Abstract
Tumor-specific T-cell tolerance represents one major mechanism of tumor-induced immune evasion. Myeloablative chemotherapy with stem cell transplantation may offer the best chance of achieving a state of minimal residual disease and, thus, minimize tumor-induced immune evasion. However, studies have shown that tumor-specific T-cell tolerance persists after transplantation. Here, we showed that CD4+CD25+ regulatory T (TReg) cells play a critical role in tumor-specific CD8+ T-cell tolerance after transplantation. Removal of TReg cells from the donor lymphocyte graft did not overcome this tolerance because of rapid conversion of donor CD4+CD25− T cells into CD4+CD25+Foxp3+ TReg cells in recipients after transplantation, and depletion of TReg cells in recipients was necessary for the reversal of tumor-specific tolerance. These results suggest that strategies capable of overcoming T-cell tolerance in recipients are required to promote antitumor immunity after transplantation. Toward this goal, we showed that dendritic cell (DC) vaccines coadministered with the TLR9 ligand, CpG could effectively overcome tumor-specific tolerance, leading to significant prolongation of tumor-free survival after transplantation. We further showed that CpG-induced type I interferon was critical for the reversal of tumor-specific tolerance in vivo. Collectively, these results may suggest effective immunotherapeutic strategies for treating cancer after stem cell transplantation.
Introduction
An ultimate goal of cancer immunotherapy is to eradicate preestablished tumors through therapeutic vaccinations.1 A variety of vaccine approaches have been studied, including those designed to prime the host to defined tumor-associated antigens when known or, more generally, to use either autologous or allogeneic tumor cells as a source of antigen for vaccination when the relevant tumor antigens are yet to be identified.2 Most of these vaccines were shown to be immunogenic and have shown impressive results in preventing tumors in murine models. However, they have, to date, shown only moderate success in treating preestablished tumors in both animals and patients in clinical trials.3 This is probably because tumor cells have developed mechanisms to avoid recognition and elimination by the immune system. These mechanisms of immune evasion include down-regulation of components of the antigen processing and presentation machinery,4,5 production of cytokines that inhibit or divert productive effector responses,6 and induction of tumor antigen-specific T-cell tolerance.7
For many hematologic malignancies, myeloablative chemotherapy and autologous stem cell transplantation may offer the best chance of achieving minimal residual disease, a state that may minimize tumor-induced immune evasion. This may serve as an ideal platform for integration of tumor vaccines. Unfortunately, the immune reconstitution stage after transplantation has been characterized as a period during which T-cell responses to antigenic stimulation are decreased because of limited thymic output.8,9 Previous studies in murine models of syngeneic transplantation have established a pivotal role of postthymic mature T cells that accompany the graft as precursors for the developing T-cell repertoire.8 It has been shown that a mature lymphocyte graft, specifically CD8+ T cells, from naive donors is necessary for mediating the antitumor effect after transplantation.10-12 However, when donor T cells derived from tumor-bearing mice, which is analogous to autologous transplantation in patients clinically, were used as a lymphocyte graft for transplantation in recipient tumor-bearing hosts, this tumor-specific T-cell response rapidly declined in association with tumor progression despite transient activation of tumor-specific T cells immediately after transplantation, probably because of homeostatic proliferation, suggesting the development of tumor-specific T-cell tolerance.11
Previous studies have shown that induction of tumor-specific T-cell tolerance in the nontransplantation setting closely resembles that of peripheral tolerance to self-antigens.13-17 Multiple mechanisms have been postulated to account for T-cell tolerance, including peripheral deletion, anergy, and regulatory T cell (TReg)–mediated suppression.18,19 Several phenotypically distinct TReg cells have been described so far.20-22 Of particular interest are thymus-derived, naturally occurring CD4+CD25+ TReg cells. These cells, which represent 5% to 10% of peripheral CD4+ T cells, have been shown to play an essential role in the prevention of autoimmunity.23,24 Recent studies have also suggested an important role of CD4+CD25+ TReg cells in suppressing antitumor immune responses.25 Depletion of these cells has been shown to promote rejection in several murine tumor models, including leukemia, lymphoma, and melanoma,26-29 and to enhance the efficacy of tumor vaccines.26 Furthermore, it has also been shown that CD4+CD25+ TReg cells isolated from patients with cancer inhibit the function of tumor-infiltrating T cells in vitro30,31 and that CD4+CD25+ TReg cells promote tumor growth in patients with ovarian cancer in vivo.32
Whether CD4+CD25+ TReg cells also play a role in posttransplantation tumor-specific tolerance remains to be defined. With the use of a model of syngeneic transplantation with influenza hemagglutinin (HA) as a model tumor antigen, we showed in this study that CD4+CD25+ TReg cells were critical for posttransplantation tumor-specific CD8+ T-cell tolerance. However, infusion of TReg cell–depleted mature lymphocyte graft from the tumor-bearing hosts could not abrogate TReg cell–mediated suppression on tumor-specific T cells. This was due to rapid conversion of donor CD4+CD25− T cells into CD4+CD25+ TReg cells in recipients after transplantation. Removal of TReg cells in recipients was necessary to overcome tumor-specific T-cell tolerance. We further showed that dendritic cell (DC) vaccines coadministered with the TLR9 ligand, CpG, in vivo could overcome this tolerance and effectively activate tumor-specific CD8+ T cells, leading to a significant prolongation of tumor-free survival. The reversal of T-cell tolerance was mediated by CpG-induced production of type I interferon (IFN) in vivo. Our finding may help design effective immunotherapeutic strategies for treating cancer in the setting of stem cell transplantation.
Methods
Mice and tumor cells
BALB/c mice (Thy1.2+) were purchased from The Jackson Laboratory (Bar Harbor, ME). The clone 4 HA-TCR transgenic mice that express a TCR recognizing a Kd-restricted HA epitope (518IYSTVASSL526)33 were kindly provided by Dr Linda Sherman (Scripps Research Institute, La Jolla, CA). The 6.5 HA-TCR transgenic mice that express a TCR recognizing an I-Ed–restricted HA epitope (110SFERFEIFPKE120) were kindly provided by Dr H. von Boehmer (Harvard University, Boston, MA).24 HA-expressing A20 B-cell lymphoma (A20-HA) cells, a gift from Dr Hyam Levitsky (Johns Hopkins University, Baltimore, MD), were grown in RPMI 1640 supplemented with 10% FBS and 400 μg/mL G418. Cells (105) in 0.2 mL PBS were used for tumor inoculation intravenously as described.34
Mice used in all experiments were between 8 and 12 weeks of age. All experiments involving the use of mice were done in accordance with protocols approved by the Animal Care and Use Committee of Duke University.
Adoptive transfer of HA-specific T cells
Naive clone 4 CD8+ and 6.5 CD4+ T cells were prepared from pooled spleens and lymph nodes of clone 4 and 6.5 HA-TCR transgenic mice, respectively. Clonotypic T cells were purified by anti-CD8 or anti-CD4 microbeads as described.34-36 Clone 4 CD8+ T cells (5 × 105) or 6.5 CD4+ T cells (2 × 106) in a total volume of 0.2 mL HBSS were adoptively transferred into recipient mice by tail vein injection.
Tumor purging and depletion of CD25+ T cells from donor splenocytes
Splenocytes from donor tumor-bearing mice were harvested and purged of A20 lymphoma cells by incubation with anti–B220-biotin (clone RA3.3A1) for 30 minutes at 4°C, followed by incubation with streptavidin-microbeads (Miltenyi Biotec, Auburn, CA) and subsequently depletion by magnetic separation as described.11 In the indicated experiments, purged splenocytes were further depleted of CD4+CD25+ TReg cells with anti-CD25 (clone 7D4) microbeads (Miltenyi Biotec). The elimination of CD4+CD25+ TReg cells was confirmed by flow cytometry with the use of a different anti-CD25 antibody (clone PC61).
Syngeneic stem cell transplantation
Bone marrow cells were harvested from the femurs and tibiae of donor tumor-bearing mice and enriched for lineage negative (Lin−) cells with the use of the StemSep mouse progenitor enrichment kit (StemCell Technologies, Vancouver, BC) for all transplants as described.12 This enrichment depleted greater than 97% of lineage-specific cells. The recipient tumor-bearing mice were irradiated with 850 cGy and injected intravenously with 0.2 mL of the graft consisting of 3 × 105 Lin−c-Kit+ bone marrow cells and 107 A20 lymphoma-purged splenocytes.
DC vaccination and CpG
DCs were generated from the bone marrow of BALB/c mice as described.34 Briefly, bone marrow cells harvested from femurs and tibiae were cultured for 5 days in the presence of murine GM-CSF (1000 U/mL) and IL-4 (500 U/mL; R&D Systems, Minneapolis, MN). On day 5, cells were matured in the presence of TNF-α (500 U/mL). On day 7, mature DCs were harvested and incubated with 10 μg/mL of the Kd-restricted HA epitope peptide, or a control irrelevant peptide, at 37°C for 1 hour. Peptide-loaded DCs (106) in 0.2 mL were used for vaccination. In some experiments, vaccinated mice were intraperitoneally injected with 20 nmol of phosphorothioate-stabilized CpG ODN (5′-TCC ATG ACG TTC CTG ATG CT)37 (Integrated DNA Technologies, Coralville, IA) in 0.1 mL PBS. In other experiments, day 5 DCs were matured in the presence of 1 nM CpG for 2 days, loaded with the HA peptide, and used for vaccination.
In vivo depletion of CD4+CD25+ TReg cells
In vivo depletion of CD25+ T cells was performed as described with some modifications.34 Briefly, 300 μg anti-CD25 mAb (clone PC61) was injected intraperitoneally daily for 3 days, beginning 7 days before vaccination. Before vaccination, blood was obtained from mice, and peripheral blood mononuclear cells were analyzed to confirm elimination of CD4+CD25+ T cells by flow cytometry with the use of a different anti-CD25 antibody (clone 7D4).
Antibodies, intracellular cytokine staining, and flow cytometry
All antibodies used for flow cytometry were purchased from BD Biosciences (San Jose, CA). Intracellular IFN-γ staining was performed as described.34,38,39 Briefly, cells were incubated in the presence of 2 μg/mL of the Kd-HA peptide and 5 μg/mL brefeldin A for 6 hours at 37°C. After washing, cells were stained with surface markers (CD8 and Thy1.1) and permeabilized to detect intracellular IFN-γ. FACSCanto (BD Biosciences) was used for flow cytometry event collection, and data were analyzed with the use of FACSDiva and CellQuest software (BD Biosciences).
In vitro suppression assay
CD4+CD25+ TReg cells were isolated from recipients 7 days after vaccination with or without CpG with the use of a FACSVantage cell sorter (BD Biosciences). Naive CD4+CD25− T cells (2 × 104), also purified by FACS sorting, were cultured with 2 × 104, 1 × 104, or 5 × 103 TReg cells in the presence of anti-CD3 antibody (1 μg/mL) and irradiated (30 Gy [3000 rad]) naive splenocytes (2 × 104) as antigen-presenting cells (APCs) for 72 hours in 96-well plates in triplicates. Cells were then pulsed with 1 μCi (0.037 MBq) per well of [3H]thymidine. Sixteen hours later, cells were harvested, and incorporation of [3H]thymidine was measured by a 1205 Betaplate liquid scintillation counter (PerkinElmer Wallac, Gaithersburg, MD).
Detection of IFN-α by enzyme-linked immunoabsorbent assay
Measurement of serum IFN-α was performed by enzyme-linked immunoabsorbent assay (ELISA) according to the manufacturer's standard protocols. The IFN-α ELISA kits were obtained from PBL Biomedical Laboratories (Piscataway, NJ).
In vivo antibody blocking
In vivo blocking of type I IFN was performed as described.40 Briefly, mice were administered intraperitoneally neutralizing antibodies to mouse IFN-α and IFN-β (10 000 IU each;, PBL Biomedical Laboratories) 6 hours before and 24 hours after vaccination. The neutralizing activity of these antibodies was verified in a cytopathic effect inhibition assay elicited by IFN-α or IFN-β as described.41
Tumor survival experiments
Syngeneic transplantation was set up in the absence of transgenic clone 4 T cells. Tumor-free survival was determined with biweekly inspection, and mice were killed after tumor development as evidenced by increasing abdominal girth and palpable abdominal masses. All killed mice had the presence of tumors (hepatic and splenic nodules and mesenteric nodal enlargement) confirmed at autopsy.
Statistical analysis
Results are expressed as mean plus or minus SD. Differences between groups were examined for statistical significance with the use of a Student t test. Statistical analysis for tumor-free survival was performed with Kaplan-Meier survival and the log-rank (Mantel-Cox) test.
Results
CD4+CD25+ TReg cells are critical for posttransplantation tumor-specific T-cell tolerance
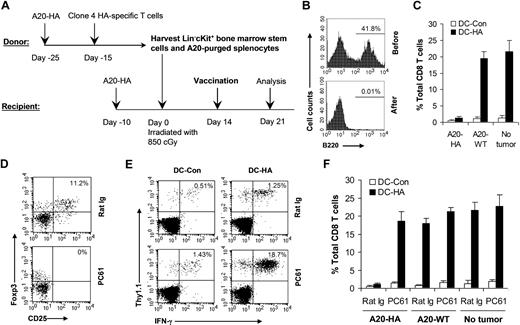
To understand the mechanism(s) underlying tumor-specific T-cell tolerance after stem cell transplantation, we established a model of syngeneic transplantation with HA as a model tumor antigen (Figure 1A). Donor BALB/c mice were inoculated with 105 A20-HA lymphoma on day −25. On day −15, 5 × 105 naive HA-specific CD8+ T cells (Thy1.1+) derived from clone 4 HA-TCR transgenic mice were transferred into these mice intravenously. On day 0, bone marrow stem cells and splenocytes were harvested from tumor-bearing donor mice. Splenocytes were further purged of B cell–derived A20 lymphoma cells. Essentially all of the B220+ B cells were removed after purging (Figure 1B). The graft, consisting of 3 × 105 Lin−c-Kit+ stem cells and 107 purged splenocytes, was injected intravenously into irradiated (850 cGy) recipient tumor-bearing BALB/c mice. Fourteen days after transplantation, mice were immunized with mature DCs pulsed with HA epitope peptide (DC-HA). These mature DCs expressed high levels of CD80, CD86, and MHC class II (data not shown). Seven days after vaccination, splenocytes were analyzed for HA-specific CD8+ T-cell response. Mice immunized with DC-HA showed only a minimal increase in IFN-γ–producing HA-specific CD8+ T cells compared with those treated with DCs pulsed with a control peptide (DC-Con; Figure 1C). However, efficient activation of HA-specific CD8+ T cells on DC-HA vaccination was observed in mice bearing wild-type A20 (A20-WT) or no tumors (Figure 1C). These results suggest that HA-specific CD8+ T-cell response is induced in A20-HA mice, but not in A20-WT or non–tumor-bearing mice after transplantation.
CD4+CD25+ TReg cells are critical for posttransplantation tumor-specific T-cell tolerance. (A) Schematic view for a syngeneic transplantation model that used clone 4 HA-specific transgenic T cells. Donor mice were transferred with A20-HA on day −25 and naive clone 4 T cells on day −15. On day −10, recipients were injected with A20-HA. On day 0, splenocytes and bone stem cells were harvested from tumor-bearing donor mice. Recipient mice were irradiated and then injected with a graft composed of Lin−c-Kit+ stem cells and A20 lymphoma–purged splenocytes. (B) Purging of A20 lymphoma cells. The percentage of B220+ B cells before and after purging is indicated. (C) Syngeneic transplantations were set up using A20-HA, A20-WT, or without tumors (No tumor). Fourteen days after transplantation, mice were vaccinated with mature DCs pulsed with either HA peptide (DC-HA) or a control peptide (DC-Con). Seven days after vaccination, splenocytes were analyzed for activation of HA-specific CD8 T cells. The mean percentage (± SD; n = 4) of IFN-γ–secreting HA-specific T cells among total CD8 T cells is shown. (D-F) Syngeneic transplantations were set up with the use of A20-HA, A20-WT, or without tumors (No tumor). Seven days after transplantation, mice were treated with either 0.3 mg PC61 or rat Ig daily for 3 days. Fourteen days after transplantation, mice were vaccinated with DC-HA or DC-Con. Seven days after vaccination, splenocytes were analyzed for the percentage of CD4+CD25+Foxp3+TReg (D); the percentage of IFN-γ–secreting HA-specific T cells (Thy1.1+IFN-γ+) among total CD8 T cells in A20-HA bearing mice is shown (E); and the mean percentage (± SD; n = 4) of IFN-γ–secreting HA-specific T cells among total CD8 T cells is shown (F). Representative results of 3 independent experiments are shown.
CD4+CD25+ TReg cells are critical for posttransplantation tumor-specific T-cell tolerance. (A) Schematic view for a syngeneic transplantation model that used clone 4 HA-specific transgenic T cells. Donor mice were transferred with A20-HA on day −25 and naive clone 4 T cells on day −15. On day −10, recipients were injected with A20-HA. On day 0, splenocytes and bone stem cells were harvested from tumor-bearing donor mice. Recipient mice were irradiated and then injected with a graft composed of Lin−c-Kit+ stem cells and A20 lymphoma–purged splenocytes. (B) Purging of A20 lymphoma cells. The percentage of B220+ B cells before and after purging is indicated. (C) Syngeneic transplantations were set up using A20-HA, A20-WT, or without tumors (No tumor). Fourteen days after transplantation, mice were vaccinated with mature DCs pulsed with either HA peptide (DC-HA) or a control peptide (DC-Con). Seven days after vaccination, splenocytes were analyzed for activation of HA-specific CD8 T cells. The mean percentage (± SD; n = 4) of IFN-γ–secreting HA-specific T cells among total CD8 T cells is shown. (D-F) Syngeneic transplantations were set up with the use of A20-HA, A20-WT, or without tumors (No tumor). Seven days after transplantation, mice were treated with either 0.3 mg PC61 or rat Ig daily for 3 days. Fourteen days after transplantation, mice were vaccinated with DC-HA or DC-Con. Seven days after vaccination, splenocytes were analyzed for the percentage of CD4+CD25+Foxp3+TReg (D); the percentage of IFN-γ–secreting HA-specific T cells (Thy1.1+IFN-γ+) among total CD8 T cells in A20-HA bearing mice is shown (E); and the mean percentage (± SD; n = 4) of IFN-γ–secreting HA-specific T cells among total CD8 T cells is shown (F). Representative results of 3 independent experiments are shown.
To test whether posttransplantation tumor-specific T-cell tolerance in recipient mice was mediated by CD4+CD25+ TReg cells, mice were treated with the depleting CD25 monoclonal antibody, PC61, or a control rat Ig. Essentially all CD4+CD25+Foxp3+ TReg cells were depleted after 3 daily injections of PC61 (Figure 1D). A20-HA–bearing mice depleted of TReg cells showed a significant (P < .001) increase in IFN-γ–producing HA-specific CD8+ T cells compared with rat Ig-treated control mice (Figure 1E,F). However, TReg cell depletion yielded no significant difference in HA-specific CD8+ T-cell response in mice bearing A20-WT or no tumors (Figure 1F), suggesting a role of antigen-specific TReg cells in mediating posttransplantation tolerance.
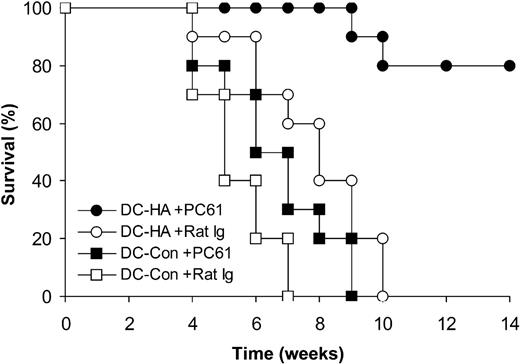
We next examined whether removal of TReg cells enhanced the efficacy of DC vaccines in treating preestablished tumors after transplantation. Because of the concern that transfer of transgenic T cells may create higher precursor frequency of antigen-specific T cells than in the natural setting, this study was performed without transfer of clone 4 HA-specific T cells. Indeed, depletion of CD4+CD25+ TReg cells with PC61 followed by vaccination with DC-HA led to a significant (P < .01) prolongation of tumor-free survival compared with rat Ig-treated control mice (Figure 2). Collectively, these results suggest that CD4+CD25+ TReg cells are critical for tumor-specific T-cell tolerance and that depletion of TReg cells in recipients promotes DC vaccine potency in treating preestablished tumors after transplantation.
In vivo depletion of CD4+CD25+ TReg cells enhances the efficacy of posttransplantation DC vaccination in treating preestablished lymphoma. Syngeneic transplantation was set up as described in Figure 1A except that no transgenic HA-specific T cells were used. Seven days after transplantation, mice were treated with either 0.3 mg PC61 or the control rat Ig daily for 3 days. Fourteen days after transplantation, mice were vaccinated with mature DCs pulsed with either HA peptide (DC-HA) or a control peptide (DC-Con) and monitored for tumor-free survival. Data (n = 10) represent percentages of tumor-free survival over time from the transplantation (day 0).
In vivo depletion of CD4+CD25+ TReg cells enhances the efficacy of posttransplantation DC vaccination in treating preestablished lymphoma. Syngeneic transplantation was set up as described in Figure 1A except that no transgenic HA-specific T cells were used. Seven days after transplantation, mice were treated with either 0.3 mg PC61 or the control rat Ig daily for 3 days. Fourteen days after transplantation, mice were vaccinated with mature DCs pulsed with either HA peptide (DC-HA) or a control peptide (DC-Con) and monitored for tumor-free survival. Data (n = 10) represent percentages of tumor-free survival over time from the transplantation (day 0).
Removal of CD4+CD25+ TReg cells in the donor lymphocyte graft does not reverse posttransplantation tolerance
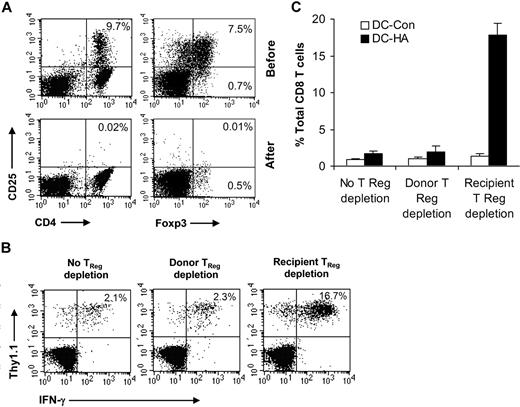
We next tested whether TReg cell–mediated tumor-specific T-cell tolerance in recipient mice after transplantation was transferred from the lymphocyte graft harvested from tumor-bearing mice. To address this question, we examined the effect of CD25+ T-cell depletion from the donor-lymphocyte graft on the posttransplantation activation of HA-specific CD8+ T cells by DC vaccines. Syngeneic transplantation was set up as described in Figure 1. On day 0, the donor splenocyte graft was depleted of CD25+ T cells with the use of anti-CD25 microbeads. This method removed greater than 99% of CD4+CD25+Foxp3+ TReg cells (Figure 3A). However, a small fraction of CD4+CD25−Foxp3+ T cells remained (Figure 3A). Mice were vaccinated 14 days after transplantation and analyzed 7 days later. Mice that received CD4+CD25+Foxp3+ TReg cell–depleted lymphocyte graft did not show an increase in HA-specific CD8+ T-cell response on vaccination with DC-HA, compared with those received unmanipulated lymphocyte graft (Figure 3B,C). However, a significant (P < .001) increase in HA-specific T cells was observed when the recipients were depleted of CD4+CD25+ TReg cells in vivo with the use of PC61 (Figure 3B,C).
Depletion of CD4+CD25+ TReg cells from the donor lymphocyte graft fails to overcome posttransplantation tolerance. (A) Syngeneic transplantations were set up as described in Figure 1A. On day 0, splenocytes were purged of A20-HA. Some A20-purged donor lymphocytes were further depleted of CD25+ T cells by microbead selection. Percentages of CD4+CD25+ T cells (left), and CD4+CD25+Foxp3+ and CD4+CD25−Foxp3+ T cells (right) before and after depletion are indicated. (B,C) On day 0, recipients were transferred with either TReg cell–depleted (Donor TReg depletion) or unmanipulated (no TReg depletion) donor lymphocytes. Some recipients that received an unmanipulated lymphocyte graft were depleted of TReg cells in vivo after transplantation by PC61 injection (Recipient TReg depletion) as described in Figure 1. Fourteen days after transplantation, mice were vaccinated with DC-HA or DC-Control. Seven days after vaccination, splenocytes were stained with anti-CD8, anti-Thy1.1, and anti–IFN-γ antibodies and subjected to FACS analysis. The percentage of IFN-γ–secreting HA-specific T cells (Thy1.1+IFN-γ+) among total CD8 T cells is indicated (B). The mean percentage (± SD; n = 4) of IFN-γ–secreting HA-specific T cells among total CD8 T cells is indicated (C). Representative results of 3 independent experiments are shown.
Depletion of CD4+CD25+ TReg cells from the donor lymphocyte graft fails to overcome posttransplantation tolerance. (A) Syngeneic transplantations were set up as described in Figure 1A. On day 0, splenocytes were purged of A20-HA. Some A20-purged donor lymphocytes were further depleted of CD25+ T cells by microbead selection. Percentages of CD4+CD25+ T cells (left), and CD4+CD25+Foxp3+ and CD4+CD25−Foxp3+ T cells (right) before and after depletion are indicated. (B,C) On day 0, recipients were transferred with either TReg cell–depleted (Donor TReg depletion) or unmanipulated (no TReg depletion) donor lymphocytes. Some recipients that received an unmanipulated lymphocyte graft were depleted of TReg cells in vivo after transplantation by PC61 injection (Recipient TReg depletion) as described in Figure 1. Fourteen days after transplantation, mice were vaccinated with DC-HA or DC-Control. Seven days after vaccination, splenocytes were stained with anti-CD8, anti-Thy1.1, and anti–IFN-γ antibodies and subjected to FACS analysis. The percentage of IFN-γ–secreting HA-specific T cells (Thy1.1+IFN-γ+) among total CD8 T cells is indicated (B). The mean percentage (± SD; n = 4) of IFN-γ–secreting HA-specific T cells among total CD8 T cells is indicated (C). Representative results of 3 independent experiments are shown.
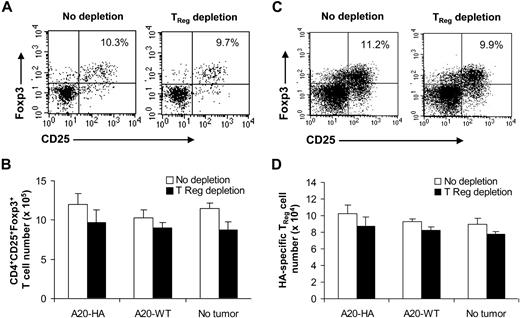
To assess why depletion of CD4+CD25+Foxp3+ TReg cells from the donor lymphocyte graft had no effect on activation of tumor-specific CD8+ T cells, we examined the status of TReg cells in recipients after transplantation. Mice that received TReg cell–depleted donor lymphocyte graft had similar numbers of CD4+CD25+Foxp3+ TReg cells to those that received an unmanipulated lymphocyte graft (Figure 4A,B), suggesting that CD4+CD25+Foxp3+ TReg cells can rapidly recover after transplantation. Similarly, CD4+CD25+Foxp3+ TReg cells could reemerge in the mice bearing A20-WT lymphoma or in non–tumor-bearing mice (Figure 4B), suggesting that TReg cell recovery after transplantation is independent of tumor antigen. Because tumor-specific TReg cells appear to mediate posttransplantation tolerance (Figure 1D), we next asked whether tumor-specific TReg cells could also rapidly repopulate after transplantation. To address this question, syngeneic transplantation was set up with the infusion of HA-specific CD4+ T cells (Thy1.1+Thy1.2+) that were derived from 6.5 HA-TCR transgenic mice into donor tumor-bearing mice on day −15 as described in Figure 1A. On day 0, the donor graft with or without depletion of CD25+ T cells was transferred into mice bearing A20-HA, A20-WT, or no tumors. Twenty-one days later, mice were analyzed for conversion of HA-specific TReg cells. Indeed, similar numbers of HA-specific TReg cells were detected in mice that received TReg cell–depleted donor lymphocyte graft compared with those that received unmanipulated lymphocytes, suggesting that tumor antigen-specific TReg cells can also rapidly repopulate after transplantation independent of tumor antigen (Figure 4C,D).
Rapid recovery of CD4+CD25+Foxp3+TReg cells in recipients that received TReg cell–depleted donor lymphocytes after transplantation. (A,B) Syngeneic transplantations were set up as described in Figure 1A. On day 0, recipients bearing A20-HA, A20-WT, or no tumors (No tumor) were transferred with either TReg cell–depleted (Donor TReg depletion) or unmanipulated (no TReg depletion) donor lymphocytes. Fourteen days after transplantation, mice were vaccinated, an, 7 days after vaccination, splenocytes were stained with anti-CD4, anti-CD25, and anti-Foxp3 antibodies. The percentage of CD4+CD25+Foxp3+TReg cells among total CD4+ T cells in A20-HA–bearing mice is indicated (A). The mean absolute number (± SD; n = 4) of CD4+CD25+Foxp3+ T cells per spleen in each group is indicated (B). (C,D) Syngeneic transplantations were set up as described with the addition of HA-specific CD4+ T cells (Thy1.1+Thy1.2+) into donor tumor-bearing mice. On day 0, recipients bearing A20-HA, A20-WT, or no tumors (No tumor) were transferred with either TReg cell–depleted (Donor TReg depletion) or unmanipulated (no TReg depletion) donor lymphocytes. Twenty-one days later, the percentage of HA-specific TReg cells (Thy1.1+Thy1.2+CD4+CD25+Foxp3+) among total clonotypic (Thy1.1+Thy1.2+) CD4+ T cells in A20-HA–bearing mice is indicated (C). The mean absolute number (± SD; n = 4) of HA-specific TReg cells per spleen in each group is indicated (D). Representative results of 2 independent experiments are shown.
Rapid recovery of CD4+CD25+Foxp3+TReg cells in recipients that received TReg cell–depleted donor lymphocytes after transplantation. (A,B) Syngeneic transplantations were set up as described in Figure 1A. On day 0, recipients bearing A20-HA, A20-WT, or no tumors (No tumor) were transferred with either TReg cell–depleted (Donor TReg depletion) or unmanipulated (no TReg depletion) donor lymphocytes. Fourteen days after transplantation, mice were vaccinated, an, 7 days after vaccination, splenocytes were stained with anti-CD4, anti-CD25, and anti-Foxp3 antibodies. The percentage of CD4+CD25+Foxp3+TReg cells among total CD4+ T cells in A20-HA–bearing mice is indicated (A). The mean absolute number (± SD; n = 4) of CD4+CD25+Foxp3+ T cells per spleen in each group is indicated (B). (C,D) Syngeneic transplantations were set up as described with the addition of HA-specific CD4+ T cells (Thy1.1+Thy1.2+) into donor tumor-bearing mice. On day 0, recipients bearing A20-HA, A20-WT, or no tumors (No tumor) were transferred with either TReg cell–depleted (Donor TReg depletion) or unmanipulated (no TReg depletion) donor lymphocytes. Twenty-one days later, the percentage of HA-specific TReg cells (Thy1.1+Thy1.2+CD4+CD25+Foxp3+) among total clonotypic (Thy1.1+Thy1.2+) CD4+ T cells in A20-HA–bearing mice is indicated (C). The mean absolute number (± SD; n = 4) of HA-specific TReg cells per spleen in each group is indicated (D). Representative results of 2 independent experiments are shown.
Rapid posttransplantation TReg cell recovery is due to conversion of donor CD4+CD25− T cells into CD4+CD25+Foxp3+ TReg cells
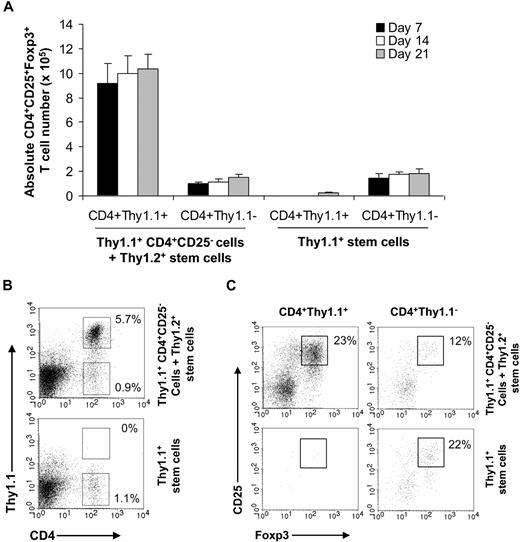
We next examined what contributed to the rapid posttransplantation recovery of CD4+CD25+Foxp3+ TReg cells. The rapid recovery of TReg cells could be due to differentiation from donor stem cells, conversion from donor CD4+CD25− T cells, or posttransplantation recovery of residual recipient TReg cells after transplantation. To address these possibilities, we transferred FACS-sorted CD4+CD25− T cells from Thy1.1+ donor tumor-bearing mice along with Thy1.2+ stem cells into irradiated Thy1.2+ tumor-bearing mice. Seven, 14, and 21 days later, splenocytes were analyzed for CD4+CD25+Foxp3+ TReg cells. Our data showed that most of the CD4+CD25+Foxp3+ TReg cells were derived from donor Thy1.1+ CD4+CD25− T cells beginning at day 7 after transplantation (Figure 5A). At day 14, approximately 23% of donor Thy1.1+CD4+ T cells were CD4+CD25+Foxp3+ TReg (Figure 5B top). We also detected a small fraction of Thy1.1− CD4+CD25+Foxp3+ TReg cells (Figure 5A,B). However, the absolute numbers of CD4+CD25+Foxp3+ TReg cells derived from the CD4+Thy1.1+ population were much higher than that from the CD4+Thy1.1− population (Figure 5A). These results suggest that rapid recovery of CD4+CD25+Foxp3+ TReg cells in the recipients after transplantation was mainly due to conversion from donor CD4+CD25− T cells.
Rapid recovery of TReg cells is due to conversion of donor CD4+CD25− T cells into CD4+CD25+Foxp3+ TReg cells in recipients after transplantation. Syngeneic transplantations were set up as described. On day 0, a cohort of recipients (Thy1.2+) received FACS-purified CD4+CD25− T cells from Thy1.1+ tumor–bearing mice and Thy1.2+ stem cells (Thy1.1+CD4+CD25− cells + Thy1.2+ stem cells). Others received only stem cells from Thy1.1+ donor mice (Thy1.1+ stem cells). Seven, 14, and 21 days later, splenocytes were stained with anti-Thy1.1, anti-CD4, anti-CD25, and anti-Foxp3 antibodies and subjected to FACS analysis. (A) The mean absolute number (± SD; n = 4) of CD4+CD25+Foxp3+ T cells per spleen in each group is indicated. (B) The percentages of CD4+Thy1.1+ and CD4+Thy1.1− T cells among total lymphocytes at day 14 are indicated. (C) The percentages of CD25+Foxp3+ T cells among CD4+Thy1.1+ or CD4+Thy1.1− T cells at day 14 are indicated. Representative results of 2 independent experiments are shown.
Rapid recovery of TReg cells is due to conversion of donor CD4+CD25− T cells into CD4+CD25+Foxp3+ TReg cells in recipients after transplantation. Syngeneic transplantations were set up as described. On day 0, a cohort of recipients (Thy1.2+) received FACS-purified CD4+CD25− T cells from Thy1.1+ tumor–bearing mice and Thy1.2+ stem cells (Thy1.1+CD4+CD25− cells + Thy1.2+ stem cells). Others received only stem cells from Thy1.1+ donor mice (Thy1.1+ stem cells). Seven, 14, and 21 days later, splenocytes were stained with anti-Thy1.1, anti-CD4, anti-CD25, and anti-Foxp3 antibodies and subjected to FACS analysis. (A) The mean absolute number (± SD; n = 4) of CD4+CD25+Foxp3+ T cells per spleen in each group is indicated. (B) The percentages of CD4+Thy1.1+ and CD4+Thy1.1− T cells among total lymphocytes at day 14 are indicated. (C) The percentages of CD25+Foxp3+ T cells among CD4+Thy1.1+ or CD4+Thy1.1− T cells at day 14 are indicated. Representative results of 2 independent experiments are shown.
To further delineate the source of Thy1.1− CD4+CD25+Foxp3+ TReg cells that were not derived from the conversion, we transferred only Thy1.1+ stem cells into irradiated Thy1.2+ tumor-bearing mice and examined CD4+CD25+Foxp3+ TReg cells. No Thy1.1+CD4+ T cells were detected at days 7 and 14 after transplantation (Figure 5A,B). Even at day 21, only a tiny fraction of CD4+CD25+Foxp3+ TReg cells were Thy1.1+ (Figure 5A). However, we identified a higher percentage of Thy1.1−CD4+ T cells that contained CD4+CD25+Foxp3+ TReg cells (Figure 5A,B), suggesting the presence of residual recipient CD4+ T cells. Thus, even at day 21 after transplantation, the thymic output of CD4+CD25+Foxp3+ TReg cells was still minimal.
In vivo coadministration of CpG enhances tumor-specific CD8+ T-cell response to posttransplantation DC vaccination
Our observation that depletion of CD4+CD25+ TReg cells in recipients, but not from the donor lymphocyte graft, was required to reverse posttransplantation TReg cell–mediated tolerance suggested that strategies capable of overcoming CD4+CD25+ TReg cell–mediated suppression in recipients would be necessary to enhance tumor-specific T-cell responses after transplantation. Although depletion of TReg cells might prove to be effective in enhancing the posttransplantation antitumor responses in humans, a potential concern is that such depletion of TReg cells could trigger the development of systemic autoimmunity as seen in patients with melanoma treated with anti–CTLA-4 Ab.42 Thus, in an effort to induce strong posttransplantation antitumor immunity without eliciting significant autoimmunity, it is compelling to explore strategies to overcome TReg cell–mediated tolerance without systemic removal of TReg cells.
Recent advances have suggested a crucial role for the innate immune system in shaping adaptive immunity.43,44 In a model of peripheral CD8 T-cell tolerance to self-antigen, we have previously shown that provision of Toll-like receptor (TLR) ligands in vivo can overcome established TReg cell–mediated CD8 tolerance in vivo by DC vaccines.34 Here, we investigated whether in vivo coinjection of the TLR9 ligand, CpG, enabled DC vaccines to overcome TReg cell–mediated tolerance after transplantation. Fourteen days after transplantation, tumor-bearing mice were immunized with DC-HA with or without coadministration of CpG. Seven days later, splenocytes were analyzed for HA-specific T-cell activation. Our results showed that coadministration of CpG significantly enhanced tumor-specific CD8+ T-cell responses (P < .001) compared with control groups (Figure 6A,B). Furthermore, DC-HA vaccine coinjected with CpG significantly (P < .01) prolonged posttransplantation tumor-free survival in the absence of transgenic T cells (Figure 6C). These results suggest that in vivo coadministration of CpG and DC vaccine can overcome posttransplantation tumor-specific T-cell tolerance in the presence of TReg cells.
Administration of CpG in vivo enhances tumor-specific CD8+ T-cell response to posttransplantation DC vaccination. (A,B) Syngeneic transplantations were set up as described in Figure 1A. Fourteen days after transplantation, mice were vaccinated with either TNF-α–matured DC-HA or DC-Con with (CpG in vivo) or without (no CpG) coadministration of CpG in vivo. Some mice received DCs matured with CpG ex vivo followed by HA pulsing (CpG ex vivo). Seven days after vaccination, splenocytes were stained with anti-CD8, anti-Thy1.1, and anti–IFN-γ antibodies and subject to FACS analysis. The percentage of IFN-γ–secreting HA-specific T cells (Thy1.1+IFN-γ+) among total CD8+ T cells is indicated (A). The mean percentage (± SD; n = 4) of IFN-γ–secreting HA-specific T cells among total CD8+ T cells is indicated (B). (C) Tumor-free survival. Syngeneic transplantations were set up in the absence of transgenic T cells. Fourteen days after transplantation, mice were vaccinated with DC-HA or DC-Con with or without coadministration of CpG in vivo and monitored for tumor-free survival. Data (n = 10) represent percentages of tumor-free survival over time from the transplant (day 0). Representative results of 2 independent experiments are shown.
Administration of CpG in vivo enhances tumor-specific CD8+ T-cell response to posttransplantation DC vaccination. (A,B) Syngeneic transplantations were set up as described in Figure 1A. Fourteen days after transplantation, mice were vaccinated with either TNF-α–matured DC-HA or DC-Con with (CpG in vivo) or without (no CpG) coadministration of CpG in vivo. Some mice received DCs matured with CpG ex vivo followed by HA pulsing (CpG ex vivo). Seven days after vaccination, splenocytes were stained with anti-CD8, anti-Thy1.1, and anti–IFN-γ antibodies and subject to FACS analysis. The percentage of IFN-γ–secreting HA-specific T cells (Thy1.1+IFN-γ+) among total CD8+ T cells is indicated (A). The mean percentage (± SD; n = 4) of IFN-γ–secreting HA-specific T cells among total CD8+ T cells is indicated (B). (C) Tumor-free survival. Syngeneic transplantations were set up in the absence of transgenic T cells. Fourteen days after transplantation, mice were vaccinated with DC-HA or DC-Con with or without coadministration of CpG in vivo and monitored for tumor-free survival. Data (n = 10) represent percentages of tumor-free survival over time from the transplant (day 0). Representative results of 2 independent experiments are shown.
CpG-induced type I IFN is critical for overcoming posttransplantation tumor-specific CD8 tolerance
We next investigated the mechanism(s) underlying CpG-dependent reversal of tumor-specific T-cell tolerance. We first examined whether the enhanced tumor-specific CD8 T-cell response was due to enhanced DC maturation on CpG stimulation. We first compared DCs matured with either CpG or TNF-α. Both CpG and TNF-α–treated DCs expressed similarly high levels (> 95%) of CD80, CD86, and MHC class II after 48 hours in culture (data not shown). Injection of ex vivo CpG-matured DC-HA did not elicit a significant HA-specific CD8 T-cell response (Figure 6A,B). This result indicates that stimulation of DCs with CpG ex vivo cannot overcome TReg cell–mediated suppression and suggests that in vivo CpG-dependent reversal of T-cell tolerance is probably not due to increased DC maturation.
We next determined whether CpG treatment in vivo altered CD4+CD25+Foxp3+ TReg cell numbers and function. Seven days after vaccination, splenocytes were analyzed for CD4+CD25+Foxp3+ TReg cells. No significant (P < .05) differences in TReg cell number were detected in mice treated with or without CpG (Figure 7A). We then examined if CpG treatment affected TReg cell function. FACS-purified TReg cells were assayed for their suppressive capacity on naive CD4+CD25− T cells with an in vitro suppression assay. We found that TReg cells isolated from CpG-treated mice suppressed the proliferation of naive CD4+CD25− T cells similarly to those from untreated mice (Figure 7B), suggesting that TReg cells isolated from CpG-treated mice possess the suppressive function by an in vitro assay.
A critical role for type I IFN in CpG-dependent reversal of tumor-specific T tolerance after transplantation. (A-C) Syngeneic transplantations were set up as described in Figure 1A. Fourteen days after transplantation, mice were vaccinated with either TNF-α–matured DC-HA or DC-Con with (CpG in vivo) or without (no CpG) coadministration of CpG in vivo. Some mice received DCs matured with CpG ex vivo followed by HA pulsing (CpG ex vivo). Seven days after vaccination, the mean absolute number (± SD; n = 4) of CD4+CD25+Foxp3+ T cells per spleen in each group is indicated (A). CD4+CD25+ TReg cells (Suppressor) were isolated by FACS sorting and assayed for their suppressive capacity on naive CD4+CD25− T cells (Responder) at the indicated ratios of suppressor to responder (S:R) using an in vitro suppression assay. Cultures were labeled with [3H]thymidine and harvested for scintillation counting. Results are expressed as mean CPM ± SD (B). Sera were harvested 12 hours after administration of CpG and measured for IFN-α by ELISA (C). (D) Fourteen days after transplantation, mice were vaccinated with DC-HA or DC-Con coadministered with CpG in vivo. Six hours before and 24 hours after vaccination, mice were treated with neutralizing antibodies to mouse IFN-α and IFN-β (IFN-αβ Ab) or a control Ab. Seven days after vaccination, splenocytes were stained with anti-CD8, anti-Thy1.1, and anti–IFN-γ antibodies, and the mean percentage (± SD; n = 4) of IFN-γ–secreting HA-specific T cells among total CD8+ T cells is indicated (D). Representative results of 2 independent experiments are shown.
A critical role for type I IFN in CpG-dependent reversal of tumor-specific T tolerance after transplantation. (A-C) Syngeneic transplantations were set up as described in Figure 1A. Fourteen days after transplantation, mice were vaccinated with either TNF-α–matured DC-HA or DC-Con with (CpG in vivo) or without (no CpG) coadministration of CpG in vivo. Some mice received DCs matured with CpG ex vivo followed by HA pulsing (CpG ex vivo). Seven days after vaccination, the mean absolute number (± SD; n = 4) of CD4+CD25+Foxp3+ T cells per spleen in each group is indicated (A). CD4+CD25+ TReg cells (Suppressor) were isolated by FACS sorting and assayed for their suppressive capacity on naive CD4+CD25− T cells (Responder) at the indicated ratios of suppressor to responder (S:R) using an in vitro suppression assay. Cultures were labeled with [3H]thymidine and harvested for scintillation counting. Results are expressed as mean CPM ± SD (B). Sera were harvested 12 hours after administration of CpG and measured for IFN-α by ELISA (C). (D) Fourteen days after transplantation, mice were vaccinated with DC-HA or DC-Con coadministered with CpG in vivo. Six hours before and 24 hours after vaccination, mice were treated with neutralizing antibodies to mouse IFN-α and IFN-β (IFN-αβ Ab) or a control Ab. Seven days after vaccination, splenocytes were stained with anti-CD8, anti-Thy1.1, and anti–IFN-γ antibodies, and the mean percentage (± SD; n = 4) of IFN-γ–secreting HA-specific T cells among total CD8+ T cells is indicated (D). Representative results of 2 independent experiments are shown.
What was then responsible for CpG-dependent reversal of tumor-specific T-cell tolerance after transplantation? It has been shown that stimulation of plasmacytoid DCs with CpG by TLR9 leads to production of high levels of type I IFN such as IFN-α and IFN-β.40,45,46 To address this, serum samples were collected 12 hours after CpG injection, and IFN-α levels were measured by ELISA. Indeed, we showed here that coadministration of CpG in vivo triggered secretion of high levels of IFN-α after transplantation (Figure 7C). No significant IFN-α production was detected in mice immunized with ex vivo CpG-treated DCs compared with the untreated control (Figure 7C). The strong correlation between the secretion of high levels of IFN-α and T-cell responses in vivo (Figures 6B, 7C) suggested that type I IFN may play a critical role in mediating the reversal of T-cell tolerance after transplantation. To test this hypothesis, we examined whether in vivo blocking of type I IFNs abolished CpG-dependent reversal of tumor-specific T-cell tolerance. Indeed, administration of neutralizing Abs to both IFN-α and IFN-β significantly reduced tumor-specific CD8+ T-cell responses (P < .001) compared with the control Ab-treated groups (Figure 7D), suggesting that reversal of tumor-specific T-cell tolerance after transplantation is critically dependent on type I IFN.
Discussion
In this study, we showed that CD4+CD25+ TReg cells played a critical role in posttransplantation tumor-specific T-cell tolerance. However, depletion of TReg cells from the lymphocyte graft harvested from tumor-bearing hosts could not reverse posttransplantation tolerance because of rapid conversion of donor CD4+CD25− T cells into CD4+CD25+Foxp3+ TReg cells. Instead, in vivo depletion of TReg cells in recipients was necessary to overcome TReg cell–mediated suppression on tumor-specific T cells. These results suggest that reversal of CD4+CD25+Foxp3+ TReg cell–mediated tolerance in recipients is necessary to enhance tumor-specific T-cell response. As a first step to achieving this goal, we showed that DC vaccines coadministered with CpG in vivo could overcome TReg cell–mediated suppression and effectively activate tumor-specific CD8+ T cells, leading to a significant prolongation of tumor-free survival. We further showed that type I IFN played a critical role in CpG-dependent reversal of tumor-specific T-cell tolerance after transplantation.
Previous studies in models of syngeneic transplantation have shown that transfer of a mature T-cell graft from the tumor-bearing mice led to transient T-cell activation and expansion immediately after transplant probably because of homeostatic proliferation.11 However, this response was not sustained, and tumor-specific T cells were ultimately tolerized, yielding tumor progression.11,12 Anergy in the absence of costimulation and physical deletion have been proposed as mechanisms for this posttransplantation tumor-specific T-cell tolerance.11,12 T-cell anergy is defined as lack of effector function such as secretion of IFN-γ, on stimulation with functionally mature APCs.47,48 Thus, although we cannot rule out the possibility of irreversible T-cell exhaustion, it would be unlikely that this tolerance was due to T-cell anergy because the ability of tumor-specific T cells to secrete IFN-γ on a per cell basis appeared not to be compromised even in the presence of TReg cells (Figure 1C). Here, we provided evidence that posttransplantation tumor-specific tolerance is mainly mediated through active suppression by CD4+CD25+ TReg cells and that depletion of TReg cells in conjunction with DC vaccination significantly prolongs tumor-free survival after transplantation. Indeed, in the non–transplantation setting, it has been well documented that CD4+CD25+ TReg cells play a critical role in suppressing antitumor immune responses.25 This is further supported by a recent report that, although both naturally occurring, thymus-derived CD4+CD25+ TReg cells and de novo tumor-induced TReg cells could contribute independently to tumor-specific tolerance, the expansion of CD4+CD25+ TReg cells significantly exceeds the induction of TReg cells from uncommitted precursors in tumor-bearing mice.49
Although active tolerance mediated by CD4+CD25+ TReg cells is transferable, we showed that removal of TReg cells from donor graft could not reverse posttransplantation tolerance, which was mainly due to rapid conversion of donor CD4+CD25− T cells into CD4+CD25+Foxp3+ TReg cells. Interestingly, the TReg cell conversion can also occur in the absence of tumor. This is in line with previous observations that both CD4+CD25−Foxp3− and CD4+CD25−Foxp3+ T cells can generate CD4+CD25+Foxp3+ TReg cells by peripheral expansion in lymphopenic mice. Thus, the residual fraction of CD4+CD25−Foxp3+ T cells in the donor lymphocyte graft could also contribute to the conversion into CD4+CD25+Foxp3+ TReg cells after transplantation. The TReg cell conversion is critically dependent on homeostatic proliferation of T cells in the lymphopenic environment.50-52 Very low proliferation or conversion occurs in nonlymphopenic mice.51 Recent studies have shown that peripheral conversion of CD4+CD25− T cells into CD4+CD25+Foxp3+ TReg cells is also dependent on both TGF-β signaling and B7 costimulation.50,53 It remains to be defined whether the same mechanisms apply to the rapid conversion into CD4+CD25+Foxp3+ TReg cells in the posttransplantation tumor setting. Delineation of signals required for posttransplantation TReg cell conversion may help design effective strategies to enhance antitumor immunity.
With the use of transgenic antigen-specific TReg cells, a recent study by Mirmonsef et al54 has suggested that antigen-specific effector CD4+ T cells could out-compete CD4+CD25+Foxp3+ TReg cells immediately after transplantation, leading to diminished TReg frequency. This is in contrast to our observations. The reasons for the discrepancy are not entirely clear but may be related to different experimental conditions or readouts. In their report, the antigen-specific TReg cells and effector CD4+ T cells were traced by congenic markers, but not CD25 or Foxp3. Thus, antigen-specific TReg cell conversion from their CD4+CD25− counterparts after transplantation was not addressed. In addition, 5 days before transplantation into recipient mice, clonotypic TReg cells and effector CD4+ T cells in donor mice were stimulated with vaccinia virus encoding the antigen to expand these cells for subsequent harvest and transfer. It is not clear whether this manipulation has an effect on TReg cell expansion and conversion in recipients after transplantation.
The observation that in vivo depletion of TReg cells in recipients was necessary for reversing TReg cell–mediated tumor-specific tolerance suggests that strategies capable of overcoming TReg cell–mediated suppression in recipients are required for promoting tumor-specific immunity after transplantation. One way to achieve this is to physically remove TReg cells in vivo as we demonstrated. A potential severe adverse effect associated with this approach is the development of systemic autoimmunity because TReg cells also play an essential role in maintaining self-tolerance.55,56 An alternative approach is to functionally overcome TReg cell–mediated suppression, rather than systemic depletion. Our finding that coadministration of the TLR9 ligand, CpG, with DC vaccines could reverse tumor-specific CD8+ T-cell tolerance in vivo suggests the feasibility of this approach. Our results may have important implications for the design of effective vaccine strategies for treating cancer in the setting of stem cell transplantation. The clinical application of the combined DC vaccines with CpG in vivo for treating hematologic malignancies will need to be tested in human clinical trials. Although no apparent toxicities were observed with a single injection of 20 nmol CpG ODN, the optimal in vivo kinetics (ie, duration, dose, etc) and the potential toxicity profile of CpG requires further investigation.
Equally important is to understand the mechanism(s) responsible for TLR9-dependent reversal of TReg cell–mediated suppression on tumor-specific CD8+ T cells after transplantation. It has been suggested that TLR-stimulated DC maturation might facilitate overcoming CD4+CD25+ TReg cell–mediated suppression in vitro.57 However, our data that CpG-stimulated DCs ex vivo could not overcome TReg cell–mediated tolerance suggest that promoting DC maturation is a less likely mechanism in vivo. Similarly, CpG treatment in vivo did not alter CD4+CD25+Foxp3+ TReg cell numbers or their suppressive function evaluated by an in vitro assay. Here, we provided evidence that ligation of TLR9 with CpG in vivo induces high levels of IFN-α and that type I IFN play a critical role in overcoming TReg cell–mediated suppression on tumor-specific CD8 T cells after transplantation. How type I IFN mediate reversal of TReg cell–mediated tolerance remains unknown. Type I IFN could act on (1) TReg cells to abrogate their suppressive function, (2) tumor-specific T cells to render them refractory to TReg cell–mediated suppression, or (3) DCs to overcome TReg cell–mediated suppression. Thus, future studies should define the mechanism(s) underlying type I IFN-dependent reversal of TReg cell–mediated suppression on tumor-specific T cells after transplantation.
In conclusion, we have shown that CD4+CD25+Foxp3+ TReg cells are critical for mediating tumor-specific tolerance after stem cell transplantation, and depletion of TReg cells in recipients is required for overcoming TReg cell–mediated suppression on tumor-specific T cells. These findings suggest that strategies capable of overcoming T-cell tolerance in recipients are necessary for enhancing antitumor immunity after transplantation. We have further shown that DC vaccines coadministered with the TLR9 ligand, CpG, in vivo can overcome tumor-specific CD8 tolerance after transplantation, and the reversal of T-cell tolerance is mediated by CpG-induced type I IFN in vivo. Overall, these results suggest new strategies for improving the outcome of cancer immunotherapy after transplantation.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by grants CA111807 (Y.Y.), CA047741 (N.J.C.), and CA136934 (Y.Y.) from the National Institutes of Health (Bethesda, MD) and by a grant from the Alliance for Cancer Gene Therapy (Stamford, CT; Y.Y.).
National Institutes of Health
Authorship
Contribution: I.H., M.Q., J.Z., and X.H. designed and performed research and collected and analyzed data; and N.J.C. and Y.Y. designed research, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Yiping Yang, Departments of Medicine and Immunology, Duke University Medical Center, Box 103005, Durham, NC 2771; e-mail: yang0029@mc.duke.edu.


![Figure 7. A critical role for type I IFN in CpG-dependent reversal of tumor-specific T tolerance after transplantation. (A-C) Syngeneic transplantations were set up as described in Figure 1A. Fourteen days after transplantation, mice were vaccinated with either TNF-α–matured DC-HA or DC-Con with (CpG in vivo) or without (no CpG) coadministration of CpG in vivo. Some mice received DCs matured with CpG ex vivo followed by HA pulsing (CpG ex vivo). Seven days after vaccination, the mean absolute number (± SD; n = 4) of CD4+CD25+Foxp3+ T cells per spleen in each group is indicated (A). CD4+CD25+ TReg cells (Suppressor) were isolated by FACS sorting and assayed for their suppressive capacity on naive CD4+CD25− T cells (Responder) at the indicated ratios of suppressor to responder (S:R) using an in vitro suppression assay. Cultures were labeled with [3H]thymidine and harvested for scintillation counting. Results are expressed as mean CPM ± SD (B). Sera were harvested 12 hours after administration of CpG and measured for IFN-α by ELISA (C). (D) Fourteen days after transplantation, mice were vaccinated with DC-HA or DC-Con coadministered with CpG in vivo. Six hours before and 24 hours after vaccination, mice were treated with neutralizing antibodies to mouse IFN-α and IFN-β (IFN-αβ Ab) or a control Ab. Seven days after vaccination, splenocytes were stained with anti-CD8, anti-Thy1.1, and anti–IFN-γ antibodies, and the mean percentage (± SD; n = 4) of IFN-γ–secreting HA-specific T cells among total CD8+ T cells is indicated (D). Representative results of 2 independent experiments are shown.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/113/21/10.1182_blood-2008-05-155150/6/m_zh89990935620007.jpeg?Expires=1764955227&Signature=pecE88UrMQ3KAmAO8xb0YZDxJwrgbPCgUf7dgvNoYOJj4D8fxihvwNVD2wQ6CiLDNVqII-8ELYA9uyQ9RZhBw~dN9wz9P4XgJoINGgTvk7PTl2ZX4EADriCigZOPERyfw9Pkgb87oA9nrJ-De8MLjp-4IXXwJTd9w~y47EIA8lheuDTzv2gUtbFDabh9THJFLMjhfz9DWCR4lYLoPLt6M8MlDs4NuuWvvclJS1lQG0qVS1sesqUcHimepGT6~rcQV9ajdRSDT23-OK4M1LWE5rEcPswD-kZ1frbutTQuOldIxsMwrSOa0JnexxMDOZMg6Kc17UFvHio40bJ2Nsx4NQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal