Abstract
Pruritus is a common symptom in patients with Philadelphia chromosome–negative myeloproliferative disorders (MPDs). The pathophysiology of MPD-associated pruritus is unclear. We have demonstrated that MPD mast cells (MCs) are involved by the malignant process. In the present study, we explored the hypothesis that MCs play an important role in the development of pruritogenesis in MPDs. We found that MPD MCs released significantly greater amounts of pruritogenic factors, including histamine, leukotrienes, and interleukin-31 (IL-31) than normal MCs. Elevated levels of IL-31 were also observed in MPD CD3+ cell-conditioned media. MPD MCs exhibited increased migratory behavior in response to stem cell factor or interleukin-8, which was associated with increased filamentous-actin content. Furthermore, the presence of pruritus in MPDs was statistically correlated with a greater number of MCs being generated by CD34+ cells, a greater number of MC colonies being formed by CD34+ cells, decreased apoptosis and prostaglandin D2 release by cultured MCs, and higher plasma levels of IL-31. These data demonstrate that functional abnormalities of MPD MCs probably lead to pruritogenesis in patients with MPDs. These studies provide cellular and molecular targets for the development of antipruritus drugs for patients with MPDs.
Introduction
Itching is defined as an unpleasant sensory experience that leads to scratching.1,2 It is frequently an important skin manifestation of several systemic diseases.1,2 Cross-talk between C neuron terminals and the spatially closely related dermal mast cells (MCs) is increasingly recognized as an important pathway in the pathophysiology of itching.1 A variety of MC mediators, including histamine, tryptase, prostaglandins (PGs), and leukotrienes (LTs), are important mediators of itching in inflammatory skin diseases.1-6 Interleukin (IL)–31, a cytokine mainly produced by activated T cells,7 is another known critical player in pruritogenesis. Transgenic mice overexpressing IL-31 present with all the hallmarks of atopic dermatitis, including severe pruritus.7 Furthermore, IL-31 has been shown to be significantly overexpressed in pruritic atopic compared with nonpruritic psoriatic skin inflammation.8
The Philadelphia chromosome–negative myeloproliferative disorders (Ph− MPDs), which include polycythemia vera (PV), essential thrombocythemia (ET), and primary myelofibrosis (PMF), are characterized by excessive proliferation of one or more terminally differentiated myeloid lineages.9,10 Pruritus is a common symptom in Ph− MPDs, occurring in approximately 50% of patients.11,12 Itching can be an agonizing aspect of the disease and may even be the presenting symptom or precede the development of hematologic manifestations by several years.12-14 Pruritus deprives patients of sleep and interferes with their social and physical activities.11-14 The itching occurs spontaneously or appears when taking a hot shower or after other sudden environmental changes. Although Ph− MPDs are recognized as clonal hematopoietic stem cell disorders frequently associated with a JAK2 mutation (JAK2V617F), the pathophysiology of the MPD-associated pruritus remains unclear.12,15,16
We have recently shown that MCs were involved by the malignant process in patients with Ph− MPDs.17 In the present study, we explored the hypothesis that MCs play an important role in pruritogenesis in Ph− MPDs. We found that cultured MPD MCs released significantly greater amounts of pruritogenic factors, including histamine, LTs, and IL-31 than that by normal MCs. Elevated levels of IL-31 were also observed in MPD CD3+ T cell–conditioned media, and the increased IL-31 transcripts were documented in MPD CD3+ T cells. MPD MCs exhibited increased migratory behavior in response to stem cell factor (SCF) or IL-8, which was associated with increased filamentous actin (F-actin) compared with normal MCs. Furthermore, the presence of pruritus in patients with MPDs was found to be statistically correlated with a greater number of MCs being generated by CD34+ cells, a greater number of MC colonies being formed by CD34+ cells, decreased apoptosis of cultured MPD MCs, decreased PGD2 release by cultured MPD MCs, and higher plasma levels of IL-31. These data demonstrate that a functional abnormality exists in MPD MCs and MCs are involved in the pathogenesis of pruritus in patients with MPDs. These studies therefore identified potential cellular or molecular targets for the development of one or more antipruritus drugs for patients with Ph− MPDs.
Methods
Patients and healthy donors
All human tissue samples were obtained after informed consent was provided according to guidelines of the Institutional Review Board of the Mount Sinai School of Medicine and in accordance with the Declaration of Helsinki. All MPD patients met the revised World Health Organization diagnostic criteria for PV or PMF.9,18 PV patients were treated with phlebotomy and low-dose aspirin, whereas PMF patients were receiving solely supportive care at the time of study. Patients with pruritus were identified by reviewing the medical records. The designation of patients as having secondary erythrocytosis (SE) required the presence of erythrocytosis, a comorbidity known to be associated with SE, JAK2 wild-type granulocytes, and a bone marrow examination that was inconsistent with a MPD. CD34+ and CD3+ cells were isolated from 30 to 50 mL peripheral blood (PB) from PMF patients or 400 mL discarded therapeutic phlebotomy units from PV patients as previously described.19 Granulocyte colony-stimulating factor–mobilized (Gmob) CD34+ cells were purchased from StemCell Technologies (Vancouver, BC). Normal CD3+ cells were isolated from healthy volunteers.
Preparation of CD34+ cells, CD3+ cells, human cultured MCs, and plasma
PB samples were layered onto Ficoll-Hypaque (1.077 g/mL), and low-density mononuclear cells (MNCs) were separated by centrifugation. CD34+ and CD3+ cells were isolated from the MNC using magnetic-activated cell isolation kits (StemCell Technologies) according to the manufacturer's instructions. Magnetically isolated CD34+ or CD3+ cells with a purity of 90% or greater were used for subsequent experiments. Plasma was also obtained from healthy volunteers, patients with SE, and patients with MPDs.
Human cultured MCs (HCMCs) were generated in vitro as we previously described.17 HCMCs with a purity of 95% or greater as determined by morphologic examination of Wright-Giemsa and Alcian blue–stained specimens were used for the planned studies.
JAK2V617F mutational analysis
The JAK2V617F allelic burden in granulocytes, HCMCs, and CD3+ cells was determined by real-time quantitative polymerase chain reaction (PCR) using the allelic discrimination method as we previously described.17,19 Patients with a JAK2V617F/JAK2total allele ratio of 50% or greater in granulocytes were classified as high-burden JAK2V617F, whereas JAK2V617F-positive patients with a JAK2V617F/JAK2total allele ratio of less than 50% were classified as low-burden JAK2V617F. Among the 22 PV and 15 PMF patients studied, 21 PV and 9 PMF patients were JAK2V617F-positive (16 JAK2V617F high-burden patients and 14 JAK2V617F low-burden patients). The JAK2V617F allelic burden in HCMCs was similar to that observed in the granulocytes,17 whereas CD3+ cells from 27 of the 30 JAK2V617F-positive MPD patients were negative for JAK2V617F and the JAK2V617F allelic burden was low in CD3+ cells from 3 JAK2V617F-positive MPD patients.
Measurement of MC mediators
HCMCs were suspended in fresh culture medium supplemented with 100 ng/mL SCF and sensitized with IgE (1 mg/mL) overnight at 37°C. The supernatants were removed by centrifugation, and cells (105 per sample) were then washed with Ca2+-free phosphate-buffered saline and suspended in warm Tyrode buffer (10 mM N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid, 137 mM NaCl, 2.7 mM KCl, 0.38 mM Na2HPO4 · 7 H2O, 5.6 mM glucose, 1.8 mM CaCl2, 1.3 mM MgSO4, and 0.03% bovine serum albumin [BSA]; pH 7.4). The IgE-sensitized cells were then challenged with or without anti–human IgE (2 mg/mL; Chemicon, Temecula, CA) for 30 minutes at either 37°C or 42°C. In some experiments, aliquots of MCs were stimulated with substance P (10 μM) for 30 minutes. After stimulation, the incubation mixtures were maintained at 4°C and centrifuged to sediment the HCMCs. The supernatants were collected, and the HCMCs were lysed in 0.1% Triton X-100 by freeze-thawing repeatedly. All samples were frozen at −20°C until analysis. The levels of histamine were analyzed by a histamine enzyme-linked immunosorbent assay (ELISA) kit (Oxford Biomedical Research, Rochester Hills, MI); concentrations of LTs were measured using leukotriene C4/D4/E4 Biotrak ELISA system (GE Healthcare, Little Chalfont, United Kingdom). IL-4 Quantikine (R&D Systems, Abingdon, United Kingdom), nerve growth factor (NGF) Emax Immunoassay System (Promega, Madison, WI), and PGD2 ELISA Kit (Cayman Chemical, Ann Arbor, MI) were used for the levels of IL-4, NGF, and PGD2, respectively. Plasma IL-31 levels were measured using an IL-31 Development Kit (PeproTech, Rocky Hill, NJ).
In separate sets of experiments, 5 × 105 MCs from each culture were treated with calcium ionophore A23187 for 1 hour at either 37°C or 42°C. The supernatant and cellular pellet fractions were prepared similarly. Levels of tryptase in the supernatant and cellular pellet fractions were measured using a MC degranulation Kit (Millipore, Billerica, MA). The percentage of histamine or tryptase release was quantitated by: histamine or tryptase in supernatant/(histamine or tryptase in supernatant + histamine or tryptase in pellet) × 100.
Preparation of media conditioned by MCs or CD3+ cells
A total of 107 CD3+ cells were incubated in 1 mL Iscove modified Dulbecco medium containing 0.1% BSA, with or without phytohemagglutinin (PHA; 2 μg/mL) at 37°C in 5% CO2 as previously described.20 Media were collected after 72 hours of incubation by centrifugation. HCMCs (106) were similarly incubated, and media was collected after 24 hours of incubation. Media conditioned by CD3+ cells or HCMCs were stored in aliquots at −80°C until analysis. The levels of IL-31 in the conditioned media were measured by ELISA.
Quantitative real-time PCR assay of IL-31 mRNA
Total RNA was extracted from MCs or CD3+ cells using a PicroPure RNA Isolation Kit (Arcturus Bioscience, Mountain View, CA). First-strand complimentary DNA (cDNA) was synthesized from total RNA with SuperScript III Reverse Transcriptase (Invitrogen, Carlsbad, CA). The sequences of the primers used for the amplification were IL-31 (162 bp): 5′-GCCAAACAACATCCACAGCC-3′ (forward), 5′-GTGCGAGGTCCATGCACTCT-3′ (reverse); β-actin (176 bp): 5′-AGCCTCGCCTTTGCCGA-3′ (forward), 5′-CTGGTGCCTGGGGCG-3′ (reverse). The quantitative real-time PCR assays were performed with the ABI Prism 7900 Sequence Detection System, and the PCR products were detected by the use of SYBR green technology (Applied Biosystems, Foster City, CA). Cycling conditions included initial denaturation at 95°C for 2 minutes, then by 45 cycles of denaturation at 95°C for 15 seconds, annealing at 55°C for 15 seconds, and extension at 72°C for 30 seconds. All assays were performed in triplicate, and a negative control was included in each assay. To quantitate IL-31 mRNA expression in relation to an internal control, an amplification standard curve of the reference gene, β-actin, was established as previously described.20,21 The IL-31 mRNA expression was quantitated by measuring the threshold cycle and presented as relative rates compared with the expression of the reference gene β-actin. The data were analyzed by 2−ΔΔCT method as previously described.22
Migration assay
The migration behavior of HCMCs was performed using 6.5-mm-diameter, 8-μm-pore transwell plates as previously described.23 Briefly, transwell filters were coated with fibronectin and washed with transwell buffer (Iscove modified Dulbecco medium with 1% BSA). A total of 105 HCMCs in 100-μL transwell buffer were then placed in the upper chamber of the transwell. A total of 500-μL transwell buffers containing 10 ng/mL SCF or IL-8 were added to the lower compartment. After incubation at 37°C for 4 hours, nonmigrated and migrated cells were harvested from the upper and lower compartments, respectively. The harvested cells in the 2 fractions were enumerated using a hemocytometer. The percentage of migrating cells was calculated by determining the ratio of the number of cells recovered from the lower compartment to the total number of cells loaded in the upper compartment.
F-actin quantitation
F-actin quantitation was performed as previously described.24 Briefly, flow cytometry was used to quantitate the F-actin content of HCMCs. A total of 5 × 104 HCMCs were suspended in 50 μL HBSS in polypropylene tubes and warmed at 37°C for 5 minutes before the addition of SCF (10 ng/mL). Cells were fixed after the specified time of SCF stimulation by the addition of 1 mL phosphate-buffered saline containing 4.6% formaldehyde and 0.1% BSA. Fixed cells were then treated with Perm/Wash buffer (BD Biosciences, San Jose, CA) for 5 minutes at room temperature. After washing, the cells were then incubated with 160 nM fluorescein isothiocyanate–phalloidin (Sigma-Aldrich, St Louis, MO) for 20 minutes at room temperature. A minimum of 10 000 MC events was recorded, and the results were reported as the mean channel fluorescence.
Statistical analysis
The results are expressed as the mean plus or minus SD of data obtained from a various number of individual experiments. Differences between percentages of migrating cells were calculated using the Wilcoxon test, whereas differences between other variables were compared using a Student t test and/or analysis of variance. Statistical significance was assumed for P values of .05 or less.
Results
Quantification of the histamine, tryptase, LTs, and PGD2 released by MPD or Gmob HCMCs
MCs were generated by incubating CD34+ cells purified from 29 MPD patients and 7 Gmob volunteers in the presence of SCF (100 ng/mL) and IL-6 (50 ng/mL) for 7 to 8 weeks.17 The HCMCs with a purity of 95% or greater were then used for experiments, including degranulation, migration, and mediator release. MC activation is often regarded as synonymous with degranulation and synthesis of its mediators. We therefore analyzed the total contents and/or the release of histamine, tryptase, LTs, and PGD2 by activated or nonactivated HCMCs from 11 MPD patients and 6 Gmob volunteers. Because patients with MPDs frequently experience severe itching when exposed to hot showers, we evaluated the release of MC mediators after incubation of MCs at either 37°C or 42°C.
MCs were sensitized with IgE overnight at 37°C. The MCs (105 cells/mL) were then challenged with or without anti-IgE for 30 minutes at either 37°C or 42°C. After sensitization with IgE, the MCs showed a substantial release of histamine, LTs, and PGD2 (Figure 1A-C); on the addition of anti-IgE, their histamine, LTs, and PGD2 release was increased (Figure 1D-F). MPD MCs released significantly greater amounts of histamine than Gmob MCs (P < .05) after both IgE sensitization and anti-IgE cross-linking; incubation of MCs at 42°C did not enhance the release of histamine by either MPD or Gmob MCs (Figure 1A,D). Significantly higher amounts of total histamine were observed in MPD MCs than that in Gmob MCs (P < .05; data not shown). The histamine release rates were, however, similar between MPD and Gmob MCs (data not shown). MPD MCs released significantly greater amounts of PGD2 than Gmob MCs (P < .05) when cells were incubated at 37°C after both IgE sensitization and anti-IgE cross-linking (Figure 1B,E). Interestingly, incubation at 42°C significantly decreased the PGD2 release by IgE-sensitized MPD MCs (P < .05) while moderately increasing the PGD2 release by IgE-sensitized Gmob MCs (P > .05) compared with incubation at 37°C (Figure 1B). In addition, MPD MCs released significantly greater amounts of LTs than Gmob MCs after anti-IgE cross-linking (P < .05; Figure 1F). Incubation at 42°C did not alter the release of LTs by either MPD or Gmob MCs (Figure 1C,F).
The levels of histamine, LTs, and PGD2 release by MPD HCMCs. HCMCs from 11 MPD patients and 6 Gmob volunteers were sensitized with IgE overnight and then were challenged with or without anti-IgE for 30 minutes at either 37°C or 42°C (105/mL). The levels of histamine (A,D), PGD2 (B,E), and LTs (C,F) in the supernatants were measured by ELISA. Each triangle represents the level of histamine in the supernatants of HCMCs sensitized with IgE (A-C) or HCMCs stimulated with IgE plus anti-IgE (D-F) at the temperature indicated. Each bar represents the mean of each sample group.
The levels of histamine, LTs, and PGD2 release by MPD HCMCs. HCMCs from 11 MPD patients and 6 Gmob volunteers were sensitized with IgE overnight and then were challenged with or without anti-IgE for 30 minutes at either 37°C or 42°C (105/mL). The levels of histamine (A,D), PGD2 (B,E), and LTs (C,F) in the supernatants were measured by ELISA. Each triangle represents the level of histamine in the supernatants of HCMCs sensitized with IgE (A-C) or HCMCs stimulated with IgE plus anti-IgE (D-F) at the temperature indicated. Each bar represents the mean of each sample group.
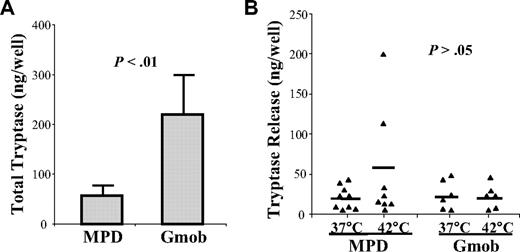
HCMCs from 9 MPD patients and 6 Gmob volunteers were also stimulated with calcium ionophore to induce tryptase release at either 37°C or 42°C. In contrast to histamine, MPD MCs contained significantly lower amounts of total tryptase than that of Gmob MCs (P < .01; Figure 2A). However, the amounts of tryptase released on stimulation were similar between MPD and Gmob MCs (P > .05). Incubation at 42°C did not alter the release of tryptase by either MPD or Gmob MCs (Figure 2B). The tryptase release rate was significantly greater in MPD MCs than Gmob MCs (P < .05; data not shown).
Measurement of the release of tryptase by MPD HCMCs. HCMCs from 9 MPD patients and 6 Gmob volunteers were stimulated with calcium ionophore to induce tryptase release for 60 minutes at either 37°C or 42°C. The levels of tryptase in the supernatants and cell pellets were measured by ELISA. (A) Each column represents the mean ± SD of the total tryptase in each group of HCMCs. (B) Each triangle represents the level of tryptase in the supernatant of HCMCs stimulated with calcium ionophore at the temperature indicated. Each bar represents the mean of each sample group.
Measurement of the release of tryptase by MPD HCMCs. HCMCs from 9 MPD patients and 6 Gmob volunteers were stimulated with calcium ionophore to induce tryptase release for 60 minutes at either 37°C or 42°C. The levels of tryptase in the supernatants and cell pellets were measured by ELISA. (A) Each column represents the mean ± SD of the total tryptase in each group of HCMCs. (B) Each triangle represents the level of tryptase in the supernatant of HCMCs stimulated with calcium ionophore at the temperature indicated. Each bar represents the mean of each sample group.
Plasma levels of IL-4, IL-31, and NGF
IL-4, IL-31, and NGF have been shown to play important roles in pruritogenesis.7,25,26 We measured the plasma levels of these factors in 22 patients with MPDs, 8 normal volunteers, and 6 patients with SE by ELISA. As shown in Figure 3, the plasma levels of IL-31 were significantly greater in MPD patients (141.3 ± 58.3 pg/mL) than normal volunteers (65.2 ± 24.1 pg/mL; P < .05) or patients with SE (69.8 ± 14.2 pg/mL; P < .05). The plasma levels of IL-4 and NGF were, however, similar for each of the 2 sources (P > .05; data not shown).
Plasma levels of IL-31. The levels of IL-31 in the plasma of healthy volunteers (n = 8), patients with MPDs (n = 22), or patients with SE (n = 6) were measured by ELISA. Each column represents the mean ± SD of the plasma levels of IL-31.
Plasma levels of IL-31. The levels of IL-31 in the plasma of healthy volunteers (n = 8), patients with MPDs (n = 22), or patients with SE (n = 6) were measured by ELISA. Each column represents the mean ± SD of the plasma levels of IL-31.
Elevated production of IL-31 by MPD CD3+ cells
IL-31 has been reported to be expressed by activated T cells.7 We analyzed IL-31 production by CD3+ cells from 17 MPD patients and 6 normal volunteers at both the protein level by ELISA and the mRNA level by real-time PCR. As shown in Figure 4A, MPD CD3+ cells (155.3 ± 42.3 pg/mL) elaborated a significantly greater amount of IL-31 than normal CD3+ cells (42.7 ± 20.4 pg/mL) in the absence of PHA. When the cells were stimulated with PHA, both MPD and normal CD3+ cells released increased levels of IL-31; MPD CD3+ cells (388.9 ± 110.3 pg/mL) again elaborated significantly higher levels of IL-31 than normal CD3+ cells (113.4 ± 52.6 pg/mL; P < .05). To confirm these data, we examined whether IL-31 transcripts were up-regulated in MPD CD3+ cells. The IL-31 mRNA expression levels in CD3+ cells were assessed by quantitative real-time PCR and normalized to β-actin. In the absence of PHA, significantly higher levels of IL-31 transcripts (15.2 ± 8.2) were observed in MPD CD3+ cells than that in normal CD3+ cells (P < .01). The levels of IL-31 transcripts were up-regulated on PHA stimulation in both MPD and normal CD3+ cells; and again, the levels of IL-31 transcripts in the PHA-stimulated MPD CD3+ cells (41.2 ± 14.3) were significantly higher than that in PHA-stimulated normal CD3+ cells (12.4 ± 7.8; Figure 4B, P < .05).
Measurement of IL-31 production by CD3+ cells from MPD or healthy volunteers. CD3+ cells (107/mL) were incubated in serum-free media containing 0.1% BSA with or without phytohemagglutinin (PHA; 2 μg/mL) at 37°C. Media was collected after 72 hours of incubation, and total RNA was extracted from each CD3+ cell population (MPD, n = 17; healthy volunteers, n = 6). (A) The levels of IL-31were assayed by ELISA. Each column represents the mean ± SD of the levels of IL-31 in each group of CD3+ cells. (B) The IL-31 mRNA expression was quantitated by measuring the threshold cycle. The data were presented as relative rates by comparing with the expression of the reference gene β-actin and were analyzed by 2−ΔΔCT method.
Measurement of IL-31 production by CD3+ cells from MPD or healthy volunteers. CD3+ cells (107/mL) were incubated in serum-free media containing 0.1% BSA with or without phytohemagglutinin (PHA; 2 μg/mL) at 37°C. Media was collected after 72 hours of incubation, and total RNA was extracted from each CD3+ cell population (MPD, n = 17; healthy volunteers, n = 6). (A) The levels of IL-31were assayed by ELISA. Each column represents the mean ± SD of the levels of IL-31 in each group of CD3+ cells. (B) The IL-31 mRNA expression was quantitated by measuring the threshold cycle. The data were presented as relative rates by comparing with the expression of the reference gene β-actin and were analyzed by 2−ΔΔCT method.
Elevated production of IL-31 by MPD MCs
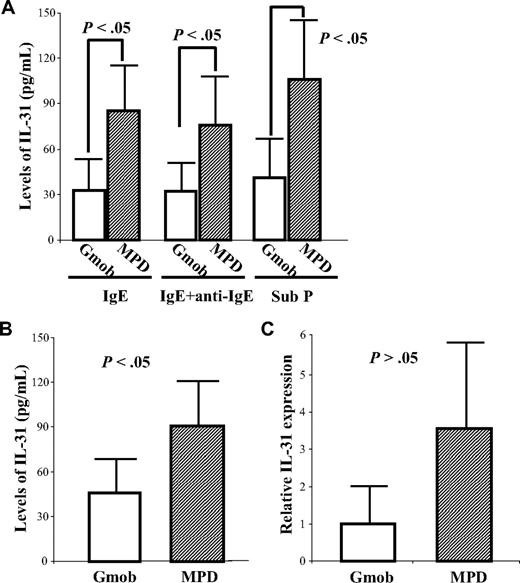
To investigate whether MCs contribute to the elevated levels of IL-31 in patients with MPDs, the secretion of IL-31 by MPD MCs (n = 19) was analyzed and compared with that by Gmob MCs (n = 8). As shown in Figure 5A, a significant amount of IL-31 was produced by Gmob MCs, indicating that MCs are another source of IL-31 besides T cells. The levels of IL-31 production by MPD MCs were significantly higher than that by Gmob MCs under stimulatory conditions, including IgE sensitization (84.9 ± 29.3 pg/mL vs 32.4 ± 22.1 pg/mL; P < .05), IgE plus anti-IgE cross-linking (75.8 ± 31.5 pg/mL vs 32.2. ± 21.5 pg/mL; P < .05), and substance P (105.3 ± 40.4 pg/mL vs 40.8 ± 27.8 pg/mL; P < .05; Figure 5A).
Elevated levels of IL-31 elaboration by MPD HCMCs. (A) HCMCs (105/mL) from 19 MPD patients and 8 Gmob volunteers were sensitized with IgE overnight and then were challenged with or without anti-IgE for 30 minutes at 37°C. A separate aliquot of MCs (105/mL) was stimulated with substance P (Sub P) for 30 minutes. The levels of IL-31 in the supernatants were measured by ELISA. (B) HCMCs (106/mL) were incubated in serum-free media for 24 hours; total RNA was then extracted from each MC population. The levels of IL-31 in the conditioned media were measured by ELISA. Each column represents the mean ± SD of the levels of IL-31 in HCMCs from each group. (C) The IL-31 mRNA expression was quantitated by measuring the threshold cycle and presented as relative rates by comparing with the expression of the reference gene β-actin. The data were analyzed by 2−ΔΔCT method.
Elevated levels of IL-31 elaboration by MPD HCMCs. (A) HCMCs (105/mL) from 19 MPD patients and 8 Gmob volunteers were sensitized with IgE overnight and then were challenged with or without anti-IgE for 30 minutes at 37°C. A separate aliquot of MCs (105/mL) was stimulated with substance P (Sub P) for 30 minutes. The levels of IL-31 in the supernatants were measured by ELISA. (B) HCMCs (106/mL) were incubated in serum-free media for 24 hours; total RNA was then extracted from each MC population. The levels of IL-31 in the conditioned media were measured by ELISA. Each column represents the mean ± SD of the levels of IL-31 in HCMCs from each group. (C) The IL-31 mRNA expression was quantitated by measuring the threshold cycle and presented as relative rates by comparing with the expression of the reference gene β-actin. The data were analyzed by 2−ΔΔCT method.
When the levels of IL-31 in the MC-conditioned media were compared, MPD MC-conditioned media (90.8 ± 30.1 pg/mL) contained greater amounts of IL-31 than Gmob MC-conditioned media (45.8 ± 22.6 pg/mL; P < .05; Figure 5B). The levels of IL-31 in media conditioned by MPD HCMCs were highly correlated with the level of IL-31 production by MPD CD3+ cells (data not shown). A considerable amount of IL-31 mRNA was also detected in both Gmob and MPD MCs by real-time PCR; however, there was no significant difference between the IL-31 transcripts in MCs from the 2 sources (Figure 5C; P > .05).
Increased migration and F-actin polymerization of MPD MCs
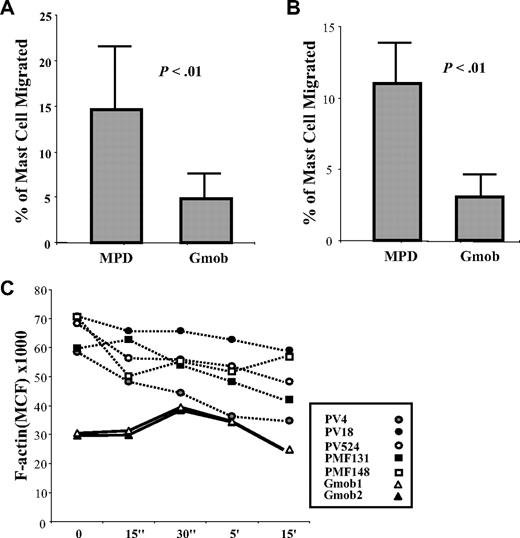
The migration of MCs and MC progenitors from blood into tissues and the migration of mature MCs within tissues are regulated by many humoral factors, including SCF and IL-8.27 We examined the SCF- or IL-8–induced migration of HCMCs from 21 MPD patients and 6 Gmob volunteers. As shown in Figure 6A, MPD MCs (14.9% ± 7.1%) exhibited significantly greater migratory ability toward SCF than Gmob MCs (4.7% ± 3.6%, P < .01). In addition, a significantly higher percentage of MPD MCs (11.3% ± 3.8%) migrated toward IL-8 compared with that of Gmob MCs (3.1% ± 1.8%; P < .01; Figure 6B).
Measurement of in vitro migration and F-actin polymerization of MPD and Gmob HCMCs. SCF (A) or IL-8 (B) induced migration of HCMCs from MPD patients (n = 21) and Gmob volunteers (n = 6). Each column represents the mean ± SD of the percentage of HCMCs from an individual PV, PMF, or Gmob volunteer, which have migrated. (C) F-actin contents in MPD (n = 5) or normal (n = 2) HCMCs. HCMCs were stimulated with 10 ng/mL SCF and fixed at the time points indicated by addition of formaldehyde. Results were expressed as mean channel fluorescence (MCF).
Measurement of in vitro migration and F-actin polymerization of MPD and Gmob HCMCs. SCF (A) or IL-8 (B) induced migration of HCMCs from MPD patients (n = 21) and Gmob volunteers (n = 6). Each column represents the mean ± SD of the percentage of HCMCs from an individual PV, PMF, or Gmob volunteer, which have migrated. (C) F-actin contents in MPD (n = 5) or normal (n = 2) HCMCs. HCMCs were stimulated with 10 ng/mL SCF and fixed at the time points indicated by addition of formaldehyde. Results were expressed as mean channel fluorescence (MCF).
Actin polymerization and depolymerization have been shown to be associated with MC migration and degranulation.24,28,29 We then compared F-actin contents in MPD and Gmob HCMCs after SCF stimulation. As shown in Figure 6C, basal levels of F-actin contents in MPD MCs were higher than that in Gmob MCs. At 30 seconds after SCF stimulation, Gmob MCs had a dramatic increase in the F-actin intensity than their base levels. The F-actin contents kept increasing and persisted in Gmob MCs up to 5 minutes of SCF stimulation. By contrast, the F-actin contents in MPD MCs were steadily reduced at each of the SCF stimulation time points examined.
Correlation of pruritus with the MPD MC functions, plasma levels of IL-31, and JAK2V617F allele burden
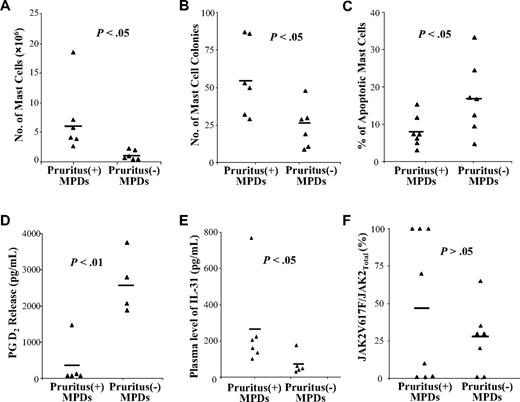
We have examined the number of MC progenitors, the production of MCs by PB CD34+ cells, and the fraction of HCMCs undergoing apoptosis from a cohort of MPD patients.17 We next correlated the presence of pruritus with the data obtained from studying these MPD patients. As shown in Figure 7, the presence of pruritus was found to be associated with a greater number of MCs generated by CD34+ cells (P < .05; Figure 7A), a greater number of MC colonies formed by CD34+ cells (P < .01; Figure 7B), decreased apoptosis of HCMCs (P < .05; Figure 7C), decreased PGD2 released by HCMCs (P < .01; Figure 7D), and higher plasma levels of IL-31 (P < .05; Figure 7E). By contrast, the presence of pruritus did not correlate with histamine, LTs, or tryptase release by MPD HCMCs (data not shown). The presence of pruritus did not correlate with the granulocyte JAK2V617F allelic burden (P > .05) but appeared to be associated with JAK2V617F homozygosity (Figure 7F).
Correlation of pruritus with the abnormal functions of MPD MCs, plasma levels of IL-31, and JAK2V617F allele burden. The correlation between patients with or without a complication of pruritus with (A) the number of MCs produced by 106 PB CD34+ cells, (B) the number of MC colonies formed by 103 PB CD34+ cells, (C) percentage of apoptotic HCMCs on overnight starvation, (D) the levels of PGD2 released by IgE + anti-IgE stimulated MCs, (E) the plasma levels of IL-31, and (F) granulocytes JAK2V617F allele burden were determined using a 2-tailed Student t test. Each bar represents the mean of each sample group.
Correlation of pruritus with the abnormal functions of MPD MCs, plasma levels of IL-31, and JAK2V617F allele burden. The correlation between patients with or without a complication of pruritus with (A) the number of MCs produced by 106 PB CD34+ cells, (B) the number of MC colonies formed by 103 PB CD34+ cells, (C) percentage of apoptotic HCMCs on overnight starvation, (D) the levels of PGD2 released by IgE + anti-IgE stimulated MCs, (E) the plasma levels of IL-31, and (F) granulocytes JAK2V617F allele burden were determined using a 2-tailed Student t test. Each bar represents the mean of each sample group.
Discussion
Ph− MPDs are clonal hematologic malignancies originating at the level of the multipotent hematopoietic stem cell.9,10 An acquired somatic mutation in JAK2, JAK2V617F has been identified in the hematopoietic cells of more than 90% of the patients with PV and 50% of the patients with ET and PMF.30-33 Using JAK2V617F, MplW515L, and chromosomal abnormalities as clonality markers, we have demonstrated that MPD MCs were involved by the malignant process in Ph− MPDs.17
A possible role of MCs in the cause of pruritus in PV has been suggested.34,35 Jackson et al have reported a correlation between the severity of pruritus and the increase of cutaneous MCs in PV.34 Recently, Abdel-Naser et al showed that the numbers of papillary dermal MCs were significantly increased in pruritic PV patients exposed to water at room temperature.35 Furthermore, obvious MC degranulation was observed in PV skin biopsy sections after warm water contact.35 Buchanan et al have shown that levels of serum tryptase were not detectable in pruritic PV patients after warm water exposure,36 and interpreted these data as evidence against MC involvement in PV-associated prutitus. We hypothesize that MPD MCs might exhibit abnormal functions that contribute to the pruritogenesis in patients with Ph− MPDs.
A variety of MC mediators, including histamine, tryptase, and LTs, are important mediators of itching in inflammatory skin diseases.1 Gilbert et al37 and Steinman et al38 have shown increased levels of histamine in the blood and urine of patients with MPDs; and the incidence of pruritus was shown to be significantly higher in patients with elevated histamine levels compared with those who had normal histamine levels. However, there were also studies that did not confirm a correlation between PV-associated pruritus and levels of blood histamine.12,34 In the present study, we showed that MPD HCMCs released increased levels of histamine and LTs than normal MCs. Histamine is not widely accepted as a key mediator of itching in Ph− MPDs as a result of only partial response to systemic antihistamines and a lack of correlation with the severity of pruritus.37 Indeed, the presence of pruritus in MPDs was not found to be correlated with the levels of histamine and LTs release by cultured MCs. Surprisingly, MPD HCMCs were found to contain decreased amounts of total tryptase, and the levels of tryptase release were similar compared with normal MCs. This is consistent with the observation of Buchanan et al, who reported that levels of serum tryptase were not detectablein PV patients with pruritus.36
PGD2 plays a role in inhibiting pruritus.39-41 Arai et al have shown that topically applied PGD2 significantly suppressed the scratching behavior of a mouse model of atopic dermatitis via their specific prostanoid DP1 receptor.39 Sugimoto et al also reported that the enhancement of scratching behavior in spontaneous dermatitis was caused by the defective production of PGD2.40,41 We found that MPD HCMCs released greater amounts of PGD2 than normal HCMCs when cells were incubated at 37°C. However, PGD2 release by MPD MCs was significantly decreased when cells were incubated at 42°C, whereas the PGD2 release by normal HCMCs was not altered. These data suggest that the PGD2 release by MPD MCs is temperature sensitive with lower production at 42°C than 37°C, which might contribute to the hot shower-induced pruritus observed in patients with Ph− MPDs. Furthermore, the presence of pruritus in MPDs was correlated with lower levels of PGD2 release by HCMCs. Interestingly, Cooper and Ahern have reported a loss of PGD2 receptors in the platelets of patients with MPDs,42 which might further impair the antipruritic effect of PGD2 in patients with MPDs.
IL-31 is mainly expressed by activated T cells, and it signals through a heterodimeric receptor composed of IL-31 receptor α and oncostatin M receptor β, which is expressed on itch-promoting cells, such as epithelial cells and keratinocytes.7 Dillon et al have shown that transgenic overexpression of IL-31 in lymphocytes induces severe pruritus and dermatitis in mice.7 We have recently reported that the deregulated production of cytokines by T cells contributed to the excessive production of hematopoietic cells in PV.20 In the present study, we found that significantly higher levels of IL-31 were produced by MPD CD3+ cells. Because T cells are polyclonal and JAK2V617F-negative in the overwhelming majority of MPD patients,19,20 the basis of this dysregulation of cytokine production appears to be independent of the JAK2V617F mutation. In addition, MPD HCMCs were found to have an increased ability of producing IL-31 as detected by ELISA. We hypothesized that the increased elaboration of IL-31 by MPD T cells and/or MCs might play a role in the pruritogenesis in patients with MPDs. Further investigation of the cellular sources and mechanisms of dysregulation of growth factor production should provide novel insights into the pathogenesis of PV.
The binding of SCF to its receptor, Kit, provides critical chemotactic signals for mature MCs and their progenitors, which facilitate homing and recruitment of MCs to various tissues.23,27 We showed that MPD HCMCs exhibited increased migratory ability toward SCF or IL-8. The aberrantly increased migratory ability of MPD MCs could result in rapid accumulation of MCs in areas with higher concentration of MC chemoattractants, such as SCF, IL-8, and SDF-1. The serum levels of IL-8 have been reported to be elevated in patients with PV,43 whereas the plasma levels of SDF-1 have been reported to be greater in patients with PMF and PV than normal volunteers.44 Polymerization and depolymerization of F-actin have been shown to drive rearrangement of the actin cytoskeleton, which is associated with the migration and degranulation of MCs.24,28,29 MPD MCs had an increased baseline F-actin content and an abnormal pattern of F-actin intensity on SCF stimulation, which might account for the abnormal migration and degranulation exhibited by the MPD MCs.
Several lines of treatment have been reported for the MPD-related itching, such as ultraviolet phototherapy, transcutaneous electrical nerve stimulation, paroxetine, cimetidine, pizotifen, aspirin, recombinant interferon-α, and interferon-α2b.16 The lack of insight into the pathogenesis of MPD-associated pruritus is partially responsible for this wide array of treatments and also for their poor efficacy.12,16,37 MCs and/or their mediators might serve as a key factor for the pruritogenesis in MPDs and are therefore potential targets for the prevention and therapy for MPD-associated pruritus. Imatinib mesylate, a tyrosine kinase inhibitor developed to treat Bcr/Abl-expressing leukemias,45 has been shown to inhibit Kit, PDGF receptor, and Abl tyrosine kinases activity. Because Kit is primarily required for the proliferation and survival of MCs, imatinib mesylate could be a useful drug to target MC-driven reactions. We have shown that erlotinib, a JAK2 inhibitor, effectively suppressed the proliferation of MPD MC progenitors,17 indicating that JAK2 inhibitors could be potential drugs to treat pruritus in MPDs by targeting MPD MCs. In addition, leukotriene receptor antagonists such as montelukast46 and PGD2 agonists such as TS-02247 could also be potential agents for the treatment of MPD-associated pruritus.
In conclusion, our study demonstrates that MPD MCs exhibited an increased production of pruritogenic factors, such as IL-31, histamine, and LTs, and a decreased elaboration of antipruritogenic factor(s), such as PGD2. The development of pruritus in Ph− MPDs could be a consequence of one or in combination of the following phenomena: (1) increased proliferation of MC progenitor cells and reduced apoptosis of MCs, (2) increased migration of MCs, which may lead to rapid accumulation of MCs surrounding the “itch receptor,” and (3) elevated elaboration of pruritogenic mediators/cytokines by MPD MCs and or T cells. These studies thus identified several cellular and molecular targets for antipruritus drug development for patients with Ph− MPDs.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Mrs Amy Rodriguez for helping us obtain patient specimens.
This work was supported by Department of Defense grant MP048007 (M.X.) and the Leukemia & Lymphoma Society of America (White Plains, NY; M.X.).
Authorship
Contribution: T.I. and J.W. performed all the experiments; J.M., R.H., and N.W. acquired the clinical information; T.I. analyzed the data and drafted the manuscript; W.Z. and Y.D. assisted in most of the experiments; and M.X. designed and supervised the studies, wrote the manuscript, and was responsible for its final draft.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Mingjiang Xu, Division of Hematology/Oncology, Tisch Cancer Institute and Department of Medicine, Mount Sinai School of Medicine, One Gustave L. Levy Place, Box 1079, New York, NY 10029-6574; e-mail: mingjiang.xu@mssm.edu.
References
Author notes
*T.I. and J.W. contributed equally to this study.