Abstract
ADAMTS13 is a multidomain protease that limits platelet thrombogenesis through the cleavage of von Willebrand factor (VWF). We previously identified 2 types of mouse Adamts13 gene: the 129/Sv-strain Adamts13 gene encodes the long-form ADAMTS13 having the same domains as human ADAMTS13, whereas the C57BL/6-strain Adamts13 gene encodes the short-form ADAMTS13 lacking the distal C-terminal domains. To assess the physiologic significance of the distal C-terminal domains of ADAMTS13, we generated and analyzed 129/Sv-genetic background congenic mice (Adamts13S/S) that carry the short-form ADAMTS13. Similar to wild-type 129/Sv mice (Adamts13L/L), Adamts13S/S did not have ultralarge VWF multimers in plasma, in contrast to 129/Sv-genetic background ADAMTS13-deficient mice (Adamts13−/−). However, in vitro thrombogenesis under flow at a shear rate of 5000 s−1 was accelerated in Adamts13S/S compared with Adamts13L/L. Both in vivo thrombus formation in ferric chloride–injured arterioles and thrombocytopenia induced by collagen plus epinephrine challenge were more dramatic in Adamts13S/S than in Adamts13L/L but less than in Adamts13−/−. These results suggested that the C-terminally truncated ADAMTS13 exhibited decreased activity in the cleavage of VWF under high shear rate. Role of the C-terminal domains may become increasingly important under prothrombotic conditions.
Introduction
ADAMTS13 is a plasma protease that specifically cleaves von Willebrand factor (VWF).1 VWF is a multimeric plasma glycoprotein that plays a critical role in platelet adhesion and aggregation on vascular lesions.2 Endothelial cells and megakaryocytes produce mainly VWF as large multimers that can exceed 20 000 kDa in mass and secrete the multimers into the circulating blood. The adhesive activity of VWF multimers depends on their molecular sizes and in particular the largest multimers, called ultralarge VWF (UL-VWF) multimers, can induce excessive platelet aggregation under shear stress. UL-VWF multimers are normally cleaved by ADAMTS13 to smaller forms, thus restraining platelet thrombus formation. The lack of ADAMTS13 activity allows UL-VWF multimers to persist in the circulation and leads to the development of thrombotic thrombocytopenic purpura (TTP).3-5
ADAMTS13 consists of multiple domains including a metalloprotease domain, a disintegrin-like domain, a thrombospondin type 1 motif (Tsp1) domain, a cysteine-rich domain, a spacer domain, 7 additional Tsp1 domains and 2 complement components C1r/C1s, urchin epidermal growth factor, and bone morphogenic protein-1 (CUB) domains in order from the N-terminus. So far, the functional roles of ADAMTS13 domains have been studied using in vitro assay systems.6-13 These studies have shown an essential role of the N-terminal region of ADAMTS13 from the metalloprotease domain to the spacer domain, on the VWF cleavage. However, the results from in vitro studies have lacked consistency on the relative importance of the C-terminal Tsp1 and CUB domains in the substrate recognition and the activity of ADAMTS13. The recombinant human ADAMTS13 mutant lacking the C-terminal Tsp1 and CUB domains maintain almost absolute VWF-cleaving activity under static conditions, indicating that the C-terminal domains are dispensable for the ADAMTS13 activity.6-8 Under flow, the same mutant is hyperactive for the cleavage of newly released VWF multimers anchored on endothelial cells, suggesting that the C-terminal domains negatively regulate the activity of ADAMTS13.10 Recombinant polypeptides and synthetic peptides derived from the first CUB domain can inhibit the cleavage of VWF multimers on endothelial cells by ADAMTS13 under flow.11 Removal of the C-terminal Tsp1 and CUB domains results in a marked decrease in VWF cleavage by ADAMTS13 in a vortex mixer and in VWF binding to ADAMTS13 in a Biacore system (Uppsala, Sweden),13 indicating that the C-terminal domains play a crucial role in the recognition and cleavage of VWF. These results prompted us to investigate the role of C-terminal domains of ADAMTS13 in vivo.
In laboratory mouse strains, 2 kinds of Adamts13 gene are present.14-16 The Adamts13 gene of the 129/Sv strain contains 29 exons like the human ADAMTS13 gene and encodes the full-length ADAMTS13 with the same domain constitutions as human ADAMTS13. On the other hand, several strains of mice, including the C57BL/6 strain, harbor the insertion of an intracisternal A-particle (IAP) retrotransposon into intron 23 of the Adamts13 gene. As a result, the distal C-terminally truncated ADAMTS13 lacking the C-terminal 2 Tsp1 domains and 2 CUB domains is predominantly expressed in these strains. Therefore, mice can be suitable animal models for evaluating functions of the distal C-terminal domains, the C-terminal 2 Tsp1 domains, and 2 CUB domains, in vivo.
In the present study, through using the spontaneous IAP insertion in mouse Adamts13 gene, we generated a congenic mouse model that had the distal C-terminally truncated ADAMTS13 on 129/Sv genetic background. While comparing with wild-type 129/Sv mice having full-length ADAMTS13 and ADAMTS13-deficient mice on the same genetic background, we analyzed platelet thrombus formation in the congenic mice to define physiologic significance of the distal C-terminal domains in ADAMTS13 functions. Our results indicate that the distal C-terminal domains of ADAMTS13 contribute to the processing of VWF multimers in vivo, and that the importance of these domains becomes obvious after suffering thrombogenic stimuli.
Methods
Animals
The 129/Sv mice were purchased from Clea Japan (Tokyo, Japan). C57BL/6 mice were purchased from Japan SLC (Hamamatsu, Japan). ADAMTS13-deficient mice on the 129/Sv genetic background were described previously.17,18 ADAMTS13-congenic mice were developed by introgressing the C57BL/6-Adamts13 gene onto the 129/Sv genetic background, as follows. C57BL/6 mice were backcrossed to 129/Sv mice for 10 generations while retaining the C57BL/6-Adamts13 gene. In the resulting N10 heterozygous mice, autosomal chromosomes were theoretically 99.9% identical to those of the 129/Sv strain and sex chromosomes were derived exclusively from the 129/Sv strain. The N10 heterozygous mice were interbred to produce homozygous mice. The Adamts13-genotype was determined by polymerase chain reaction (PCR) with HotStarTaq DNA polymerase (QIAGEN, Hilden, Germany). The amplification was carried out using primers: the intron 23-specific forward primer, 5′-ACCTCTCAAGTGTTTGGGATGCTA-3′, the IAP-specific reverse primer, 5′-TCAGCGCCATCTTGTGACGGCGAA-3′, and the primer downstream of the IAP target site, 5′-TGCCAGATGGCCATGATTAACTCT-3′. For the experiments, all animals were matched for age and sex. All animal procedures were approved by the Animal Care and Use Committees of the National Cardiovascular Center Research Institute and Immune Disease Institute.
Northern blot analysis
Total RNA was extracted from liver using ISOGEN reagent (Nippon Gene, Tokyo, Japan) and poly(A)+ RNA was purified using PolyATtract mRNA Isolation Systems (Promega, Madison, WI). The alkaline phosphatase–labeled probe was synthesized from mouse Adamts13 cDNA (1.3 kb) using AlkPhos Direct labeling module (GE Healthcare, Little Chalfont, United Kingdom). Poly(A)+ RNA was separated on a 1% agarose gel containing 2% formaldehyde and transferred to a nylon membrane. The probe was hybridized to the blot and detected using CDP-Star detection reagent (GE Healthcare).
Blood sampling
Blood was collected from the retro-orbital plexus into tubes containing a 0.1 volume of 3.8% sodium citrate. Blood cell counts and hematocrit were determined using an automatic cell counter (KX-21NV; Sysmex, Kobe, Japan). Plasma was prepared from blood by centrifugation at 800g for 15 minutes.
Determination of plasma ADAMTS13 activity
Plasma ADAMTS13 activity was measured using GST-mVWF73-H, a recombinant mouse VWF73 peptide flanked by N-terminal glutathione S-transferase (GST) and C-terminal His6 tags, as described previously.14 In brief, GST-mVWF73-H (500 ng) was incubated with 0.8 μL plasma in 40 μL reaction buffer (10 mM N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid, 150 mM NaCl, 5 mM CaCl2, and 0.005% Tween 20, pH 7.4) at 37°C for 1 hour. The reaction was stopped by adding 10 μL sodium dodecyl sulfate (SDS) sample buffer (50 mM tris(hydeoxymethyl)aminomethane-HCl, 10 mM EDTA, 10% SDS, 250 mM dithiothreitol, 30% glycerol, and 0.1% bromophenol blue, pH 6.8). The samples were subjected to SDS–polyacrylamide gel electrophoresis and Western blot using a rabbit anti-GST antibody (Invitrogen, Carlsbad, CA) and a peroxidase-labeled anti–rabbit IgG antibody (KPL, Gaithersburg, MD). Activity was also determined using a fluorogenic human VWF73 peptide of FRETS-VWF73 (Peptide Institute, Minoh, Japan).19,20 FRETS-VWF73 (2 μM) was incubated with 4 μL plasma in 200 μL assay buffer (5 mM bis(2-hydroxyethyl)-amino-tris(hydeoxymethyl)methane, 25 mM CaCl2, and 0.005% Tween 20, pH 6.0) at 30°C. Increases in fluorescence were measured using a 350-nm excitation filter and a 440-nm emission filter in a fluorescence photometer (Mx3000P; Stratagene, La Jolla, CA).
VWF multimer analysis
Plasma VWF multimer patterns were analyzed as described previously.17 Plasma samples in SDS sample buffer were electrophoresed on a 1% agarose gel (Agarose IEF; GE Healthcare) at a constant current of 15 mA at 4°C. After transfer to a nitrocellulose membrane, the membrane was incubated in peroxidase-conjugated rabbit anti–human VWF (1:500; Dako, Glostrup, Denmark) in 5% skim milk to detect VWF multimers. Bound antibody was detected with Western Lighting Chemiluminescence Reagent Plus (PerkinElmer, Waltham, MA) on an image analyzer (LAS-3000; Fujifilm, Tokyo, Japan). The chemiluminescent intensities of each lane were scanned using Image Gauge software (version 4.2.2; Fujifilm); the relative intensity profiles were shown.
Parallel plate flow chamber assay
Platelet thrombus formation in flowing blood on immobilized collagen was analyzed using a parallel plate flow chamber as described previously.17,21 Acid-insoluble type I collagen–coated glass coverslips were placed in a flow chamber. The chamber was mounted on a fluorescence microscope (Axiovert 200M; Carl Zeiss, Oberkochen, Germany) equipped with a CCD camera system (DXC-390; Sony, Tokyo, Japan). Blood was collected into tubes containing argatroban (240 μM; Mitsubishi Chemical, Tokyo, Japan). The fluorescent dye mepacrine (10 μM; Sigma-Aldrich, St Louis, MO) was added to the blood. Whole blood samples were aspirated through the chamber and across the collagen-coated coverslip at a constant wall shear rate. To analyze the cumulative thrombus volume, image sets at 1.0-μm z-axis intervals within a defined area (156.4 μm × 119.6 μm) were captured using MetaMorph software (version 6.1.4; Universal Imaging, West Chester, PA). After blind deconvolution of image sets processed by AutoDeblur software package (version 8.0.2; AutoQuant Imaging, Troy, NY), 3D volumetric measurements of thrombi were accomplished using VoxBlast software (version 3.0; Vartek, Fairfield, IA).
Intravital microscopy
Intravital microscopy was performed as described previously.22,23 Platelets were isolated from platelet-rich plasma and fluorescently labeled with calcein AM (2.5 μg/mL; Invitrogen). Recipient mice were anesthetized and labeled platelets were infused through retro-orbital plexus. The mesentery was gently exteriorized through a midline abdominal incision and arterioles of 100- to 150-μm diameters were visualized with a fluorescence microscope and a CCD camera system. The shear rate was calculated using an optical Doppler velocimeter as described.24 Filter paper saturated with 10% ferric chloride was applied for 5 minutes on an arteriole by topical application. Thrombus formation in the arteriole was monitored for 40 minutes after injury or until complete occlusion occurred and lasted for more than 30 seconds. The following 2 parameters were evaluated: time to first thrombus formation, defined as the time required for formation of a thrombus larger than 30 μm, and occlusion time, defined as the time required for cessation of blood flow for at least 30 seconds.
Collagen plus epinephrine–induced thrombosis model
Statistical analysis
Statistical significance was assessed by the one-way analysis of variance followed by the Bonferroni multiple comparison tests. Differences were considered to be significant at P values less than .05.
Results
Generation of Adamts13S/S mice
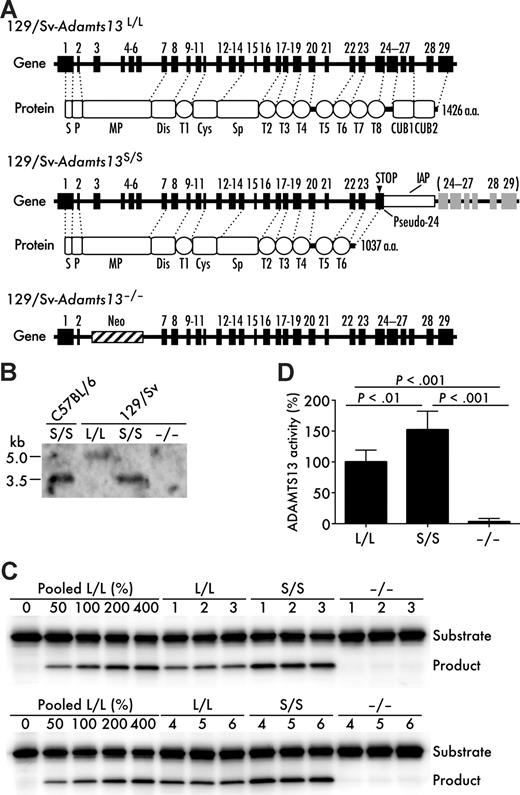
To address the functional implication of the distal C-terminal domains of ADAMTS13 in vivo, we generated and characterized a congenic mouse model that has the C-terminally truncated form of ADAMTS13 on 129/Sv genetic background (Figure 1A). We confirmed the presence of IAP insertion in the Adamts13 gene of the congenic (Adamts13S/S) mice by PCR (data not shown) and detected an IAP chimeric transcript (∼ 3.5 kb) by Northern blotting of RNA from liver (Figure 1B), primary site of synthesis.14 An IAP-free ADAMTS13 mRNA (∼ 5 kb) was detected in wild-type 129/Sv (Adamts13L/L) mice and no ADAMTS13 mRNA was detected in ADAMTS13-deficient (Adamts13−/−) mice on 129/Sv genetic background (Figure 1B). Adamts13S/S mouse plasma exhibited higher cleaving activity for both GST-mVWF73-H and FRETS-VWF73 than Adamts13L/L mouse plasma, whereas the activity in Adamts13−/− mouse plasma was below detection limits (Figure 1C,D). Therefore, the distal C-terminal domains of ADAMTS13 were not necessary for the cleavage of the VWF73-based peptide substrate as observed previously.8,14 Platelet counts were not different among the genotypes (Adamts13L/L, 744 ± 180 × 109/L; Adamts13S/S, 693 ± 44 × 109/L; Adamts13−/−, 672 ± 39 × 109/L; mean ± SD, n = 8). Both Adamts13S/S mice and Adamts13−/− mice were viable and showed no TTP-like symptoms throughout the study.
Generation of Adamts13S/S mice with 129/Sv-genetic background. (A) Gene and protein structure of ADAMTS13 in the wild-type (Adamts13L/L) 129/Sv mice, the Adamts13S/S mice on 129/Sv genetic background, and the Adamts13−/− mice on 129/Sv genetic background. An intracisternal A-particle (IAP) insertion into intron 23 creates a pseudo-exon 24 including a premature stop codon. ADAMTS13 with a truncated C-terminus is expressed mainly in Adamts13S/S mice. S indicates signal peptide; P, propeptide; MP, metalloprotease domain; Dis, disintegrin-like domain; T (numbered 1-8), thrombospondin type 1 motif domain; Cys, cysteine-rich domain; Sp, spacer domain; and CUB, complement components C1r/C1s, urchin epidermal growth factor, and bone morphogenic protein-1 domain. (B) Expression of Adamts13 mRNA in liver. Poly(A)+ RNA isolated from liver of indicated mice was probed with a 1.3-kb Adamts13 cDNA corresponding to exons 3 to 13. (C) GST-mVWF73-H assay. Plasma ADAMTS13 activity of indicated mice was measured using a recombinant mouse VWF73 peptide, GST-mVWF73-H. Results from 6 mice for each genotype are shown. Standard reactions using graded amounts of pooled plasma from 10 Adamts13L/L mice were performed simultaneously. (D) FRETS-VWF73 assay. Plasma ADAMTS13 activity in indicated mice was determined using a fluorogenic human VWF73 peptide, FRETS-VWF73. Data are mean ± SD of 6 mice for each genotype. The average activity measured in Adamts13L/L mice was arbitrarily defined as 100%.
Generation of Adamts13S/S mice with 129/Sv-genetic background. (A) Gene and protein structure of ADAMTS13 in the wild-type (Adamts13L/L) 129/Sv mice, the Adamts13S/S mice on 129/Sv genetic background, and the Adamts13−/− mice on 129/Sv genetic background. An intracisternal A-particle (IAP) insertion into intron 23 creates a pseudo-exon 24 including a premature stop codon. ADAMTS13 with a truncated C-terminus is expressed mainly in Adamts13S/S mice. S indicates signal peptide; P, propeptide; MP, metalloprotease domain; Dis, disintegrin-like domain; T (numbered 1-8), thrombospondin type 1 motif domain; Cys, cysteine-rich domain; Sp, spacer domain; and CUB, complement components C1r/C1s, urchin epidermal growth factor, and bone morphogenic protein-1 domain. (B) Expression of Adamts13 mRNA in liver. Poly(A)+ RNA isolated from liver of indicated mice was probed with a 1.3-kb Adamts13 cDNA corresponding to exons 3 to 13. (C) GST-mVWF73-H assay. Plasma ADAMTS13 activity of indicated mice was measured using a recombinant mouse VWF73 peptide, GST-mVWF73-H. Results from 6 mice for each genotype are shown. Standard reactions using graded amounts of pooled plasma from 10 Adamts13L/L mice were performed simultaneously. (D) FRETS-VWF73 assay. Plasma ADAMTS13 activity in indicated mice was determined using a fluorogenic human VWF73 peptide, FRETS-VWF73. Data are mean ± SD of 6 mice for each genotype. The average activity measured in Adamts13L/L mice was arbitrarily defined as 100%.
Adamts13S/S mice have normal VWF multimers
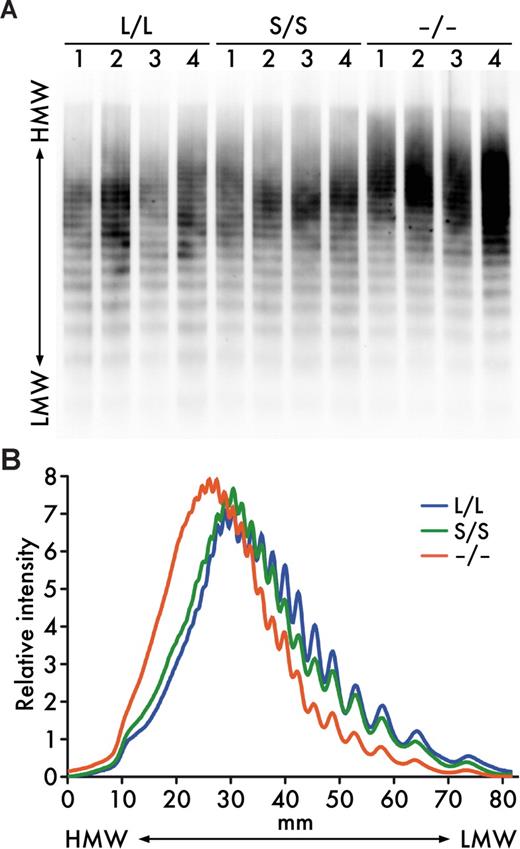
As previously reported,17 UL-VWF multimers persisted in plasma of Adamts13−/− mice on 129/Sv-genetic background (Figure 2). Thus, ADAMTS13 activity is important for the size regulation of VWF multimers in mice at least on this genetic background. However, the VWF multimer patterns in Adamts13S/S mice were indistinguishable from those in Adamts13L/L mice (Figure 2). These results suggest that the distal C-terminally truncated form of mouse ADAMTS13 exhibits VWF-cleaving activity sufficient for maintenance of normal size distribution of plasma VWF multimers under steady state in vivo.
Plasma VWF multimers. (A) VWF multimer patterns. Plasma samples (1 μL/lane) from Adamts13L/L, Adamts13S/S, and Adamts13−/− mice were electrophoresed on SDS-agarose gels and transferred to nitrocellulose membranes. VWF multimers were detected with anti-VWF antibodies. (B) Relative intensities of plasma VWF multimers. The chemiluminescent intensities of the VWF multimer patterns (A) were scanned using image analysis software. An average of multiple lanes from 4 mice for each genotype is shown. HMW indicates high molecular weight; LMW, low molecular weight.
Plasma VWF multimers. (A) VWF multimer patterns. Plasma samples (1 μL/lane) from Adamts13L/L, Adamts13S/S, and Adamts13−/− mice were electrophoresed on SDS-agarose gels and transferred to nitrocellulose membranes. VWF multimers were detected with anti-VWF antibodies. (B) Relative intensities of plasma VWF multimers. The chemiluminescent intensities of the VWF multimer patterns (A) were scanned using image analysis software. An average of multiple lanes from 4 mice for each genotype is shown. HMW indicates high molecular weight; LMW, low molecular weight.
In vitro thrombogenesis is increased in Adamts13S/S mice only at a high shear rate
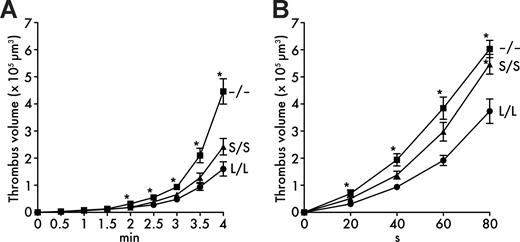
When whole blood was perfused over a collagen-coated surface in a parallel plate flow chamber at a shear rate of 1000 s−1, platelet thrombus formation was significantly promoted in Adamts13−/− mice (Figure 3A) compared with Adamts13L/L mice, consistent with the presence of UL-VWF multimers in plasma of Adamts13−/− mice. However, whole blood thrombus formation at 1000 s−1 was not significantly different between Adamts13S/S mice and Adamts13L/L mice (Figure 3A), indicating that the distal C-terminally truncated form of mouse ADAMTS13 does not completely lose the activity.
In vitro thrombogenesis on collagen surface under flow. (A) Thrombus formation at 1000 s−1. Whole blood from Adamts13L/L, Adamts13S/S, or Adamts13−/− mice containing mepacrine-labeled platelets was perfused over an acid-insoluble type I collagen–coated surface at a wall shear rate of 1000 s−1. The cumulative thrombus volume, analyzed using a multidimensional imaging system, was measured every 0.5 minutes until 4 minutes. Data are the mean ± SEM of 25 mice for each genotype. (B) Thrombus formation at 5000 s−1. Whole-blood samples from indicated mice were perfused over an acid-insoluble type I collagen–coated surface at a wall shear rate of 5000 s−1. The cumulative thrombus volume was measured every 20 seconds until 80 seconds. Blood from 2 mice was pooled and used for experiments. Data are the mean ± SEM of 15 samples for each genotype. *P < .05 in comparison with Adamts13L/L mice.
In vitro thrombogenesis on collagen surface under flow. (A) Thrombus formation at 1000 s−1. Whole blood from Adamts13L/L, Adamts13S/S, or Adamts13−/− mice containing mepacrine-labeled platelets was perfused over an acid-insoluble type I collagen–coated surface at a wall shear rate of 1000 s−1. The cumulative thrombus volume, analyzed using a multidimensional imaging system, was measured every 0.5 minutes until 4 minutes. Data are the mean ± SEM of 25 mice for each genotype. (B) Thrombus formation at 5000 s−1. Whole-blood samples from indicated mice were perfused over an acid-insoluble type I collagen–coated surface at a wall shear rate of 5000 s−1. The cumulative thrombus volume was measured every 20 seconds until 80 seconds. Blood from 2 mice was pooled and used for experiments. Data are the mean ± SEM of 15 samples for each genotype. *P < .05 in comparison with Adamts13L/L mice.
As fluid shear rate increases progressively, the interaction between VWF and platelet GPIbα becomes more important in platelet thrombus formation.26 It has been reported that thrombus formation in mouse blood on collagen surface is completely dependent on the VWF-GPIbα interaction above a threshold shear rate between 2000 s−1 and 5000 s−1.27 In addition, ADAMTS13 cleaves VWF and down-regulates thrombus formation in shear rate–dependent manner.28 Based on these observations, we further examined thrombus formation at a higher shear rate of 5000 s−1. As expected, thrombus formation in Adamts13−/− mice was significantly elevated compared with Adamts13L/L mice at 5000 s−1 (Figure 3B). In addition, we found accelerated thrombus formation in Adamts13S/S mice compared with Adamts13L/L mice at this higher shear rate (Figure 3B). These results suggest that the distal C-terminally truncated form of mouse ADAMTS13 has reduced activity compared with the full-length form and does not sufficiently limit thrombus formation under high shear rate in vitro.
In vivo thrombus growth is accelerated in Adamts13S/S mice
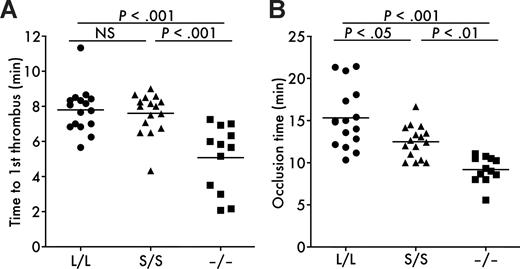
To examine whether the truncation of the distal C-terminal domains in ADAMTS13 affects thrombus formation in vivo, we carried out intravital microscopy experiments in a model of experimental arteriolar thrombosis. In this model, vascular injury was induced by topical application of ferric chloride on a mesenteric arteriole, which provoked the generation of free radicals leading to the endothelial disruption.23 The diameter and shear rate of studied arterioles were 118.0 plus or minus 13.1 μm and 1362 plus or minus 219 s−1 (mean ± SD, n = 16) for Adamts13L/L mice, 122.8 plus or minus 11.1 μm and 1394 plus or minus 136 s−1 (n = 16) for Adamts13S/S mice, and 115.6 plus or minus 10.8 μm and 1405 plus or minus 225 s−1 (n = 12) for Adamts13−/− mice and not significantly different among the groups. Both time to first thrombus (Figure 4A) and occlusion time (Figure 4B) after injury in Adamts13−/− mice (time to first thrombus = 5.1 ± 1.9 minutes, occlusion time = 9.2 ± 1.6 minutes; mean ± SD) were significantly decreased compared with Adamts13L/L mice (time to first thrombus = 7.8 ± 1.3 minutes, occlusion time = 15.3 ± 3.6 minutes), indicating that ADAMTS13 contributes to down-regulation of thrombogenesis at the site of arteriolar injury in 129/Sv mice. In the case of Adamts13S/S mice, time to first thrombus after injury (7.6 ± 1.2 minutes) was not different from Adamts13L/L mice. However, the initial thrombi grew rapidly to occlusive size in Adamts13S/S mice and occlusion time was significantly shorter in Adamts13S/S mice (12.5 ± 1.9 minutes) compared with Adamts13L/L mice (Figure 4B). These results suggest that the distal C-terminally truncated form of mouse ADAMTS13 is less active in down-regulating thrombus growth in vivo compared with full-length ADAMTS13.
In vivo thrombogenesis in ferric chloride–injured mesenteric arterioles. (A) Time to first thrombus formation. Calcein AM–labeled platelets representing approximately 2.5% of total platelets were observed in mesenteric arterioles of live mice after injury with 10% ferric chloride. The time required for formation of a thrombus more than 30 μm was measured. (B) Occlusion time. The time required for a complete stop of blood flow was measured after injury with 10% ferric chloride. Symbols represent data from a single mouse. Bars represent the mean values of groups (n = 16 for Adamts13L/L mice, n = 16 for Adamts13S/S mice, and n = 12 for Adamts13−/− mice).
In vivo thrombogenesis in ferric chloride–injured mesenteric arterioles. (A) Time to first thrombus formation. Calcein AM–labeled platelets representing approximately 2.5% of total platelets were observed in mesenteric arterioles of live mice after injury with 10% ferric chloride. The time required for formation of a thrombus more than 30 μm was measured. (B) Occlusion time. The time required for a complete stop of blood flow was measured after injury with 10% ferric chloride. Symbols represent data from a single mouse. Bars represent the mean values of groups (n = 16 for Adamts13L/L mice, n = 16 for Adamts13S/S mice, and n = 12 for Adamts13−/− mice).
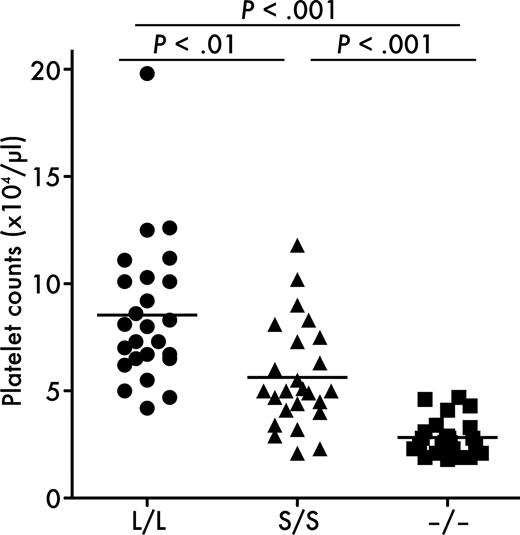
To elucidate the consequences of the lack of the distal C-terminal domains in ADAMTS13 on systemic thrombosis, we performed collagen plus epinephrine infusion model experiments. In this model, widespread intravascular thrombosis was induced by intravenous infusion of collagen fibrils in combination with epinephrine, and the incorporation of platelets into thrombi was monitored by the reduction in circulating platelet counts.29 Consistent with our previous observation,17 platelet counts after the infusion were significantly lower in Adamts13−/− mice (28 ± 8 × 109/L, mean ± SD) than in Adamts13L/L mice (85 ± 33 × 109/L), suggesting that ADAMTS13 contributes to inhibition of platelet aggregation in this experimental system (Figure 5). Platelet counts after the infusion in Adamts13S/S mice (56 ± 24 × 109/L) were significantly higher than in Adamts13−/− mice and lower than in Adamts13L/L mice (Figure 5), whereas platelet counts of untreated mice were not different among the groups (Adamts13L/L, 666 ± 44 × 109/L; Adamts13S/S, 770 ± 65 × 109/L; Adamts13−/−, 710 ± 49 × 109/L, mean ± SD of 4 mice). These findings complement accelerated thrombus growth in Adamts13S/S mice compared with Adamts13L/L mice, indicating that the distal C-terminally truncated form of mouse ADAMTS13 has significantly reduced activity in vivo.
Platelet counts after collagen plus epinephrine infusion. Mice were injected with 600 ng/g collagen plus 60 ng/g epinephrine via tail vein and platelet counts were measured 5 minutes after injection. Symbols represent platelet counts from a single mouse. Bars represent the mean values of 25 mice in each group. Platelet counts of untreated mice were not different among the groups.
Platelet counts after collagen plus epinephrine infusion. Mice were injected with 600 ng/g collagen plus 60 ng/g epinephrine via tail vein and platelet counts were measured 5 minutes after injection. Symbols represent platelet counts from a single mouse. Bars represent the mean values of 25 mice in each group. Platelet counts of untreated mice were not different among the groups.
Discussion
It is now evident that genetic background is an important phenotypic determinant in mutant mice with hemostatic defects. For instance, mice carrying the factor V Leiden (R504Q) mutation have shown increased perinatal thrombotic mortality on the mixed 129/Sv and C57BL/6J background relative to C57BL/6J background.30 Similar effects of genetic backgrounds on phenotypes have been observed in other mutants such as the thrombomodulin G404P-mutant mice,31 the fibrinogen-deficient mice,32 and the tissue factor–deficient mice.33 In ADAMTS13-deficient mice, genetic backgrounds have also been shown to significantly affect their thrombotic phenotypes.34 Thus, phenotypes of ADAMTS13 mutant mice should be compared with control mice on the appropriate and uniform strain background. We have previously demonstrated that ADAMTS13 deficiency in mice results in a prothrombotic state with accumulation of UL-VWF multimers on 129/Sv background.17 Therefore in this study, we applied a spontaneous mutation in the Adamts13 gene of C57BL/6 mice onto 129/Sv mice by 10-generation backcrossing, and obtained the congenic mice that were expected to have 99.9% 129/Sv genome and primarily expressed the distal C-terminally truncated ADAMTS13. Then, we compared their phenotypes with positive and negative control animals: the wild-type 129/Sv mice and the 129/Sv-background ADAMTS13-deficient mice. By this approach, we minimized the background effects and defined the significance of the distal C-terminal domains of ADAMTS13 in mice.
Plasma of the congenic mice exhibited higher cleaving activity against GST-mVWF73-H and FRETS-VWF73 compared with plasma of the wild-type mice. We previously observed that the recombinant distal C-terminally truncated mouse ADAMTS13 cleaves GST-mVWF73-H to a similar extent compared with the full-length form.14 The other group reported that the recombinant distal C-terminally truncated mouse ADAMTS13 is slightly less active in cleaving GST-mVWF73-H than the full-length form.15 These findings suggest that the distal C-terminally truncated ADAMTS13 in mouse plasma has equivalent or slightly lower specific activity against VWF73-based substrates compared with the full-length ADAMTS13. Thus, the data in the present study imply that the distal C-terminally truncated ADAMTS13 is more abundant than the full-length form in circulating blood in 129/Sv mice. Preferential expression of the distal C-terminally truncated mouse ADAMTS13 has also been found in HeLa cells14 and HEK 293T cells.15 Unfortunately, because we have failed to determine the ADAMTS13 antigen levels in mouse plasma, it remains unclear whether the distal C-terminal truncation of ADAMTS13 actually increases plasma levels of the enzyme. Despite the congenic mice having higher in vitro ADAMTS13 activity in plasma, they showed prothrombotic phenotypes, suggesting the importance of the distal C-terminal domains in ADAMTS13 activity in vivo.
We reconfirmed that ADAMTS13 deficiency in 129/Sv mice allowed the accumulation of UL-VWF multimers in plasma (Figure 2), therefore, promising the essential contribution of ADAMTS13 on preventing the accumulation of UL-VWF multimers in 129/Sv mice. Under these situations, lack of the distal C-terminal domains of ADAMTS13 in 129/Sv mice did not increase plasma VWF multimer sizes (Figure 2), showing that the distal C-terminally truncated ADAMTS13 maintained the VWF-cleaving activity in vivo. Although the distal C-terminally truncated form of mouse ADAMTS13 was reported to show considerably lower activity than the full-length form for purified human VWF multimers under in vitro static conditions,15 our results show that the distal C-terminal truncation of mouse ADAMTS13 allows retention of normal size distribution of plasma VWF multimers in vivo at least under steady state.
In the parallel-plate flow chamber experiments, ADAMTS13 deficiency in 129/Sv mice markedly enhanced thrombogenic responses (Figure 3), indicating that ADAMTS13 is critical for limiting platelet thrombus formation under whole blood flow conditions. In the same experimental conditions, the distal C-terminal truncation of ADAMTS13 in 129/Sv mice did not promote thrombogenesis at 1000 s−1 (Figure 3A) but significantly promoted thrombogenesis at 5000 s−1 (Figure 3B). It is conceivable that the distal C-terminally truncated ADAMTS13 is active but not fully competent to cleave VWF within growing thrombus under flow. Because both the interaction of GPIbα-VWF and the cleavage of VWF by ADAMTS13 are facilitated by increasing fluid shear rate, the function of the distal C-terminal domains may become vital to down-regulate thrombogenesis under high shear conditions. Actually, similar results were obtained in the in vivo arteriolar injury model experiments (Figure 4). The distal C-terminal truncation of ADAMTS13 in 129/Sv mice did not affect the time to first thrombus formation in the arterioles where fluid shear rates were around 1500 s−1 (Figure 4A). However, when thrombus grew to a larger size, the arteriolar lumen was narrowed, which resulted in increase in shear rates.23 Then, the distal C-terminal truncation of ADAMTS13 significantly reduced the occlusion time compared with full-length ADAMTS13 (Figure 4B). Therefore, the distal C-terminal domains are important for ADAMTS13 to sufficiently down-regulate thrombogenesis under high shear rate in vivo as well as in vitro. After the induction of systemic platelet aggregation by challenge with a mixture of collagen and epinephrine, consumptive thrombocytopenia was also enhanced by the distal C-terminal truncation of ADAMTS13 in 129/Sv mice (Figure 5), supporting the idea that the distal C-terminal domains are required for optimal down-regulation of platelet aggregation in vivo. The complete deficiency of ADAMTS13 in 129/Sv significantly accelerated thrombus growth to injured vessel wall and systemic thrombi compared with 129/Sv mice with truncation of the distal C-terminal domains in ADAMTS13 (Figures 4,5). Therefore, we can conclude that the distal C-terminally truncated ADAMTS13 has significantly decreased activity in limiting thrombosis in vivo.
The binding of platelets to VWF is reported to accelerate the cleavage of VWF by ADAMTS13 under static35 and flow36 conditions in vitro. It has also been shown that ADAMTS13 can cleave platelet-bound VWF multimers37 and limit thrombus formation through the cleavage of VWF at the surface of forming thrombi28 in in vitro flow chamber systems. Therefore, in our experimental settings, ADAMTS13 attenuates thrombus growth, possibly through the cleavage of VWF multimers bound on the surface of platelet-rich thrombi under high shear rate. The distal C-terminal domains may be necessary for ADAMTS13 to efficiently recognize and cleave platelet-bound VWF multimers on a growing thrombus. Conceivably, the distal C-terminal domains may contribute to the interaction with unidentified ADAMTS13-binding cofactors localized on the surface of platelets or subendothelium, and this interaction may be necessary for ADAMTS13 to control VWF-mediated thrombus formation. However, we cannot rule out the possibility that the distal C-terminal domains of ADAMTS13 contribute to the prevention of thrombosis independent from the VWF-cleaving activity of ADAMTS13, nevertheless VWF has been suggested as the only relevant substrate for ADAMTS1338 and functions of ADAMTS13 other than its VWF-cleaving activity have yet to be reported.
The distal C-terminally truncated ADAMTS13 is expressed in a lot of mouse strains including the BALB/c, C3H/He, C57BL/6, and DBA/2 strains as substitute for the full-length form.14,15 Our present results suggest that thrombotic response in these strains would be increased, at least partially, by their incomplete ADAMTS13 activity. This should be taken into account when studying genetically modified mice with heterogeneous genetic background.
In summary, our results define the role of the distal C-terminal domains in ADAMTS13 in vivo. Deletion of the C-terminal 2 Tsp1 and 2 CUB domains permits normal size distribution of plasma VWF multimers under steady state, but exacerbates platelet thrombosis after thrombogenic stimulation in mice. Thus, the distal C-terminally truncated ADAMTS13 is not fully active in vivo. These distal C-terminal domains of ADAMTS13 may play a role in the efficient processing of VWF multimers during platelet thrombus growth, and thus their functions may become increasingly important when vascular damage is induced.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Ms Miyuki Kuroi and Ms Yuko Nobe (National Cardiovascular Center Research Institute), and Ms Meghan Walsh (Immune Disease Institute) for technical assistance.
This work was supported in part by grants-in-aid from the Ministry of Health, Labor, and Welfare of Japan, Tokyo, Japan (T.M.); the Ministry of Education, Culture, Sports, Science, and Technology of Japan, Tokyo, Japan (F.B., K.K., and T.M.); the Japan Society for the Promotion of Science, Tokyo, Japan (K.K. and T.M.); and from the Program for Promotion of Fundamental Studies in Health Sciences of the National Institute of Biochemical Innovation of Japan, Ibaraki, Japan (T.M.); a Sponsored Research Agreement from Baxter Bioscience, Vienna, Austria (A.K.C. and D.D.W.) and a National Heart, Lung, and Blood Institute of the National Institutes of Health grant P01-HL066105 (D.D.W.).
National Institutes of Health
Authorship
Contribution: F.B. designed research, performed experiments, analyzed data, and wrote the paper; A.K.C. performed experiments, contributed vital analytical tools, and analyzed the data; K.K. designed research, performed experiments, and wrote the paper; J.Y. performed experiments and analyzed data; S.M. and D.D.W. contributed vital analytical tools and interpreted the data; and T.M. designed research and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Toshiyuki Miyata, National Cardiovascular Center Research Institute, 5-7-1 Fujishirodai, Suita, Osaka 565-8565, Japan; e-mail: miyata@ri.ncvc.go.jp.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal