Abstract
Retroviral vectors containing internal promoters, chromatin insulators, and self-inactivating (SIN) long terminal repeats (LTRs) may have significantly reduced genotoxicity relative to the conventional retroviral vectors used in recent, otherwise successful clinical trials. Large-scale production of such vectors is problematic, however, as the introduction of SIN vectors into packaging cells cannot be accomplished with the traditional method of viral transduction. We have derived a set of packaging cell lines for HIV-based lentiviral vectors and developed a novel concatemeric array transfection technique for the introduction of SIN vector genomes devoid of enhancer and promoter sequences in the LTR. We used this method to derive a producer cell clone for a SIN lentiviral vector expressing green fluorescent protein, which when grown in a bioreactor generated more than 20 L of supernatant with titers above 107 transducing units (TU) per milliliter. Further refinement of our technique enabled the rapid generation of whole populations of stably transformed cells that produced similar titers. Finally, we describe the construction of an insulated, SIN lentiviral vector encoding the human interleukin 2 receptor common γ chain (IL2RG) gene and the efficient derivation of cloned producer cells that generate supernatants with titers greater than 5 × 107 TU/mL and that are suitable for use in a clinical trial for X-linked severe combined immunodeficiency (SCID-X1).
Introduction
HIV-based lentiviral vectors are rapidly becoming the retrovirus vector system of choice for research and clinical gene transfer applications. The enhanced ability of lentiviral vectors to transduce both quiescent stem cells1 and nondividing terminally differentiated cells2 has led to the development of a wide range of therapeutic gene delivery vectors,3 as well as promising research tools such as short hairpin RNA gene knockdown libraries4 and vectors for induction of pluripotency in terminally differentiated cells.5 Early gamma-retroviral clinical gene therapy vectors restored immune function in patients with X-linked severe combined immunodeficiency (SCID-X1), but they were subsequently found to cause proliferative disorders via transactivation of protooncogenes.6,7 Newer lentiviral vector designs may significantly reduce that risk, and they await clinical testing for final validation of their predicted safety. Clinical-scale production of these vectors, however, is problematic, as the generation of stable producer cell lines is made significantly more difficult by their self-inactivating (SIN) long terminal repeats (LTRs). As a result, most clinical-grade production of lentiviral vectors is currently being performed using cumbersome transient transfection processes.
Insertional mutagenesis by previous gamma-retroviral gene therapy vectors occurred when strong viral enhancers within the LTR activated genes (eg, LMO2) surrounding the integrated vector.6,7 SIN vector designs completely eliminate the viral enhancers and promoters in the LTR, and when coupled with appropriate internal promoters having less or no enhancer activity, they have been shown to significantly reduce oncogene activation.8-10 Chromatin insulator sequences have also been inserted into SIN LTRs and appear to protect neighboring genes from residual transactivation from the internal promoters.11 When inserted into the LMO2 locus in Jurkat cells, lentiviral vector genomes containing an internal EF1α promoter flanked by SIN LTRs and chicken HS4 chromatin insulators caused only minimal transactivation of the LMO2 promoter.12
Clinical-scale production of such safety-modified vectors would be greatly facilitated by stable producer cell lines, which allow convenient generation of standardized, large-volume supernatants for downstream process optimization and preclinical studies. Although there have been numerous reports of lentiviral packaging cell lines,13-22 all high-titer (> 107 transducing units per milliliter [TU/mL]) stable producer lines described in these publications were created by the traditional method of viral transduction of packaging cell lines using non-SIN vector supernatants, which efficiently creates populations of cells with vector genomes integrated at sites favorable for active transcription, and in multiple copies per cell. SIN vector genomes, by virtue of the inactivating deletion in the LTR, are thus incompatible with this method. “Conditional SIN” vectors,22 which contain regulatable enhancers and promoters in the LTR, partially solve this problem, but they are a compromise in vector design and have not yet been rigorously tested either with insulators or in newly developed insertional mutagenesis assays. Fully SIN vector genomes (ie, those with no enhancers in the LTRs) must be introduced into packaging cells by nonretroviral methods, typically by cotransfection of a vector plasmid and a selectable drug resistance plasmid, which integrate inefficiently but can be selected and expanded. When this method is directly compared with viral transduction methods, the average titer of resulting producer clones is more than 40-fold decreased, and the highest titer–producing clone (at 107 TU/mL) rapidly lost titer with passage.18
In this report, we describe the creation of a new series of lentiviral packaging cell lines, and we present a solution to the problem of SIN vector genome addition to these cells. By optimizing the structure of the transfected vector genome expression cassette, we have significantly increased the frequency of high-titer producer cells within transfected and selected cell populations. We also describe a novel self-inactivating and insulated EF1α-driven clinical gene therapy vector for SCID-X1, as well as the efficient derivation of stable producer cell lines for this vector that generate titers exceeding 5 × 107 TU/mL.
Methods
Plasmid DNA constructions
SIN murine leukemia virus (MLV) vectors are based on the construct pSFG-tcLuc-ECT3.23 All lentiviral vectors are based on the construct pCL20c-MSCV-GFP.24 Further details can be found in the online data supplement (available on the Blood website; see the Supplemental Materials link at the top of the online article).
Cell lines
A Master Cell Bank vial of HEK293T/17 cells was provided by Children's GMP, LLC (Memphis, TN) and expanded for use in all subsequent manipulations. All culturing of 293T, HeLa, and U2OS cell lines was performed with D10 (Dulbecco modified Eagle medium [DMEM; Cambrex, East Rutherford, NJ] supplemented with 10% fetal calf serum [HyClone, Logan, UT] and 2 mM l-alanyl-l-glutamine [Cellgro, Herndon, VA]). All lines containing the tTA repressor were maintained with 1 ng/mL doxycycline (Clontech Laboratories, Palo Alto, CA). Puromycin, at 2 μg/mL, and Zeocin (Invitrogen, Carlsbad, CA), at 100 μg/mL, were used to select for expression of the pac and ble genes, respectively. ED7R cells (provided by Adrian Thrasher, Institute of Child Health, London, United Kingdom) were cultured in RPMI 1640 plus 10% fetal calf serum.
Lentiviral vector production
293T cells were transfected with 4 plasmids: 12 μg CL20 vector plasmid (pCL20c-MSCV-GFP24 for green fluorescent protein [GFP] vectors or pCL20i4r-EF1a-hgcOPT for common γ chain, γc), 6 μg pCAGG-HIVgpco (gagpol), 2 μg pCAG4-RTR2 (rev-tat),25 and 2 μg pHDM.G (vesicular stomatitis virus glycoprotein G, VSV-G). To make HIV vectors from packaging cell lines, cells were washed twice with phosphate-buffered saline (PBS) before seeding, and the following day cells were transfected as normally with the omission of plasmids coding for genes already present in the cell. For production from full producer cells without transfection, the same procedure was followed but without transfection and posttransfection washes. Alternatively, 1.5 × 106 cells were seeded in a 10-cm dish, and 3 days later cells were washed twice and supplemented with fresh media. Unless otherwise specified, vector was harvested 72 hours after induction.
Vector titration
All titer values reported, other than those for γc vectors, were performed using 105 HeLa cells seeded in a 6-well dish (Corning, Corning, NY) 24 hours before transduction. Frozen aliquots of vector were thawed, diluted, and applied along with 8 μg/mL polybrene in a total volume of 1 mL D10, incubated for 24 hours before supplementing with an additional 1 mL D10, and incubated for 72 hours before flow cytometry to assess percentage transduction. Only transductions yielding less than 20% positive cells were used in calculations. For ED7R cell transductions with γc vectors, 4 × 105 cells were mixed in a 6-well dish with supernatant, 6 μg/mL polybrene (Sigma-Aldrich, St Louis, MO), and 2 mL media, and dishes were centrifuged at 715g for 1 hour. After 96 hours of culture, cells were incubated with monoclonal antibody tuGh4 (BD Pharmingen, San Diego, CA) before flow cytometry to assess the percentage of γc-expressing cells. The extrapolated titers of Figure 7A were calculated using the following formula: Titer = {log[1 − (% interleukin 2 receptor common γ chain (IL2RG)+/100)]/[log(4 × 105 − 1) − log(4 × 105)]| × 200, which accommodates for the high probability of multiply transduced cells in many of the transductions. When identical vector preparations are titered using the HeLa and ED7R methods, the HeLa titer is approximately 2- to 4-fold lower than the ED7R titer (data not shown).
Murine bone marrow transduction and transplantation
Arf−/−γc−/− mice were treated with 5-fluorouracil 5 days before harvest of marrow from tibias and femurs. Cells were cultured in cytokines26 for 24 hours before incubation of 4.7 × 106 cells with 6.7 × 107 TU vector and 6 μg/mL polybrene in a total volume of 2 mL in RetroNectin-coated plates (Takara Bio, Shiga, Japan). Twenty-four hours later, cells were collected and transplanted into γc−/−Rag2−/− mice (Taconic, Germantown, NY; irradiated at 600 cGy), using 1 million cells per mouse. Mononuclear cells from peripheral blood were collected at 10 weeks after transplantation, stained with antibodies specific for the antigens indicated in Figure 6, and analyzed by flow cytometry. All animal experiments were approved by the St Jude Children's Research Hospital Institutional Animal Care and Use Committee.
Concatemer transfection
Blunt ligations contained a gel-purified PmeI-StuI fragment of pTL20-MSCV-GFP, mixed in a 25:1 molar ratio with a gel-purified PvuII-SnaBI fragment of pLT-PGK-ble. After ligation using a Quick Ligation kit (New England Biolabs, Beverly, MA), DNA was purified using phenol-chloroform-isoamyl alcohol extraction and ethanol precipitation and transfected into GPRG (gagpol, rev, and VSV-G) cells. For directional ligations, pTL20i4r2-MSCV-YFP was digested with SfiI, and pLT-PGK-ble-v3 was digested with PflMI. Both fragments were purified and similarly ligated and transfected into GPRG cells. Three days after transfection, cells were split into Zeocin (Invitrogen)–containing media.
Further details can be found in the online Supplemental Materials.
Results
Derivation of lentiviral packaging cell lines
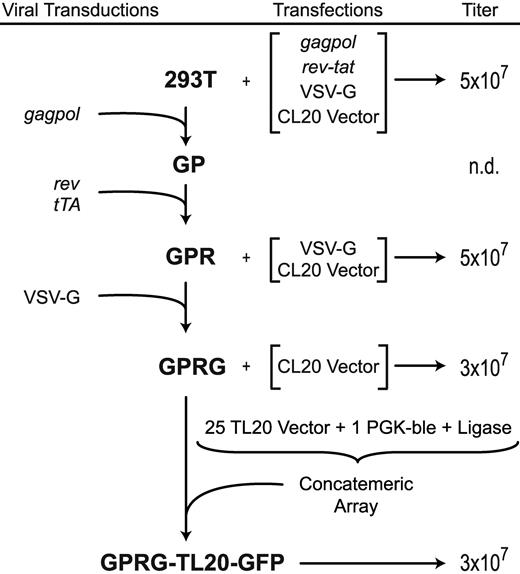
To make lentiviral packaging cells, we transduced 293T cells with gamma-retroviral vectors bearing codon-optimized genes coding for the necessary HIV vector components, similar to the method described by Ikeda et al,18 but using SIN MLV vectors and doxycycline regulation for expression of rev and the VSV-G envelope gene (Figure 1). Gamma-retroviral vectors were introduced sequentially, as outlined schematically in Figure 2, and clones were screened based on infectious vector titer of supernatants after transient transfection of the remaining components. The cell lines were given designations corresponding to the integrated genes (GP for gagpol, GPR for gagpol and rev, and GPRG for gagpol, rev, and VSV-G). A GPRT line (gagpol, rev, and tat, which was also expressed from a doxycycline-regulatable vector), was also created for research applications with vectors dependent on HIV Tat (data not shown). Western blotting using antibodies specific for HIV capsid, Rev, and the VSV-G protein (Figure S1) demonstrates abundant Gag expression in both cell lysates and supernatants, as well as tightly regulated Rev and VSV-G expression. After induction and transient transfection with plasmids encoding the remaining components necessary for producing complete vector particles, titers from all lines significantly exceeded 107 TU/mL (Figure 2). The GPR cell line was passaged for more than one year, with no detectable loss in titer production (data not shown). The GPRG line produced titers between 1 and 5 × 107 TU/mL for 3 months of continuous passage before declining gradually (data not shown).
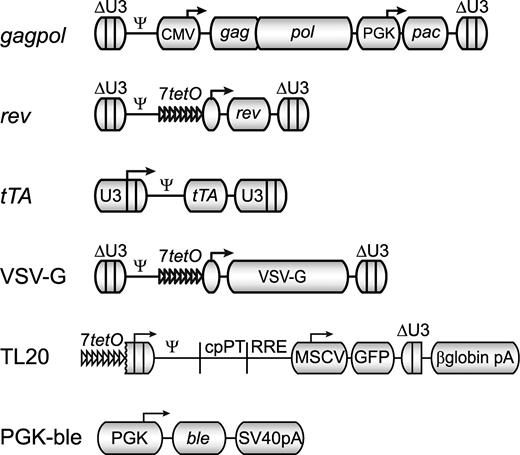
Schematic maps of constructs used in the construction of packaging and producer cell lines. gagpol, rev, rTA, and VSV-G are γ-retroviral vector genomes, shown in their integrated, proviral form; ΔU3 denotes a SIN LTR, U3 denotes a non-SIN LTR, 7tetO indicates a doxycycline repressible promoter, and pac (puromycin N-acetyltransferase) confers resistance to puromycin. TL20 and PGK-ble are transfected DNA expression cassettes for a lentiviral vector genome and bleomycin resistance, respectively. The lentiviral vector genome produced from the doxycycline repressible TL20 cassette is identical to that produced by the CL20 vector plasmid used in transient transfections.
Schematic maps of constructs used in the construction of packaging and producer cell lines. gagpol, rev, rTA, and VSV-G are γ-retroviral vector genomes, shown in their integrated, proviral form; ΔU3 denotes a SIN LTR, U3 denotes a non-SIN LTR, 7tetO indicates a doxycycline repressible promoter, and pac (puromycin N-acetyltransferase) confers resistance to puromycin. TL20 and PGK-ble are transfected DNA expression cassettes for a lentiviral vector genome and bleomycin resistance, respectively. The lentiviral vector genome produced from the doxycycline repressible TL20 cassette is identical to that produced by the CL20 vector plasmid used in transient transfections.
Flow chart showing genealogy of cell lines and their titer output after induction and/or transient transfection. Transiently transfected components are indicated by brackets (see “Methods” for plasmid designations). Cell line names are in bold, and viral transductions with constructs diagrammed in Figure 1 are indicated on the left. Unconcentrated supernatant titers, in HeLa TU/mL, are shown on the right.
Flow chart showing genealogy of cell lines and their titer output after induction and/or transient transfection. Transiently transfected components are indicated by brackets (see “Methods” for plasmid designations). Cell line names are in bold, and viral transductions with constructs diagrammed in Figure 1 are indicated on the left. Unconcentrated supernatant titers, in HeLa TU/mL, are shown on the right.
Concatemer array transfection for stable SIN vector producer cell construction
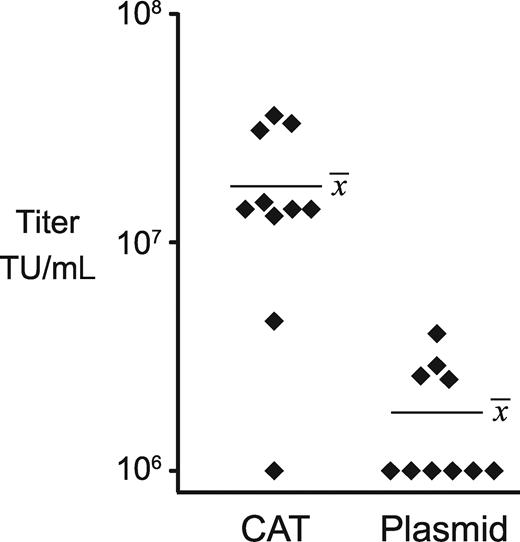
In an effort to develop methods that did not rely on viral transduction to introduce SIN vector genomes, we used traditional transfection and selection methods but attempted to optimize the structure of the transfected DNA to promote high copy number integration. The genomic promoter for the SIN lentiviral vector plasmid, pCL20c-MSCV-GFP,24 was modified by replacing the cytomegalovirus (CMV) enhancer with 7 tet operators to make a doxycycline-regulated vector genomic RNA expression cassette (TL20, Figure 1). We separately constructed a bleomycin resistance (ble) cassette driven by a weak PGK promoter (PGK-ble, Figure 1). We then compared 2 methods for transfection of these DNAs into the GPRG packaging cell line. In the first method we mixed the vector plasmid with the PGK-ble plasmid at a 25:1 molar ratio before transfection. In the second method we created a concatemeric array by excising the 2 expression cassettes from their plasmid backbones and ligating them in vitro at a 25:1 molar ratio. After transfecting these DNAs into GPRG cells, selecting with drug, and isolating clones by limiting dilution, induced supernatant titers were determined for each of a set of 10 clones. As can be seen in Figure 3, concatemer-transfected clones yielded significantly higher titers than did plasmid-transfected clones (P = .0002; mean, 1.76 × 107 vs 1.8 × 106 TU/mL), and this was the only method that reliably generated clones with titers more than107 TU/mL. After expanding our highest titer clone, designated GPRG-TL20-GFP, and freezing cells in liquid nitrogen, this line could be reliably thawed, propagated, and induced to yield 3 × 107 TU/mL. Induced titers remained above 107 TU/mL for 3 months of continuous passage (data not shown). Genomic DNA purified from this line, when analyzed by quantitative real-time polymerase chain reaction (PCR), contained approximately 200 copies of the vector packaging sequence per cell equivalent.
Distribution of producer clone titers using 2 forms of DNA. Each symbol represents the titer of an individual clone generated by concatemeric array transfection (CAT) or plasmid transfection. The mean titer for each set is indicated.
Distribution of producer clone titers using 2 forms of DNA. Each symbol represents the titer of an individual clone generated by concatemeric array transfection (CAT) or plasmid transfection. The mean titer for each set is indicated.
Large-scale production of a SIN lentiviral vector
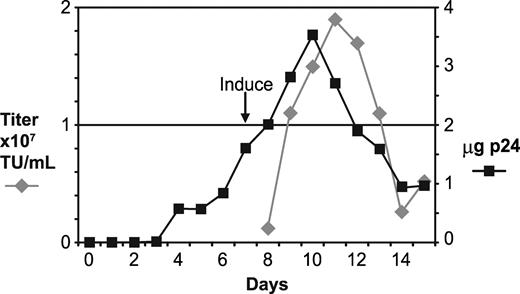
A frozen cell stock of GPRG-TL20-GFP was thawed and expanded, and 5 × 108 cells were seeded into a 5-liter WAVE Bioreactor (GE Healthcare Life Sciences, Piscataway, NJ), using fibrous discs as support for the adherent cells. Particle production from the expanding cell population, as measured by p24 antigen levels, increased gradually to a peak of 3.5 μg/mL (Figure 4). Seven days after seeding, the cells were induced by washing free of doxycycline, and media was subsequently replaced daily to harvest virus. Infectious vector titers peaked at 1.9 × 107 TU/mL on the fourth day after induction. Five consecutive harvests of approximately 4.5 liters each had infectious titers greater than 107 TU/mL. A portion of this material was concentrated approximately 18-fold by tangential flow filtration and was used to transduce HeLa cells at high multiplicity of infection (MOI). Southern blotting analysis of genomic DNA purified from these cells and probed with vector sequences revealed a single band of the expected size, indicating that vector genomes produced from this line were homogeneous and faithfully transmitted (lane 1 of Figure S2).
Large-scale production using the GPRG-TL20-GFP cell line. A 5-liter WAVE Bioreactor was seeded with 5 × 108 cells at day 0 and cultured for 2 weeks. At day 7, cells were washed free of doxycycline. Aliquots of media were harvested daily and assayed for infectious vector (left axis) and p24 antigen, from which the net daily gain in p24 concentration was calculated and plotted (right axis).
Large-scale production using the GPRG-TL20-GFP cell line. A 5-liter WAVE Bioreactor was seeded with 5 × 108 cells at day 0 and cultured for 2 weeks. At day 7, cells were washed free of doxycycline. Aliquots of media were harvested daily and assayed for infectious vector (left axis) and p24 antigen, from which the net daily gain in p24 concentration was calculated and plotted (right axis).
Directional concatemeric array transfection for optimal DNA stability in vivo
We sought to improve on our concatemeric array transfection process by forcing directional ligation of the cassettes in the in vitro ligation reactions. New restriction enzyme sites were introduced flanking the vector and ble cassettes to allow creation of fragments having 3 nucleotide nonpalindromic overhangs, which can only ligate from head to tail. A 400-bp chromatin insulator fragment27 was also embedded in the SIN LTR of the vector plasmid to further test our ability to make stable cell lines with safety-modified SIN vector genomes. In vitro–ligated concatemers were prepared with this new vector DNA, as well as with the blunt-ended fragments used in the construction of GPRG-TL20-GFP. After ligation, concatemer DNA was digested with EcoRI and the products were analyzed on an agarose gel (Figure S3) to confirm that ligations occurred as statistically predicted (diagrammed in Figure 5A). Blunt-ended ligations yielded a mixture of fragments with an approximately 1:2:1 ratio of head-to-head–, head-to-tail–, and tail-to-tail–linked vector cassettes, as well as a wide range of less frequent ligation products containing the ble cassette. Directional ligation, on the other hand, yielded predominantly head-to-tail events, represented by monomer length vector after EcoRI digestion, as well as a trace quantity of a smaller species formed by the rare insertion of the ble cassette.
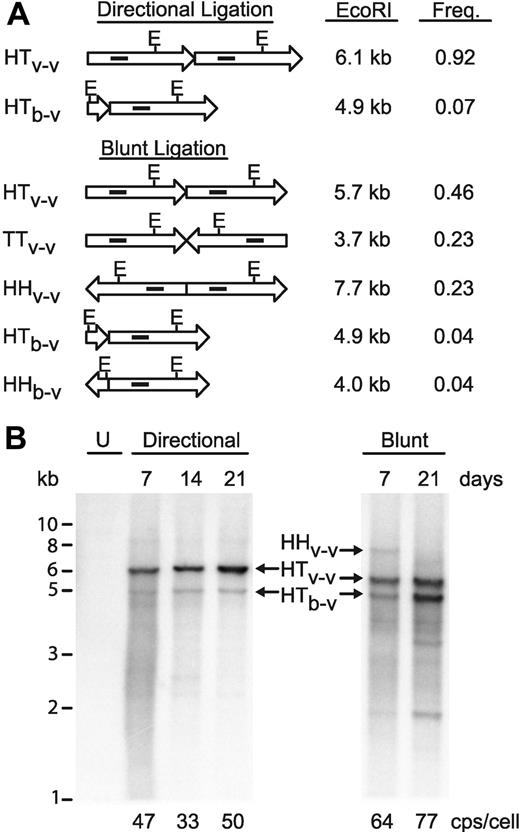
Optimized concatemeric array transfection for efficient stable producer cell engineering. (A) Diagrammatic representation of vector (long arrows) and ble (short arrows) ligation events, with the theoretically expected molar frequency of that event in the reaction and the fragment sizes after EcoRI digestion. E, location of EcoRI sites. Solid interior rectangles indicate approximate location of probe for Southern blot in panel B. Note that the 3.7-kb TTv-v fragment does not contain a probe target and so is not visualized on the Southern blot. HH, head-to-head; TT, tail-to-tail; HT, head-to-tail; v-v, vector-to-vector ligation; b-v, ble-to-vector ligation. (B) Southern blot analysis of EcoRI-digested, concatemerized vector DNA after selection in GPRG packaging cells for the indicated number of days, probed with a fragment of the vector genome (indicated in panel A). U, untransfected GPRG cells. Sizes from standards are indicated (in kb) at left. Designations in the center correspond to those in panel A. Real-time PCR quantitated vector copy numbers for each population are indicated at the bottom (in copies/cell).
Optimized concatemeric array transfection for efficient stable producer cell engineering. (A) Diagrammatic representation of vector (long arrows) and ble (short arrows) ligation events, with the theoretically expected molar frequency of that event in the reaction and the fragment sizes after EcoRI digestion. E, location of EcoRI sites. Solid interior rectangles indicate approximate location of probe for Southern blot in panel B. Note that the 3.7-kb TTv-v fragment does not contain a probe target and so is not visualized on the Southern blot. HH, head-to-head; TT, tail-to-tail; HT, head-to-tail; v-v, vector-to-vector ligation; b-v, ble-to-vector ligation. (B) Southern blot analysis of EcoRI-digested, concatemerized vector DNA after selection in GPRG packaging cells for the indicated number of days, probed with a fragment of the vector genome (indicated in panel A). U, untransfected GPRG cells. Sizes from standards are indicated (in kb) at left. Designations in the center correspond to those in panel A. Real-time PCR quantitated vector copy numbers for each population are indicated at the bottom (in copies/cell).
GPRG cells were transfected with the 2 concatemer preparations, selected with drug, and monitored during culture, under selection, for 3 weeks. The in vivo fate of the concatemerized vector genome expression cassettes was monitored by Southern blotting of EcoRI-digested genomic DNA, purified from the cells at varying times. As shown in Figure 5B, the directional concatemers are significantly more stable in vivo, with consistent maintenance of high vector-to-resistance cassette ratios and the absence of obvious deletion events. Although nondirectionally concatemerized DNA was present at similar, if not higher, copy numbers (indicated at bottom of Figure 5), it showed clear evidence of vector genome deletions. For example, the 7.7-kb head-to-head ligation product (HHv-v), approximately one-fourth of the transfected DNA, is barely detected at 7 days and is completely absent at 21 days. The 4.9-kb head-to-tail ble vector fragment (HTb-v) appears to be gradually enriched over time, such that at 21 days postselection it is more abundant than the head-to-tail vector-vector ligation band (HTv-v), even though in the transfected DNA this ble vector fragment was barely detectable (Figure S3). Also appearing during selection are shorter fragments (eg, at 2 kb, Figure 5B), which hybridize to the vector probe but are shorter than monomer EcoRI cut vector, and therefore most probably represent partially deleted vector genomes.
We hypothesized that efficient and stable introduction of vector genome cassettes would convert all cells in the culture to high-titer producer cells. We therefore repeated the directional concatemer transfection and assessed vector productivity in drug-selected cell populations by periodically seeding cells in a separate culture dish and washing them free of doxycycline. Remarkably, after just 14 days of selection, titers of induced supernatants approached 107 TU/mL and climbed gradually during the following 21 days of passage to peak at 3 × 107 TU/mL. These high-titer supernatants were used to transduce HeLa cells at high MOI, and Southern blotting of genomic DNA from these cells confirmed homogeneous and correctly transmitted genomes, without rearrangement (Figure S2 lane 3).
A SIN, insulated clinical gene therapy vector for SCID-X1 gene therapy
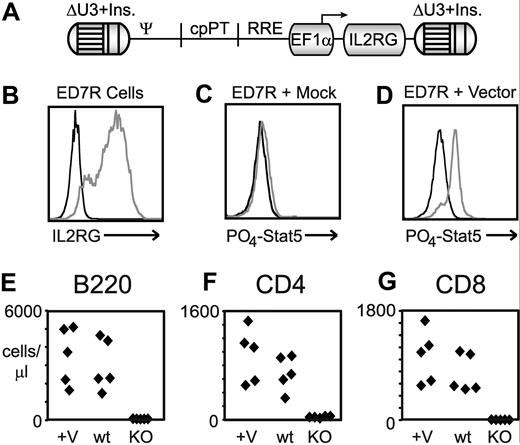
SCID-X1 remains an excellent candidate for gene therapy, as the therapeutic efficacy in 2 prior clinical trials was clearly evident,28,29 and newer, safety-modified vector designs8,12 have the potential to ameliorate the genotoxic side effects observed in those trials.6,7 Our candidate lentiviral vector (diagrammed in Figure 6A) contains an internal EF1α promoter driving expression of a codon-optimized human γc cDNA (the sequence of this cassette is presented as Figure S4) and a full deletion of both promoter and enhancer sequences in the 3′ LTR. Initial versions of this vector contained 2 repeats of the 250-bp core element of the chicken HS4 chromatin insulator30 within the SIN LTRs, which were subsequently replaced with a single 400-bp fragment based on the work of Aker et al.27 To test the functionality of this EF1α vector cassette, vector particles were incubated with ED7R cells, a human T-cell line that does not express the common γc gene.31 As shown in Figure 6B, robust surface expression of the γc protein can be seen after staining with a human common γc-specific antibody. The transduced ED7R cells were incubated with and without IL-2 and subsequently stained with an antibody recognizing phosphorylated Stat5, a target of kinases activated by IL-2 via the common γc receptor. As can be seen in Figure 6C,D, efficient Stat5 phosphorylation was only detected when ED7R cells were both transduced with γc vector and incubated with IL-2, confirming functional signaling via the vector-mediated γc receptor expression. The ability of this EF1α vector cassette to correct a murine model of common γc deficiency was tested by transducing IL2RG−/− murine bone marrow with the vector before transplantation into lethally irradiated recipients. As can be seen in Figure 6E and G, the absolute cell counts in B- and T-cell lineages for the vector-corrected mice at 10 weeks after transplantation are comparable with wild-type controls.
EF1α-driven human common γc vectors for clinical gene therapy. (A) Schematic map of the EF1α-hgcOPT vector expressing codon-optimized human γc cDNA. (B-D) In vitro correction of ED7R cells after transduction with EF1α-driven common γc vector containing the 400-bp insulator. (B) Staining with antibody specific for human common γc after transduction with vector (gray line) or mock (black line) supernatants. Staining with antibody specific for phosphorylated Stat5 is shown for mock (C) and vector-transduced (D) cells either with (gray line) or without (black line) stimulation by IL-2. (E-G) In vivo correction of SCID-X1 mouse bone marrow with EF1α vector containing 2 × 250-bp insulator elements. Absolute lymphocyte cell counts determined by staining for the indicated antigen for B cells (E), CD4+ T cells (F), and CD8+ T cells (G) at 10 weeks post transplantation are shown for both vector (+V) and mock (KO) transduced IL2RG−/− bone marrow–transplanted mice, compared with wild-type (wt) mice. Each diamond represents 1 mouse.
EF1α-driven human common γc vectors for clinical gene therapy. (A) Schematic map of the EF1α-hgcOPT vector expressing codon-optimized human γc cDNA. (B-D) In vitro correction of ED7R cells after transduction with EF1α-driven common γc vector containing the 400-bp insulator. (B) Staining with antibody specific for human common γc after transduction with vector (gray line) or mock (black line) supernatants. Staining with antibody specific for phosphorylated Stat5 is shown for mock (C) and vector-transduced (D) cells either with (gray line) or without (black line) stimulation by IL-2. (E-G) In vivo correction of SCID-X1 mouse bone marrow with EF1α vector containing 2 × 250-bp insulator elements. Absolute lymphocyte cell counts determined by staining for the indicated antigen for B cells (E), CD4+ T cells (F), and CD8+ T cells (G) at 10 weeks post transplantation are shown for both vector (+V) and mock (KO) transduced IL2RG−/− bone marrow–transplanted mice, compared with wild-type (wt) mice. Each diamond represents 1 mouse.
Stable producer clones for a SIN, clinical gene therapy vector
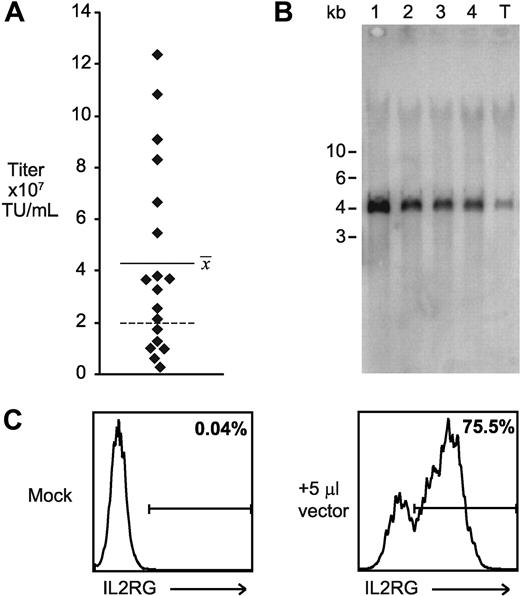
After validation of the performance of the EF1α vectors, directional concatemers were generated with the vector containing the 400-bp chromatin insulator and transfected into GPRG cells. After selection with drug, 20 isolated colonies were picked, expanded, and induced to produce vector supernatants, which were screened by transduction of ED7R cells. The range of calculated titers was 3 × 106 to 1 × 108 TU/mL, with a mean for all clones of 4 × 107 TU/mL (plotted in Figure 7A). Remarkably, more than half of all clones produced higher titers than the best titer obtained with the same vector genome using 4-plasmid transfection of 293T cells (2 × 107 TU/mL, indicated in Figure 7A as a dashed line). A flow cytometry histogram resulting from incubation of 5 μL supernatant with 4 × 105 cells is shown in Figure 7C. To confirm the integrity of the transmitted genomes, supernatants from the 4 best clones and a transient transfection were again incubated with ED7R cells, and subsequently genomic DNA from the cells was purified and analyzed by Southern blotting. As shown in Figure 7B, all vectors transmitted faithfully with a single homogeneous product in transduced cells. Quantitative analysis of the Southern image confirmed a 5-fold increase in titer for the best clone over that obtained by transient transfection.
Construction of high-titer clinical vector producer cells. (A) Distribution of calculated ED7R cell titer of supernatants from 20 independent producer clones for SCID-X1 clinical vector. Each point represents an individual clone, and the horizontal bar shows the average of the titers from all of the clones. The dashed line indicates the highest titer achieved for an identical vector prepared using 4-plasmid transient transfection of 293T cells. (B) Southern blot analysis of ED7R genomic DNA after transduction with 50 μL supernatant from the top 4 cloned producer cell lines (lanes 1-4, in order of decreasing titer) and from transiently transfected 293T cells (lane T). The SbfI enzyme used cuts once in each copy of the chromatin insulator, and it is expected to generate a 4-kb fragment spanning the complete provirus. (C) Flow cytometry histograms of human common γc antibody binding to ED7R cells 4 days after incubation with either fresh media (Mock), or 5 μL supernatant produced by the top performing stable producer cell clone.
Construction of high-titer clinical vector producer cells. (A) Distribution of calculated ED7R cell titer of supernatants from 20 independent producer clones for SCID-X1 clinical vector. Each point represents an individual clone, and the horizontal bar shows the average of the titers from all of the clones. The dashed line indicates the highest titer achieved for an identical vector prepared using 4-plasmid transient transfection of 293T cells. (B) Southern blot analysis of ED7R genomic DNA after transduction with 50 μL supernatant from the top 4 cloned producer cell lines (lanes 1-4, in order of decreasing titer) and from transiently transfected 293T cells (lane T). The SbfI enzyme used cuts once in each copy of the chromatin insulator, and it is expected to generate a 4-kb fragment spanning the complete provirus. (C) Flow cytometry histograms of human common γc antibody binding to ED7R cells 4 days after incubation with either fresh media (Mock), or 5 μL supernatant produced by the top performing stable producer cell clone.
The suitability of the best clone for clinical-scale production was further demonstrated after transfer to a certified good manufacturing practice (GMP) production facility and expansion into a 150-vial Master Cell Bank. After thawing a vial of cells from this bank, and growth in culture for 28 days in the presence of doxycycline and the absence of other antibiotics, cells were seeded into a 1-liter WAVE Bioreactor bag with FibraCel disks. After 8 days of expansion, cells were induced by removal of doxycycline and supernatants were harvested daily by complete media changes. ED7R infectious titers ranged between 4 × 107 and 2 × 108 TU/mL for 6 consecutive harvests. Supernatants from this producer clone were confirmed to be free of replication-competent virus by transducing U2OS cells at high MOI (∼ 22), which were subsequently passaged for 4 weeks before testing for both p24 antigen and reverse transcriptase activity using a sensitive polymerase-enhanced reverse transcriptase (PERT) assay,32 both of which were not detected.
Discussion
We show in this report the efficient generation of high-titer, SIN, lentiviral vector producer cell lines suitable for use in a clinical vector production environment. Although many previous reports have described lentiviral vector producer cell lines, they have uniformly been limited by a dependence on viral transduction methods to introduce the vector genome expression cassette into packaging cells. As such, previous methods have been unsuitable for the production of SIN vector genomes, which critically enhance the safety profile of candidate clinical gene therapy vectors. Our new concatemeric array transfection system overcomes this hurdle and enables stable production of SIN lentiviral vectors at both high titers (> 107 TU/mL) and on a large scale, dramatically enhancing our ability to conduct a wide range of clinical trials for gene therapy.
In the construction of our packaging cell line, we were guided by previous reports describing the use of gamma-retroviral vectors for the stable introduction of helper genetic elements,18 codon optimization of open reading frames,18,19 and the tet regulatory system.14,17,19,20 We used self-inactivating gamma-retroviral vectors (which reduce the risk of helper gene transmission, as observed by others18 ) and the Tet-off system, which allows harvest of bulk supernatants free of exogenous antibiotics. We opted to first introduce the popular VSV-G envelope gene into GPR due to the higher average titers and excellent particle stability it provides. The resulting GPRG line generates high titers, both after transfection of vector genome plasmids (Figure 1) and from stable lines generated with it (Figures 2,Figure 3–4 and 7). In our hands these lines have provided more than adequate stability for generation of clinical producer cells, Master Cell Banks, and large-scale, bioreactor supernatants in a GMP environment.
To introduce SIN vector genomes into our packaging cells, we developed a new method, termed “concatemeric array transfection,” which uses an in vitro ligation strategy to create large arrays of linked vector genome expression cassettes, with drug resistance cassettes being only rarely interspersed among them. Transfection of this concatemer into our GPRG packaging cells and selection with drug allowed the isolation of high-titer producer cell clones, the best of which produced on average 3 × 107 TU/mL. The subsequent use of nonpalindromic restriction site overhangs forced directional ligations and further improved the concatemer method by preventing genomic instability resulting from the direct inverted repeats produced by blunt ligations (Figure 5B). Although one clear advantage of concatemeric array transfection is that it promotes multicopy integration of vector genome cassettes, we note that this and other features of the method are shared with viral transduction with non-SIN vectors. For one, we specifically removed bacterial plasmid sequences, which are known to reduce gene expression in mammalian cells33 and are not transmitted by lentiviral vectors. In addition, unlike the constitutive genomic RNA expression provided by typical transient transfection vector plasmids, in our constructs vector genome synthesis is regulated, as it is in non-SIN lentiviral vectors (where the LTR transcription is Rev and Tat dependent) and in conditional SIN vectors (eg, regulated by doxycycline20 ).
Regardless of the precise mechanistic advantage of the concatemer method, when combined with our packaging cells it allowed the derivation of high-titer producer cell lines for SIN lentiviral vectors, an accomplishment not previously reported. The GPRG-TL20-GFP line was sufficiently stable to generate more than 20 liters of supernatant in a bioreactor production run, and this product transmitted uniform genomes without rearrangment. Our optimized concatemer method is so efficient, in fact, that we can rapidly generate frozen cell banks of producer populations without cloning, which can be thawed and expanded into cell numbers sufficient for 25-liter bioreactor production, while maintaining induced titers exceeding 107 TU/mL, as well as uniform, correctly sized genome structure (Figure S2).
Another significant advantage of our method is that it places no requirements on the structure of the vector genome, other than the absence of certain restriction enzyme sites, and thus it can be broadly applied to nearly all clinical and research vector production applications. In a clinically relevant application, we showed the efficient generation of cloned, high-titer producer cell lines for a promising candidate vector for the treatment of SCID-X1. This EF1α-driven vector is similar in design to vectors proven safer in protooncogene activation assays,9,12 and it efficiently complements γc deficiency in both human cells and a murine model of the disease (Figure 6B-G). Although our latest version of this vector clearly warrants testing in a clinical setting, the SIN design renders stable producer cell line generation problematic with previous methods. Using directional concatemeric array transfection, we derived at least 4 candidate producer clones following a limited screening of only 20 clones, and the best clone was shown to be sufficiently stable to allow creation of a clinical Master Cell Bank, which robustly produced vector in a bioreactor after 1 month of continuous culture in the absence of antibiotic selection. With the robust productive capacity and clinical suitability of these cell lines, we hereby accomplish a critical milestone in the effort to capitalize on the success of the previous clinical trials, while avoiding the genotoxic side effects. The broad applicability of the concatemeric array transfection method should also enable the clinical manufacture of many other promising lentiviral gene therapy vectors.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We acknowledge Richard Mulligan, Adrian Thrasher, and Christopher Baum for the generous gifts of reagents; Matthew Wielgosz, Taihe Lu, Rebecca Banks-Spivey, Kimberly Berg, and Rhonda Clark for technical assistance; Jie Yang for statistical assistance; and Arthur Nienhuis for careful reading of the manuscript.
This work was supported by National Heart, Lung, and Blood Institute grant 5 PO1 HL-53749, 2V54HL070590-06, the Assisi Foundation of Memphis, the American Lebanese Syrian Associated Charities, and in part by the intramural program of the National Institute of Allergy and Infectious Diseases.
National Institutes of Health
Authorship
Contribution: R.E.T., S.Z., H.L.M., B.P.S., and J.T.G. designed research; R.E.T., A.A.O., A.C., T.L., M.G., S.S.D.R., M.M., and J.T.G. performed research; R.E.T., S.Z., M.G., and A.C. collected data; R.E.T., S.Z., B.P.S., and J.T.G. analyzed and interpreted data; and J.T.G. wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: John T. Gray, Division of Experimental Hematology, St Jude Children's Research Hospital, 262 Danny Thomas Pl, Memphis, TN 38105; e-mail: john.gray@stjude.org.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal