Abstract
Alpha hemoglobin stabilizing protein (AHSP) reversibly binds nascent α globin to maintain its native structure and facilitate its incorporation into hemoglobin A. Previous studies indicate that some naturally occurring human α globin mutations may destabilize the protein by inhibiting its interactions with AHSP. However, these mutations could also affect hemoglobin A production through AHSP-independent effects, including reduced binding to β globin. We analyzed 6 human α globin variants with altered AHSP contact surfaces. Alpha globin amino acid substitutions H103Y, H103R, F117S, and P119S impaired interactions with both AHSP and β globin. These mutations are destabilizing in biochemical assays and are associated with microcytosis and anemia in humans. By contrast, K99E and K99N α globins bind β globin normally but exhibit attenuated binding to AHSP. These mutations impair protein folding and expression in vitro and appear to be mildly destabilizing in vivo. In Escherichia coli and erythroid cells, α globin K99E stability is rescued on coexpression with AHSP mutants in which binding to the abnormal globin chain is restored. Our results better define the biochemical properties of some α globin variants and support the hypothesis that AHSP promotes α globin chain stability during human erythropoiesis.
Introduction
Hemoglobin A (HbA), the adult blood oxygen carrier, is a heterotetramer composed of α and β globin proteins, each bound to a heme prosthetic group. Mutations in globin genes cause numerous blood disorders by affecting the production, stability, and/or functional properties of Hb tetramers. Thus, missense mutations in α globin or β globin genes (also designated HBA and HBB, respectively) can alter oxygen affinity to perturb its transport or increase the susceptibility of HbA to oxidation. Missense mutations that inhibit globin folding, interactions with heme or subunit assembly through either of 2 interaction interfaces, termed α1β1 or α1β2, also render the proteins more susceptible to denaturation and proteolytic degradation.
The discovery of α hemoglobin stabilizing protein (AHSP) potentially provides new insights into certain human anemias associated with HbA instability. AHSP is an erythroid protein that binds free α globin but not β globin or HbA.1 In mice, AHSP enhances the stability of free α globin and augments its incorporation into HbA.1-4 AHSP null murine erythrocytes contain globin precipitates and exhibit decreased life spans. In human K562 erythroleukemia cells, AHSP deficiency induces α globin precipitation and apoptosis.5
The role of AHSP in human erythropoiesis and disease is not clear because naturally occurring gene mutations are only beginning to be studied.6,7 It is also possible that some α globin gene mutations destabilize the corresponding protein by inhibiting its interaction with AHSP. Some α globin variants contain amino acid substitutions at the AHSP-binding interface, which has been mapped by structural studies (see Globin Gene Server, http://globin.cse.psu.edu).8,9 Several reports describe clinically significant α globin mutations that inhibit α globin-AHSP interactions, including antiterminator mutations Hb Pakse and Hb Constant Springs10,11 and missense mutations F117S (Hb Foggia)12 and P119S (Hb Groene-Hart).13 These studies suggest new mechanisms for hemoglobinopathies by identifying α globin variants that may not be stabilized by AHSP in vivo. These mutations might directly destabilize α globin by partially or fully abrogating its interactions with AHSP or they could produce deleterious effects through several AHSP-independent mechanisms. First, AHSP-α globin interactions may be qualitatively different and destabilize the native α globin fold. Second, because AHSP interacts with α G and H helical surfaces that overlap the heterodimer α1β1 interface, α globin mutations may disrupt both AHSP and β globin binding. Third, α globin mutations may destabilize the protein through mechanisms that inhibit proper protein folding. Thus, additional studies are required to understand the biochemical consequences of α globin mutations that are predicted to affect interactions with AHSP.
We studied 6 known α globin variants with amino acid substitutions at the AHSP-binding interface and 2 control mutations that are not expected to alter AHSP interactions. We examined these mutant globins for binding to AHSP, stabilization by AHSP during expression in vitro and in Escherichia coli, and direct interaction with β globin. Only mutations at position K99 affect AHSP interaction exclusively. These K99 mutations destabilize α globin during expression, consistent with prior studies that demonstrate a role for AHSP in Hb production.1-3 Four other mutations involving amino acid positions H103, F117, and P119 inhibit binding to both β globin and AHSP, suggesting that destabilization occurs through multiple effects. Our results better define the role for AHSP in human erythropoiesis and illustrate new ways by which α globin gene missense mutations can affect Hb assembly and stability.
Methods
Proteins
Recombinant AHSP protein was synthesized and purified as described.14 HbA was purified from human erythrocytes and separated into holo α and β globin chains as described by Bucci et al.14,15 Holo α and β globin chains purified from human erythrocytes contain the full complement of heme and were either saturated with oxygen before use or converted to the more stable cyanomet form. Alpha globin and AHSP cDNAs encoding variant proteins were generated by polymerase chain reaction (PCR) mutagenesis according to standard methods. The analysis of protein structures is described in supplemental methods (available on the Blood website; see the Supplemental Materials link at the top of the online article).
In vitro TNT to produce apo and holo α globins
Radiolabeled α globin was generated in a 25-, 50-, or 100-μL reaction volume (depending on the experiment) using the In Vitro Transcription and Translation (TNT) wheat germ kit (Promega, Madison, WI) according to the manufacturer's instructions. The TNT reaction was performed in the presence of 35S-methionine and 35S-cysteine (PerkinElmer Life and Analytical Sciences, Waltham, MA, concentration 14.3 mCi/mL, specific activity > 1000 Ci/mmol). CN-hemin was added (0.5 μM) as a stable monomeric form of oxidized hemin16,17 to promote the production of holo globins. Hemin was not included in the TNT reactions used to prepare α globins for the limited proteolysis assay (Figure 4). A stock hemin (Sigma-Aldrich, St Louis, MO) solution (5 mM) was prepared by dissolving the powder in 1 M NaOH, adjusting the pH to 7.0 with 1 M HCl. Precipitates were removed by centrifugation. These were minimal if the solution was used immediately before large aggregates could form.17 The CN-hemin stock solution contained 50 μM hemin and 1% potassium cyanide.
GST pull-down
Radiolabeled wild-type (WT) and mutant α globins were generated by TNT in a 100-μL reaction, and the volume was expanded to 200 μL by addition of 50 mM Tris (pH 7), 150 mM NaCl. A total of 5 μL was withdrawn and analyzed as INPUT. The remaining reaction was incubated with 20 μL glutathione-Sepharose (GST) 4B beads (GE Healthcare, Little Chalfont, United Kingdom; 50% slurry) that were previously preincubated with either GST or GST-fused AHSP proteins and washed. The mixture was rotated overnight at 4°C and then washed 3 times with 50 mM Tris (pH 7), 150 mM NaCl. The beads were boiled with 2× sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) loading buffer and the supernatants fractionated by SDS-PAGE. Radiolabeled protein was visualized by autoradiography.
Monomer shift assay/IEF
This assay assesses formation of hemoglobin by measuring the ability of purified native holo-β globin to alter the mobility of 35S-labeled WT and mutant α globins on isoelectric focusing (IEF) gels. Approximately 3 ng of 35S-labeled α globin containing bound CN-hemin was generated in each TNT reaction (∼7.5 nM in a 25-μL reaction).18,19 The reaction was incubated with 20 nM unlabeled purified human oxy-β globin for 10 minutes at room temperature and then loaded onto precast agarose gels (FR9120 Hemoglobin Test Kit; pH range, 6-8; PerkinElmer Life and Analytical Sciences). Electrophoresis was performed on an LKB 2117 Multiphor at 1500 V for 65 minutes. Labeled proteins were visualized by autoradiography, and unlabeled proteins were visualized by Coomassie blue staining. IEF markers (pH range, 3-10) were obtained from Invitrogen (Carlsbad, CA).
Limited proteolysis
Coexpression of α globin with AHSP or β globin in E coli
This assay is based on the principle that in E coli newly synthesized α globin chains are stabilized by interaction with either β globin or AHSP.13,21,22 We used vector pETDuet-1 (Novagen, Madison, WI) to coexpress 2 proteins in E coli BL21-DE3 cells.23 Bacterial cultures grown overnight were diluted 1:20 with fresh Luria Broth (LB), cultured for 2 hours at 37°C, and then globin expression was induced by addition of 0.5 M isopropyl β-D-1-thiogalactopyranoside (IPTG). Hemin (5 μM) was added to facilitate hemoglobin synthesis. The cells were incubated for 10 hours at 30°C and then lysed in BC500 buffer (20 mM Tris, pH 8, 500 mM KCl, 20% glycerol, 1% Igepal, 0.5 M ethylenediaminetetraacetic acid) and centrifuged at 10 000g to remove insoluble material. A total of 1 μg of supernatant was fractionated on 15% SDS-PAGE gels and analyzed by Western blotting.
Western blotting
Western blotting was performed using Transblot membrane (Bio-Rad, Hercules, CA). Bound antibody was detected using Supersignal West Pico (Pierce Chemical, Rockford, IL). Anti–human AHSP antiserum was prepared by injecting purified full-length recombinant protein into rabbits and used for Western blotting at a dilution of 1:1000. Anti–human α globin antiserum was generated by injecting purified carbon monoxide–treated protein into rabbits and used for Western blotting at a dilution of 1:200. Rabbit anti–human HbA antibody was purchased from ICN Pharmaceuticals (Costa Mesa, CA) and used at a dilution of 1:500.
Coexpression of α globin K99E and AHSP in MEL cells
pcDNA3 plasmid (Invitrogen) encoding α globin K99E was transfected into murine erythroleukemia (MEL) cells using Lipofectamine LTX and Plus reagent (Invitrogen). Cells were selected for stable integration of the vector by culturing in G418 (3 mg/mL) for 2 weeks. Then the G418-resistant cells were transduced with MIGR1 retrovirus24 encoding WT or mutant AHSP cDNAs linked to a puromycin resistance cassette and selected in puromycin (0.5 μg/mL) for an additional 3 weeks. Cells were lysed by incubating for 30 minutes at 4°C in 50 mM Tris pH 7, 450 mM NaCl, 0.5% Igepal, clarified by centrifugation, and soluble protein in the supernatant was analyzed by Western blotting.
Reverse-transcribed PCR to detect ectopically expressed human α globin in MEL cells
RNA was isolated using Trizol (Invitrogen). Complementary DNA synthesis was performed using the Superscript II First Strand kit (Invitrogen). Human α globin cDNA primers: forward: 5′GCTGGAATTC CTGGTCCCCACAGA3′; reverse: 5′TCGAGCGGCCGCACTCAGACTTTA3′; murine Gapdh cDNA primers: forward: 5′AGGTTGTCTCCTGCGACTTCA3′, reverse: 5′CCAGGAAATGAGCTTGACAAA. The conditions for PCR were: 95°C, 3 minutes; 95°C, 1 minute; 58°C, 30 seconds; 72°C, 1 minute/25 cycles; 72°C, 5 minutes. The PCR productions were fractionated on a 1% agarose gel.
Results
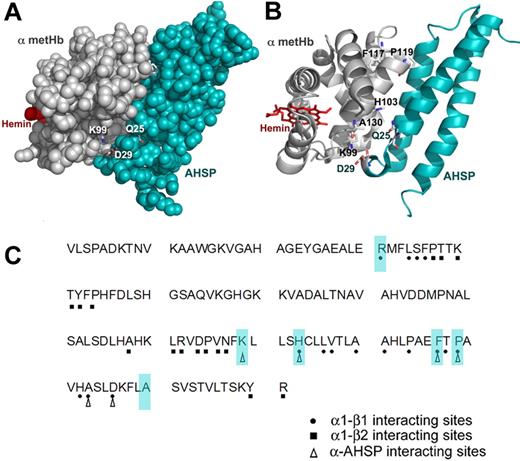
The α globin mutations studied here are summarized in Table 1.8,12,25-35 We used the Globin Gene Server, published case reports, and crystallographic studies to identify naturally occurring α globin gene mutations that alter amino acids at AHSP contact sites (Figure 1A).8 Some of these mutations occur at the α1β1 interface and may also affect β globin binding (Table 1; Figure 1)8 (and www.rcsb.org). As controls, we analyzed the α globin substitution A130D, which is outside of the binding interfaces for both β globin and AHSP, and R31S, which is localized at the α1β1 interface but not at the AHSP-α globin interface.
Clinical and biochemical features of naturally occurring α globin missense mutations analyzed in this study
| Mutation . | References . | Name . | Clinical features . | Predicted protein contacts . | ||||
|---|---|---|---|---|---|---|---|---|
| Mutated allele . | Percentage variant measured* . | Anemia . | Other features . | AHSP . | β globin (α1β1) . | |||
| R31S | 26-28 | Prato | α2 | 15-19 | Mild | Mild anisocytosis and hypochromia; variant Hb tetramer is mildly unstable in isopropanol | No | Yes |
| K99E | 25,41 | Turriff | α1, (α2)† | 10.5, 22 | No | Variant Hb tetramer comigrates with HbA1c; nascent chain is unstable in biosynthetic labeling studies (<5% total α globin) | Yes | No |
| K99N | 29 | Beziers | α1 | 15.8 | No | Comigrates with HbA1c | Yes | No |
| H103Y | 30 | Lombard | α2 | 8.4 | Mild | Yes | Yes | |
| H103R | 31 | Contaldo | ND | 20.4 | Moderate‡ | Microcytosis and hypochromia; Heinz bodies present α/β synthetic ratio 0.7, indicating possible concomitant α thalassemia in proband | Yes | Yes |
| F117S | 12 | Foggia | α2 | 0 | No | Mild microcytosis | Yes | Yes |
| P119S | 32-34 | Groene-Hart | α1 | 0 | Mild§ | Variant protein undetectable in hemolysate; microcytosis and hypochromia | Yes | Yes |
| A130D | 35 | Yuda | ND | 30 | None | Low oxygen affinity Hb | No | No |
| Mutation . | References . | Name . | Clinical features . | Predicted protein contacts . | ||||
|---|---|---|---|---|---|---|---|---|
| Mutated allele . | Percentage variant measured* . | Anemia . | Other features . | AHSP . | β globin (α1β1) . | |||
| R31S | 26-28 | Prato | α2 | 15-19 | Mild | Mild anisocytosis and hypochromia; variant Hb tetramer is mildly unstable in isopropanol | No | Yes |
| K99E | 25,41 | Turriff | α1, (α2)† | 10.5, 22 | No | Variant Hb tetramer comigrates with HbA1c; nascent chain is unstable in biosynthetic labeling studies (<5% total α globin) | Yes | No |
| K99N | 29 | Beziers | α1 | 15.8 | No | Comigrates with HbA1c | Yes | No |
| H103Y | 30 | Lombard | α2 | 8.4 | Mild | Yes | Yes | |
| H103R | 31 | Contaldo | ND | 20.4 | Moderate‡ | Microcytosis and hypochromia; Heinz bodies present α/β synthetic ratio 0.7, indicating possible concomitant α thalassemia in proband | Yes | Yes |
| F117S | 12 | Foggia | α2 | 0 | No | Mild microcytosis | Yes | Yes |
| P119S | 32-34 | Groene-Hart | α1 | 0 | Mild§ | Variant protein undetectable in hemolysate; microcytosis and hypochromia | Yes | Yes |
| A130D | 35 | Yuda | ND | 30 | None | Low oxygen affinity Hb | No | No |
Unless otherwise noted, all mutations were described in heterozygotes. R31S and A130D were used in this study as controls. Predicted protein contacts are derived from previously published structural data8 (and www.rcsb.org).
ND indicates not determined.
Values represent the percentage variant Hb tetramer (ααxββ) measured in circulation. For variants with normal production and protein stability, expected values are 44% to 46% for heterozygous α2 encoded alleles and 24% to 28% for heterozygous α1-encoded alleles (supplemental data). Values below these normal ranges suggest protein instability.
This mutation was presumed to occur in the α2 allele, given its relatively high expression.
Coexisting α thal-1 deletion.
Associated with variable mild anemia in the simple heterozygous state. Homozygous state associated with mild anemia. Heterozygosity appears to contribute to anemia when combined with deletional α thalassemia.
The α globin-AHSP and α1β1 interfaces. The structure was taken from Feng et al9 (PDB 1z8u), and the drawings were made in PyMol. (A) Space-filling model of the α globin-AHSP interaction with α globin depicted in silver and AHSP in teal. The key α globin residues K99, AHSP D29, and AHSP Q25 are shown as sticks with Corey-Pauling-Koltun (CPK) coloring. (B) Ribbon drawing of the α globin-AHSP interface. The amino acids that were mutated are depicted as sticks with CPK coloring. R31 is not shown because it is on the contralateral faces of the diagrams in panels A and B. The α globin residues under investigation here, except R31 and A130, all contact AHSP. (C) The primary amino acid sequence of human α globin. The triangles show predicted AHSP-binding sites. The circles show residues that come into close contact with β globin at the α1β1 interface. The squares show β globin contact sites at the α1β2 interface. Amino acids examined by mutational analysis in this study are shaded.
The α globin-AHSP and α1β1 interfaces. The structure was taken from Feng et al9 (PDB 1z8u), and the drawings were made in PyMol. (A) Space-filling model of the α globin-AHSP interaction with α globin depicted in silver and AHSP in teal. The key α globin residues K99, AHSP D29, and AHSP Q25 are shown as sticks with Corey-Pauling-Koltun (CPK) coloring. (B) Ribbon drawing of the α globin-AHSP interface. The amino acids that were mutated are depicted as sticks with CPK coloring. R31 is not shown because it is on the contralateral faces of the diagrams in panels A and B. The α globin residues under investigation here, except R31 and A130, all contact AHSP. (C) The primary amino acid sequence of human α globin. The triangles show predicted AHSP-binding sites. The circles show residues that come into close contact with β globin at the α1β1 interface. The squares show β globin contact sites at the α1β2 interface. Amino acids examined by mutational analysis in this study are shaded.
Two functional α globin alleles, termed α2 and α1, exist in tandem on the short arm of human chromosome 16. In general, most simple heterozygous α globin missense mutations are clinically insignificant because only one of 4 alleles is altered. Such mutations probably cause anemia when present at homozygosity or in the context of other α globin mutations, most commonly deletional forms of α thalassemia (Table 1). The clinical and biochemical phenotypes depend on the affected allele because α2 is approximately 2-fold more active than α1 (Table 1).36,37
Selected α globin mutations impair binding to AHSP
Transcription coupled translation (TNT) was used to synthesize radiolabeled α chains and then assessed their ability to bind recombinant AHSP fused to GST in “pull-down” experiments (Figure 2). The TNT reaction was performed with CN hemin present to promote synthesis of stable holo (heme-bound) α globin. Equal amounts of labeled α globin protein were incubated with Sepharose beads linked to GST alone or GST-AHSP. The beads were washed to remove excess proteins, and bound α globin was eluted with detergent and analyzed by SDS-PAGE followed by autoradiography. The α globin-AHSP interface mutants K99E, K99N, F117S, H103Y, H103R, and P119S all fail to bind AHSP-GST beads. The R31S and A130D mutants, which contain substitutions outside of AHSP contact regions, did bind AHSP-GST beads, although to a lesser extent than WT α globin. These data are in accord with the AHSP-α globin crystal structure and with prior biochemical studies (Figure 1).13
In vitro interactions of WT and mutant α globins with AHSP-GST. 35S-radiolabeled mutant α globins were synthesized by transcription coupled translation (TNT) with CN-hemin present, incubated with GST or GST-AHSP Sepharose beads, fractionated by SDS-PAGE, and visualized by autoradiography. Input controls (not incubated with beads) are shown in the top panel.
In vitro interactions of WT and mutant α globins with AHSP-GST. 35S-radiolabeled mutant α globins were synthesized by transcription coupled translation (TNT) with CN-hemin present, incubated with GST or GST-AHSP Sepharose beads, fractionated by SDS-PAGE, and visualized by autoradiography. Input controls (not incubated with beads) are shown in the top panel.
Some α globin mutations impair binding to both AHSP and β globin
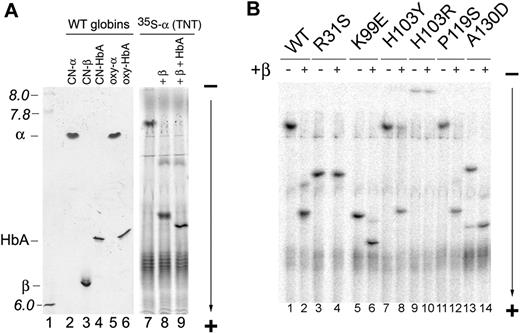
AHSP binds α globin on a surface that overlaps with the α1β1 interface.8 Thus, missense mutations in this region of α globin could alter binding to both AHSP and β globin. The side chains of α globin positions 103, 117, and 119 are part of both the AHSP and β globin interfaces (Figure 1).8 To study effects on the α1β1 interaction, we used TNT to generate radiolabeled holo (CN) α globins, added approximately 2-fold excess unlabeled oxy-β globin (∼ 20 nM), and analyzed Hb complexes by IEF (Figure 3). The equilibrium dissociation constants for Hb dimer to monomer and tetramer to dimer are approximately 2 × 10−12 M and 2 × 10−6 M, respectively.38,39 Thus, αβ dimers are probably the primary Hb species in experiments with TNT-derived α globin (∼ 10−8 M). These Hb samples migrate similarly as complexes stained with Coomassie, which were run at much higher concentrations, indicating that tetramers and dimers have similar isoelectric points (pIs) (Figure 3A lanes 4, 6, and 8). 35S-labeled α globin derived from TNT does have a slightly increased pI compared with α globin purified from human erythrocytes, which may be the result of the presence of uncleaved N-terminal methionine or another posttranslational modification unique to the TNT reaction (Figure 3A lanes 2, 5, and 7). Significant decreases in the Bohr effect (less proton release) are observed when hemoglobin is expressed in E coli with the “extra” M at the N-terminus, suggesting alterations in the pKa of amino acid side chains near the N-termini of HbA subunits.21,40 When excess HbA (∼ 3 μM) is added to the radiolabeled β/α globin mixture (Figure 3A lanes 8 and 9), the pI decreases, presumably because of formation of hybrid tetramers containing both TNT and human erythrocyte-derived α globin.
Monomer shift assay to examine interactions of mutant α globins with β globin. (A) IEF (pH 6-8) of purified and in vitro-synthesized WT hemoglobins. (Left panel) Hemoglobins purified from human blood were fractionated by IEF and stained with Coomassie blue. Each lane contains 10 μg of purified protein. (Right panel) WT 35S-α globin was synthesized by TNT with CN-hemin present and then unlabeled oxygenated β Hb subunit (20 nM final concentration) with or without HbA (5 μg = 3.1 μM final concentration in 25 μL) were added. The mixtures were fractionated by IEF and 35S-labeled α globin complexes were detected by autoradiography. pH markers and the positions of purified hemoglobins are indicated on the left. As described in the text, purified α globin (lanes 2 and 6) migrates slightly faster than TNT-synthesized 35S-α globin (lane 7) probably the result of posttranslational modification(s) that are specific to the TNT reaction. Also note that addition of excess HbA drives 35S-α globin-β globin dimers into mixed HbA tetramers, which have a slightly lower pI than the αβ dimers (compare lanes 8 and 9). (B) WT and mutant 35S-radiolabeled α globins were synthesized by TNT with CN-hemin present, and then unlabeled oxygenated β Hb subunit (20 nM) was added. The mixtures were fractionated by IEF (pH 6-8) and radiolabeled α globin (either free or in the context of α1β1 heterodimers) was visualized by autoradiography. (−) indicates no β globin added; and (+), β globin added.
Monomer shift assay to examine interactions of mutant α globins with β globin. (A) IEF (pH 6-8) of purified and in vitro-synthesized WT hemoglobins. (Left panel) Hemoglobins purified from human blood were fractionated by IEF and stained with Coomassie blue. Each lane contains 10 μg of purified protein. (Right panel) WT 35S-α globin was synthesized by TNT with CN-hemin present and then unlabeled oxygenated β Hb subunit (20 nM final concentration) with or without HbA (5 μg = 3.1 μM final concentration in 25 μL) were added. The mixtures were fractionated by IEF and 35S-labeled α globin complexes were detected by autoradiography. pH markers and the positions of purified hemoglobins are indicated on the left. As described in the text, purified α globin (lanes 2 and 6) migrates slightly faster than TNT-synthesized 35S-α globin (lane 7) probably the result of posttranslational modification(s) that are specific to the TNT reaction. Also note that addition of excess HbA drives 35S-α globin-β globin dimers into mixed HbA tetramers, which have a slightly lower pI than the αβ dimers (compare lanes 8 and 9). (B) WT and mutant 35S-radiolabeled α globins were synthesized by TNT with CN-hemin present, and then unlabeled oxygenated β Hb subunit (20 nM) was added. The mixtures were fractionated by IEF (pH 6-8) and radiolabeled α globin (either free or in the context of α1β1 heterodimers) was visualized by autoradiography. (−) indicates no β globin added; and (+), β globin added.
WT α globin monomer has a relatively high pI and migrates close to the IEF origin (Figure 3A lanes 2 and 5). WT β globin is more acidic and migrates near the bottom of the gel (Figure 3A lane 3), whereas HbA has an intermediate pI, approximately 6.7 (Figure 3A lanes 4 and 6). Thus, addition of unlabeled β globin to 35S-labeled free α globin increases its IEF mobility by forming αβ dimers (Figure 3A lanes 7 and 8). Many of the α globin mutations alter the pI of the monomer because of alternately charged amino acid side chains. For example, the R31S and A130D α mutations cause the same net decrease in pI from loss of a positive and gain of a negative charge, respectively (Figure 3B). The K99E substitutions results in a further shift to lower pI because of net change of −2 in charge (Figure 3B). However, in all cases, formation of a heterodimer with β globin is both expected and observed to shift the IEF band toward a more acidic pI.
Monomeric WT α globin shifted completely to a heterodimer after addition of unlabeled β globin (Figure 3A lanes 7 and 8 and 3B lanes 1 and 2). α globins K99E (Figure 3B lanes 5 and 6) and P119S behaved similarly (Figure 3B lanes 11 and 12), indicating efficient binding to β globin. Under the same conditions, a small amount of α globin H103Y remained unbound (Figure 3B lanes 7 and 8), suggesting reduced affinity for β globin. In contrast, addition of β globin did not alter the mobility of α globin mutant R31S (Figure 3B lanes 3 and 4), indicating markedly reduced affinity for β globin. The α globin mutant H103R failed to form prominent bands, most probably because introduction of a basic amino acid added a positive charge, raising the pI to a point beyond the pH range of the IEF gel (Figure 3B lane 9). However, failure of this mutant protein to run into the gel after addition of excess β globin suggests strongly that the α1β1 interaction is impaired (Figure 3B lane 10).
Mutant α globins exhibit variable resistance to protein degradation in the presence of AHSP or β subunits
AHSP may act as a molecular chaperone to help newly synthesized α globin fold properly.3 We analyzed this function of AHSP by exploiting the principle that most proteins are relatively protease-resistant when folded into their native structures, compared with unfolded states.20 We synthesized WT or mutant α globins, with or without recombinant AHSP present, and then treated the mixtures with trypsin and assessed the extent of proteolysis by SDS-PAGE and autoradiography (Figure 4A). WT α globin bound to AHSP is relatively trypsin-resistant.3 This protection is not simply because AHSP physically restricts access to protease but must indicate greater stability of the folded state because most predicted trypsin cleavage sites on α globin are distant from the AHSP-binding interface.3 In contrast to WT, the α globin mutants K99E, K99N, H103Y, H103R, F117S, and P119S, which failed to bind GST-AHSP in pull-down experiments, were not protected from trypsin digestion by preincubation with AHSP. These results suggest that direct physical interaction is required for AHSP to enhance resistance to proteolysis. Although the α globin R31S substitution is located outside of the crystallographically observed AHSP contact sites, the corresponding mutant protein appears to bind AHSP at reduced affinity (Figure 2) and is not protected from trypsin digestion by preincubation with AHSP (Figure 4A). It is possible that the R31S mutation alters α globin structure globally to increase susceptibility to proteolysis and also reduce its affinity for AHSP.
Limited proteolysis to examine the effects of AHSP and β globin on folding of nascent α globin. (A) 35S-radiolabeled α globins were synthesized by TNT with or without recombinant AHSP (4 μg/mL) and then treated with 10 μg/mL trypsin for the indicated times. Digestion was stopped by addition of SDS-PAGE loading dye. The extent of proteolysis was determined by SDS-PAGE electrophoresis followed by autoradiography. (B) 35S-radiolabeled α globin was synthesized by TNT with 40 ng/mL β globin (2.5 nM) present and then treated with 15 μg/mL trypsin for the indicated times and processed as described in panel A. CN-heme was not added to the TNT reactions in these experiments.
Limited proteolysis to examine the effects of AHSP and β globin on folding of nascent α globin. (A) 35S-radiolabeled α globins were synthesized by TNT with or without recombinant AHSP (4 μg/mL) and then treated with 10 μg/mL trypsin for the indicated times. Digestion was stopped by addition of SDS-PAGE loading dye. The extent of proteolysis was determined by SDS-PAGE electrophoresis followed by autoradiography. (B) 35S-radiolabeled α globin was synthesized by TNT with 40 ng/mL β globin (2.5 nM) present and then treated with 15 μg/mL trypsin for the indicated times and processed as described in panel A. CN-heme was not added to the TNT reactions in these experiments.
Protection from trypsin digestion by purified β globin was also tested in the absence of AHSP (Figure 4B). WT α globin and the K99E mutant, which both bind β globin in vitro (Figure 3B), were strongly protected from proteolysis by β globin (Figure 4B). Thus, the α K99E mutation appears to inhibit binding to AHSP selectively. In contrast, α globin P119S was proteolyzed rapidly in the presence of added β globin or AHSP (Figure 4A,B). This result differs with the IEF monomer shift assay, which indicated that α globin P119S can form α1β1 dimers (Figure 3B). Thus, this mutation only partially inhibits interactions with β globin; and when heme is present, a dimer can be formed. Proteolysis of apo-α globin may be a more sensitive assay to detect weakening of the α1β1 interaction when only the β partner contains heme. Alternatively, α globin P119S may form α1β1 dimers at normal affinity but fail to achieve its native protein fold, thereby rendering the α subunit more sensitive to trypsin, even when bound to β globin. In either case, the limited proteolysis assay suggests that α globin P119S cannot be stabilized normally by β globin. Hence, the deleterious effects of this mutation are almost certainly the result of both failed interactions with AHSP and β subunits. This conclusion is supported further by our studies of P119S α globin expression in E coli.
AHSP and β subunits differentially enhance heterologous expression yields of mutant α globins in E coli
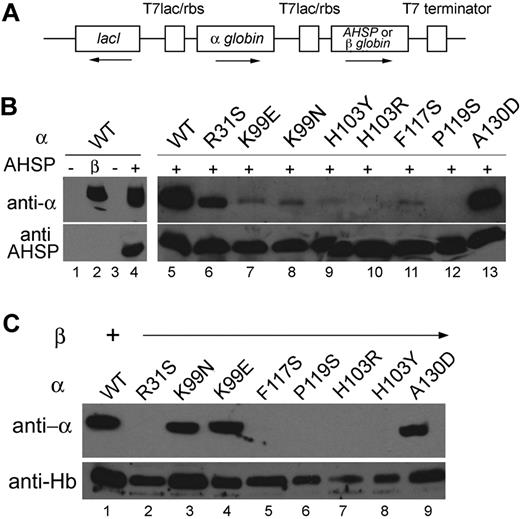
We studied mutant α globin interactions with AHSP and β globin during expression in E coli (Figure 5). A single IPTG-inducible bicistronic vector allows production of α globin and its interaction partner at approximately equimolar ratios (Figure 5A).23 We supplemented the cultures with hemin, induced protein synthesis for 12 hours, lysed the bacteria, extracted soluble protein, and monitored the synthesis of recombinant proteins by Western blotting (Figure 5B). As reported previously,13 α globin alone cannot be produced at high levels in E coli because of inherent protein instability and degradation by bacterial proteases (Figure 5B lanes 1 and 3). However, α globin synthesis is significantly enhanced by coexpression of either AHSP or β globin (Figure 5B lanes 2 and 4). Using this assay system, we examined whether the production of mutant α globins could be augmented by coexpression with AHSP (Figure 5B). As expected, AHSP enhanced the production of α globins R31S and A130D, which interacted with AHSP-GST beads in the pull-down assay (Figure 2). The production of very small, but detectable, amounts of α globin mutants K99E, K99N, H103Y, and F117S were observed when AHSP was coexpressed in E coli, even though these α mutants did not bind to GST-AHSP beads. Thus, the in vivo bacterial coexpression assay has a higher sensitivity for detecting weak AHSP-α globin interactions. In contrast, the α globin mutants H103R and P119S, which also did not interact with AHSP-GST (Figure 2), were not detected in E coli during coexpression with AHSP (Figure 5B lanes 10 and 12). Together, the GST-pull-down and in E coli coexpression assays demonstrate that binding to AHSP is strongly disrupted by the α P119S and H103R substitutions and more moderately impaired by α K99E, K99N, F117S, and H103Y.
Stabilization of mutant α globins by AHSP or β globin in E coli. (A) The pETDuet-1 vector was used to coexpress α globin and AHSP or β globin from a single plasmid in E coli. This vector contains 2 multiple cloning sites, each of which is preceded by an IPTG-inducible T7 promoter/lac operator and a ribosome-binding site (rbs), followed by a single T7 terminator. The plasmids were transformed into E coli, and IPTG was used to induce protein expression. The cells were lysed, cytosolic fractions were separated by SDS-PAGE, and protein expression was measured by Western blotting with the appropriate antibodies. (B) Western blots showing coexpression of AHSP and various α globin mutant proteins in E coli. (C) Western blots showing coexpression of WT β globin and various α globin mutant proteins in E coli. Expressed β globin was detected using an antibody against HbA.
Stabilization of mutant α globins by AHSP or β globin in E coli. (A) The pETDuet-1 vector was used to coexpress α globin and AHSP or β globin from a single plasmid in E coli. This vector contains 2 multiple cloning sites, each of which is preceded by an IPTG-inducible T7 promoter/lac operator and a ribosome-binding site (rbs), followed by a single T7 terminator. The plasmids were transformed into E coli, and IPTG was used to induce protein expression. The cells were lysed, cytosolic fractions were separated by SDS-PAGE, and protein expression was measured by Western blotting with the appropriate antibodies. (B) Western blots showing coexpression of AHSP and various α globin mutant proteins in E coli. (C) Western blots showing coexpression of WT β globin and various α globin mutant proteins in E coli. Expressed β globin was detected using an antibody against HbA.
Coexpression of β globin enhances production of WT α globin in E coli.21 Coexpressed β globin also enhanced the production of K99E, K99N, and A130 D α globins (Figure 5C). In contrast, β globin did not allow expression of the α globin mutants R31S, H103Y, H103R, F117S, and P119S. The α globin mutants R31S, H103Y, and H103R also failed to interact with β globin in the IEF monomer shift assay (Figure 3B). In contrast, α globin P119S physically interacted with β globin in the IEF monomer shift assay (Figure 3B). However, β globin did not protect the α P119S mutant from trypsin digestion, suggesting functionally impaired α1β1 interaction or partially unfolded α chains within the dimer (Figure 4B). This interpretation is further supported by the inability of β globin to enhance expression of α globin P119S in E coli.
Restored binding to AHSP rescues stability of α globin K99E
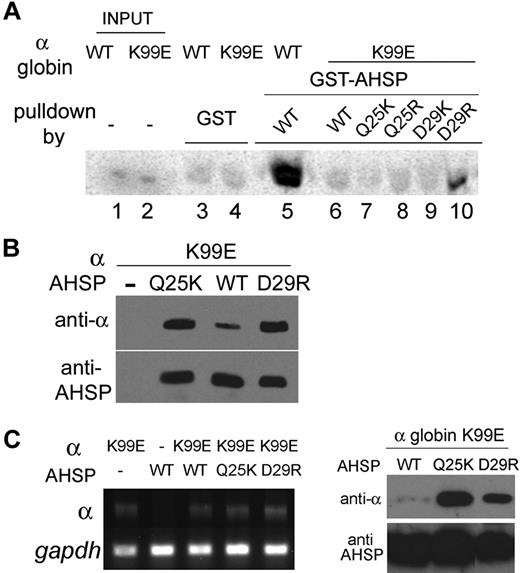
Mutations affecting amino acid K99 destabilize α globin predominantly by inhibiting interactions with AHSP, whereas binding to β globin remains intact. We hypothesized that it should be possible to construct a revertant AHSP that restores binding to K99E α globin and reestablishes its stability in coexpression assays. In WT α globin, the Nϵ atom of K99 is close to the terminal atoms of the side chains of AHSP Q25 and AHSP D29, suggesting the presence of favorable electrostatic interactions between these amino acids (Figure 6A). Mutation of lysine 99 to glutamate introduces a charge switch from positive to negative, disfavoring interaction with the native residue D29 of AHSP. Thus, we constructed mutants to alter the charge on the complementary surface of AHSP and restore binding to α globin K99E (Figure 6 right panel). AHSP Q25 or D29 was changed to K or R, and these mutant proteins were tested to determine whether they could stabilize α globin K99E and enhance its expression (Figure 7).
Structures of WT and mutant α globin-AHSP interfaces. The structure of the complex was adapted from Feng et al9 (PDB 1z8u), and the drawings were made in PyMol. (A) Close-up ribbon diagram of the WT α globin-AHSP interface (α globin in silver and AHSP in teal; α K99 and AHSP Q25 and D29 side chains are depicted as CPK-colored sticks). The electrostatic interactions between α globin K99 and AHSP D29 stabilize the α globin-AHSP interface. The α K99E mutant disrupts these favorable electrostatic interactions. (B) Close-up ribbon diagram of a theoretical model of the same interface but with α K99E and AHSP D29R mutations. As is suggested by data in this work, favorable electrostatic interactions between the negatively charged α globin E99 side chain and the positively charged AHSP revertant R29 can restore heterodimerization.
Structures of WT and mutant α globin-AHSP interfaces. The structure of the complex was adapted from Feng et al9 (PDB 1z8u), and the drawings were made in PyMol. (A) Close-up ribbon diagram of the WT α globin-AHSP interface (α globin in silver and AHSP in teal; α K99 and AHSP Q25 and D29 side chains are depicted as CPK-colored sticks). The electrostatic interactions between α globin K99 and AHSP D29 stabilize the α globin-AHSP interface. The α K99E mutant disrupts these favorable electrostatic interactions. (B) Close-up ribbon diagram of a theoretical model of the same interface but with α K99E and AHSP D29R mutations. As is suggested by data in this work, favorable electrostatic interactions between the negatively charged α globin E99 side chain and the positively charged AHSP revertant R29 can restore heterodimerization.
Restoration of AHSP binding stabilizes α globin K99E. (A) GST pull-down assay. Radiolabeled WT or K99E α globins were incubated with Sepharose beads linked to GST-WT AHSP or GST-AHSP altered specificity mutants, and AHSP-bound α globin was visualized as described in Figure 2. (B) Bicistronic vectors encoding α globin K99E and the indicated forms of AHSP were introduced into E coli, and Western blotting was used to examine protein expression, as described in Figure 5. (C) Human α globin K99E and various forms of AHSP were stably expressed in MEL cells. (Left panel) Agarose gel electrophoresis of reverse transcribed PCR products generated using specific primers for human α globin and mouse Gapdh. Note that human α globin cDNA expression is roughly equivalent in all MEL lines, except for the negative control in lane 2. (Right panel) Western blot analysis of cytosolic extracts from MEL cells expressing α globin K99E and various AHSP mutants. Note that expression of K99E α globin protein is specifically enhanced by coexpression of altered specificity AHSP mutants Q25K and D29R.
Restoration of AHSP binding stabilizes α globin K99E. (A) GST pull-down assay. Radiolabeled WT or K99E α globins were incubated with Sepharose beads linked to GST-WT AHSP or GST-AHSP altered specificity mutants, and AHSP-bound α globin was visualized as described in Figure 2. (B) Bicistronic vectors encoding α globin K99E and the indicated forms of AHSP were introduced into E coli, and Western blotting was used to examine protein expression, as described in Figure 5. (C) Human α globin K99E and various forms of AHSP were stably expressed in MEL cells. (Left panel) Agarose gel electrophoresis of reverse transcribed PCR products generated using specific primers for human α globin and mouse Gapdh. Note that human α globin cDNA expression is roughly equivalent in all MEL lines, except for the negative control in lane 2. (Right panel) Western blot analysis of cytosolic extracts from MEL cells expressing α globin K99E and various AHSP mutants. Note that expression of K99E α globin protein is specifically enhanced by coexpression of altered specificity AHSP mutants Q25K and D29R.
The AHSP D29R mutation restored binding to α globin K99E significantly, as measured by the GST pull-down assay (Figure 7A lane 10). Coexpressed AHSP Q25K and D29R also augmented the production of α globin K99E in E coli compared with WT AHSP (Figure 7B). The different behavior of AHSP Q25K in the 2 assays used here (Figure 7A vs Figure 7B) is consistent with earlier findings indicating a lower sensitivity in the GST pull-down vs the E coli coexpression assays for detecting weak α globin-AHSP interactions.
We next tested whether the AHSP mutations that restore binding to α globin K99E can enhance its expression in erythroid cells. We prepared pools of MEL cells with stably integrated expression constructs encoding α globin K99E and the altered specificity forms of AHSP. The α globin K99E mRNA was expressed at approximately equal levels in all lines tested (Figure 7C left panel). Cytosolic extracts were examined by Western blotting for human α globin and AHSP protein expression (Figure 7C right panel). In agreement with our findings in E coli, both AHSP mutants Q25K and D29R enhanced α globin K99E expression in MEL cells. Most importantly, these AHSP mutants stabilized α globin K99E to a much greater extent than WT AHSP, confirming that the K99E mutation destabilizes α globin specifically by inhibiting interaction with AHSP. These results also demonstrate that favorable electrostatic interactions between the α K99 side chain and AHSP Q25 and D29 contribute strongly to heterodimerization.
Discussion
Our working model of AHSP function is based on previous studies of Ahsp−/− mice, which indicate that AHSP binds nascent apo or holo α globins to augment their stability en route to HbA formation.1,3,4 Interaction of the AHSP-α globin complex with β globin displaces AHSP to form α1β1 heterodimers, which then combine via α1β2 contacts to form HbA tetramer. AHSP is not essential for HbA formation but does optimize the process by maximizing the pool of productive α globin subunits. Once incorporated into HbA, α globin no longer requires AHSP, consistent with the latter's proposed role as a molecular chaperone.
Naturally occurring α globin missense mutations could destabilize the nascent protein by inhibiting its interactions with AHSP. Such mutations were identified through structural studies that define the α globin-AHSP interface, ie, α globins P119S and F117S.12,13 These mutations inhibit interaction with AHSP in E coli13 and yeast,8 suggesting α globin destabilization in human erythrocytes. However, a single mutation in α globin could inhibit both AHSP and β globin binding because these contact surfaces overlap. Alpha globin mutations at the AHSP interface could also destabilize indirectly through global effects on protein structure that do not alter the binding process per se.
To address these issues, we selected 6 naturally occurring human α globin missense mutations predicted to inhibit interaction with AHSP. We used biochemical and cell-based assays to examine how these mutations affect binding to AHSP and β globin and subsequent protein stabilization. The results indicate that alterations at α globin amino acid positions 103, 117, and 119 affect both β globin and AHSP interactions, providing at least 2 mechanisms for their destabilizing effects (Table 2). In contrast, mutations at position 99 appear to weaken AHSP interactions selectively. This effect is only partial as WT AHSP can facilitate weak heterologous expression of K99 α globin mutants in E coli.
Methods used to examine variant α globin interactions
| Interaction/method . | WT . | R31S . | K99E . | K99N . | H103Y . | H103R . | F117S . | P119S . | A130D . |
|---|---|---|---|---|---|---|---|---|---|
| AHSP-α | |||||||||
| Coexpression in E coli | ++ | ++ | + | + | + | − | + | − | ++ |
| GST pulldown | ++ | + | − | − | − | − | − | − | ++ |
| Limited proteolysis | ++ | − | − | − | − | − | − | − | + |
| α1β1 | |||||||||
| Monomer shift assay | ++ | − | ++ | ++ | + | − | ND | ++ | ++ |
| Coexpression in E coli | ++ | − | ++ | ++ | − | − | − | − | ++ |
| Summary of biochemical effects | |||||||||
| Binding to AHSP | ++ | ++ | + | + | + | − | + | − | ++ |
| Binding to β globin | ++ | − | ++ | ++ | + | − | + | + | ++ |
| Interaction/method . | WT . | R31S . | K99E . | K99N . | H103Y . | H103R . | F117S . | P119S . | A130D . |
|---|---|---|---|---|---|---|---|---|---|
| AHSP-α | |||||||||
| Coexpression in E coli | ++ | ++ | + | + | + | − | + | − | ++ |
| GST pulldown | ++ | + | − | − | − | − | − | − | ++ |
| Limited proteolysis | ++ | − | − | − | − | − | − | − | + |
| α1β1 | |||||||||
| Monomer shift assay | ++ | − | ++ | ++ | + | − | ND | ++ | ++ |
| Coexpression in E coli | ++ | − | ++ | ++ | − | − | − | − | ++ |
| Summary of biochemical effects | |||||||||
| Binding to AHSP | ++ | ++ | + | + | + | − | + | − | ++ |
| Binding to β globin | ++ | − | ++ | ++ | + | − | + | + | ++ |
++ indicates normal interaction; +, reduced interaction; −, no interaction detected; and ND, not determined.
It is also possible that some of the mutations we studied block binding to AHSP and β globin indirectly by inhibiting the incorporation of CN-hemin, which promotes native folding of apo α globin. This possibility seems less likely because (1) all of the α globin mutations alter AHSP contact residues and are distant from the heme binding pocket; (2) AHSP binds apo α globin, which lacks heme14 ; (3) the limited proteolysis assay (Figure 4) detects AHSP-apo α globin interactions independent of heme3 ; and (4) the ability of complementary AHSP mutations to restore interaction with α globin K99E suggests that the mutations at this position do not inhibit native folding.
Our model of Hb assembly and AHSP function predicts additional features of the α globin variants. For example, impaired binding to AHSP should inhibit the stability of nascent α globin but have no effect on the protein once it is incorporated into HbA tetramer. In this case, the variant globin (termed x) would be relatively underrepresented in erythrocytes or in biosynthetic labeling studies. However, stabilities of variant Hb tetramers (ααxβ2), as measured by chemical, acid, or heat denaturation profiles, should be normal. In contrast, impaired interaction with β globin should destabilize the mutant α globin in its monomeric form and inhibit formation of Hb dimers and tetramers. Unfortunately, the appropriate clinical laboratory tests were not performed for all the reported cases of persons carrying these variant α chains. In the future, such studies would be helpful for identifying differential interactions of mutant α globins with β globin versus AHSP.
In their heterozygous forms, none of the mutations studied here are expected to have strong clinical effects because only 1 of 4 α globin alleles (either α1 or α2) is affected. Some of the mutations are reported to produce anemia when present at homozygosity or in combination with deletional α thalassemia (Table 1). Mutations present on the α2 allele are expected to have a relatively stronger effect than those on the α1 allele because the α2:α1 protein synthetic ratio is between 2:1 and 2.5:1.36,37 In general, α globin mutations that inhibit binding to β globin are expected to be more severe than those that exclusively impair AHSP interactions because formation of the α1β1 heterodimer is essential for HbA assembly and stability, whereas AHSP is nonessential, at least in mice.1
Among the mutations that we studied, α globins H103R, H103Y, F117S, and P119S, which exhibit impaired interactions with both β globin and AHSP, appear to cause the most significant clinical effects (Table 1). The α globin mutants R31S and H103R did not interact with β globin in the IEF mobility shift assay (Figure 3B) or in E coli (Figure 5C), yet small amounts of circulating Hb tetramers containing these mutant globin chains were detected in human subjects (Table 1). Because formation of α1β1 dimers is prerequisite for tetramer formation, interactions between these mutant α globins and β globin cannot be completely abolished. In circulating erythrocytes, these weaker α1β1 interactions will be stabilized in the context of Hb tetramers and by the high concentrations of globins present in red cells (∼ 10−2 M) versus that in our IEF assay (10−8 M).
A key issue is whether AHSP is required for efficient Hb production and erythropoiesis in humans. Among the α globin variants analyzed, only those involving K99 allowed us to address this question unambiguously. These substitutions (K99E and K99N) partially block binding to AHSP but have no effect on binding to β globin. Expression of AHSP mutants with increased affinity for α globin K99E enhances the mutant protein production in E coli and erythroid cells. Therefore, the K99 substitutions destabilize specifically by inhibiting interactions with AHSP. These rescue experiments demonstrate that the K99E mutation specifically disrupts strong electrostatic interactions with residues Q25 and D29 in AHSP.
Human α globin K99 mutations have been detected only in their heterozygous forms and are not reported to cause significant anemia or microcytosis.25,41 These α chain variants comigrate with HbA1c and were discovered incidentally during diabetic screening. However, there is some evidence that the Hb Turriff (K99E) mutation is mildly destabilizing. This mutant protein is slightly underrepresented in hemolysates of one person who is heterozygous for the mutation presumed to be on the α2 allele (22% circulating variant-containing Hb heterotetramer αxαββ/ααxββ vs 44% to 46% expected; Table 1; Supplemental Methods). In another subject, an α1 gene-encoded K99E variant composed 10.5% circulating variant tetramer, which contrasts to the predicted level of 24% to 28% for a stable variant (Table 1; supplemental data). Globin chain synthetic studies on this patient also indicated protein instability, as the mutant α globin was present in only 5% of the total α chains produced by in vitro translation.41 This contrasts with the expected value of 14% to 17% for an α1-encoded globin protein with normal stability (supplemental data; Equations 1-4).
In conclusion, our studies illustrate several complexities in interpreting the effects of naturally occurring α globin mutations predicted to affect binding to β globin and AHSP. The major dilemma is the similarity of the α subunit interfaces for these heterologous protein interactions. Mutations at positions 103, 117, and 119 inhibit interactions with both AHSP and β globin by altering the αG helix. In contrast, α K99E has little effect on binding to β globin but markedly weakens interactions with AHSP. Our studies of α K99E, combined with the mild instability in carriers, support the hypothesis that AHSP augments human Hb assembly by enhancing the stability of nascent α globin during Hb assembly. Observations that α K99E variant can still be incorporated into HbA of human carriers, albeit at lower than expected levels, are compatible with our findings that complete loss of AHSP does not markedly inhibit HbA production in mice. The phenotype of K99E subjects may underestimate the importance of AHSP because interactions between this mutant globin and AHSP are not completely abolished. However, the importance of the α globin K99-AHSP D29 electrostatic interaction is clear because binding of α K99E subunits to AHSP is restored by the complimentary AHSP D29R mutations. In future work, we hope to quantify the significance of these types of specific interactions for the entire α globin-AHSP interface and define their relationships to β globin binding.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Kazuhiko Adachi, Stephen Liebhaber, and Eugene Khandros for helpful suggestions and careful reading of the manuscript.
This work was supported by the National Institutes of Health (grants DK061692, M.J.W.; HL087427, M.J.W.; HL47020, J.S.O.; GM35649, J.S.O.; Welch grant C-0612, J.S.O). X.Y. is supported by an American Heart Association Predoctoral Fellowship Award. M.J.W. is a Leukemia & Lymphoma Society Scholar. T.L.M. is supported by the Biotechnology Training Grant GM008362 from the National Institutes of Health.
National Institutes of Health
Authorship
Contribution: X.Y., T.L.M., and A.B. designed and performed experiments, analyzed data, and wrote the paper; and A.J.G., J.S.O., and M.J.W. designed experiments, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: J.S.O. and M.J.W. have a patent application for using AHSP to increase the expression of recombinant hemoglobin in E coli: “Enhancing Recombinant Hemoglobin (rHb) Production by Co-Expression with Alpha Hemoglobin Stabilizing Protein (AHSP).” US Patent Application 11/685,986 (PCT/US2005/033028), under review. Inventors: John S. Olson and Mitchell J. Weiss.
Correspondence: Mitchell J. Weiss, 3615 Civic Center Blvd, 316B ARC, Philadelphia, PA 19104; e-mail: weissmi@email.chop.edu.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal