Abstract
Cell surface–associated proteolysis plays a crucial role in the migration of mononuclear phagocytes to sites of inflammation. The glycolytic enzyme enolase-1 (ENO-1) binds plasminogen at the cell surface, enhancing local plasmin production. This study addressed the role played by ENO-1 in lipopolysaccharide (LPS)–driven chemokine-directed monocyte migration and matrix invasion in vitro, as well as recruitment of monocytes to the alveolar compartment in vivo. LPS rapidly up-regulated ENO-1 cell-surface expression on human blood monocytes and U937 cells due to protein translocation from cytosolic pools, which increased plasmin generation, enhanced monocyte migration through epithelial monolayers, and promoted matrix degradation. These effects were abrogated by antibodies directed against the plasminogen binding site of ENO-1. Overexpression of ENO-1 in U937 cells increased their migratory and matrix-penetrating capacity, which was suppressed by overexpression of a truncated ENO-1 variant lacking the plasminogen binding site (ENO-1ΔPLG). In vivo, intratracheal LPS application in mice promoted alveolar recruitment of monocytic cells that overexpressed ENO-1, but not of cells overexpressing ENO-1ΔPLG. Consistent with these data, pneumonia-patients exhibited increased ENO-1 cell-surface expression on blood monocytes and intense ENO-1 staining of mononuclear cells in the alveolar space. These data suggest an important mechanism of inflammatory cell invasion mediated by increased cell-surface expression of ENO-1.
Introduction
Monocytes are circulating mononuclear phagocytes with a broad spectrum of activities and functions. In pathologic conditions associated with acute or chronic inflammation, monocytes migrate into the affected tissues and differentiate into tissue macrophages.1,2 Thus, monocytes and tissue macrophages are critical cellular components of the host defense system against infectious diseases, including forms of pneumonia, and have important functions in both native and acquired immunity. They are responsible for the phagocytosis and clearance of invading microorganisms, regulate antimicrobial programs of gene expression, are a potent source of inflammatory cytokines, and interact with T cells through cell-cell or cytokine-mediated mechanisms, thereby facilitating acquired immunity to specific pathogens.1–7 In atherosclerosis, monocytes infiltrate atherosclerotic plaques, accumulate lipid-rich material, and differentiate into macrophage-type foam cells.8 Moreover, tumor-infiltrating monocytes may play a role in tumor progression and metastasis by different mechanisms, including regulation of angiogenesis.9 Many of these activities depend upon the ability of monocytes to localize and regulate cell surface–associated proteolytic activity.
The enzymes of the fibrinolytic system represent one of the most broadly distributed cell surface–associated protease systems. The accumulation of plasminogen (PLG) and its activators, namely urokinase (u-PA) and tissue-plasminogen activator (t-PA), at the cell surface augments u-PA and t-PA catalytic efficiency, and protects these enzymes from inactivation by inhibitors.10–12 Plasmin, which is generated from PLG by limited proteolysis, plays a key role in the degradation of a provisional matrix composed of fibrin and other extracellular matrix components. These pericellular proteolytic activities facilitate cell migration and invasion.13–15 Cell-surface receptors for PLG and its activators provide a mechanism for cells to harness and regulate the activities of these proteases.16 Binding sites for PLG, t-PA, and u-PA have been identified on a variety of cell types, including monocytes, lymphocytes, granulocytes, fibroblasts, and endothelial cells.12,17 These sites include enolase-1 (ENO-1),18,19 annexin II,20,21 p11,22 histone H2B (H2B),23 and gangliosides.24 ENO-1 is a key glycolytic enzyme that acts as a 2-phospho-D-glycerate hydrolase in the cytoplasm of prokaryotic and eukaryotic cells. On the cell surface, interaction of PLG with ENO-1 enhances its activation by t-PA and protects PLG from inhibition by α2-plasmin inhibitor (α2PI). The PLG–ENO-1 interaction is mediated by binding of PLG kringle domains to the C-terminal lysine residues of ENO-1.18,19 As a consequence, PLG binding to ENO-1 is inhibited by lysine analogs, such as ϵ-aminocaproic acid or tranexamic acid (TXA). Moreover, treatment of cells with carboxypeptidase B strongly reduces cell surface–dependent binding and activation of PLG.18,25
Many pathogens (including group A Streptococci, Streptococcus pneumoniae) use extracellular ENO-1 to capture plasmin(ogen), potentially allowing bacteria to acquire surface-associated proteolytic activity.26,27 Consequently, bacterial invasion and dissemination in the infected host is markedly facilitated.28 Despite the well-documented contribution of ENO-1 to the migration of microorganisms, the role of ENO-1 in the migration and invasion of inflammatory cells to sites of infection and inflammation is largely unknown. Therefore, in the present study we asked whether ENO-1 cell-surface expression on human blood monocytes and the U937 monocytic cell line can be modulated by inflammatory stimuli, and whether ENO-1–dependent plasmin generation may contribute to monocyte migration and recruitment to the inflamed lung.
Methods
A detailed description of routine methodologies is provided in Document S1 (available on the Blood website; see the Supplemental Materials link at the top of the online article). Only nonstandard procedures and specialized materials are described here.
Patient samples
Investigations were approved by the local institutional review board at the University of Giessen Lung Center. Informed consent was obtained from the patients or their next-of-kin in accordance with the Declaration of Helsinki. Diagnosis of pneumonia was settled on the basis of typical clinical symptoms (eg, fever, tachycardia, dyspnea, tachypnea, cough) and auscultatory findings (including rales and egophony), the presence of new lung infiltrates on chest X-rays, and microbiologic identification of pathogens in the lower respiratory tract by bronchoscopy. Human peripheral blood monocytes (PBMos) were isolated from pneumonia patients and healthy volunteers by density-gradient separation.29 Cell purity was greater than 95% (determined by flow cytometry), and cell viability was greater than 95% (determined by trypan blue exclusion). Lung specimens were obtained by autopsy from 5 pneumonia patients and 5 control patients (4 patients who died of myocardial infarction and 1 patient who died from drug intoxication). In the case of control patients, pathologic conditions of the lung were ruled out by histologic examination of lung tissue sections.
Cell-associated fibrinolytic activity
The U937 cells or PBMos (1.5 × 106/mL) in phosphate-buffered saline (PBS) were preincubated with 15 μM Lys-PLG (Chromogenix, Mölndal, Sweden) for 1 hour at 37°C and washed with PBS. Plasmin generation was measured at 405 nm as a function of time after addition of 3 nM t-PA (American Diagnostica, Pfungstadt, Germany) and 0.5 mM chromogenic substrate S-2251 (Chromogenix) in 200 μL, using an EL 808 microtiter plate reader (BioTek Instruments, Highland Park, VT). In some experiments, the U937 cells or PBMos were incubated with 15 μM Lys-PLG in the presence of 10 μg/mL of a mouse monoclonal anti–ENO-1 neutralizing antibody (from Dr M. Ranson, Department of Biological Sciences, Institute for Molecular Recognition, University of Wollongong, Wollongong, Australia), 10 μg/mL IgG control (BD Biosciences, Franklin Lakes, NJ), 10 mM tranexamic acid (TXA; Sigma-Aldrich, Taufkirchen, Germany), or 30 μM α2-PI (American Diagnostica).
Migration, transmigration, and matrix invasion assays
The U937 cells or PBMos (105) were preincubated with 15 μM Lys-PLG for 1 hour at 37°C. Afterward, 3 nM t-PA was added, and cell aliquots were applied to microwell inserts with a 5-μm pore size membrane (Greiner Bio-One, Frickenhausen, Germany) that were either uncoated (migration assay), coated with a monolayer of A549 cells (transmigration assay), or coated with Matrigel (BD Biosciences; matrix invasion assay) as previously described.21 The integrity of A549 cell monolayers was assessed microscopically. The inserts were placed into the wells containing serum-free RPMI, with or without 50 ng/mL MCP-1 (R&D Systems, Wiesbaden, Germany) and incubated for 12 hours (migration and transmigration assays) or for 18 hours (matrix invasion assay) at 37°C. The cells that migrated into the lower well were counted using CasyCounter TT (Schaerfe Systems, Reutlingen, Germany). In some experiments, U937 cells were stimulated with 5 μg/mL LPS for 6 hours and then preincubated with 15 μM Lys-PLG for 1 hour in the absence or presence of 10 μg/mL anti–ENO-1 neutralizing antibody (provided by Dr M. Ranson), 10 μg/mL IgG control, or 10 mM TXA. To assess the number of cells adherent on transwell filters, the upper side of the filters was stained with 0.2% crystal violet (Sigma-Aldrich) in 2% ethanol. Excess stain was washed away. Membranes were inspected by light microscopy, where cell density on the membrane surface was reflected by blue-colored foci.
Animal experiments
Animal experiments were performed in accordance with national guidelines and were approved by the local institutional review board at University of Giessen Lung Center. Six-week-old BALB/c mice were used (n = 10 for each group). Escherichia coli LPS (0.5 mg/kg body weight [b.w.]; Sigma-Aldrich) was administered as an aerosol via microsprayer, as recently described.30 After 24 hours, mice received intravenous injections of fluorescently labeled mock-transfected as well as wild-type (WT) ENO-1 (ENO-1WT)– or ENO-1ΔPLG–overexpressing U937 cells (5 × 106 in a total volume of 100 μL PBS). The cells were fluorescently labeled using PKH26-PCL (Sigma-Aldrich) as described previously.31 After 5 hours, mice were killed with a lethal dose of ketamine and xylazine. The lungs were either subjected to bronchoalveolar lavage (BAL) or were prepared, together with spleens, for histologic examination, as recently described in detail.30 Lung cryosections (5 μm) were stained with hematoxylin-eosin and evaluated for evidence of cellular infiltrates. To examine the recruitment of PKH26-PCL-positive cells to the lung or spleen, 5-μm cryosections were fixed with 4% paraformaldehyde for 10 minutes and then either mounted with Vectashield mounting medium (Vector Laboratories, Burlingame, CA) or blocked with 3% bovine serum albumin (BSA)–PBS for 1 hour at 4°C. To asses PLG binding to the cell surface of PKH26-PCL–positive cells, the lung cryosections were incubated with an anti-PLG antibody (1:200; Abcam) overnight at 4°C. For identification of endothelial and alveolar epithelial cells, overnight incubation at 4°C was performed with an anti–von Willebrand factor (VWF; 1:100; Dako) or an anti–pro-surfactant protein C antibody (pro-SP-C; 1:400; Millipore, Billerica, MA), respectively. Slides were incubated with fluorescein isothiocyanate (FITC)–conjugated secondary antibody and mounted with Vectashield mounting medium. Nuclei were visualized by DAPI (4,6 diamidino-2-phenylindole) staining. The images were captured by a Leica DMR microscope with 20×, 40×/1.25-0.75 oil objective or 63×/1.32-0.6 oil objective at room temperature and photographed using MetaMorph 7.0 (Molecular Devices, Downingtown, PA). The images were processed with Adobe Photoshop 9.0 (Adobe Systems, San Jose, CA). Where necessary, fluorescence images were quantified by comparing the number of red-stained cells with the number of blue-stained cells in a given field. All images illustrated are representative of at least 6 other areas per section, seen on at least 3 independent sections. To examine localization of ENO-1 and PLG on the cell surface of U937 cells, in some experiments LPS-treated mice received unlabeled ENO-1WT overexpressing cells. The cells were recovered from BAL fluid by fluorescence-activated cell sorting (FACS) 5 hours after application and were stained for ENO-1 and PLG.
Statistics
All data are given as mean plus or minus SD. Statistical significance was assessed using one-way analysis of variance (ANOVA) followed by Tukey–honestly significant differences (HSD) post hoc test, and paired samples were analyzed using the paired 2-tailed Student t test. A level of P less than .05 was considered statistically significant.
Results
LPS increased cell-surface expression of ENO-1 on normal PBMos
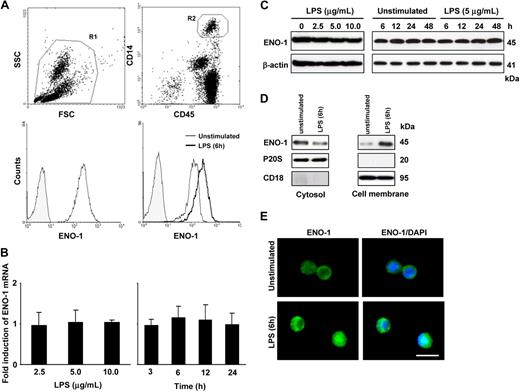
The cell-surface expression of ENO-1 on PBMos was examined by flow cytometry. The PBMo population, identified as CD45+/CD14+ cells, uniformly expressed ENO-1 on the cell surface (Figure 1A). The LPS stimulation of PBMos resulted in increased cell-surface localization of ENO-1 (Figure 1A), but did not alter the total expression of ENO-1 mRNA or protein (Figure 1B,C). To assess whether increased surface density of ENO-1 might be due to protein translocation from cytosolic pools upon stimulation, cellular proteins from PBMos were separated into cytosolic and membrane fractions, and ENO-1 levels were assessed by Western blot. Under basal conditions, ENO-1 was detected predominantly in the cytosolic fraction, however, after LPS stimulation, ENO-1 was primarily associated with the membrane fraction. Translocation of ENO-1 occurred rapidly (within 1 hour after LPS exposure; not shown) and was most pronounced at 6 hours (Figure 1D). Pronounced cell-surface expression of ENO-1 in response to LPS stimulation could also be observed by immunofluorescence (Figure 1E).
LPS stimulates the translocation to the cell surface, but does not alter total cellular abundance of ENO-1 in human PBMos. (A) Flow cytometric analysis of human blood cells (R1; top left) for ENO-1 expression in PBMos identified as CD45/CD14-positive cells (R2; top right); histogram overlay illustrating ENO-1 cell-surface expression (open histogram) in PBMos and isotype control (shaded histogram; bottom left); histogram overlay of ENO-1 cell-surface expression (open histograms) on PBMos after stimulation with 5 μg/mL LPS for 6 hours (bold line) versus unstimulated (thin line) and isotype control (shaded histogram; bottom right). (B,C) Dose-response (left panels) and time course (right panels) of ENO-1 expression in PBMos after LPS stimulation as assessed by (B) real-time polymerase chain reaction (PCR) and (C) Western blot. Real-time PCR results are expressed as the fold-increase in ENO-1 expression (normalized for β-actin expression) versus values obtained for unstimulated cells, and are mean ± SD; n = 5. The Western blot illustrated is from 1 representative experiment of 4. (D) Western blot demonstrating ENO-1 cellular localization after stimulation of PBMos with 5 μg/mL LPS for 6 hours. Cells were surface-biotinylated and then lysed, and membrane proteins were separated from cytosolic fractions using streptavidin beads. The purity of cytosolic and cell membrane fractions was assessed by probing the samples for CD18 and P20S (the 20S subunit of the proteasome), respectively. The Western blot illustrated is from 1 representative experiment of 4. (E) Immunofluorescence for the detection of ENO-1 on unstimulated and LPS-treated (5 μg/mL, 6 hours) PBMos. Original magnification 63×/1.32-0.6 oil objective. Scale bar, 5 μm.
LPS stimulates the translocation to the cell surface, but does not alter total cellular abundance of ENO-1 in human PBMos. (A) Flow cytometric analysis of human blood cells (R1; top left) for ENO-1 expression in PBMos identified as CD45/CD14-positive cells (R2; top right); histogram overlay illustrating ENO-1 cell-surface expression (open histogram) in PBMos and isotype control (shaded histogram; bottom left); histogram overlay of ENO-1 cell-surface expression (open histograms) on PBMos after stimulation with 5 μg/mL LPS for 6 hours (bold line) versus unstimulated (thin line) and isotype control (shaded histogram; bottom right). (B,C) Dose-response (left panels) and time course (right panels) of ENO-1 expression in PBMos after LPS stimulation as assessed by (B) real-time polymerase chain reaction (PCR) and (C) Western blot. Real-time PCR results are expressed as the fold-increase in ENO-1 expression (normalized for β-actin expression) versus values obtained for unstimulated cells, and are mean ± SD; n = 5. The Western blot illustrated is from 1 representative experiment of 4. (D) Western blot demonstrating ENO-1 cellular localization after stimulation of PBMos with 5 μg/mL LPS for 6 hours. Cells were surface-biotinylated and then lysed, and membrane proteins were separated from cytosolic fractions using streptavidin beads. The purity of cytosolic and cell membrane fractions was assessed by probing the samples for CD18 and P20S (the 20S subunit of the proteasome), respectively. The Western blot illustrated is from 1 representative experiment of 4. (E) Immunofluorescence for the detection of ENO-1 on unstimulated and LPS-treated (5 μg/mL, 6 hours) PBMos. Original magnification 63×/1.32-0.6 oil objective. Scale bar, 5 μm.
LPS induced the translocation of ENO-1 to the cell surface of U937 cells
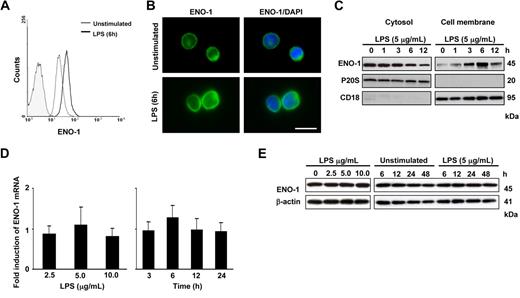
Similar to PBMos, U937 cells expressed cell-surface ENO-1, and cell-surface expression was increased after LPS stimulation (Figure 2A,B). Western blot analysis of cell membrane and cytosolic fractions from U937 cells revealed maximal ENO-1 translocation to the cell membrane after 6 hours of stimulation (Figure 2C), while the total mRNA or protein expression of ENO-1 remained unaltered (Figure 2D,E). It was previously noted that plasminogen binding is elevated at the cell surface of apoptotic or necrotic cells.32 To exclude the possibility that the observed increase in ENO-1 cell-surface expression was due to apoptosis or necrosis, these 2 parameters were assessed in U937 cells and in PBMos after LPS challenge. No appreciable increase in the number of apoptotic U937 cells and PBMos was observed after a 6-hour LPS stimulation when the strongest increase in Eno-1 surface expression was noted (Figure S1). No appreciable changes in the population of necrotic cells were observed after this 6-hour LPS stimulation either (data not shown). After 24-hour LPS stimulation, a small increase in apoptosis was observed in U937 cells (Figure S1). However, in all subsequent experiments, cells were stimulated with LPS for 6 hours.
LPS increases the cell-surface abundance of ENO-1 on U937 cells. (A) Histogram overlay of ENO-1 cell-surface expression (open histograms) on U937 cells after stimulation with 5 μg/mL LPS for 6 hours (bold line) versus unstimulated (thin line) and isotype control (shaded histogram). (B) Immunofluorescence for ENO-1 on unstimulated and LPS (5 μg/mL, 6 hours)–treated U937 cells. Original magnification 63×/1.32-0.6 oil objective. Bar size 5 μm. (C) Western blot demonstrating the cellular localization of ENO-1 after stimulation with 5 μg/mL LPS for 0 to 12 hours. Cells were surface-biotinylated, and streptavidin beads were used to separate membrane proteins from proteins resident in the cytosol. The purity of cell membrane and cytosolic fractions was assessed by probing the samples for P20S and CD18, respectively. The Western blot illustrated is from 1 representative experiment of 4. (D,E) Dose response (left panels) and time course (right panels) of ENO-1 expression in U937 cells after LPS stimulation as assessed by (D) real-time PCR and (E) Western blot. Real-time PCR results are expressed as the fold-increase in ENO-1 expression (normalized for β-actin expression) versus values obtained for unstimulated cells and are mean ± SD; n = 5. The Western blot illustrated is from 1 representative experiment of 4.
LPS increases the cell-surface abundance of ENO-1 on U937 cells. (A) Histogram overlay of ENO-1 cell-surface expression (open histograms) on U937 cells after stimulation with 5 μg/mL LPS for 6 hours (bold line) versus unstimulated (thin line) and isotype control (shaded histogram). (B) Immunofluorescence for ENO-1 on unstimulated and LPS (5 μg/mL, 6 hours)–treated U937 cells. Original magnification 63×/1.32-0.6 oil objective. Bar size 5 μm. (C) Western blot demonstrating the cellular localization of ENO-1 after stimulation with 5 μg/mL LPS for 0 to 12 hours. Cells were surface-biotinylated, and streptavidin beads were used to separate membrane proteins from proteins resident in the cytosol. The purity of cell membrane and cytosolic fractions was assessed by probing the samples for P20S and CD18, respectively. The Western blot illustrated is from 1 representative experiment of 4. (D,E) Dose response (left panels) and time course (right panels) of ENO-1 expression in U937 cells after LPS stimulation as assessed by (D) real-time PCR and (E) Western blot. Real-time PCR results are expressed as the fold-increase in ENO-1 expression (normalized for β-actin expression) versus values obtained for unstimulated cells and are mean ± SD; n = 5. The Western blot illustrated is from 1 representative experiment of 4.
Increased ENO-1 cell-surface localization led to enhanced pericellular proteolysis in U937 cells and PBMos
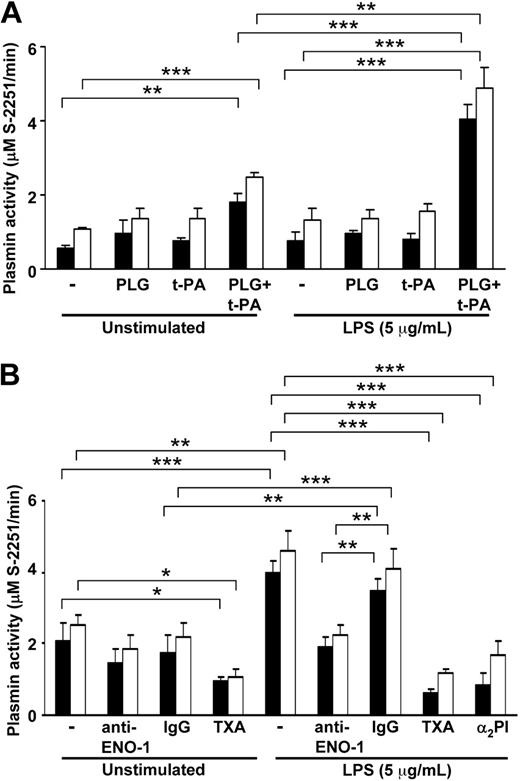
Cell-surface ENO-1 may act as a docking protein for PLG.18,19 Consequently, we explored whether the LPS-induced increase in ENO-1 cell-surface abundance might result in increased pericellular proteolytic activity. The t-PA–dependent plasmin generation at the monocyte cell surface was evaluated by measuring hydrolysis of S-2251. Low basal proteolytic activity was found in unstimulated U937 cells and PBMos in the presence of 3 nM t-PA and 15 μM Lys-PLG that was mildly affected by the presence of an antibody directed against the PLG binding site of ENO-1 (Figure 3A,B). After stimulation with LPS, cell-surface plasmin activity was increased by approximately 2-fold in both U937 cells and PBMos (Figure 3A). An antibody directed against the PLG binding site of ENO-1, but not an isotype control antibody, reduced plasmin proteolytic activity in both U937 cells and PBMos to basal levels observed in unstimulated cells (Figure 3B). The lysine analog TXA blocked the bulk of plasmin formation by unstimulated and LPS-treated cells (Figure 3B). Similarly, addition of α2-PI together with PLG also blocked the bulk of plasmin formation by LPS-treated cells (Figure 3B). This observation is in line with a previous study demonstrating that α2-PI–derived peptides can block PLG binding to the surface of U937 cells.33 Titration experiments, where the Lys-PLG concentration was varied between 2.5 μM (approximating normal physiologic PLG concentrations34 ) and 15 μM, yielded similar results (not shown). Together, these results indicate that the LPS-induced increase in cell-surface ENO-1 density on U937 cells and PBMos was largely responsible for the elevated cell-surface plasmin formation in both cell types under these conditions.
LPS-induced ENO-1 cell-surface expression results in increased plasmin generation at the cell surface of U937 cells and human blood monocytes. (A) U937 cells (■) or human blood monocytes (□) exposed to 5 μg/mL LPS for 6 hours, as well as unstimulated cells, were preincubated with 15 μM Lys-PLG for 1 hour at 37°C followed by addition of 3 nM t-PA, as indicated. Plasmin proteolytic activity was followed as the conversion of the chromogenic substrate S-2251 (0.5 mM) at 405 nm. Data represent mean values ± SD from 4 independent experiments, each performed in quintuplicate, **P < .01; ***P < .001. (B) U937 cells (■) or human blood monocytes (□) exposed to 5 μg/mL LPS for 6 hours as well as unstimulated cells were preincubated with 15 μM Lys-PLG in the absence (−) or presence of an anti–ENO-1 neutralizing antibody (10 μg/mL), IgG control (10 μg/mL), tranexamic acid (TXA; 10 mM), or α2PI (30 μM). Afterward, 3 nM t-PA and 0.5 mM chromogenic substrate S-2251 were added. Plasmin proteolytic activity was followed as the conversion of the S-2251 substrate at 405 nm. Data represent mean values ± SD from 4 independent experiments, each performed in quintuplicate; *P < .05; **P < .01; ***P < .001.
LPS-induced ENO-1 cell-surface expression results in increased plasmin generation at the cell surface of U937 cells and human blood monocytes. (A) U937 cells (■) or human blood monocytes (□) exposed to 5 μg/mL LPS for 6 hours, as well as unstimulated cells, were preincubated with 15 μM Lys-PLG for 1 hour at 37°C followed by addition of 3 nM t-PA, as indicated. Plasmin proteolytic activity was followed as the conversion of the chromogenic substrate S-2251 (0.5 mM) at 405 nm. Data represent mean values ± SD from 4 independent experiments, each performed in quintuplicate, **P < .01; ***P < .001. (B) U937 cells (■) or human blood monocytes (□) exposed to 5 μg/mL LPS for 6 hours as well as unstimulated cells were preincubated with 15 μM Lys-PLG in the absence (−) or presence of an anti–ENO-1 neutralizing antibody (10 μg/mL), IgG control (10 μg/mL), tranexamic acid (TXA; 10 mM), or α2PI (30 μM). Afterward, 3 nM t-PA and 0.5 mM chromogenic substrate S-2251 were added. Plasmin proteolytic activity was followed as the conversion of the S-2251 substrate at 405 nm. Data represent mean values ± SD from 4 independent experiments, each performed in quintuplicate; *P < .05; **P < .01; ***P < .001.
ENO-1–dependent directed invasion and migration of U937 cells and PBMos
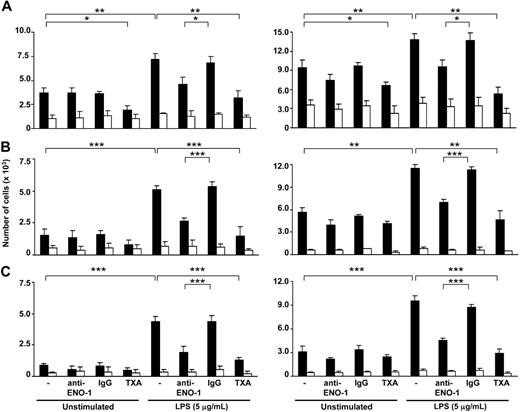
The importance of ENO-1–induced cell-surface plasmin activity in the directed migration, transmigration through epithelial monolayers, and matrix invasion of LPS-stimulated U937 cells and PBMos toward the chemokine MCP-1 was examined. In unstimulated cells, inhibition of ENO-1 with an anti–ENO-1 antibody did not significantly alter the directed migration of U937 cells or PBMos through 5-μm polycarbonate filters (Figure 4A). Transmigration through a monolayer of human lung A549 epithelial cells and cell invasion into Matrigel also remained unaffected (Figure 4B,C). Preincubation of U937 cells or PBMos with LPS resulted in a significant increase in directed migration through the polycarbonate membrane, transmigration through a A549 cell monolayer, and Matrigel invasion (Figure 4A-C). The presence of an anti–ENO-1 antibody almost completely abrogated this LPS-induced increase in directed migration, transmigration, and invasion in both cell types (Figure 4A-C).
ENO-1 mediates the directed migration, transmigration, and invasion of LPS-activated U937 cells and human blood monocytes. U937 cells (left panel) or human blood monocytes (right panel) exposed to 5 μg/mL LPS for 6 hours as well as unstimulated cells were preincubated with 15 μM Lys-PLG in the absence (−) or presence of anti–ENO-1 antibodies (10 μg/mL), IgG control (10 μg/mL), or tranexamic acid (TXA; 10 mM). Subsequently, 3 nM t-PA was added, and the cells seeded onto inserts were analyzed for directed migration through a porous (5-μm) polycarbonate membrane (A), directed transmigration through a monolayer of A549 cells (B), or invasion through Matrigel (C). The inserts were placed into wells without (□) or with MCP-1 (■) and incubated for 12 or 18 hours at 37°C. The cells present in the lower chamber were counted. Data represent mean values ± SD from 4 independent experiments, each performed in quintuplicate; *P < .05; **P < .01; ***P < .001.
ENO-1 mediates the directed migration, transmigration, and invasion of LPS-activated U937 cells and human blood monocytes. U937 cells (left panel) or human blood monocytes (right panel) exposed to 5 μg/mL LPS for 6 hours as well as unstimulated cells were preincubated with 15 μM Lys-PLG in the absence (−) or presence of anti–ENO-1 antibodies (10 μg/mL), IgG control (10 μg/mL), or tranexamic acid (TXA; 10 mM). Subsequently, 3 nM t-PA was added, and the cells seeded onto inserts were analyzed for directed migration through a porous (5-μm) polycarbonate membrane (A), directed transmigration through a monolayer of A549 cells (B), or invasion through Matrigel (C). The inserts were placed into wells without (□) or with MCP-1 (■) and incubated for 12 or 18 hours at 37°C. The cells present in the lower chamber were counted. Data represent mean values ± SD from 4 independent experiments, each performed in quintuplicate; *P < .05; **P < .01; ***P < .001.
The impact of the LPS-induced increase in ENO-1 cell-surface expression on the directed migration of U937 cells through the polycarbonate membrane was unexpected and was, therefore, confirmed by visual inspection of transwell filters (Figure S2), where the increase in ENO-1 cell-surface expression by LPS resulted in fewer U937 cells attached to the upper side of the transwell membrane compared with unstimulated cells, which is consistent with the increased directed migration seen with these cells in response to LPS stimulation. We have further demonstrated that plasmin formation is required for this process, because in the absence of Lys-PLG and t-PA, LPS stimulation of U937 cells and PBMos did not impact the directed migration of cells toward MCP-1 (Figure S3). Together, these data demonstrate the importance of ENO-1 in the recruitment of cell-surface plasmin activity, leading to increased migratory, transmigratory, and invasive properties of LPS-stimulated U937 cells and PBMos.
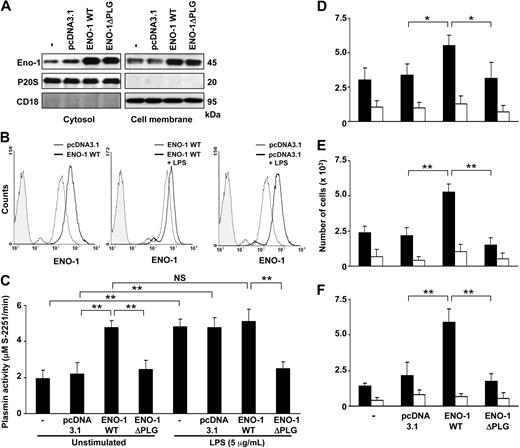
Overexpression of ENO-1 potentiated the directed migration, transmigration, and invasion of U937 cells
To further assess the importance of ENO-1 in the physiologic processes of monocytes, wild-type ENO-1 (ENO-1WT) or an ENO-1 variant in which the PLG binding site was removed (ENO-1ΔPLG) were overexpressed in U937 cells. Overexpression of ENO-1 resulted in increased cytosolic and cell-membrane ENO-1 levels (Figure 5A). Enhanced cell-surface localization was also confirmed by flow cytometry (Figure 5B left panel). In contrast to U937 cells that had been transfected with an empty control vector (Figure 5B right panel), the LPS stimulation of ENO-1WT–transfected cells produced only a small increase in cell-surface density of ENO-1 (Figure 5B middle panel). This indicates that overexpression of ENO-1WT in U937 cells saturates the amount of this protein on the cell surface. Accordingly, overexpression of ENO-1WT in unstimulated U937 cells resulted in strongly increased plasmin proteolytic activity; however, plasmin generation was—in contrast to unstimulated, untransfected, or empty vector-transfected cells—only slightly further enhanced by LPS stimulation (Figure 5C). Transfection of U937 cells with ENO-1ΔPLG did not result in increased plasmin proteolytic activity irrespective of the presence of LPS. Next, the impact of overexpressing ENO-1 on the directed migration, transmigration, and invasion properties of U937 cells was examined. In the presence of PLG and t-PA, overexpression of ENO-1WT resulted in significantly increased directed migration through the polycarbonate membrane (Figure 5D), transmigration through a A549 cell monolayer (Figure 5E), and invasion into Matrigel (Figure 5F) of U937 cells in response to MCP-1, compared with mock-transfected cells or to ENO-1ΔPLG–expressing cells. The unexpected impact of ENO-1WT overexpression on the directed migration through polycarbonate membranes was confirmed by visual inspection of transwell filters (Figure S4), where overexpression of ENO-1WT resulted in fewer cells attached to the upper side of the membrane compared with mock- or ENO-1ΔPLG–transfected cells, which is consistent with the increased directed migration seen with ENO-1WT overexpression.
Overexpression of ENO-1 by U937 cells increased cell-surface plasmin generation and enhanced directed migratory, transmigratory, and invasive properties. (A) ENO-1 expression was examined in stably transfected U937 cells overexpressing ENO-1 wild-type (ENO-1WT) or ENO-1 lacking the plasminogen binding site (ENO-1ΔPLG), as well as in mock-transfected (pcDNA3.1) or native (−) cells. The ENO-1 located in the cytosol (left panels) or associated with the cell membrane (right panels) was determined by Western blot. The purity of cytosolic and membrane fractions was assessed by probing the samples for CD18 and P20S, respectively. The Western blot illustrated is from 1 representative experiment of 4. (B) Comparison of ENO-1 cell-surface expression by U937 stable transfectants using flow cytometry. (Left panel) Unstimulated cells transfected with pcDNA3.1 (thin line) versus ENO-1WT (bold line); (middle panel) ENO-1WT–transfected cells stimulated with LPS (5 μg/mL, 6 hours; bold line) versus unstimulated (thin line); (right panel) pcDNA3.1-transfected cells stimulated with LPS (5 μg/mL, 6 hours; bold line) versus unstimulated (thin line). Isotype controls are represented by the shaded histograms. One representative experiment of 3 is illustrated. (C) Plasmin proteolytic activity as measured by S-2251 degradation by stably transfected U937 cells as indicated, either in the absence or presence of LPS (5 μg/mL LPS, 6 hours). Data represent mean values ± SD from 4 independent experiments, each performed in quintuplicate. Directed migration (D), transmigration (E), and invasion (F) of unstimulated stably transfected U937 cells toward the chemokine MCP-1 (■), compared with basal activity in absence of MCP-1 (□). The experiments were conducted in analogy with those described in Figure 4. Data represent mean values ± SD from 4 independent experiments, each performed in quintuplicate. NS indicates not significant; *P < .05; **P < .01.
Overexpression of ENO-1 by U937 cells increased cell-surface plasmin generation and enhanced directed migratory, transmigratory, and invasive properties. (A) ENO-1 expression was examined in stably transfected U937 cells overexpressing ENO-1 wild-type (ENO-1WT) or ENO-1 lacking the plasminogen binding site (ENO-1ΔPLG), as well as in mock-transfected (pcDNA3.1) or native (−) cells. The ENO-1 located in the cytosol (left panels) or associated with the cell membrane (right panels) was determined by Western blot. The purity of cytosolic and membrane fractions was assessed by probing the samples for CD18 and P20S, respectively. The Western blot illustrated is from 1 representative experiment of 4. (B) Comparison of ENO-1 cell-surface expression by U937 stable transfectants using flow cytometry. (Left panel) Unstimulated cells transfected with pcDNA3.1 (thin line) versus ENO-1WT (bold line); (middle panel) ENO-1WT–transfected cells stimulated with LPS (5 μg/mL, 6 hours; bold line) versus unstimulated (thin line); (right panel) pcDNA3.1-transfected cells stimulated with LPS (5 μg/mL, 6 hours; bold line) versus unstimulated (thin line). Isotype controls are represented by the shaded histograms. One representative experiment of 3 is illustrated. (C) Plasmin proteolytic activity as measured by S-2251 degradation by stably transfected U937 cells as indicated, either in the absence or presence of LPS (5 μg/mL LPS, 6 hours). Data represent mean values ± SD from 4 independent experiments, each performed in quintuplicate. Directed migration (D), transmigration (E), and invasion (F) of unstimulated stably transfected U937 cells toward the chemokine MCP-1 (■), compared with basal activity in absence of MCP-1 (□). The experiments were conducted in analogy with those described in Figure 4. Data represent mean values ± SD from 4 independent experiments, each performed in quintuplicate. NS indicates not significant; *P < .05; **P < .01.
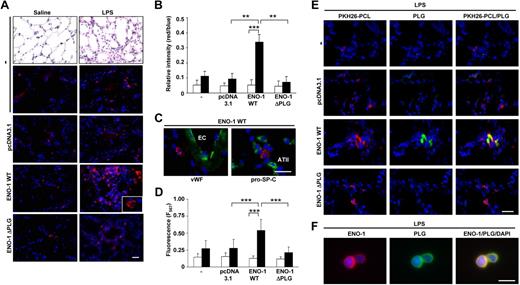
ENO-1 overexpression increased recruitment of monocytic cells to the acutely inflamed lung
As overexpression of ENO-1 enhanced the directed migration, transmigration, and invasion of U937 cells in vitro, the role of ENO-1 on monocyte recruitment in vivo was examined. We assessed the impact of ENO-1 on monocytic cell recruitment to the alveolar compartment of the acutely inflamed lung. For this, U937 cells were fluorescently labeled with PKH26-PCL and injected intravenously 24 hours after intratracheal LPS administration. Mouse lungs were analyzed 5 hours after injection of the cells. In contrast to cells transfected with the pcDNA3.1 control vector or cells overexpressing ENO-1 that lacked the PLG binding site (ENO-1ΔPLG), pronounced recruitment to the acutely inflamed lungs of cells overexpressing ENO-1WT was observed (Figure 6A), which was confirmed by quantitative evaluation of the lung sections (Figure 6B). Further evaluation of lung sections from LPS-challenged mice receiving monocytic cells overexpressing ENO-1WT revealed accumulation of these cells within alveolar walls (Figure 6C). Moreover, LPS-treated mice receiving monocytic cells overexpressing ENO-1WT demonstrated an increased fluorescent signal from PKH26-PCL–labeled cells in bronchoalveolar lavage (BAL) fluid, whereas animals that received mock-transfected cells or cells expressing the ΔPLG variant of ENO-1 remained at basal levels (Figure 6D). Recruited U937 cells overexpressing ENO-1WT in the lungs of LPS-treated mice exhibited strong staining for plasminogen (Figure 6E), as did U937 cells recovered by BAL from treated mice (Figure 6F). No differences were observed in the accumulation of U937 cells in the spleens of mice after intratracheal LPS application, irrespective of whether these cells expressed ENO-1WT or the ΔPLG variant of ENO-1 (Figure S5).
ENO-1 overexpression facilitates recruitment of monocytic cells to the alveolar compartment of LPS-treated mice. BALB/c mice were challenged intratracheally with either saline or LPS (0.5 mg/kg b.w.). After 24 hours, mice received an intravenous injection of PKH26-PCL–labeled (red color) stably transfected U937 cells (5 × 106 in a total volume of 100 μL PBS). Mice were then killed 5 hours after injection. (A) Representative lung tissue sections from saline- (left panel) or LPS- (right panel) treated mice injected with untransfected cells (−), mock-transfected cells (pcDNA3.1), cells overexpressing ENO-1 wild-type (ENO-1WT), or cells overexpressing ENO-1 lacking the plasminogen binding site (ENO-1ΔPLG). Original magnification ×20. Bar size 20 μm. Insert, original magnification 63×/1.32-0.6 oil objective. (B) Relative intensity of red/blue staining presented in panel A. Relative intensity of red/blue staining was determined by counting 4 randomly chosen fields per section using 3 independent sections for each group. □ represents saline-treated animals, while ■ represents LPS-treated mice. **P < .01; ***P < .001 (C) Localization of PKH26-PCL–positive monocytic cells overexpressing ENO-1WT (red color) within the alveolar wall. Sections were immunostained with the endothelial marker von Willebrand factor (VWF; green color; left panel) or with the alveolar epithelial type II cell marker prosurfactant protein-C (pro-SP-C; green color; right panel). EC indicates endothelial cells; and ATII, alveolar epithelial type II cells. Original magnification 63×/1.32-0.6 oil objective. Bar size 5 μm. (D) Fluorescence signal from PKH26-PCL–labeled monocytic cells present in bronchoalveolar lavage fluids obtained from saline- (□) or LPS- (■) treated mice. Mean values ± SD are reported; with n = 10 for each group; ***P < .001. (E) Representative lung tissue sections from LPS-treated mice injected with PKH26-PCL–labeled (red color) untransfected cells (−), mock-transfected cells (pcDNA3.1), cells overexpressing ENO-1 wild-type (ENO-1WT), or cells overexpressing ENO-1 lacking the plasminogen binding site (ENO-1ΔPLG) stained for PLG (green color). Original magnification 40×/1.25-0.75 oil objective. Bar size 10 μm. (F) Cellular localization of ENO-1 and PLG in U937 cells overexpressing ENO-1 recovered from bronchoalveolar lavage fluid of LPS-treated mice. Original magnification 63×/1.32-0.6 oil objective. Bar size 5 μm.
ENO-1 overexpression facilitates recruitment of monocytic cells to the alveolar compartment of LPS-treated mice. BALB/c mice were challenged intratracheally with either saline or LPS (0.5 mg/kg b.w.). After 24 hours, mice received an intravenous injection of PKH26-PCL–labeled (red color) stably transfected U937 cells (5 × 106 in a total volume of 100 μL PBS). Mice were then killed 5 hours after injection. (A) Representative lung tissue sections from saline- (left panel) or LPS- (right panel) treated mice injected with untransfected cells (−), mock-transfected cells (pcDNA3.1), cells overexpressing ENO-1 wild-type (ENO-1WT), or cells overexpressing ENO-1 lacking the plasminogen binding site (ENO-1ΔPLG). Original magnification ×20. Bar size 20 μm. Insert, original magnification 63×/1.32-0.6 oil objective. (B) Relative intensity of red/blue staining presented in panel A. Relative intensity of red/blue staining was determined by counting 4 randomly chosen fields per section using 3 independent sections for each group. □ represents saline-treated animals, while ■ represents LPS-treated mice. **P < .01; ***P < .001 (C) Localization of PKH26-PCL–positive monocytic cells overexpressing ENO-1WT (red color) within the alveolar wall. Sections were immunostained with the endothelial marker von Willebrand factor (VWF; green color; left panel) or with the alveolar epithelial type II cell marker prosurfactant protein-C (pro-SP-C; green color; right panel). EC indicates endothelial cells; and ATII, alveolar epithelial type II cells. Original magnification 63×/1.32-0.6 oil objective. Bar size 5 μm. (D) Fluorescence signal from PKH26-PCL–labeled monocytic cells present in bronchoalveolar lavage fluids obtained from saline- (□) or LPS- (■) treated mice. Mean values ± SD are reported; with n = 10 for each group; ***P < .001. (E) Representative lung tissue sections from LPS-treated mice injected with PKH26-PCL–labeled (red color) untransfected cells (−), mock-transfected cells (pcDNA3.1), cells overexpressing ENO-1 wild-type (ENO-1WT), or cells overexpressing ENO-1 lacking the plasminogen binding site (ENO-1ΔPLG) stained for PLG (green color). Original magnification 40×/1.25-0.75 oil objective. Bar size 10 μm. (F) Cellular localization of ENO-1 and PLG in U937 cells overexpressing ENO-1 recovered from bronchoalveolar lavage fluid of LPS-treated mice. Original magnification 63×/1.32-0.6 oil objective. Bar size 5 μm.
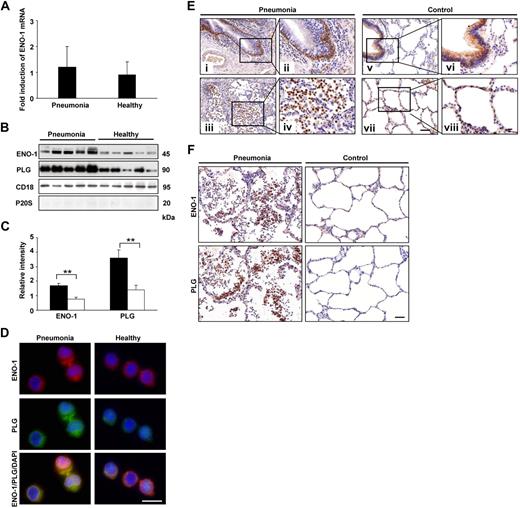
Pneumonia patients exhibit elevated levels of ENO-1 on the surface of PBMos and intense ENO-1 staining on mononuclear cells in the alveolar compartment
To support the conclusions drawn from our in vitro cell culture and in vivo animal model experiments, the expression of ENO-1 in PBMos and lung tissue obtained from pneumonia patients was examined. The ENO-1 mRNA levels in PBMos revealed no differences in ENO-1 expression between pneumonia patients and healthy controls (Figure 7A). However, similar to our in vitro data demonstrating increased cell-surface localization of ENO-1 in response to LPS stimulation, membrane fractions of PBMos from pneumonia patients exhibited a significantly elevated abundance of ENO-1 compared with healthy controls (Figure 7B,C). The elevated abundance of ENO-1 was associated with significantly increased levels of PLG in cell-membrane fractions of PBMos from pneumonia patients (Figure 7B,C). Dual staining with anti–ENO-1 and anti-PLG antibodies also suggested an increased abundance of ENO-1 and PLG, and a strong colocalization in PBMos from pneumonia patients (Figure 7D). Lung sections from pneumonia patients revealed intense ENO-1 staining in basal bronchial epithelial cells (Figure 7Ei,ii) as well as in mononuclear cells in the alveolar compartment (Figure 7Eiii,iv). In control lungs, ENO-1 staining was observed exclusively in bronchial epithelial cells (Figure 7Ev-viii). Moreover, intense ENO-1 staining on lung mononuclear cells in pneumonia patients correlated with strong staining for PLG on these cells (Figure 7F).
ENO-1 expression on blood monocytes and in lung tissue from pneumonia patients. (A) ENO-1 mRNA expression in blood monocytes obtained from pneumonia patients and healthy controls was assessed by real-time PCR. Results are expressed as fold-increase of the ratio of ENO-1/β-actin over control values (healthy controls) and are mean ± SD; n = 5. (B) Western blot illustrating cell-membrane localization of ENO-1 and PLG on blood monocytes from pneumonia patients and healthy controls. Streptavidin beads were used to separate biotinylated membrane proteins from nonbiotinylated cytosolic proteins. The purity of the cell-membrane fraction was assessed by probing the samples for P20S. CD18 was used as loading control. (C) Densitometric analysis of panel B. □ represents healthy subjects, while ■ represents pneumonia patients. **P < .01. (D) Cellular localization of ENO-1 and PLG in monocytes isolated from the blood of pneumonia patients and healthy controls. Original magnification 63×/1.32-0.6 oil objective. Bar size 5 μm. (E) Representative lung tissue sections from pneumonia patients (i-iv) and controls (v-viii) were stained for ENO-1. In lungs from pneumonia patients, strong immunoreactivity for ENO-1 was observed in basal bronchial epithelial cells (i,ii) and mononuclear cells (iii,iv). In lungs from control patients, ENO-1 staining was observed in bronchial epithelial cells (v-viii). Original magnification ×20 (i,iii,v,vii), or ×40 (ii,iv,vi,viii). Bar size 20 μm. Lung sections from 1 representative pneumonia patient and 1 control patient of 5 per group are illustrated. (F) Representative lung tissue sections from pneumonia patients and control patients stained for ENO-1 and PLG. Original magnification ×40. Bar size 20 μm.
ENO-1 expression on blood monocytes and in lung tissue from pneumonia patients. (A) ENO-1 mRNA expression in blood monocytes obtained from pneumonia patients and healthy controls was assessed by real-time PCR. Results are expressed as fold-increase of the ratio of ENO-1/β-actin over control values (healthy controls) and are mean ± SD; n = 5. (B) Western blot illustrating cell-membrane localization of ENO-1 and PLG on blood monocytes from pneumonia patients and healthy controls. Streptavidin beads were used to separate biotinylated membrane proteins from nonbiotinylated cytosolic proteins. The purity of the cell-membrane fraction was assessed by probing the samples for P20S. CD18 was used as loading control. (C) Densitometric analysis of panel B. □ represents healthy subjects, while ■ represents pneumonia patients. **P < .01. (D) Cellular localization of ENO-1 and PLG in monocytes isolated from the blood of pneumonia patients and healthy controls. Original magnification 63×/1.32-0.6 oil objective. Bar size 5 μm. (E) Representative lung tissue sections from pneumonia patients (i-iv) and controls (v-viii) were stained for ENO-1. In lungs from pneumonia patients, strong immunoreactivity for ENO-1 was observed in basal bronchial epithelial cells (i,ii) and mononuclear cells (iii,iv). In lungs from control patients, ENO-1 staining was observed in bronchial epithelial cells (v-viii). Original magnification ×20 (i,iii,v,vii), or ×40 (ii,iv,vi,viii). Bar size 20 μm. Lung sections from 1 representative pneumonia patient and 1 control patient of 5 per group are illustrated. (F) Representative lung tissue sections from pneumonia patients and control patients stained for ENO-1 and PLG. Original magnification ×40. Bar size 20 μm.
Discussion
Plasmin is a key enzyme of intravascular fibrinolysis, but it is also known to have a broad spectrum of extravascular functions related to cell invasion processes, where the degradation of the extracellular matrix is a prerequisite for cell migration across the matrix barrier.13–15 The recruitment of PLG to the cell surface potentiates local plasmin generation and endows the cells with pericellular proteolytic activity.10–17 These properties depend upon the capacity of PLG to bind to C-terminal lysine residues in its receptors. Therefore, the blockade (by TXA) or removal (by plasma carboxypeptidase B, pCPB) of C-terminal lysine residues severely impairs plasmin generation and cell mobility.18,25 The prominent role of the PLG system and PLG-PLG receptor interaction in the recruitment of inflammatory cells has been demonstrated in different inflammatory models conducted in PLG−/−35,36 or pCPB−/−37 mice.
Despite the well-documented presence of ENO-1 on the surface of monocytic cells,18,19 the importance of ENO-1 in the migration and invasion of these cells—in particular, when they are exposed to an inflammatory stimulus—has not been addressed in detail to date. Our data demonstrate for the first time that LPS induces an increase in the cell-surface expression of ENO-1 on monocytic cells and PBMos, which results in enhanced plasmin proteolytic activity at the cell surface. Consequently, the ENO-1–mediated increase in plasmin formation promotes the directed migration and matrix invasion of monocytic cells and facilitates their infiltration into the inflamed lung.
Previous studies have reported the differential regulation of PLG receptors by various stimuli, which indicates that changing environmental conditions may alter the surface proteolytic capacities of cells.38,39 In this regard, Felez et al38 demonstrated elevated cell-surface PLG receptor expression on human monocytic cell lines after stimulation with phorbol ester or vitamin D-3. Of note, in the case of the human monocytic THP-1 cell line, the up-regulation of PLG receptors on nonadherent THP-1 cells required the presence of an adherent population of cells and was not observed when other inflammatory stimuli were used, including tumor necrosis factor-α (TNF-α), interleukin-1α (IL-1α), and LPS.38 In contrast, our results demonstrated that LPS markedly up-regulated ENO-1 density on the surface of U937 cells, which were maintained in suspension. These differences most likely arise from the distinct cell lines investigated and/or the different time points used to analyze PLG receptor expression. Moreover, Felez et al38 examined the changes in the total number of PLG binding sites on the cell surface, whereas our study was strictly focused on ENO-1. We then expanded these observations, demonstrating that PBMos share the capacity to up-regulate cell-surface expression of ENO-1 after LPS stimulation.
The LPS-induced increase in ENO-1 cell-surface expression on U937 cells and PBMos occurred rapidly, within 1 hour after LPS exposure. The rapid increase in cell surface–associated ENO-1 strongly suggests the possibility of an enhanced transport of the molecule from the cytosol to the cell surface. Thus, ENO-1, which is already present in large quantities in the cytoplasm, may be actively trafficked to the cell surface, allowing monocytes to rapidly react to inflammatory stimuli. In line with these ideas, we demonstrated a significant increase in the amount of ENO-1 associated with the cell-membrane fractions of U937 cells and human PBMos in response to LPS challenge, while the ENO-1 content of the cytosolic fraction decreased, and no change in total ENO-1 abundance was noted, at the mRNA or protein level. However, these observations do not exclude the possibility that activation of monocytes not only increased the amount of ENO-1 on the cell surface but also induced a conformational change in ENO-1, thereby enhancing the accessibility of C-terminal lysine residues for PLG. Notably, no change in the amount of the PLG receptor annexin II at the cell surface was detected during the 6-hour stimulation period (M.W., unpublished data, January 2008). A possible explanation may be due to the short stimulation time. Consistent with the literature, the amount of annexin II on the cell surface of PBMos can change within days and may correlate with the differentiation of monocytes to macrophages,40 which were previously shown to exhibit an additional increase in annexin II surface expression upon inflammatory stimulation with thioglycolate.40 In contrast, we observed that the cell-surface density of ENO-1 on monocytes or monocytic cells is rather decreasing with the differentiation to macrophages (M.W., unpublished data, January 2008).
To investigate the functional consequences of the modulation of ENO-1 in monocytic cells and PBMos after inflammatory stimulation, 4 different assays were used. We demonstrated that the LPS-induced tPA-dependent increase in cell-surface plasmin activity in U937 cells and PBMos was largely attributable to increased cell-surface expression of ENO-1 under these conditions, because the presence of an anti–ENO-1 antibody restored proteolytic activity to the basal levels observed in unstimulated cells. These observations were corroborated by the finding that overexpression of ENO-1WT in unstimulated U937 cells, which saturated the amount of ENO-1 on the cell surface, increased plasmin activity to a similar degree to that seen in LPS-stimulated cells. This effect was not observed when an ENO-1 variant, in which the PLG binding site had been deleted, was used instead. Furthermore, we demonstrate that increased cell-surface expression of ENO-1 contributes significantly to the enhanced directed transmigration through lung epithelial cells and matrix invasion of LPS-stimulated U937 cells and PBMos. Once again, these observations were confirmed in additional experiments where a strong increase in transmigration through lung epithelial cells, as well as matrix invasion of ENO-1–overexpressing U937 cells, was observed. Interestingly, both the LPS stimulation (which increased ENO-1 cell-surface expression) and ENO-1 overexpression also enhanced the directed migration of monocytes through the pores of a polycarbonate membrane. This observation was unexpected because no degradation of extracellular matrix proteins is required for this process, although we demonstrated that plasmin formation is essential. Thus, ENO-1–mediated plasmin formation may directly activate the monocytes and, thereby, stimulate their migration. Indeed, plasmin specifically interacts with cell-surface receptors such as integrins and protease-activated receptors (PARs),41–43 activates intracellular signaling pathways in different cells (for example, induction of the p38 MAPK and JAK/STAT pathways in human monocytes),41,44,45 and plays a critical role in the actin cytoskeleton organization of monocytes and other cells, thereby significantly enhancing the spreading and mobility of these cells.41,42,45 These processes require an intact catalytic center of plasmin. Thus, plasmin generation may well have contributed to the observed enhancement in the migration of monocytes with increased ENO-1 cell-surface expression through the pores of polycarbonate membrane. Together, these observations clearly suggest that increased cell-surface expression of ENO-1 plays a crucial role in enhanced plasmin generation and cell mobility of monocytic cells when these cells are exposed to an inflammatory stimulus such as LPS. However, plasmin formation as well as directed migration and invasion of unstimulated U937 cells or PBMos were only slightly inhibited by anti–ENO-1 antibodies, which is in line with a previous study reporting a minor role of membrane-associated ENO-1 in PLG binding on native U937 cells.25 These observations indicate that other proteins may also serve as PLG binding sites, which may be involved in pericellular proteolysis and associated processes as well, and that the relative importance of cell-surface PLG receptors may depend on whether or not cells were exposed to inflammatory stimuli. The degree of differentiation to macrophages may also influence the spectrum of cell-surface PLG receptors and their contribution to pericellular proteolytic activity. In this regard, it has previously been shown that annexin II may promote PLG activation and migration of unstimulated THP-1 monocytic cells,21 PBMos,40 and cells of the murine macrophage RAW 264.7 cell line.21,39 Furthermore, a pronounced impairment of plasmin generation and Matrigel invasion by native RAW 264.7 macrophages in the presence of antibodies against the PLG receptor H2B has also been demonstrated.39
In view of our evidence describing a major role for ENO-1 in cell-surface plasmin generation and the migration and invasion of inflammatory stimulated monocytes in vitro, we investigated whether this same role could be demonstrated in vivo using the well-established mouse model of endotoxin-induced lung inflammation and specimens from patients with acute inflammatory lung diseases. A timely and comprehensive monocyte response under these conditions is crucial for clearing infection and controlling the inflammatory process. We have now demonstrated a pronounced increase in the recruitment of ENO-1–overexpressing monocytic cells to acutely inflamed mouse lungs, compared with mock-transfected cells and cells overexpressing the ΔPLG variant of ENO-1. These data are in line with previous studies demonstrating that resident alveolar macrophages are rapidly replaced by recruited monocytes in endotoxin-induced lung inflammation,46 and they expand those studies to include a novel, crucial role for ENO-1 in this recruitment process. Several potential mechanisms exist by which elevated cell-surface expression of ENO-1, and the corresponding increase in cell-surface plasmin generation, may promote monocyte recruitment to the alveolar space of inflamed lungs. Plasmin itself may directly degrade extracellular matrix proteins, as well as fibrin deposits that develop after tissue injury, thereby abolishing physical barriers.13–15 In addition, enhanced ENO-1-mediated plasmin formation may indirectly promote monocyte recruitment by activating other matrix-degrading proteases such as matrix metalloproteinases, by regulating the expression, secretion, and activation of cytokines involved in the inflammatory process, or by inducing cellular signaling that may alter the migratory and invasive properties of monocytes.43,44,47–50
Of note, Das et al recently demonstrated the importance of the PLG receptor histone H2B, rather than ENO-1, in macrophage recruitment into the peritoneum.39 However, a different inflammatory stimulus was used in our model versus their model (LPS vs thioglycolate). Moreover, the mechanisms of inflammatory cell recruitment and the contribution of different PLG receptors to this process may vary, depending on the organ or tissue target.
Our data provide the first evidence that ENO-1–mediated recruitment of monocytes to the acutely inflamed lung may be relevant in humans. In pneumonia patients, in contrast to control patients, intense ENO-1 staining was observed in association with mononuclear cells of the alveolar compartment. Furthermore, PBMos from pneumonia patients revealed elevated cell-surface expression of ENO-1 (and PLG) compared with healthy controls, which fits nicely with our observation of increased ENO-1 cell-surface localization on PBMos in response to LPS challenge.
In conclusion, our study demonstrates that inflammatory stimulation of monocytic cells and PBMos induces rapid translocation of ENO-1 to the cell surface, increasing local plasmin generation and, thereby, providing cells with enhanced migratory, transmigratory, and invasive properties, promoting their recruitment to the inflamed lung. Thus, the present study identified an important and functionally relevant mechanism of inflammatory cell invasion to sites of inflamed tissue mediated by increased cell-surface expression of ENO-1.
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Gisela Mueller and Horst Thiele for excellent technical assistance.
This study was funded by grants from the Deutsche Forschungsgemeinschaft (DFG; KFO 118, SPP11/90) and the Excellence Cluster Cardiopulmonary System (ECCPS).
Authorship
Contribution: M.W. designed and performed research, analyzed data, and wrote the manuscript; L.M.M. performed FACS analysis and analyzed data; R.E.M. analyzed data and wrote the manuscript; I.H. performed animal experiments; A.G. and J.L. provided clinical materials; P.M. provided clinical materials, analyzed data, and wrote the manuscript; and K.T.P. designed and coordinated research and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Malgorzata Wygrecka, Department of Biochemistry, Faculty of Medicine, Justus Liebig University Giessen, Friedrichstrasse 24, D-35392 Giessen, Germany; e-mail: malgorzata.wygrecka@innere.med.uni-giessen.de.
References
Author notes
*P.M. and K.T.P. contributed equally to this work.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal