In this issue of Blood, Hollink and colleagues establish WT1 mutations as a worthy addition to a growing list of molecular abnormalities that promise to improve the biologic understanding and treatment of intermediate-risk AML.
Acute myeloid leukemia (AML) is a clinically and genetically heterogeneous disease that accounts for 20% and 70% of acute leukemia in children and adults, respectively. Currently, cytogenetic analysis at diagnosis allows for stratification of AML cases into a favorable group [t(8;21), inv(16) and t(15;17)], an unfavorable group [t(6;9), abnl(3q), -7/del(7q), -5/del(5q)], and an intermediate group (normal cytogenetics or other cytogenetic abnormalities). For the favorable and unfavorable groups, risk-dependent treatment decisions, such as whether to consolidate with hematopoietic stem cell transplantation (HSCT) in first remission, have become part of standard practice. Data upon which to base such decisions have been lacking in the intermediate- risk group, however, and 60% to 70% of cases of adult and childhood AML fall into this group.
This situation has changed in recent years with the discovery of a number of molecular abnormalities that are found primarily in the intermediate-risk group and are undetectable by standard cytogenetics. Perhaps the most significant of these is the FLT3/internal tandem duplication (FLT3/ITD), since it identifies a group of patients that not only is at high risk of relapse1 but may also benefit from novel agents that target FLT3 signaling.2 Other important abnormalities include mutations in NPM13 and CEBPA,4 each of which has been shown to identify a group of patients with a more favorable prognosis. A more recent candidate for inclusion in this list of mutations is WT1, which occurs in about 10% of cases of intermediate-risk adult AML and is associated with unfavorable prognosis. This article by Hollink et al is the first to fully characterize WT1 mutations in childhood AML.5
A major strength of this study is the use of complementary techniques to thoroughly analyze WT1 at the genomic and transcript levels. Thirty-five of 298 cases (12%) harbored WT1 mutations, most of which were frameshift insertions in exon 7. In about half of the cases, biallelic WT1 involvement could be demonstrated, due either to mutations of both alleles or to loss of heterozygosity (LOH). In cases with only one mutation, expression of both the mutant and wild-type alleles at the RNA level was confirmed, but the authors were unfortunately not able to assess expression at the protein level. Thus, the important question as to whether the wild-type protein is expressed and functional in these cases remains unanswered. This study was able to demonstrate the stability of WT1 mutations in paired diagnostic and relapse samples, making a strong case that these mutations are a primary leukemogenic event. Moreover, WT1 mutations were occasionally gained at relapse and may therefore represent a marker of disease progression.
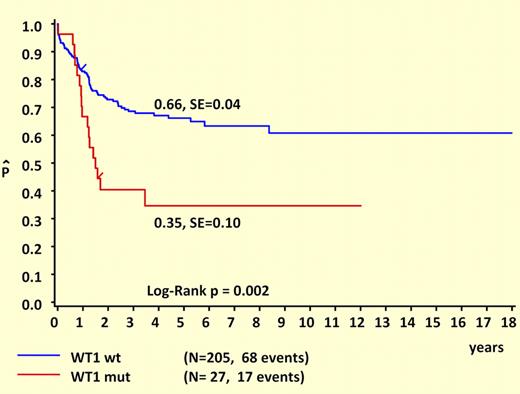
Another major strength of this study is its comprehensive clinical and prognostic analysis. Like NPM1 and FLT3/ITD mutations, WT1 mutations are almost never seen in patients younger than 3 years at diagnosis, suggesting either a prolonged latency or an age-related resistance to the initial acquisition of these lesions. Remarkably, WT1 and NPM1 mutations are mutually exclusive and are both strongly associated with FLT3/ITD mutations. It is tempting to speculate that these cases are linked by a common underlying mutation that predisposes to DNA replication errors. What “unlinks” WT1 and NPM1 are their different prognostic influences. This study makes it clear that WT1 mutations are associated with unfavorable outcomes in childhood AML, as shown in the figure. Another important difference between WT1 and NPM1 mutations is that WT1 mutations retain prognostic significance in the presence of FLT3/ITD. While the favorable influence of NPM1 mutations appears to be “trumped” by FLT3/ITD, this study suggests that the unfavorable influence of FLT3/ITD may be “trumped” by wild-type WT1, since the outcome for FLT3/ITD+ patients who lacked WT1 mutations was not significantly worse than for patients with wild-type FLT3.
Survival curves of childhood AML patients with and without WT1 mutations. Kaplan-Meier estimates for 5-year pOS. See the complete figure in the article beginning on page 5951.
Survival curves of childhood AML patients with and without WT1 mutations. Kaplan-Meier estimates for 5-year pOS. See the complete figure in the article beginning on page 5951.
What this study lacks is evidence supporting a causative link between WT1 mutations and the comparatively poor response to therapy. Of particular interest would be evidence at a functional cellular level that WT1 mutational status is associated with parameters that might predict for poor clinical outcome, such as in vitro chemoresistance or differences in apoptotic responses to DNA damage. Such findings might pave the way for WT1 mutations to serve not only as prognostic markers but as potential molecular targets for novel antileukemic therapies.
Conflict-of-interest disclosure: The author declares no competing financial interests. ■

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal