Abstract
Migration toward chemoattractants is a hallmark of T-cell trafficking and is essential to produce an efficient immune response. Here, we have analyzed the function of the Rac activator Tiam1 in the control of T-cell trafficking and transendothelial migration. We found that Tiam1 is required for chemokine- and S1P-induced Rac activation and subsequent cell migration. As a result, Tiam1-deficient T cells show reduced chemotaxis in vitro, and impaired homing, egress, and contact hypersensitivity in vivo. Analysis of the T-cell transendothelial migration cascade revealed that PKCζ/Tiam1/Rac signaling is dispensable for T-cell arrest but is essential for the stabilization of polarization and efficient crawling of T cells on endothelial cells. T cells that lack Tiam1 predominantly transmigrate through individual endothelial cells (transcellular migration) rather than at endothelial junctions (paracellular migration), suggesting that T cells are able to change their route of transendothelial migration according to their polarization status and crawling capacity.
Introduction
T cells traffic through the body and pass the secondary lymphoid organs (SLOs), seeking for a cell presenting specific antigens. The entry of T cells to SLOs (homing) and exit from SLOs (egress) is a tightly regulated process required for T cells to allow a coordinated response in time and space.1 The migration of T cells toward chemoattractants is an essential requirement for their trafficking. During homing, homeostatic chemokines CCL19, CCL21 (SLC), CXCL12 (SDF1α), and CXCL13, which are expressed on high endothelial venules (HEVs) of peripheral lymph nodes (pLNs), are essential to attract leukocytes.2 Egress from pLNs is controlled by the lipid mediator S1P, in part because of its capacity to induce T-cell migration.3
To home to pLNs and to reach inflammation sites, leukocytes have to cross a natural barrier corresponding to HEVs or postcapillary venules, which involves a stepwise cascade of events including rolling, adhesion, and transendothelial migration of leukocytes.4 Additional steps have been recently defined in the transmigration cascade such as tethering, slow rolling, strengthening of adhesion, spreading, and crawling (reviewed in Ley et al5 ). Leukocytes can use 2 routes to cross the endothelial barrier (ie, the paracellular and the transcellular route). The most commonly used route is the paracellular route, when leukocytes transmigrate between endothelial cells at sites of cell junctions.6 During transcellular migration, leukocytes cross the endothelial barrier by transmigrating directly through individual endothelial cells. This pathway identified in various in vitro models7-10 is used by neutrophils and activated T cells particularly to reach sites of inflammation and to cross the blood-brain barrier.11
Attractants together with shear flow are essential to trigger transendothelial migration and cause integrin activation, polarization, directed cell migration, and diapedesis. Small GTPases of the Rho family, including Cdc42, Rac, and RhoA, regulate and coordinate the cytoskeleton remodeling required for adhesion, polarization, and migration of cells.12 Their activation is controlled by guanine nucleotide exchange factors (GEFs) that promote the exchange of GDP for GTP and also select upstream and downstream partners.13,14 Rac has been shown to be crucial for transendothelial migration.15 The Rac activator Vav1 is required for T-cell adhesion and chemotaxis in vitro, but no Vav1-dependent defects in T-cell trafficking have been observed so far.16,17 Dock2, another Rac-specific GEF, is crucial for T-cell trafficking,18,19 but the presence of lymph nodes in Dock2-deficient mice suggests that other Rac-specific GEFs contribute to T-cell trafficking.
Here we have studied the function of Tiam1 in T-cell trafficking and transendothelial migration. Tiam1 has been identified as a T-lymphoma invasion and metastasis-inducing gene by retroviral insertional mutagenesis20 and encodes a GEF specific for Rac.21 Whereas expression of a hyperactive form of Tiam1 induces invasion of otherwise noninvasive T-lymphoma cells, its actual function in primary T cells is largely unknown. Here we show that Tiam1 controls T-cell polarization and is required for chemokine- and S1P-induced T-cell migration in vitro and efficient T-cell homing and egress in vivo. Furthermore, Tiam1-deficient mice are delayed in contact hypersensitivity, showing the requirement of Tiam1-dependent polarization and migration for efficient T-cell response. When crossing the endothelial monolayer, Tiam1-deficient T cells switch from paracellular to transcellular migration, suggesting that T cells can switch their transmigration route depending on their polarization status.
Methods
Cell culture and retroviral transduction of T-cell blasts
The murine brain-derived endothelial cell line (bEnd.3; gift from Dr D. Vestweber, Max-Planck-Institute of Molecular Biomedicine, Münster, Germany) was cultured in DMEM supplemented with 10% fetal calf serum (FCS). T cells were isolated from lymph nodes and spleen of 6- to 12-week-old WT (FVB) and Tiam1−/− mice.22 Negative selection was carried out using a pan T-cell isolation kit (magnetic-activated cell sorting [MACS]; Miltenyi Biotec, Auburn, CA). T-cell purity was more than 95% as determined by flow cytometry. T-cell blasts were prepared by culturing splenocytes derived from WT and Tiam1−/− mice with 5 μg/mL concanavalin A for 3 days, followed by expansion of nonadherent cells with 25 U/mL mouse IL-2 (Peprotech, Rocky Hill, NJ). For retroviral transduction of pIB-GFP-actin (kindly provided by Dr M. F. Krummel, University of California, San Francisco, CA), virus was prepared using the Phoenix retrovirus packaging cells as described.23 T-cell blasts (3 × 106) were incubated with 1 mL virus-containing supernatant in the presence of 8 μg/mL polybrene (Sigma-Aldrich, St Louis, MO) and spin-infected for 2 hours at 800g. A second round of infection was performed after 16 hours and subsequently cells were allowed to grow for 24 hours. Infection efficiency was between 30% and 40%.
Antibodies and reagents
The following antibodies were used in immunoblottings and immunofluorescent stainings. Antibodies against PKCζ (C-20), Tiam1 (C-16), and PECAM-1 (M-20) were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Antibody against Rac1 was from Upstate Technology (Lake Placid, NY), against phospho-PKCζ/λ (Thr 410/403) was from Cell Signaling Technology (Beverly, MA), and against ICAM-1 was from Chemicon International (Temecula, CA). Antibody against LFA-1 (M17-4) was provided by E. Roos (The Netherlands Cancer Institute). All the conjugated secondary antibodies for immunofluorescent staining were purchased from Invitrogen (Frederick, MD). Recombinant S1P, SLC, and SDF1α were purchased from Peprotech. The PKCζ pseudosubstrate inhibitor and Src inhibitor PP2 were purchased from Calbiochem (San Diego, CA) and Sigma-Aldrich, respectively.
Homing assay
Naive T lymphocytes were fluorescently labeled with 0.5 μM green cell tracker CMFDA or 1.5 μM orange cell tracker CMTMR for 2 hours at 37°C. Of each population, 10 × 106 labeled cells were admixed and injected intravenously in FVB mice. After 2 hours, recipient mice were killed, blood was collected, and SLOs were isolated. Percentages of green- and red-labeled adoptively transferred cells were determined by flow cytometry. The ratio of the input population (input WT cells divided by input Tiam1−/− cells) was used as a correction factor as described.24 A homing ratio was calculated for each organ as followed: (number of Tiam1−/− adoptively transferred cells divided by number WT adoptively transferred cells) × (correction factor input). One pLN from each mouse was snap frozen for immunohistologic analysis.
Egress assay
Assessment of egress from pLNs was performed essentially as described.25 Naive T lymphocytes were fluorescently labeled using 0.5 μM CMFDA or 1.5 μM CMTMR for 2 hours at 37°C. Of each population, 25 × 106 cells were admixed in 200 μL PBS and injected intravenously in FVB mice. Twenty-four hours after lymphocyte transfer, L-selectin blocking Ab (Mel-14 [Biolegend, San Diego, CA], 100 μg/mouse) was injected intravenously to prevent further homing. Mice were killed 0, 12, and 24 hours after mAb injection and pLNs were isolated. The relative numbers of adoptively transferred T cells were determined using flow cytometry. For each organ, absolute numbers were also deduced. A pLN retention ratio was determined for each time point as the ratio of (number of recovered Tiam1−/− T lymphocytes divided by number of recovered WT lymphocytes) × correctionfactor for the input population ratio. Twenty-four hours after adoptive transfer, some of the mice were killed and pLNs were snap frozen for immunohistologic analysis.
Contact hypersensitivity
WT and Tiam1−/− mice were immunized by applying 0.5% FITC solubilized in 1:1 acetone/dibutyl pthalate (400 μL) on their shaved abdomens. After 6 days, baseline ear thickness was measured and 10 μL 0.5% FITC solution was applied to both left ear surfaces. Acetone/dibutyl pthalate solution was applied on the right ear as negative control. On days 1 and 4 after challenge, the thickness of each ear was measured. The data are expressed as changes in ear thickness (ear thickness 24 hours after elicitation minus baseline ear thickness). From each mouse, one of the ears was snap frozen for immunohistologic analysis. From each experiment, 2 mice were killed 3 days after exposure of the abdomen to hapten, and draining LNs were isolated. Single-cell suspensions were counted, stained with anti-CD11c conjugated with APC (BD Biosciences, San Diego, CA), and analyzed by flow cytometry. Animal experiments were performed strictly according to the laws of the Netherlands and were approved by the Animal Commission of The Netherlands Cancer Institute.
Histology
For homing and egress experiments, 10-μm sections of frozen pLNs were fixed for 10 minutes in 2% paraformaldehyde at room temperature (RT), washed in PBS, blocked with FCS, and stained with anti-PNAd Ab (MECA-79 antibody; BD Biosciences). As a secondary Ab, biotinylated anti–rat Ig was used, followed by Cy5-conjugated streptavidin. For contact hypersensitivity experiments, 5-μm sections of frozen ears were acetone-fixed for 10 minutes, washed in PBS, blocked with FCS, and stained with biotinylated anti-CD3 (145-2C11 antibody; BD Biosciences), followed by Cy5-conjugated streptavidin. The number of T cells infiltrating the inflamed ears was quantified as the number of CD3+ cells present per field using a 10×/0.4 NA phase-contrast objective. All sections were analyzed using a Leica confocal microscope (Heidelberg, Germany).
Transmigration under shear flow
Experiments were performed essentially as described.26 Briefly, bEnd.3 cells were plated at confluence on 30-mm coverslips coated with fibronectin (50 μg/mL in PBS) and stimulated for 16 hours with TNFα (500 U/mL). The bEnd.3 cell monolayers were subsequently overlaid with SDF1α (2 μg/mL) for 5 minutes. After extensive washing, coverslips were mounted on a laminar flow chamber (C&L Instruments, Hershey, PA). T-cell blasts or naive T cells were untreated or treated with 2 μM PP2 and/or 2 μM PKCζ inhibitor for 1 hour. After washing, T cells were resuspended at 0.5 × 106/mL in flow buffer (20 mM Hepes, 132 mM NaCl, 6 mM KCl, 1 mM MgSO4, 1.2 mM KH2PO4, 5 mM glucose, 1 mM CaCl2, 0.5% BSA). Perfusion of T cells into the flow chambers was performed at 0.25 dyne/cm2 for 2 minutes over the monolayer to allow accumulation of leukocytes. The flow rate was then increased to 2 dyne/cm2 (physiologic shear flow) for a period of 15 minutes. Images were recorded at 1 frame per 10 seconds using a 20×/0.4 NA phase-contrast objective with a microscope-linked CCD camera (DFC 350 FX; Leica). Motion analysis was performed manually. Lymphocytes arrested during the accumulation phase and resisting detachment from the endothelial surface were subdivided into 3 categories: (1) Lymphocytes that remained stationary throughout the assay were considered stationary. (2) Lymphocytes that crawled at least 2 cell diameters without detaching or transmigrating were considered crawling. (3) Lymphocytes that underwent stepwise darkening in phase contrast were considered transmigrating cells (diapedesis). Finally, T cells that showed a leading and trailing edge after 15-minute tracking were considered polarized, independently of their migration status.
Arrest, locomotion, and polarization under shear flow
ICAM-1 proteins (5 μg/mL) were coated on coverslips OVN at 4°C. T-cell blasts were resuspended at 0.5 × 106/mL in PBS+/+ (PBS containing 1 mM CaCl2 and 1 mM MgCl2), and perfused through the flow chamber at 2 dyne/cm2. All flow experiments were conducted at 37°C and were recorded at 1 frame per 5 to 10 seconds with a 10×/0.4 NA phase-contrast objective and a microscope-linked CCD camera (DFC 350 FX; Leica). Adherent cells were defined as cells remaining attached to the substrate for the complete flow period. The number of adherent cells per field was quantified over time. Migration analysis on purified ligands was conducted during the same time. Cells that showed after 10 minutes a leading and trailing edge and GFP-actin accumulation at the leading edge were considered polarized, independently of their migration status.
Determination of route of diapedesis
BEnd.3 cells were plated at 90% confluence on fibronectin-coated coverslips (50 μg/mL). After 24 hours, cells were activated by 500 U/mL TNFα overnight. T-cell blasts (5 × 106 in PBS+/+) were added to the SDF1α-bearing endothelial monolayer for 30 minutes at 37°C. Samples were fixed in 4% PFA at RT for 15 minutes, and saturated in PBS 5% FCS for 20 minutes. T cells undergoing transendothelial migration were identified as LFA-1–positive cells that extended down to the basal sections at the endothelial cell substrate, as already described.27 Additional immunofluorescent stainings were performed to determine the distribution of T cells relative to the endothelial cells in the X-Y and Z dimensions (for detailed explanation see Figure S1, available on the Blood website; see the Supplemental Materials link at the top of the online article). After identifying the transmigrating cells, the route of diapedesis was essentially determined as follows: cells were stained with LFA-1 to identify the T cells and with PECAM-1 and ICAM-1 to identify endothelial cell perimeter and the transmigration pore, respectively. Appropriate fluorescent-labeled secondary antibodies were subsequently used. Diapedesis not in close proximity to endothelial junctions was identified as transcellular. Diapedesis at endothelial cell-cell contacts was scored as paracellular.7,9
Electron microscopy
BEnd.3 cells were plated at 90% confluence on fibronectin-coated (50 μg/mL) Thermanox plastic coverslips (Nunc, Rochester, NY). After 24 hours, cells were activated by 500 U/mL TNFα overnight. T-cell blasts (5 × 106 in PBS+/+) were added to the SDF1α-bearing endothelial monolayer for 30 minutes at 37°C. Cells were fixed in Karnovsky fixative and postfixed in 1% osmiumtetroxide, processed through dehydration for flat-embedding in embed812/NMA, sectioned, stained with 1% uranylacetate, and examined with a Philips CM10 Electron Microscope (Eindhoven, The Netherlands).
Chemotaxis assay
The inner and outer surface of transwells (5-μm pore size; Costar, Cambridge, MA) were coated with 0.5% ovalbumin (Ova) for 2 hours at RT. Purified T cells (106 in 150 μL RPMI 0.1% Ova) were treated with 2 μM PKCζ inhibitor for 1 hour when indicated and loaded in an Ova-coated transwell, which was placed into a 24-well plate containing 250 μL RPMI supplemented with 0.1% Ova and SDF1α, SLC (100 ng/mL), or S1P (15 nM), as indicated. After 1 hour at 37°C, the cells that migrated into the lower chamber were collected and counted.
Cell lysis and immunoblotting
Lysates were prepared in standard NP40 lysis buffer (10% glycerol, 50 mM Tris-HCl [pH 7.4], 1% NP40, 150 mM NaCl, 20 mM NaF, 2 mM MgCl2, 1 mM Na3VO4, 1 mg/mL protease inhibitor) for 10 minutes at 4°C and centrifuged at 16 000g for 10 minutes at 4°C. Lysates were denatured with sodium dodecyl sulfate (SDS) and separated by SDS–polyacrylamide gel electrophoresis (PAGE). For immunoblotting, membranes were blocked with 5% BSA, probed with specific antibodies, and incubated with the appropriate horseradish peroxidase–conjugated secondary antibodies. Immunoreactive bands were visualized by enhanced chemiluminescence (Pierce, Rockford, IL).
Rac activity assay
Rac activity was determined as described previously.28 T cells (10 × 106) were starved for 18 hours in IMDM medium with 0.5% BSA, stimulated as indicated, and lysed in standard NP40 buffer containing a biotinylated Rac1/Cdc42-interactive binding motif peptide of PAK1. Active Rac was precipitated with streptavidin-agarose beads.
Statistical analysis
Data were expressed as mean plus or minus SD. Comparisons between groups were analyzed by using the Student t test with Microsoft Excel Software (Redmond, WA). Data were considered significant when P values were less than .05.
Results
Tiam1-mediated Rac activity is necessary for chemokine- and S1P-induced T-cell migration
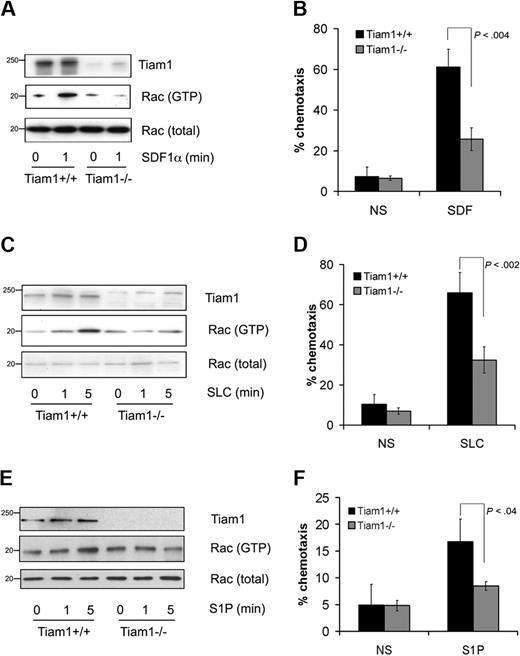
To investigate whether Tiam1 was required for Rac activation induced by attractants, we used Tiam1-deficient (Tiam1−/−) T cells and analyzed Rac activation in response to various extracellular stimuli. Whereas basal Rac activity was not significantly different between wild-type (WT) and Tiam1−/− T cells (Figure 1A,C,E), Tiam1−/− cells were defective in SDF1α-induced Rac activation (Figure 1A) leading to impaired chemotaxis (Figure 1B). Tiam1 was also necessary for Rac activation and migration induced by the chemokine SLC (Figure 1C,D). We wondered whether other types of attractant for T cells could also signal via Tiam1. The lipid S1P is a crucial component regulating T-cell egress from pLNs and functions as an attractant for T cells (for review, see Cyster29 ). Similarly as found for chemokines, S1P-mediated Rac activation (Figure 1E) and migration (Figure 1F) appeared also impaired in Tiam1−/− T cells compared with WT T cells. From these results, we conclude that Tiam1 is essential for chemokine- and S1P-mediated Rac activation that controls chemotactic cell migration in T cells.
Tiam1 is required for chemokine- and S1P-induced Rac activation and chemotaxis. (A) Lymphocytes from WT and Tiam1−/− mice were either nonstimulated or stimulated with 250 ng/mL SDF1α for the indicated time period. Subsequently Rac activity was determined. (Top panel) Tiam1. (Bottom panels) GTP-bound Rac and total Rac. Sizes are indicated in kilodaltons. Representative results of 3 independent experiments are shown. (B) Chemotaxis of WT and Tiam1−/− T lymphocytes toward SDF1α (100 ng/mL) was measured in transwells. After 1 hour, cells present in the lower chamber were counted. Results are derived from 3 independent experiments and presented as the percentage of the input cells. Error bars indicate SD; p indicates P value. (C) Lymphocytes from WT and Tiam1−/− mice were either nonstimulated or stimulated with 250 ng/mL SLC for the indicated time period. Subsequently Rac activity was determined. (Top panel) Tiam1. (Bottom panels) GTP-bound Rac and total Rac. Sizes are indicated in kilodaltons. Representative results of 2 independent experiments are shown. (D) Chemotaxis of WT and Tiam1−/− T lymphocytes toward SLC (100 ng/mL) was measured in transwells. After 1 hour, cells present in the lower chamber were counted. Results are derived from 2 independent experiments and presented as the percentage of the input cells. Error bars indicate SD; p indicates P value. (E) Lymphocytes from WT and Tiam1−/− mice were either nonstimulated or stimulated with 150 nM S1P for the indicated time period. Subsequently Rac activity was determined. (Top panel) Tiam1. (Bottom panels) GTP-bound Rac and total Rac. Sizes are indicated in kilodaltons. Representative results of 3 independent experiments are shown. (F) Chemotaxis of WT and Tiam1−/− T lymphocytes toward S1P (15 nM) was measured in transwells. After 1 hour, cells present in the lower chamber were counted. Results are derived from 3 independent experiments and presented as the percentage of the input cells. Error bars indicate SD; p, P value.
Tiam1 is required for chemokine- and S1P-induced Rac activation and chemotaxis. (A) Lymphocytes from WT and Tiam1−/− mice were either nonstimulated or stimulated with 250 ng/mL SDF1α for the indicated time period. Subsequently Rac activity was determined. (Top panel) Tiam1. (Bottom panels) GTP-bound Rac and total Rac. Sizes are indicated in kilodaltons. Representative results of 3 independent experiments are shown. (B) Chemotaxis of WT and Tiam1−/− T lymphocytes toward SDF1α (100 ng/mL) was measured in transwells. After 1 hour, cells present in the lower chamber were counted. Results are derived from 3 independent experiments and presented as the percentage of the input cells. Error bars indicate SD; p indicates P value. (C) Lymphocytes from WT and Tiam1−/− mice were either nonstimulated or stimulated with 250 ng/mL SLC for the indicated time period. Subsequently Rac activity was determined. (Top panel) Tiam1. (Bottom panels) GTP-bound Rac and total Rac. Sizes are indicated in kilodaltons. Representative results of 2 independent experiments are shown. (D) Chemotaxis of WT and Tiam1−/− T lymphocytes toward SLC (100 ng/mL) was measured in transwells. After 1 hour, cells present in the lower chamber were counted. Results are derived from 2 independent experiments and presented as the percentage of the input cells. Error bars indicate SD; p indicates P value. (E) Lymphocytes from WT and Tiam1−/− mice were either nonstimulated or stimulated with 150 nM S1P for the indicated time period. Subsequently Rac activity was determined. (Top panel) Tiam1. (Bottom panels) GTP-bound Rac and total Rac. Sizes are indicated in kilodaltons. Representative results of 3 independent experiments are shown. (F) Chemotaxis of WT and Tiam1−/− T lymphocytes toward S1P (15 nM) was measured in transwells. After 1 hour, cells present in the lower chamber were counted. Results are derived from 3 independent experiments and presented as the percentage of the input cells. Error bars indicate SD; p, P value.
Tiam1 acts in conjunction with the PKCζ to promote S1P-induced Rac activation and migration
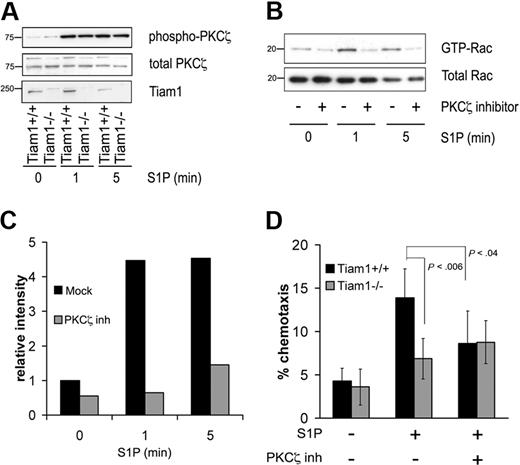
We previously found that Tiam1 regulates Rac activation and T-cell migration induced by the chemokine SDF1α downstream of the Par/PKCζ polarity complex.30 As PKCζ activation is required for Tiam1-mediated Rac activation, we investigated whether PKCζ could be activated in response to S1P by analyzing the phosphorylation status of PKCζ (Thr410). S1P increased PKCζ phosphorylation in both WT and Tiam1−/− T cells (Figure 2A), demonstrating that the PKCζ is activated by S1P independently of Tiam1. However, inhibition of PKCζ downstream signaling by a myristoylated PKCζ pseudosubstrate (PKCζ inhibitor)31 abrogated S1P-induced Rac activation in WT cells, indicating that PKCζ functions upstream of Tiam1 and is required for S1P-induced Rac activation (Figure 2B,C). We finally determined whether PKCζ activity was necessary for S1P-mediated T-cell migration. Inhibition of PKCζ downstream signaling reduced chemotactic migration toward S1P of WT cells, whereas it had no effect on residual chemotactic migration of Tiam1−/− T cells (Figure 2D).
Tiam1 cooperates with PKCζ to regulate S1P-induced Rac activation and chemotaxis. (A) WT and Tiam1−/− T cells were either nonstimulated or stimulated with 150 nM S1P for the indicated time periods. Subsequently PKCζ phosphorylation status was determined. (Top panel) Phosphorylated PKCζ. (Middle panel) Total PKCζ. (Bottom panel) Tiam1. Sizes are indicated in kilodaltons. (B) WT T cells were either nontreated or treated with 2 μM PKCζ inhibitor for 1 hour and subsequently stimulated with 150 nM S1P for the indicated time periods. Subsequently, Rac activity status was determined. (Top panel) GTP-bound Rac. (Bottom panel) Total Rac. Sizes are indicated in kilodaltons. (C) Quantification of the Rac activation as shown in panel B. Rac activity value in nonstimulated WT T cells is set to 1. (D) Chemotaxis of WT and Tiam1−/− lymphocytes either treated or untreated with 2 μM PKCζ inhibitor was measured in response to 15 nM S1P after 1 hour. The results are presented as the percentage of the input cells and are based on 3 independent experiments. Error bars indicate SD; p, P value.
Tiam1 cooperates with PKCζ to regulate S1P-induced Rac activation and chemotaxis. (A) WT and Tiam1−/− T cells were either nonstimulated or stimulated with 150 nM S1P for the indicated time periods. Subsequently PKCζ phosphorylation status was determined. (Top panel) Phosphorylated PKCζ. (Middle panel) Total PKCζ. (Bottom panel) Tiam1. Sizes are indicated in kilodaltons. (B) WT T cells were either nontreated or treated with 2 μM PKCζ inhibitor for 1 hour and subsequently stimulated with 150 nM S1P for the indicated time periods. Subsequently, Rac activity status was determined. (Top panel) GTP-bound Rac. (Bottom panel) Total Rac. Sizes are indicated in kilodaltons. (C) Quantification of the Rac activation as shown in panel B. Rac activity value in nonstimulated WT T cells is set to 1. (D) Chemotaxis of WT and Tiam1−/− lymphocytes either treated or untreated with 2 μM PKCζ inhibitor was measured in response to 15 nM S1P after 1 hour. The results are presented as the percentage of the input cells and are based on 3 independent experiments. Error bars indicate SD; p, P value.
These data show that PKCζ activity is required for Tiam1-mediated Rac activation and that this pathway regulates S1P-mediated chemotactic migration of T cells.
Tiam1 is required for efficient T-cell trafficking
Chemokine signaling is required for T-cell adhesion and migration,32,33 processes that are essential for T cells to home to secondary lymphoid organs (SLOs).34 By contrast, S1P-mediated signaling has been implicated in the egress of T cells from pLNs. Our in vitro studies showed that Tiam1 functions in both chemokine- and S1P-mediated T-cell migration and we investigated therefore the function of Tiam1 in T-cell trafficking in vivo.
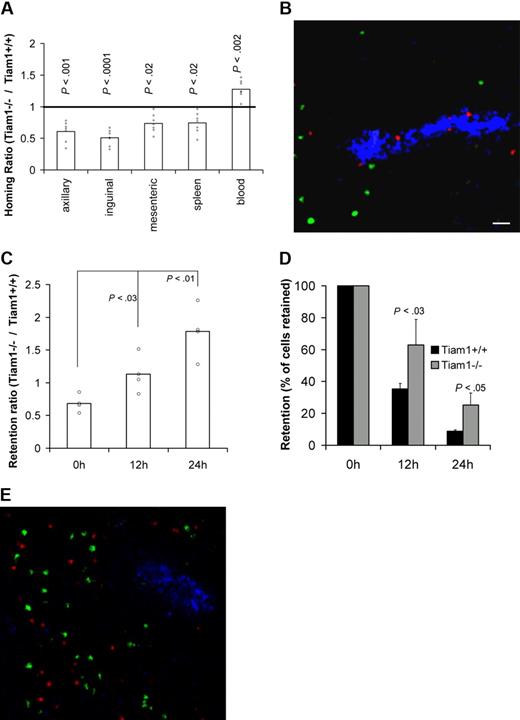
We first analyzed the requirement of Tiam1 for T-cell homing by analyzing the capacity of adoptively transferred WT and Tiam1−/− T cells to reach SLOs in WT mice. Two hours after adoptive transfer, approximately 2 times more WT T cells were found in pLNs compared with Tiam1−/− T cells (Figure 3A). Moreover, we found that higher numbers of WT cells reached the mesenteric lymph nodes and spleen compared with Tiam1−/− T cells (Figure 3A). Consistent with these data, lower numbers of WT cells remained in the circulation. The same difference in homing was observed for CD4+ and CD8+ cells (Figure S2), indicating that a lack of Tiam1 delays homing of both T-cell subpopulations. Cryosections of pLNs from homing experiment revealed that Tiam1−/− cells were concentrated within or close to HEVs, whereas WT T cells were already present within the pLNs (Figure 3B). This supports our conclusion that Tiam1−/− cells enter pLNs less efficiently than WT T cells. From these data, we conclude that Tiam1 deficiency delays short-term homing of T cells, particularly to pLNs.
Tiam1 is required for homing and egress of T cells. (A) T-cell homing: CFDA-labeled WT and CMTMR-labeled Tiam1−/− naive T cells were mixed and injected intravenously in a WT mouse. Mice were killed 2 hours after transfer and the percentage of labeled cells in different organs was determined. Results are expressed as a homing ratio, corresponding to the ratio between number of Tiam1−/− and WT cells. Bars are means. All data points are depicted by a dot; P values between WT and Tiam1−/− T cells are depicted for every organ tested. (B) Representative cryosection of a pLN after a homing experiment performed in panel A. Adoptively transferred WT T cells appear in green and Tiam1−/− T cells, in red. HEVs (blue) are visualized using MECA-79 Ab. Scale bar represents 20 μm. (C-E) T-cell egress: mixed adoptively transferred CMFDA-labeled WT and CMTMR-labeled Tiam1−/− T cells were allowed to home to pLNs for 24 hours. Homing was then blocked by Mel-14 Ab injection. Mice were killed 0, 12, and 24 hours after blocking of homing. The relative number of adoptively transferred cells present in pLNs was calculated by FACS analysis. (C) Ratio between the number of Tiam1−/− and WT cells present in pLNs at different time points. Bars are means. All data points are depicted by a dot. Three independent experiments were performed; P values of 12- and 24-hour time points compared with 0 hours are depicted. (D) Kinetics of WT and Tiam1−/− cell numbers present in pLNs during the egress assay. Results are expressed as a percentage of the initial population present at time point 0. Three independent experiments were performed. Values indicate mean ± SD; P values: comparison of the relative decrease of WT and Tiam1−/− T-cell numbers at the same time point. (E) Representative cryosection of a pLN 24 hours after adoptive transfer of WT (green) and Tiam1−/− T cells (red). HEVs (blue) are visualized using MECA-79 Ab.
Tiam1 is required for homing and egress of T cells. (A) T-cell homing: CFDA-labeled WT and CMTMR-labeled Tiam1−/− naive T cells were mixed and injected intravenously in a WT mouse. Mice were killed 2 hours after transfer and the percentage of labeled cells in different organs was determined. Results are expressed as a homing ratio, corresponding to the ratio between number of Tiam1−/− and WT cells. Bars are means. All data points are depicted by a dot; P values between WT and Tiam1−/− T cells are depicted for every organ tested. (B) Representative cryosection of a pLN after a homing experiment performed in panel A. Adoptively transferred WT T cells appear in green and Tiam1−/− T cells, in red. HEVs (blue) are visualized using MECA-79 Ab. Scale bar represents 20 μm. (C-E) T-cell egress: mixed adoptively transferred CMFDA-labeled WT and CMTMR-labeled Tiam1−/− T cells were allowed to home to pLNs for 24 hours. Homing was then blocked by Mel-14 Ab injection. Mice were killed 0, 12, and 24 hours after blocking of homing. The relative number of adoptively transferred cells present in pLNs was calculated by FACS analysis. (C) Ratio between the number of Tiam1−/− and WT cells present in pLNs at different time points. Bars are means. All data points are depicted by a dot. Three independent experiments were performed; P values of 12- and 24-hour time points compared with 0 hours are depicted. (D) Kinetics of WT and Tiam1−/− cell numbers present in pLNs during the egress assay. Results are expressed as a percentage of the initial population present at time point 0. Three independent experiments were performed. Values indicate mean ± SD; P values: comparison of the relative decrease of WT and Tiam1−/− T-cell numbers at the same time point. (E) Representative cryosection of a pLN 24 hours after adoptive transfer of WT (green) and Tiam1−/− T cells (red). HEVs (blue) are visualized using MECA-79 Ab.
Subsequently, we analyzed whether the egress of T cells from pLNs was also affected by Tiam1 deficiency. We performed egress experiments in which further homing was blocked with anti–L-selectin mAb injection 24 hours after adoptive transfer of WT and Tiam1−/− T cells. We determined the ratio between the percentage of Tiam1−/− and WT T cells retained in pLNs after blocking homing. If the egress rate of Tiam1−/− T cells is slower than that of WT T cells, the retention ratio should increase overtime. After 24 hours of homing (time point 0), we found a retention ratio of approximately 0.7 (Figure 3C). When blocking homing, this retention ratio gradually increased to 1.8 at 24 hours (Figure 3C). Analysis of the number of cells retained in pLNs at different time points confirmed that Tiam1−/− cells had a lower egress rate than WT T cells (Figure 3D). The dwell time (time when 50% of the initial population had left pLNs) was 11.8 hours and 16 hours for WT and Tiam1−/− T cells, respectively. The defect in egress of Tiam1−/− T cells was not caused by a retention of these cells in the HEVs, as Tiam1−/− cells freely migrated within the pLNs 24 hours after adoptive transfer (Figure 3E).
From these data, we conclude that Tiam1-deficient T cells are delayed in both homing and egress. Apparently, the established defects in chemokine- and S1P-induced Rac activation and chemotaxis of Tiam1-deficient T cells in vitro lead to less efficient homing and egress of these cells in vivo.
Tiam1 is required for contact hypersensitivity
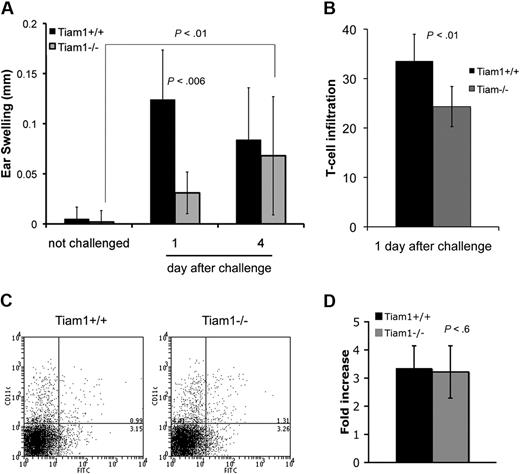
As migration of T cells is required not only for steady-state trafficking but also for reaching sites of inflammation during immune responses, we suspected a function of Tiam1 during T-cell–mediated immune responses. To test this hypothesis, we performed contact hypersensitivity experiments on WT and Tiam1−/− mice and compared ear swelling induced by FITC sensitization. As shown in Figure 4A, 1 day after challenge, Tiam1−/− mice showed significantly decreased ear swelling compared with WT mice (Figure 4A), which was accompanied by a reduced T-cell infiltration in the ear (Figure 4B). Interestingly, 4 days after challenge, whereas WT mice showed a decrease in ear swelling, Tiam1−/− mice mounted up a response, and showed also significant ear swelling (Figure 4A). This delay in contact hypersensitivity response suggests that T-cell recruitment, rather than activation, is affected by Tiam1 deficiency. Indeed, hapten uptake and recruitment of antigen-presenting cells to the draining LN was not impaired since the percentage of CD11c- and FITC-positive cells in the inguinal LN was identical between WT and Tiam1−/− mice 3 days after sensitization (Figure 4C). Furthermore, LN amplification after hapten sensitization was not impaired in Tiam1−/− mice (Figure 4D), arguing for a normal initiation of the immune response in those mice. Together, these data suggest that the ear swelling defect observed in Tiam1−/− mice is due to a reduced capacity of T cells to leave the LN and/or to reach the inflammation site as a result of impaired Tiam1-mediated Rac activation.
Tiam1 functions during contact hypersensitivity. (A) WT and Tiam1−/− mice were sensitized with 0.5% FITC solution. After 6 days, 10 μL 0.5% FITC solution or control solution was applied to both ears and the thickness of each ear was measured on days 1 and 4 after challenge. Values are means ± SD (n = 10); p indicates P value. (B) Infiltrating T cells in ears of WT and Tiam1−/− mice 1 day after FITC challenge. T-cell infiltrates are presented as the number of CD3+ T cells per unit surface area in the FITC-challenged ear. Values are means ± SD (n = 6). (C,D) WT and Tiam1−/− mice were sensitized with 0.5% FITC solution. On day 3, draining LNs were isolated and single cell suspensions were stained for CD11c and counted. (C) Representative FACS analysis showing Cd11c/FITC double-positive cells in draining LNs. (D) Increased LN cell number after hapten sensitization. Results are expressed as fold increase (LN cell number of sensitized mouse/LN cell number of control mouse). Values are means ± SD (n = 4).
Tiam1 functions during contact hypersensitivity. (A) WT and Tiam1−/− mice were sensitized with 0.5% FITC solution. After 6 days, 10 μL 0.5% FITC solution or control solution was applied to both ears and the thickness of each ear was measured on days 1 and 4 after challenge. Values are means ± SD (n = 10); p indicates P value. (B) Infiltrating T cells in ears of WT and Tiam1−/− mice 1 day after FITC challenge. T-cell infiltrates are presented as the number of CD3+ T cells per unit surface area in the FITC-challenged ear. Values are means ± SD (n = 6). (C,D) WT and Tiam1−/− mice were sensitized with 0.5% FITC solution. On day 3, draining LNs were isolated and single cell suspensions were stained for CD11c and counted. (C) Representative FACS analysis showing Cd11c/FITC double-positive cells in draining LNs. (D) Increased LN cell number after hapten sensitization. Results are expressed as fold increase (LN cell number of sensitized mouse/LN cell number of control mouse). Values are means ± SD (n = 4).
Tiam1 is optional for T-cell arrest but essential for T-cell migration
During processes of homing to pLNs and recruitment to inflammation site, T cells have to cross a natural barrier constituted by specialized endothelial cells. Chemokines are important in attracting T cells, but are mainly required during transendothelial migration for arrest, migration, and diapedesis.5,35 Therefore, we wondered in which chemokine-dependent steps necessary for transendothelial migration Tiam1 was required.
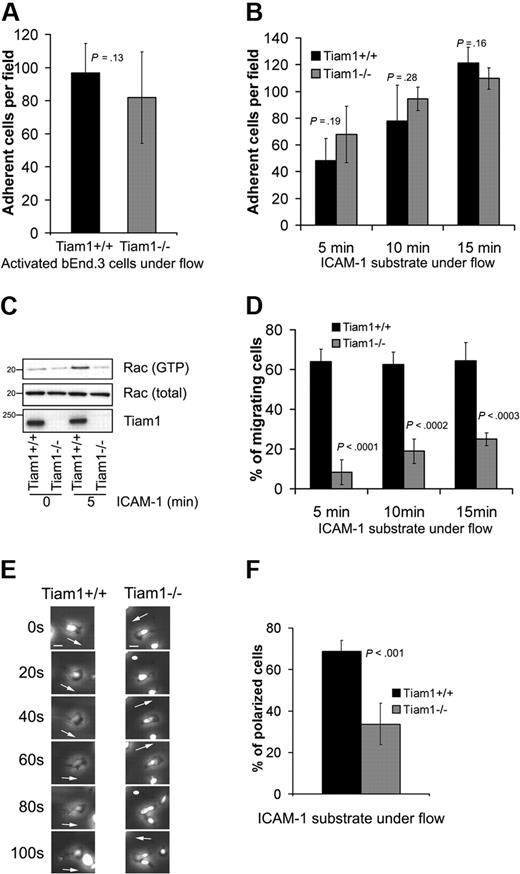
We first determined the ability of naive WT and Tiam1−/− T cells to arrest and adhere to a TNFα-activated endothelial monolayer under physiologic shear flow. No significant differences in adhesion capacity to the endothelial monolayer were found between WT and Tiam1−/− T cells (Figure 5A). We also analyzed the ability of activated T cells to adhere to an ICAM-1 substrate under shear flow condition in the presence of Ca2+/Mg2+. We could not detect significant differences between adhesion capacity of WT and Tiam1−/− T cells (Figure 5B). Intriguingly, although Tiam1−/− T cells adhered similarly as WT cells, they were unable to activate Rac upon adhesion to an ICAM-1 substrate (Figure 5C), suggesting that disturbed Tiam1-mediated Rac activation is not essential for T-cell arrest on ICAM-1 under shear flow condition.
Tiam1 is dispensable for T-cell arrest but essential for subsequent T-cell migration. (A) WT and Tiam1−/− T cells were subjected to shear flow for 15 minutes after an accumulation phase on activated bEnd.3 monolayer. The average number of adherent cells per field (×20 objective) at the end time point was determined from 4 independent experiments (2 fields per experiment). Error bars indicate SD; p, P value. No significant difference was observed between WT and Tiam1−/− T cells. (B) WT and Tiam1−/− T-cell blasts were subjected to shear flow on ICAM-1 substrate. At the indicated time points, average number of adherent cells per field (×10 objective) was calculated from 3 independent experiments (2 fields per experiment). Error bars indicate SD; p, P value between number of adherent WT and Tiam1−/− T cells for the same time point. No significant difference was observed. (C) Blasts from WT and Tiam1−/− mice were either nonstimulated or stimulated by adhesion to ICAM-1 for 5 minutes. Subsequently, Rac activity was determined. (Top panel) GTP-bound Rac. (Middle panel) Total Rac. (Bottom panel) Tiam1. (D) Locomotion of adherent WT and Tiam1−/− blasts on an ICAM-1 substrate under shear flow was recorded for 15 minutes, and the percentage of migrating cells was determined at the indicated time points. At least 3 independent experiments were performed and 2 fields for each time point per experiment were recorded. Values indicate mean plus or minus SD; p, P value between percentage of migrating WT and Tiam1−/− T cells, for each time point. (E) Representative confocal images of adherent WT and Tiam1−/− T-cell blasts on an ICAM-1 substrate under shear flow. Images are recorded every 20 seconds. Scale bar represents 5 μm. Arrows indicate the direction of polarization. Note the switches in direction of polarization of Tiam1−/− T cells. (F) Polarization of WT or Tiam1−/− T-cell blasts was monitored after 10 minutes on ICAM-1–coated coverslips under shear flow. At least 100 cells were monitored in each group. Two independent experiments were performed and 2 fields per condition were analyzed. Values indicate mean ± SD; p, P value between percentage of polarized WT and Tiam1−/− T cells.
Tiam1 is dispensable for T-cell arrest but essential for subsequent T-cell migration. (A) WT and Tiam1−/− T cells were subjected to shear flow for 15 minutes after an accumulation phase on activated bEnd.3 monolayer. The average number of adherent cells per field (×20 objective) at the end time point was determined from 4 independent experiments (2 fields per experiment). Error bars indicate SD; p, P value. No significant difference was observed between WT and Tiam1−/− T cells. (B) WT and Tiam1−/− T-cell blasts were subjected to shear flow on ICAM-1 substrate. At the indicated time points, average number of adherent cells per field (×10 objective) was calculated from 3 independent experiments (2 fields per experiment). Error bars indicate SD; p, P value between number of adherent WT and Tiam1−/− T cells for the same time point. No significant difference was observed. (C) Blasts from WT and Tiam1−/− mice were either nonstimulated or stimulated by adhesion to ICAM-1 for 5 minutes. Subsequently, Rac activity was determined. (Top panel) GTP-bound Rac. (Middle panel) Total Rac. (Bottom panel) Tiam1. (D) Locomotion of adherent WT and Tiam1−/− blasts on an ICAM-1 substrate under shear flow was recorded for 15 minutes, and the percentage of migrating cells was determined at the indicated time points. At least 3 independent experiments were performed and 2 fields for each time point per experiment were recorded. Values indicate mean plus or minus SD; p, P value between percentage of migrating WT and Tiam1−/− T cells, for each time point. (E) Representative confocal images of adherent WT and Tiam1−/− T-cell blasts on an ICAM-1 substrate under shear flow. Images are recorded every 20 seconds. Scale bar represents 5 μm. Arrows indicate the direction of polarization. Note the switches in direction of polarization of Tiam1−/− T cells. (F) Polarization of WT or Tiam1−/− T-cell blasts was monitored after 10 minutes on ICAM-1–coated coverslips under shear flow. At least 100 cells were monitored in each group. Two independent experiments were performed and 2 fields per condition were analyzed. Values indicate mean ± SD; p, P value between percentage of polarized WT and Tiam1−/− T cells.
To further understand what Tiam1-mediated Rac activation was responsible for, we analyzed the migratory capacity of WT and Tiam1−/− T cells under shear flow condition. Shortly after their arrest, most of the control WT T cells (> 65%) started to migrate on ICAM-1, whereas only a small portion of the Tiam1−/− T cells (∼ 20% after 15 minutes) were able to do so (Figure 5D). Time-lapse imaging revealed that after arrest most of the WT T cells became stably front-rear polarized (Figure 5E) and started to migrate in one direction (Video S1). This was confirmed by real-time localization of GFP-actin, showing stable lamellipodium formation at the leading edge of migrating WT cells (Figure S3A). In contrast, the polarization of Tiam1−/− T cells was much more transient and unstable. At the arrest site, Tiam1−/− T cells frequently changed their polarity and position of lamellipodium, as seen in phase-contrast images (Figure 5E) and images of GFP-actin–expressing cells (Figure S3A). This defect resulted in less efficient directional migration (Figure S3B, Video S2) and in a decrease in overall migration speed (Figure S3C). Consistent with this finding, the percentage of polarized cells on ICAM-1 after 15 minutes under shear flow conditions was 2 times higher in WT cells compared with Tiam1−/− T cells (Figure 5F).
Together, these data indicate that the impaired Rac activation observed in Tiam1−/− T cells upon adhesion is required for stable polarization and subsequent efficient migration rather than adhesion.
Tiam1 and PKCζ activity are essential for crawling during T-cell transendothelial migration
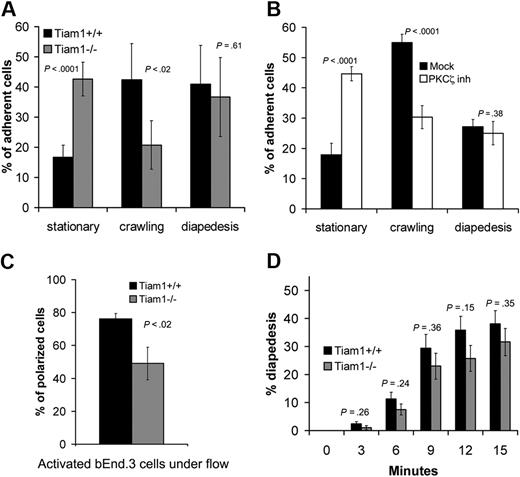
To further investigate the function of Tiam1 during transendothelial migration, we used an approach allowing us to study diapedesis independently from crawling.26 The behavior of WT and Tiam1−/− T-cell blasts on TNFα-activated endothelial monolayers was analyzed under shear flow condition. Approximately 16% of the adherent WT T cells remained nonmotile (stationary), 42% of the cells migrated on top of the monolayer without transmigrating (crawling), whereas 40% of the cells succeeded in transmigration (diapedesis; Figure 6A). In contrast, only a small proportion (20%) of Tiam1−/− T cells were able to crawl on top of endothelial monolayers, consistent with the finding that Tiam1 is required forefficient migration on ICAM-1 (Figure 5D). As a consequence, we found an increased percentage (42%) of Tiam1−/− T cells in the stationary phase. Tiam1−/− T cells showed no obvious difference in diapedesis capacity compared with WT cells (Figure 6A). Similar results were found with naive T cells (Figure S4), indicating that Tiam1 deficiency affected both naive and activated T cells.
Tiam1 is required for T-cell crawling. T-cell blasts were subjected to shear flow for 15 minutes after accumulation on activated bEnd.3 monolayer. Four independent experiments were performed and 2 fields per condition for every experiment were recorded. (A,B) The behavior of single adherent T-cell blasts was analyzed and classified as stationary, crawling (without transendothelial migration), or transmigrating (diapedesis condition). Values indicate mean ± SD. (A) Analysis of WT and Tiam1−/− T cells; p indicates P value between the percentage of WT and Tiam1−/− T cells. (B) Analysis of WT T cells treated with or without a PKCζ inhibitor (2 μM); p indicates P value between percentage of treated and nontreated WT T cells. (C) Polarization of WT or Tiam1−/− T-cell blasts was monitored after 15 minutes. At least 50 cells were monitored in each group. Values indicate mean ± SD; p, P value between percentage of polarized WT and Tiam1−/− T cells. (D) The percentage of WT and Tiam1−/− T cells undergoing diapedesis was analyzed over time (every 3 minutes). Values indicate mean ± SD; p, P value between percentage of WT and Tiam1−/− T cells undergoing diapedesis at the same time point. No significant difference in diapedesis was observed between WT and Tiam1−/− T cells.
Tiam1 is required for T-cell crawling. T-cell blasts were subjected to shear flow for 15 minutes after accumulation on activated bEnd.3 monolayer. Four independent experiments were performed and 2 fields per condition for every experiment were recorded. (A,B) The behavior of single adherent T-cell blasts was analyzed and classified as stationary, crawling (without transendothelial migration), or transmigrating (diapedesis condition). Values indicate mean ± SD. (A) Analysis of WT and Tiam1−/− T cells; p indicates P value between the percentage of WT and Tiam1−/− T cells. (B) Analysis of WT T cells treated with or without a PKCζ inhibitor (2 μM); p indicates P value between percentage of treated and nontreated WT T cells. (C) Polarization of WT or Tiam1−/− T-cell blasts was monitored after 15 minutes. At least 50 cells were monitored in each group. Values indicate mean ± SD; p, P value between percentage of polarized WT and Tiam1−/− T cells. (D) The percentage of WT and Tiam1−/− T cells undergoing diapedesis was analyzed over time (every 3 minutes). Values indicate mean ± SD; p, P value between percentage of WT and Tiam1−/− T cells undergoing diapedesis at the same time point. No significant difference in diapedesis was observed between WT and Tiam1−/− T cells.
As PKCζ, a component of the Par polarity complex, acts upstream of Tiam1 and Rac1 (Figure 2), we also inhibited PKCζ signaling in WT cells. Similarly as found for Tiam1−/− cells, WT T cells treated with the PKCζ inhibitor showed impaired crawling on the endothelial monolayer but showed normal transmigration (Figure 6B), suggesting that Tiam1 and PKCζ are acting in the same signaling pathway to control T-cell polarization and crawling on endothelial monolayers. Indeed, we found also reduced percentage of polarized Tiam1−/− cells compared with WT cells on top of the endothelial monolayer (Figure 6C). Although the percentage of diapedesis over time was slightly higher for WT T cells, we could not find significant differences between WT and Tiam1−/− T cells (Figure 6D). Moreover, the average time for one single cell to cross the endothelial monolayer was not different between the 2 genotypes (Figure S5). Together, we conclude that Tiam1 and PKCζ are specifically required for stable polarization and crawling of T cells on top of an endothelial monolayer.
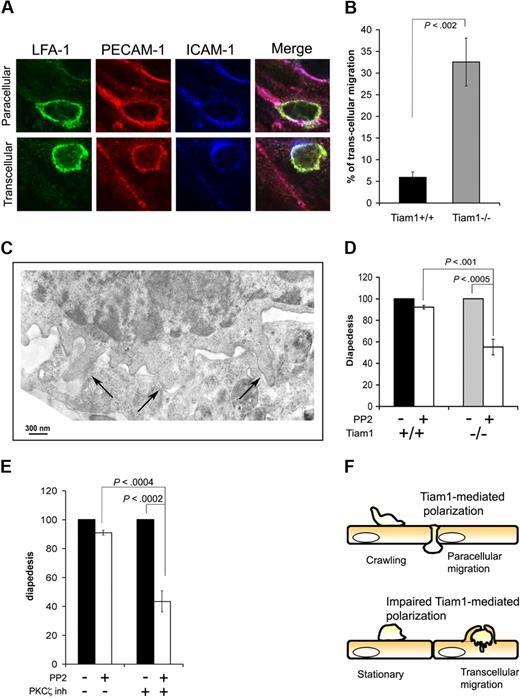
Inhibition of PKCζ/Tiam1 signaling leads to a switch from paracellular to transcellular T-cell diapedesis
Leukocytes can use 2 different routes to cross the endothelial barrier (ie, the paracellular and the transcellular route).5,36 Paracellular migration is the most frequently route used for transendothelial migration, and implies that leukocytes transmigrate between endothelial cells at cell junctions. When using the transcellular route, leukocytes cross the endothelial barrier by transmigrating directly through individual endothelial cells. As we found no major differences in percentage of WT and Tiam1−/− T cells undergoing diapedesis, we investigated whether disturbed PKCζ/Tiam1/Rac signaling affected the route of transendothelial migration. By fixed-cell imaging, we blindly quantified the percentage of cells transmigrating through the endothelial cytoplasm or at endothelial junctions. Endothelial cell junctions and the transmigration pore were visualized by PECAM-1 and ICAM-1 staining, whereas T cells were visualized by LFA-1 staining as depicted in Figure 7A. Using this method of quantification, most of WT T cells used the paracellular route (Figure 7B). Intriguingly, more than 30% of the Tiam1−/− T cells crossed the endothelial monolayer via the transcellular route (Figure 7B), suggesting that impaired Tiam1-dependent polarization caused this shift in transmigration route. Recent studies have demonstrated that transcellular, but not paracellular, migration is dependent on Src-mediated formation of invasive podosomes on T cells.7 Indeed, electron microscopic studies revealed the presence of podosomes particularly in Tiam1−/− cells before diapedesis (Figure 7C), suggesting that transcellular migration in Tiam1−/− T cells is dependent on podosome formation. We treated therefore T cells with the Src inhibitor PP2 and quantified the percentage of adherent T cells undergoing diapedesis under shear flow. Treatment of WT T cells with the Src inhibitor PP2 only slightly decreased the percentage of transmigrating cells (Figure 7D), consistent with the low percentage of WT cells (∼ 5%) using the transcellular route. In contrast, diapedesis was approximately 40% reduced in Src-inhibited Tiam1−/− T cells, consistent with the higher percentage of Tiam1−/− cells using the transcellular route. These results further support the conclusion drawn from visual analysis (Figure 7B) that transcellular migration is enhanced in Tiam1−/− T cells.
Impaired PKCζ/Tiam1/Rac signaling induces a switch from paracellular to transcellular migration. (A,B) Activated WT and Tiam1−/− T cells were incubated on top of a monolayer of TNFα-activated bEnd.3 cells overlaid with SDF1α for 30 minutes. Fixed samples were subjected to LFA-1, ICAM-1, and PECAM-1 staining. (A) Representative examples of immunofluorescence images showing paracellular and transcellular migration of a T cell. Scale bars represent 5 μm. (B) Percentage of WT and Tiam1−/− T cells showing diapedesis via the transcellular route. Three independent experiments were performed. Values are means ± SD; p indicates P value between WT and Tiam1−/− T cells. (C) Tiam1−/− T cells migrating on activated bEnd.3 were fixed after 30 minutes and processed for transmission electron microscopy (EM). Image shows typical podosome structures invading the endothelial cell (→). (D,E) Naive T cells were either untreated or treated 2 μm PP2for 1 hour. After accumulation on activated bEnd.3 monolayer, T cells were subjected to shear flow for 15 minutes. Percentages of adherent cells undergoing diapedesis during this period were evaluated. Diapedesis of PP2-treated T cells was expressed as a percentage of control cells (untreated cells = 100%). Two fields for each condition were recorded in 4 independent experiments. Values indicate mean ± SD. (D) Comparison of diapedesis route used by WT and Tiam1−/− T cells upon PP2 treatment; p indicates P value between percentage WT and Tiam1−/− T cells undergoing diapedesis. (E) WT T cells were untreated or treated for 1 hour at 37°C with a PKCζ inhibitor (2 μM) before shear flow experiments; p indicates P value between percentage of untreated WT cells and PKCζ inhibitor–treated WT cells undergoing diapedesis. (F) Model illustrating that crawling and the route of transendothelial migration depends on PKCζ/Tiam1-dependent polarization and crawling. Tiam1 is dispensable for T-cell adhesion to endothelial monolayers but is required for polarization and subsequent T-cell crawling on top of endothelial monolayers. T cells with intact PKCζ/Tiam1 signaling preferentially transmigrate through endothelial junctions (paracellular migration). Loss of Tiam1 and disturbed PKCζ signaling in T cells prevents chemokine-induced Rac activation leading to unstable polarization and thereby impaired T-cell crawling on top of endothelial monolayers. As a result, these T cells preferentially cross the endothelial monolayer through individual endothelial cells (transcellular migration).
Impaired PKCζ/Tiam1/Rac signaling induces a switch from paracellular to transcellular migration. (A,B) Activated WT and Tiam1−/− T cells were incubated on top of a monolayer of TNFα-activated bEnd.3 cells overlaid with SDF1α for 30 minutes. Fixed samples were subjected to LFA-1, ICAM-1, and PECAM-1 staining. (A) Representative examples of immunofluorescence images showing paracellular and transcellular migration of a T cell. Scale bars represent 5 μm. (B) Percentage of WT and Tiam1−/− T cells showing diapedesis via the transcellular route. Three independent experiments were performed. Values are means ± SD; p indicates P value between WT and Tiam1−/− T cells. (C) Tiam1−/− T cells migrating on activated bEnd.3 were fixed after 30 minutes and processed for transmission electron microscopy (EM). Image shows typical podosome structures invading the endothelial cell (→). (D,E) Naive T cells were either untreated or treated 2 μm PP2for 1 hour. After accumulation on activated bEnd.3 monolayer, T cells were subjected to shear flow for 15 minutes. Percentages of adherent cells undergoing diapedesis during this period were evaluated. Diapedesis of PP2-treated T cells was expressed as a percentage of control cells (untreated cells = 100%). Two fields for each condition were recorded in 4 independent experiments. Values indicate mean ± SD. (D) Comparison of diapedesis route used by WT and Tiam1−/− T cells upon PP2 treatment; p indicates P value between percentage WT and Tiam1−/− T cells undergoing diapedesis. (E) WT T cells were untreated or treated for 1 hour at 37°C with a PKCζ inhibitor (2 μM) before shear flow experiments; p indicates P value between percentage of untreated WT cells and PKCζ inhibitor–treated WT cells undergoing diapedesis. (F) Model illustrating that crawling and the route of transendothelial migration depends on PKCζ/Tiam1-dependent polarization and crawling. Tiam1 is dispensable for T-cell adhesion to endothelial monolayers but is required for polarization and subsequent T-cell crawling on top of endothelial monolayers. T cells with intact PKCζ/Tiam1 signaling preferentially transmigrate through endothelial junctions (paracellular migration). Loss of Tiam1 and disturbed PKCζ signaling in T cells prevents chemokine-induced Rac activation leading to unstable polarization and thereby impaired T-cell crawling on top of endothelial monolayers. As a result, these T cells preferentially cross the endothelial monolayer through individual endothelial cells (transcellular migration).
Similarly as the effects of PKCζ inhibition on stabilization of polarization and T-cell crawling, inhibition of PKCζ also led to a strong reduction in diapedesis upon Src inhibition in WT but not in Tiam1−/− T cells (Figure 7E). This indicates that upon PKCζ inhibition WT cells preferentially use the transcellular route during diapedesis similarly as Tiam1-deficient T cells. Together we conclude that T cells with impaired PKCζ/Tiam1/Rac signaling show unstable polarization and impaired crawling, and shift from paracellular to transcellular migration during the process of transendothelial migration (Figure 7F).
Discussion
Trafficking of immune cells relies on their capacity to migrate toward a chemotactic gradient, or in other terms, to undergo chemotaxis.37 Here, we found that the Rac activator Tiam1 in conjunction with PKCζ are required for efficient chemokine- and S1P-induced Rac activation and migration and thereby are essential for efficient T-cell trafficking. Indeed, Tiam1-deficient T cells show impaired homing and egress while the recruitment to sites of inflammation is strongly delayed. During T-cell trafficking, Tiam1-mediated Rac activation is not required for T-cell arrest but is essential for stable polarization and crawling of T cells on top of endothelial monolayers. Intriguingly, a large percentage of T cells with impaired PKCζ/Tiam1/Rac signaling switch from paracellular to transcellular migration (Figure 7F), suggesting that stable polarization is a prerequisite for transmigration at endothelial junctions.
We and others have shown that Tiam1 associates with Par3 of the Par polarity complex (consisting of Par3, Par6, and atypical PKCζ) and thereby regulates various polarization processes such as axon specification in neuronal cells,38 epithelial apical-basal cell polarization,28,39,40 and front-rear polarization of keratinocytes and T cells.30,41 Here we found that T cells lacking Tiam1 or having impaired PKCζ signaling show short-lived polarization whereby cells frequently change their direction of polarity, preventing efficient migration on ICAM1 or endothelial monolayers. These data strongly suggest that Tiam1 and the Par complex regulate the maintenance and stability of polarization in T cells. As a consequence of the polarization and migration defects, Tiam1-deficient T cells switch the route of transendothelial migration and show a delay in homing, egress, and recruitment to inflammatory sites.
It is likely that other Rac activators contribute to the different steps of T-cell trafficking. In vitro studies indicate that Dock2 is required for crawling endothelial monolayers but is not essential for T-cell arrest and diapedesis.26 The striking similarities between the function of Dock2 and Tiam1 suggest that they act in the same signaling pathway. However, Tiam1 activation is controlled by Rap1 activity independently of Dock2,30 whereas Dock2 deletion does not influence chemokine-induced Rap activation.19 This suggests that Tiam1 and Dock2 initiate 2 separate signaling pathways that possibly converge and that are both essential for T-cell migration.42 The residual migration seen in both Dock2−/− and Tiam1−/− T cells could be controlled by the redundant function of these different Rac activators.
Tiam1 deficiency did not affect the adhesion of T cells under shear flow. This is consistent with the findings that PKCζ is not involved in LFA-1 high-affinity state and T-cell arrest on HEVs.43 Apparently PKCζ/Tiam1 signaling is dispensable for T-cell adhesion, suggesting that other Rac activators may control integrin-mediated adhesion of T cells.44 Likely candidates are Rac activators of the Vav family. Vav1 activates Rac upon LFA-1 stimulation45 and has been implicated in T-cell adhesion mediated by the integrin α4β1.16 Together these data suggest that different activators of Rac have specific functions in the various steps of T-cell trafficking.
Crawling of leukocytes seems to be important for seeking appropriate sites for transendothelial migration. Mac1-deficient neutrophils are impaired in crawling and show delayed transmigration across HEVs, preferentially through the transcellular pathway.46 These observations are consistent with our conclusions that delayed homing, impaired ear swelling, and the switch to transcellular migration are the result of the inability of Tiam1-deficient cells to respond properly to chemokine stimuli required for stable polarization and crawling.
Diapedesis is a critical component of T-cell trafficking necessary for immune function. For transcellular migration, ICAM1, PECAM1, caveolae, and vimentin are required on the endothelial cells as well as Src-dependent formation of invasive podosomes on the leukocytes.7,9,10,47 The type of vascular bed may determine the conditions and the type of leukocytes that use transcellular migration.7 Transcellular migration has been observed in the microvasculature, notably at the blood-brain barrier11 and at HEVs.11,48 Electron microscopic studies revealed the formation of podosomes in Tiam1−/− T cells in vitro before diapedesis. These data are consistent with the recently observed requirement of podosome formation for transcellular migration. Podosome formation is dependent on Src7 and indeed inhibition of Src strongly reduced diapedesis of Tiam1−/− but not WT T cells, indicating Tiam1−/− T cells preferentially use the transcellular route of diapedesis. The shift from paracellular to transcellular migration likely contributes to the delayed homing and egress observed in Tiam1-deficient T cells. We cannot rule out the possibility that Tiam1−/− T cells are more predisposed to make podosomes than WT cells while contacting endothelial cells, thereby enhancing transcellular migration. However, it is more conceivable that the reduced polarization and crawling capacity of Tiam1-deficient cells makes it more difficult to find suitable transmigration sites at endothelial junctions, as suggested earlier.46 In that scenario Tiam1-deficient T cells could specifically initiate alternatives by the formation of podosomes allowing their transmigration through individual endothelial cells.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank coworkers from The Netherlands Cancer Institute (NKI) mouse facility for their assistance with animal experiments, and P. Hordijk and F. van Alphen (Sanquin, Amsterdam, The Netherlands) for their help with the shear flow experiments. Furthermore, we thank S. Iden and S. Mertens for critical advice on the paper.
This work is supported by grants from the Dutch Cancer Society (Amsterdam, The Netherlands; J.G.C.).
Authorship
Contribution: A.G. conceptualized the study, performed all experiments (unless otherwise specified), analyzed the data, drew the conclusions, and wrote the article; R.A.v.d.K. helped with mice breeding and contact hypersensitivity experiments; H.J. performed the EM experiments; S.I.E. quantified the T-cell migration data from the videos; and J.G.C. supervised the work and contributed to the writing of this article.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: John G. Collard, The Netherlands Cancer Institute, Division of Cell Biology, Plesmanlaan 121, 1066 CX Amsterdam, The Netherlands; e-mail: j.collard@nki.nl.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal