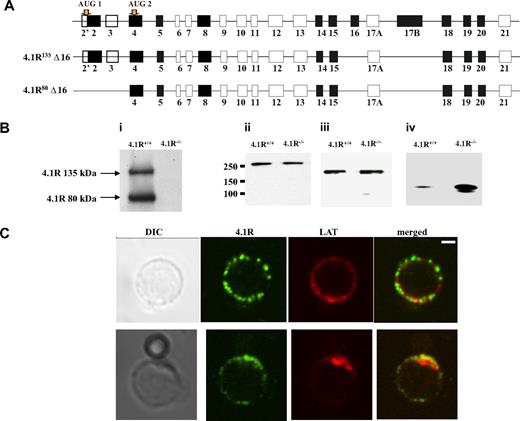
In situ hybridization experiment showed strong expression of 4.1R in mouse thymus.13 Isoforms of 4.1R have previously been shown to be present in primary human T cells and in human T-cell lines.36 Because there is a complexity in which 4.1R isoforms are expressed in nonerythroid cells, we first cloned 4.1R from CD4+ T cells by reverse transcription PCR with primers initiating from ATG-1 and ATG-2. Six clones of each kind were sequenced, and all give the same exon compositions that are shown in Figure 1A. Like 4.1R isoforms isolated from human MOLT4 T cells, all clones lack exon 16. However, different from MOLT4 T cells in which isoforms lacking exon 4, exon 5, exon 18, or exon 19 were observed, none of our clones miss these exons.37 Moreover, Western blot analysis of CD4+ T cells with the use of anti-4.1R exon 13 showed 2 bands with the molecular weight of approximately 135 kDa and approximately 80 kDa (Figure 1Bi), corresponding to 4.1R starting from ATG-1 and ATG-2, respectively. In addition, other 3 members of protein 4.1 family are also expressed in the CD4+ T cells, and, interestingly, although 4.1B and 4.1G are unchanged (Figure 1Bii,iii), 4.1N is significantly up-regulated in 4.1R−/− cells (Figure 1Biv).
Expression and localization of 4.1R in mouse CD4+ T cells. (A) Exon composition of 4.1R isoforms. Schematic representation of the exon map of 4.1R is displayed in the top panel. Two translation initiation sites are indicated. Alternatively spliced exons are shown in black, constitutive exon in gray, and noncoding exons in open boxes. Exon compositions of 4.1R 135 kDa and 80 kDa are shown in the middle and bottom panels, respectively. (B) Western blot analysis of protein 4.1 members. CD4+ T cells (106 cells) purified from 4.1R+/+ or 4.1R−/− T lymph nodes were subjected to immunoblot analysis with polyclonal rabbit antibodies against 4.1R exon 13 (i), 4.1B headpiece (ii), 4.1G headpiece (iii), and 4.1N headpiece (iv). The positions of approximately 135 kDa and approximately 80 kDa 4.1R are indicated. Note that, although 4.1B and 4.1G are unchanged, 4.1N expression is up-regulated in 4.1R−/− CD4+ T cells. (C) Localization of 4.1R in T cells. Unstimulated and stimulated primary mouse T cells were doubled stained with anti-4.1R exon 13 and anti-LAT antibodies and analyzed by confocal microscopy. In unstimulated samples, protein 4.1R is evenly distributed in discrete puncta around the cell membrane where it dose not colocalize with LAT. After stimulation, both protein 4.1R and LAT become enriched at the interface between T cell and bead where they colocalize. Scale bar, 2 μm.
Expression and localization of 4.1R in mouse CD4+ T cells. (A) Exon composition of 4.1R isoforms. Schematic representation of the exon map of 4.1R is displayed in the top panel. Two translation initiation sites are indicated. Alternatively spliced exons are shown in black, constitutive exon in gray, and noncoding exons in open boxes. Exon compositions of 4.1R 135 kDa and 80 kDa are shown in the middle and bottom panels, respectively. (B) Western blot analysis of protein 4.1 members. CD4+ T cells (106 cells) purified from 4.1R+/+ or 4.1R−/− T lymph nodes were subjected to immunoblot analysis with polyclonal rabbit antibodies against 4.1R exon 13 (i), 4.1B headpiece (ii), 4.1G headpiece (iii), and 4.1N headpiece (iv). The positions of approximately 135 kDa and approximately 80 kDa 4.1R are indicated. Note that, although 4.1B and 4.1G are unchanged, 4.1N expression is up-regulated in 4.1R−/− CD4+ T cells. (C) Localization of 4.1R in T cells. Unstimulated and stimulated primary mouse T cells were doubled stained with anti-4.1R exon 13 and anti-LAT antibodies and analyzed by confocal microscopy. In unstimulated samples, protein 4.1R is evenly distributed in discrete puncta around the cell membrane where it dose not colocalize with LAT. After stimulation, both protein 4.1R and LAT become enriched at the interface between T cell and bead where they colocalize. Scale bar, 2 μm.
4.1R is recruited to the immunologic synapse
We then examined the localization of 4.1R in T cells. Immunofluorescence staining of 4.1R shows that in resting T cell 4.1R is evenly distributed in a punctuate pattern on the membrane where it dose not colocalize with LAT. Interestingly, in T cells stimulated by CD3/CD8-coated bead, 4.1R is recruited to the immunologic synapse and colocalized with LAT, a immunologic synapse marker (Figure 1C). The localization of 4.1R at the immunologic synapse was further supported by the finding that, although 4.1R is uniformly distributed on the membrane of the resting primary T cells from 5c.c7 transgenic mice (Figure S1A, available on the Blood website; see the Supplemental Materials link at the top of the online article), it is recruited to the T cell/APC interface when 5c.c7 T cells were stimulated with peptide-loaded CH27 cells (Figure S1B). No staining was observed in 4.1R−/− T cells (data not shown) under the conditions we used. These results suggest that 4.1R may positively or negatively regulate TCR-mediated signal transduction.
Absence of gross defects in the lymphoid organs of 4.1R−/− mice
In addition to the expression in CD4+ T cells, 4.1R is also expressed in thymocytes (Figure S2A), B cells, and cells of myeloid origin from 4.1R+/+ mice (data not shown). However, examination of the central and peripheral lymphoid organs showed a normal representation of these cell types. The numbers of thymic T cells and of splenic T and B cells in 4.1R−/− mice were similar to those in 4.1R+/+ littermate controls. The IgM and IgD expression pattern on peripheral B cells, as well as the CD4+ as against CD8+ peripheral T-cell populations, the developmental profile of B cells in the BM and T cells in the thymus, the expression level of the activation makers CD69 and CD25, and the memory B- and T-cell phenotype (B220/CD44/CD62L or CD3/CD44/CD62L) were indistinguishable between 4.1R+/+ and 4.1R−/− mice. Peritoneal CD5+ B cells and myeloid cells in all tissues analyzed were also unaffected. Thus, 4.1R-deficient mice did not show any gross developmental defects in either lymphoid or myeloid compartments in bone marrow, spleen, lymph nodes, thymus, and peritoneum. The data of the detailed phenotyping of T cells in major lymphoid organs are shown in Table S1.
Hyperactivation of 4.1R−/− CD4+ T cells
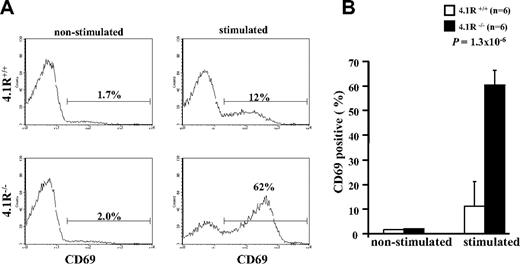
To determine whether 4.1R deficiency affects function of mature T cells, we first examined their activation (by staining of activation marker CD69) on T-cell receptor stimulation. The representative profiles of CD69 expression are displayed in Figure 2A. It shows that, although the expression of CD69 in both 4.1R+/+ and 4.1R−/− CD4+ T cells was almost undetectable before stimulation, after stimulation with anti-CD3ϵ antibody the percentage of CD69-expressing cells in 4.1R+/+ and 4.1R−/− CD4+ T cells is increased to 12% and 62%, respectively. Data from 6 experiments were plotted and are shown in Figure 2B, which shows a greater than 5-fold increase (11% ± 1.6% for 4.1R+/+ cells and 60% ± 5.7% for 4.1R−/− cells; n = 6; P < .013) in 4.1R−/− cells. Thus, 4.1R negatively regulates mature T-cell activation.
Hyperactivation of 4.1R−/− T cells. Purified 4.1R+/+ or 4.1R−/− CD4+ T cells were stimulated with control IgG or anti-CD3ϵ (1 μg/mL) for 16 hours, and the surface expression of CD69 was assessed by flow cytometry. The representative profiles of CD69 expression of 4.1R+/+ and 4.1R−/− CD4+ T cells were shown (A), and the quantitative analysis was shown (B). Note a significantly higher percentage of 4.1R−/− CD4+ T cells express CD69. Error bars indicate SD.
Hyperactivation of 4.1R−/− T cells. Purified 4.1R+/+ or 4.1R−/− CD4+ T cells were stimulated with control IgG or anti-CD3ϵ (1 μg/mL) for 16 hours, and the surface expression of CD69 was assessed by flow cytometry. The representative profiles of CD69 expression of 4.1R+/+ and 4.1R−/− CD4+ T cells were shown (A), and the quantitative analysis was shown (B). Note a significantly higher percentage of 4.1R−/− CD4+ T cells express CD69. Error bars indicate SD.
Hyperproliferation of 4.1R−/−CD4+ T cells
We next compared the proliferation responses between 4.1R+/+ and 4.1R−/− CD4+ T cells when stimulated with anti-CD3 antibody. Figure 3A shows that the uptake of 3H-thymidine by 4.1R−/− CD4+ T cells was significantly higher than that by 4.1R+/+ T CD4+ cells. No altered proliferation was observed in 4.1R+/− CD4+ T cells (data not shown). To determine whether the increased 3H-thymidine incorporation is due to increased proliferation or survival, we performed CFSE (carboxy-fluorescein-diacetate succinimidyl ester) dilution experiment, a method that provides a convenient means to analyze the kinetics of cell division.38 Figure 3B displays the representative CFSE profiles of both 4.1R+/+ and 4.1R−/− CD4+ T cells, and it clearly shows the increased division rate of 4.1R−/− CD4+ T cells. Figure 3C summarizes the results from 4 CSFE labeling experiments, and it shows that 4.1R−/− T CD4+ cells divide significantly faster than T cells from 4.1R+/+ because there are clearly less cells in stages D0 (undivided) and D1 (1 division) and more cells in D3 (3 divisions) and D4 (4 divisions) within the same experimental time frame. Together these data show that 4.1R negatively regulates T-cell proliferation.
Hyperproliferation of 4.1R−/− T cells. (A) [3H] thymidine incorporation of CD4+ T cells. CD4+ T cells (105 per well) purified from lymph nodes of 4.1R+/+ mice or 4.1R−/− mice were stimulated with various concentrations of plate-bound anti-CD3ϵ as indicated. Proliferation was determined by [3H] thymidine uptake. The experiments were preformed for 6 times, and the data shown represent the mean value of triplicate samples from 1 experiment. (B,C) In vitro T-cell expansion. T-cell expansion in vitro was assessed by CFSE dilution as described in “In vitro T-cell expansion using CFSE.” Note fewer cells in D0 (undivided) and D1 (1 division) and more cells in D3 (3 divisions) and D4 (4 divisions) of 4.1R−/− CD4+ T cells compared with 4.1R+/+ CD4+ T cells, showing faster division of 4.1R−/− CD4+ T cells.
Hyperproliferation of 4.1R−/− T cells. (A) [3H] thymidine incorporation of CD4+ T cells. CD4+ T cells (105 per well) purified from lymph nodes of 4.1R+/+ mice or 4.1R−/− mice were stimulated with various concentrations of plate-bound anti-CD3ϵ as indicated. Proliferation was determined by [3H] thymidine uptake. The experiments were preformed for 6 times, and the data shown represent the mean value of triplicate samples from 1 experiment. (B,C) In vitro T-cell expansion. T-cell expansion in vitro was assessed by CFSE dilution as described in “In vitro T-cell expansion using CFSE.” Note fewer cells in D0 (undivided) and D1 (1 division) and more cells in D3 (3 divisions) and D4 (4 divisions) of 4.1R−/− CD4+ T cells compared with 4.1R+/+ CD4+ T cells, showing faster division of 4.1R−/− CD4+ T cells.
Increased production of cytokines of 4.1R−/− CD4+ T cells
We then compared secretion of cytokines between 4.1R+/+ and 4.1R−/− CD4+ T cells. Figure 4A,B shows that the secretion of IL-2 and IFN-γ as assessed by ELISA was markedly higher from 4.1R−/− CD4+ T cells. To determine whether the increased cytokine production is due to more cells in the culture or due to individual cells producing more, we performed intracellular staining of IL-2. As shown in Figure 4C, expression of IL-2 was not detectable in nonstimulated 4.1R+/+ and 4.1R−/− CD4+ T cells; after stimulation the percentage of IL-2–expressing cells in 4.1R+/+ and 4.1R−/− CD4+ T cells is increased to 21% and 41%, respectively. Data from 4 experiments were plotted and are shown in Figure 4D, which shows approximately 2-fold increase (23% ± 2.3% for 4.1R+/+ cells and 37% ± 3.7% for 4.1R−/− cells; n = 4, P < .001).
Increased cytokine production of 4.1R−/− T cells. (A,B) Measurements of IL-2 and IFNγ by ELISA. CD4+ T cells were stimulated with 5 μg/mL plate-bound anti-CD3ϵ similarly as above. IL-2 (A) and IFNγ (B) in the media were measured with ELISA. The experiments were performed 6 times, and the data shown represent the mean value of triplicate samples from 1 experiment. Error bars indicate SD. (C,D) Intracellular staining of IL-2. The representative profiles of IL-2 expression of 4.1R+/+ and 4.1R−/− CD4+ T are shown (C), and the quantitative analysis is shown (D). Note a significantly higher percentage of 4.1R−/− CD4+ T cells express IL-2.
Increased cytokine production of 4.1R−/− T cells. (A,B) Measurements of IL-2 and IFNγ by ELISA. CD4+ T cells were stimulated with 5 μg/mL plate-bound anti-CD3ϵ similarly as above. IL-2 (A) and IFNγ (B) in the media were measured with ELISA. The experiments were performed 6 times, and the data shown represent the mean value of triplicate samples from 1 experiment. Error bars indicate SD. (C,D) Intracellular staining of IL-2. The representative profiles of IL-2 expression of 4.1R+/+ and 4.1R−/− CD4+ T are shown (C), and the quantitative analysis is shown (D). Note a significantly higher percentage of 4.1R−/− CD4+ T cells express IL-2.
Enhanced signaling in 4.1R−/− mature T cells
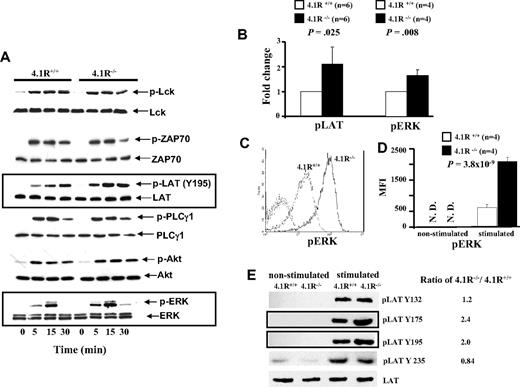
Because activation, proliferation, and cytokine secretion of T cells require activation of TCR-mediated signal transduction, we examined whether signals transduced by the TCR were enhanced in 4.1R-deficient T cells. Figure 5A shows the representative results of Western blotting analysis. It shows that after stimulation by anti-CD3ϵ antibody, there was no discernible difference in the level of phosphorylation of Lck or of ZAP-70 between 4.1R+/+ and 4.1R−/− T cells. By contrast, 4.1R−/− T cells exhibited greatly enhanced phosphorylation of LAT (Y195), relative to that in 4.1R+/+ T cells. Phosphorylation of LAT activates 3 major downstream pathways: the PLC-γ1, PI3 kinase, and MAP kinase pathways. We therefore examined the activation of PLC-γ1, Akt of PI3K pathway, and ERK of the MAP kinase pathway in T cells. Although we found no differences in the activation of either PLC-γ or the Akt of PI3K pathway between 4.1R+/+ and 4.1R−/− T cells, we observed a significant increase in phosphorylation of ERK in 4.1R−/− T cells. Quantitative analysis of phosphorylation levels (stimulated for 15 minutes) of LAT and ERK was shown in Figure 5B, which shows a modest but significant increase of phosphorylation of both LAT (n = 6; P = .025) and ERK (n = 4; P = .008) in 4.1R−/− T cells. The phosphorylation of ERK was further examined by intracellular staining with anti-pERK antibody. Figure 5C shows the representative staining profiles of pERK in 4.1R+/+ and 4.1R−/− T cells. It can be seen, again, that phosphorylation of ERK is significantly increased in 4.1R−/− T cells. Data from 6 experiments were plotted and shown in Figure 5D, which shows approximately 3-fold increase (mean fluorescence intensity, 635 ± 87 for 4.1R+/+ cells and 2087 ± 128 for 4.1R−/− cells; n = 6, P < .001). These findings clearly show that 4.1R negatively regulates signaling from the TCR through LAT to the MAP kinase pathway.
Biochemical analysis of signal transduction pathways in 4.1R+/+ and 4.1R−/− CD4+ T cells. (A) Western blot analysis of signal molecules. 4.1R+/+ or 4.1R−/− T cells were stimulated with anti-CD3ϵ (5 μg/mL) for various time periods, and indicated proteins were immunoblotted for the level of expression and for the extent of phosphorylation. Note the significantly enhanced phosphorylation of LAT and ERK in 4.1R−/− T cells. (B) Quantitative analysis of pLAT and pERK. The phosphorylation levels of LAT or ERK after 15 minutes of stimulation were quantified with the use of ImageJ software (National Institutes of Health, Bethesda, MD). Note the significantly increased phosphorylation of both LAT and ERK. (C,D) Intracellular staining of pERK. Intracellular staining of pERK was described in “Intracellular staining.” The representative profiles of pERK of 4.1R+/+ and 4.1R−/− CD4+ T cells are shown (C), and the quantitative analysis is shown (D). Note a significantly higher expression of pERK in 4.1R−/− CD4+ T cells. (E) Western blot analysis of phosphor-residues of LAT. 4.1R+/+ or 4.1R−/− T cells were stimulated with anti-CD3ϵ (5 μg/mL) for 15 minutes, and Western blot analysis was performed using anti-LAT phosphor-residue–specific antibodies as indicated. Note the significantly enhanced phosphorylation of Y175 and Y195 but not Y132 and Y235 of LAT in 4.1R−/− T cells. Error bars indicate SD.
Biochemical analysis of signal transduction pathways in 4.1R+/+ and 4.1R−/− CD4+ T cells. (A) Western blot analysis of signal molecules. 4.1R+/+ or 4.1R−/− T cells were stimulated with anti-CD3ϵ (5 μg/mL) for various time periods, and indicated proteins were immunoblotted for the level of expression and for the extent of phosphorylation. Note the significantly enhanced phosphorylation of LAT and ERK in 4.1R−/− T cells. (B) Quantitative analysis of pLAT and pERK. The phosphorylation levels of LAT or ERK after 15 minutes of stimulation were quantified with the use of ImageJ software (National Institutes of Health, Bethesda, MD). Note the significantly increased phosphorylation of both LAT and ERK. (C,D) Intracellular staining of pERK. Intracellular staining of pERK was described in “Intracellular staining.” The representative profiles of pERK of 4.1R+/+ and 4.1R−/− CD4+ T cells are shown (C), and the quantitative analysis is shown (D). Note a significantly higher expression of pERK in 4.1R−/− CD4+ T cells. (E) Western blot analysis of phosphor-residues of LAT. 4.1R+/+ or 4.1R−/− T cells were stimulated with anti-CD3ϵ (5 μg/mL) for 15 minutes, and Western blot analysis was performed using anti-LAT phosphor-residue–specific antibodies as indicated. Note the significantly enhanced phosphorylation of Y175 and Y195 but not Y132 and Y235 of LAT in 4.1R−/− T cells. Error bars indicate SD.
Enhanced phosphorylation Y175 and Y195 of LAT in 4.1R−/− T cells
In response to TCR engagement, several tyrosines of LAT are phosphorylated.39 In human LAT, these include Y132, Y171, Y191, and Y226, and they correspond to Y132, Y175, Y195, and Y235 in mouse LAT. We have shown in Figure 5A,B that phosphorylation of Y195 was significantly increased in 4.1R−/− T cells. To examine whether phosphorylation of other tyrosine residues are affected in the absence of 4.1R, we performed Western blotting analysis with phosphor-residue–specific antibodies against Y132, Y175, and Y235. As shown in Figure 5E, in addition to increased phosphorylation of Y195, increased phosphorylation of Y175, but not of Y132 and Y235, was observed in 4.1R−/− T cells. The results show that 4.1R specifically modulates phosphorylation of residues Y175 and Y195 of mouse LAT. It should be noted that phosphorylation of Y171 and Y191 of human LAT (which are equivalent to Y175 and Y195 of mouse LAT) are involved in activation of the MAP kinase pathway.39,40 Thus, the selective hyperphosphorylation of Y175 and Y195 of LAT in 4.1R−/− mouse T cells explains the above finding that phosphorylation of ERK but not PLCγ or ATK is affected because of deficiency of 4.1R.
Enhanced signaling of 4.1R−/− thymocytes
As shown in Figure S2A, 4.1R is also expressed in the thymocytes. We next examined the effect of 4.1R deficiency on thymocytes. Figure S2B shows that, like mature T cells, on stimulation by anti-CD3ϵ antibody, enhanced phosphorylation of LAT and ERK, but not of Akt, was also observed in 4.1R−/− thymocytes. Figure S2C shows the quantitative analysis of phosphorylation levels of LAT and ERK, both were increased approximately 2 times in 4.1R−/− T cells. The implication is that 4.1R negatively regulates activation of developing T cells just like mature T cells, using the same signal transduction pathway.
Association of 4.1R with LAT in situ
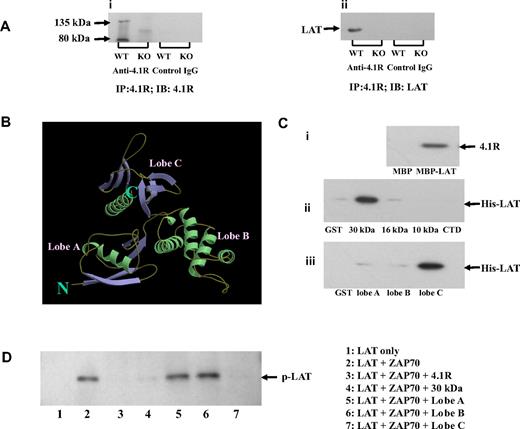
To understand the molecular mechanism of hyperphosphorylation of LAT in 4.1R−/− T cells, we first explored the possibility that 4.1R may associate with the transmembrane adapter LAT in T cells. We performed coimmunoprecipitation assays to test for an interaction between 4.1R and LAT and found that LAT was indeed coprecipitated by anti-4.1R antibody with 4.1R from 4.1R+/+ but not from 4.1R−/− T cells (Figure 6Ai,ii). Preimmune antibody caused no coprecipitation. These results show the association between LAT and 4.1R in situ.
Association of 4.1R with LAT and effect of 4.1R on LAT phosphorylation. (A) Association of 4.1R with LAT in situ. 4.1R was immunoprecipitated from 4.1R+/+ or 4.1R−/− T cells using anti-4.1R or preimmune IgG. 4.1R or LAT in the immunoprecipitate was detected using anti-4.1R antibody (i) or anti-LAT antibody (ii), respectively. (B) Crystal structure of membrane domain of 4.1R. Note the 3-lobe structure of the 30-kDa membrane binding domain. (C) Direct binding of 4.1R with LAT and inhibition of LAT phosphorylation by 4.1R in vitro. (i) Binding of 4.1R to cytoplasmic domain of LAT. 4.1R was incubated for 30 minutes at room temperature with MBP-tagged cytoplasmic domain of LAT, and binding was assessed by pull-down assay. 4.1R binding was detected by blotting with anti-4.1R exon 13 antibody after SDS-PAGE. (ii) Binding of cytoplasmic domain of LAT to recombinant 4.1R fragments. Recombinant His-tagged cytoplasmic domain of LAT was incubated with GST-tagged 4.1R fragments. Binding was assayed as above, using anti-His antibody for detection. (iii) Binding of cytoplasmic domain of LAT to subdomains of 4.1R 30-kDa membrane binding domain. Binding assays were performed as above. The minimum binding was mapped to lobe C of the 30-kDa domain. (D) Inhibition of in vitro LAT phosphorylation by 4.1R. Cytoplasmic domain of LAT was phosphorylated by ZAP-70 in the absence or presence of 4.1R or 4.1R fragments. The phosphorylation was detected by Western blot analysis with the use of anti-phosphotyrosine antibody 4G10. Note the phosphorylation was inhibited by 4.1R, 30 kDa of 4.1R, and lobe C of 30-kDa domain.
Association of 4.1R with LAT and effect of 4.1R on LAT phosphorylation. (A) Association of 4.1R with LAT in situ. 4.1R was immunoprecipitated from 4.1R+/+ or 4.1R−/− T cells using anti-4.1R or preimmune IgG. 4.1R or LAT in the immunoprecipitate was detected using anti-4.1R antibody (i) or anti-LAT antibody (ii), respectively. (B) Crystal structure of membrane domain of 4.1R. Note the 3-lobe structure of the 30-kDa membrane binding domain. (C) Direct binding of 4.1R with LAT and inhibition of LAT phosphorylation by 4.1R in vitro. (i) Binding of 4.1R to cytoplasmic domain of LAT. 4.1R was incubated for 30 minutes at room temperature with MBP-tagged cytoplasmic domain of LAT, and binding was assessed by pull-down assay. 4.1R binding was detected by blotting with anti-4.1R exon 13 antibody after SDS-PAGE. (ii) Binding of cytoplasmic domain of LAT to recombinant 4.1R fragments. Recombinant His-tagged cytoplasmic domain of LAT was incubated with GST-tagged 4.1R fragments. Binding was assayed as above, using anti-His antibody for detection. (iii) Binding of cytoplasmic domain of LAT to subdomains of 4.1R 30-kDa membrane binding domain. Binding assays were performed as above. The minimum binding was mapped to lobe C of the 30-kDa domain. (D) Inhibition of in vitro LAT phosphorylation by 4.1R. Cytoplasmic domain of LAT was phosphorylated by ZAP-70 in the absence or presence of 4.1R or 4.1R fragments. The phosphorylation was detected by Western blot analysis with the use of anti-phosphotyrosine antibody 4G10. Note the phosphorylation was inhibited by 4.1R, 30 kDa of 4.1R, and lobe C of 30-kDa domain.
4.1R binds directly to LAT and inhibits its phosphorylation by ZAP-70
We next examined the possibility that 4.1R interacts directly with LAT to inhibit its phosphorylation by ZAP-70. A direct interaction was indeed shown by pull-down assays, using recombinant proteins. The quality of recombinant proteins used in this study are shown in Figure S3. As shown in Figure 6Ci, 4.1R binds to the MBP-tagged cytoplasmic domain of LAT, but not to MBP alone. Furthermore, the cytoplasmic domain of LAT binds specifically to the 30-kDa membrane-binding domain of 4.1R, but to neither of the other domains (Figure 6Cii). The 30-kDa domain is composed of 3 lobes that bind to different transmembrane proteins (Figure 6B).17 By the use of expressed subfragments of the 30-kDa domain we showed that the binding site for LAT is contained in lobe C (Figure 6Ciii). Kinetic analysis of using surface plasma surface assay showed that the interaction between LAT and lobe C fits a 1:1 binding model and that the binding affinity is 1.2 × 10−7 M. The binding profile between lobe C of 4.1R and cytoplasmic domain is displayed in Figure S4. We further explored whether binding of 4.1R to LAT inhibits phosphorylation of the latter by ZAP-70. In vitro phosphorylation assays showed that the cytoplasmic domain of LAT is phosphorylated by ZAP-70 and that this phosphorylation is inhibited by 4.1R, its 30-kDa domain or lobe C of the 30-kDa domain (Figure 6D). Lobe A and lobe B, by contrast, were both inactive.
Enhanced T-cell–dependent immune response in 4.1R−/− mice
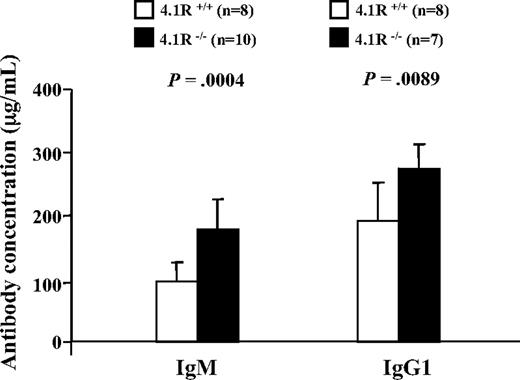
To determine whether 4.1R deficiency also influences T-cell function in vivo, we compared the T cell–dependent antibody response after immunization with NP-KLH in 4.1R+/+ and 4.1R−/− mice. The mice were bled on day 10 after immunization for measurement of IgM and on day 23 for measurement of IgG1. Significantly higher levels of both antibodies were observed in 4.1R−/− than in wild-type mice (Figure 7), indicative of enhanced T cell–dependent antibody responses in vivo.
Enhanced humoral response to NP-KLH in 4.1R−/− mice. Ten 4.1R+/+ mice and 10 4.1R−/− mice were immunized with NP-KLH. The concentrations of anti-NP–specific IgM at day 10 after primary immunization and IgG1 at day 23 after primary immunization (10 days after a booster injection on day 13) were measured. Error bars indicate SD.
Enhanced humoral response to NP-KLH in 4.1R−/− mice. Ten 4.1R+/+ mice and 10 4.1R−/− mice were immunized with NP-KLH. The concentrations of anti-NP–specific IgM at day 10 after primary immunization and IgG1 at day 23 after primary immunization (10 days after a booster injection on day 13) were measured. Error bars indicate SD.
Discussion
4.1R is the prototypic member of a large superfamily of proteins, of which more than 40 members have so far been identified. It is subdivided into 5 groups, comprising the 4.1 proteins (4.1R, 4.1G, 4.1N, and 4.1B), the ERM family (ezrin, radixin, and moesin), talin-related molecules, protein tyrosine phosphatase proteins, and NBL4 (a novel band-4.1-like protein).41 Recent data indicate that ERM proteins may play important roles in T-cell biology.28-30 All these studies, however, have been conducted on cell lines, and the physiologic actions of the proteins in vivo have therefore remained unknown. In the present study, with the use of 4.1R knockout mice, we have shown that 4.1R negatively regulates T-cell activation and proliferation both in vitro and in vivo. We further identified the molecular mechanism for this novel function of 4.1R in T cells.
In addition to 4.1R, it has been previously documented that 4.1G is also expressed in thymus and human T-cell line.42 We now show that 4.1B and 4.1N are also expressed in CD4+ T cells. Moreover, although the expression levels of 4.1B and 4.1G are unaltered in 4.1R-deficient T cells, 4.1N is significantly up-regulated, suggesting that 4.1N may compensate for the function of 4.1R. Although it is not clear if 4.1R and 4.1N have redundant function in CD4+ T cells, we would like to speculate that double knockout of 4.1R and 4.1N in T cells would lead to more enhanced T-cell proliferation.
One important outcome of the present study is that phosphorylation of LAT is regulated by 4.1R both in situ and in vitro. Although phosphorylation and de-phosphorylation of LAT are critical for regulation of TCR-mediated signal transduction, the molecular mechanism associated with this process is still poorly understood. It has been shown that in human LAT, phosphorylation of Y132 is important for activation of PLCγ, whereas Y171, Y191, and Y226 are responsible for Grb2 binding.39,40 It is also known that Grb2 activates MAP kinase pathway. Thus, it is interesting to note that in 4.1R−/− mouse T cell, phosphorylation of Y175 and Y195 but not Y132 was significantly increased, which in turn leads to hyperphosphorylation of ERK, one of MAK kinase pathway molecules. To our knowledge, no other nonphosphatase-negative regulator of LAT has been reported to date.
Changes in TCR-mediated signal transduction in 4.1R−/− thymocytes might have been expected to affect thymocyte selection. Yet 4.1R knockout mice did not display changes in thymocyte populations as defined by expression of surface markers, such as CD3, CD4, CD5, and CD8. One possible explanation is that these observations could not exclude the possibility that the lack of 4.1R leads to changes in thymic selection, which are masked by the heterogeneity of the TCR repertoire.
Although the membrane-binding domains of the proteins of the ERM and 4.1 families share a high degree of sequence homology, their functions appear to differ strikingly. Thus, in particular, they evidently exert opposite effects on cell proliferation. Although there is increasing evidence that members of the 4.1 family suppress cell growth and act as tumor suppressors,43-47 ezrin promotes cell growth and may play a role in tumor metastasis.48,49 These disparities may be attributable to the different binding partners of these proteins or possibly to different effects of posttranslational modifications on their functions. For example, it has been found that phosphorylation unmasks binding sites in ERM proteins, which are thereby activated.22 By contrast, phosphorylation of 4.1R in erythroid cells weakens its interactions with its binding partners.23,50,51 The function of 4.1R, but not of ERM proteins, is also regulated by Ca2+/calmodulin, as well as by phosphatidylserine.52,53 In regard to the immunologic synapse, it has been shown that ERM proteins are excluded from regions of cell-cell contact,29,54 whereas we show here that 4.1R is recruited to the immunologic synapse on stimulation.
In addition to the suppressive action of 4.1R on activation of both mature and developing T cells on T cell–receptor stimulation, we have observed hyperactivation of B cells in 4.1R−/− mice (our unpublished data, July 2007). This is consistent with the inference that members of the 4.1 family can function as tumor suppressors.43-47
Classically, 4.1R and the other family members of this protein have been shown to play important functional roles through their interactions with skeletal proteins such as spectrin and actin. The findings from the present study have identified yet another important role for 4.1R in signal transduction and cell proliferation. More complete understanding of the detailed mechanisms involved in this process await further studies.
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Wenzhi Tian for helping us in initiating the project, Avery August and Karina Yazdanbakhsh for constructive suggestions, and Morgan Huse for providing primary T-cell blasts from 5c.c7 T-cell receptor–transgenic mice and CH27 B-cell lymphoma (IEk-positive) cells.
This work was supported by the National Institutes of Health (Bethesda, MD) grants DK 26263, DK 32094, and HL31579 (to M.N.) and by a grant from the MetLife Foundation (New York, NY; to G.H.).
National Institutes of Health
Authorship
Contribution: Q.K., Y.Y., R.H., X.P., S.H., X.Z., X.G., and G.H. performed research and analyzed the data; N.M. designed the experiments and edited the paper; and X.A. designed experiments, analyzed the data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Xiuli An, Red Cell Physiology Laboratory, New York Blood Center, 310 East 67th St, New York, NY 10065; e-mail: xan@nybloodcenter.org.
References
Author notes
*Q.K., Y.Y., X.P., and R.H. contributed equally to this study.

![Figure 3. Hyperproliferation of 4.1R−/− T cells. (A) [3H] thymidine incorporation of CD4+ T cells. CD4+ T cells (105 per well) purified from lymph nodes of 4.1R+/+ mice or 4.1R−/− mice were stimulated with various concentrations of plate-bound anti-CD3ϵ as indicated. Proliferation was determined by [3H] thymidine uptake. The experiments were preformed for 6 times, and the data shown represent the mean value of triplicate samples from 1 experiment. (B,C) In vitro T-cell expansion. T-cell expansion in vitro was assessed by CFSE dilution as described in “In vitro T-cell expansion using CFSE.” Note fewer cells in D0 (undivided) and D1 (1 division) and more cells in D3 (3 divisions) and D4 (4 divisions) of 4.1R−/− CD4+ T cells compared with 4.1R+/+ CD4+ T cells, showing faster division of 4.1R−/− CD4+ T cells.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/113/24/10.1182_blood-2008-10-182329/4/m_zh80170934120003.jpeg?Expires=1771132963&Signature=GV0bQCQM6Qeh3Q7vfaXZV6N2e-b949mF~pCnjo2xm98kV0Ea8VLAhStlOr2rEaDyZhKi6HCd9ndZAROZ1CeuJFenWfMj8OoyozGF~tl4-67osZS8OIR3Ezo~d2mYbhTR5rdD6mc9WI6Tfltudj3WJlZCOg6I2eaj1rnzWojU1df9BQJGmuHV1wvafRu9jWzYx3iFezhjtEFeB0cKIhWP7V8BLvTBSU3Yf~gQ2phERmYcer0nZl0H3cz4wC2OJkXMqmVPzKJf9qr5JkIJZNZwvAFkvgNxEre-Q3MU2s7h9NYF-~vIEfAv0~OKNLaOKzRUHH6O6WD3hl-CHg19ViNCoQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal