Heparin-induced thrombocytopenia (HIT) is caused by platelet-activating antibodies that recognize PF4/heparin complexes. Uncertainties remain regarding HIT immunobiology, including the temporal relation of antibody formation to onset of thrombocytopenia, and whether immunoglobulin class switching occurs. Using serial plasma samples from 2 heparin thromboprophylaxis trials, we determined the time of onset, antibody levels, and immunoglobulin class distributions (IgG, IgA, IgM) for 12 patients with HIT and 36 patients who formed anti-PF4/heparin antibodies, but did not develop HIT (“seropositive non-HIT controls”). In patients with HIT, anti-PF4/heparin antibodies became detectable 4 days (median) after starting heparin; antibody detection preceded the platelet count decline by 2 days (median). Patients with HIT produced higher levels of IgG antibodies, but similar IgA and IgM levels, compared with seropositive non-HIT controls. Among all 48 seroconverting patients, the first day of a positive antibody test (median, day 6) did not differ among the immunoglobulin classes. Thus, the HIT immune response does not exhibit the classic paradigm of IgM class precedence/immunoglobulin class switching; rather, relatively rapid formation of IgG antibodies is observed, sometimes with concomitant IgA and IgM formation. Compared with seropositive non-HIT controls, HIT patients develop significantly higher anti-PF4/heparin IgG levels that are detectable before the onset of thrombocytopenia.
Introduction
Heparin-induced thrombocytopenia (HIT) is an adverse drug reaction caused by platelet-activating antibodies that recognize complexes of platelet factor 4 (PF4) bound to heparin.1,2 In most patients, IgG class antibodies3,–5 are formed that bind to PF4/heparin complexes6 on platelet surfaces,7 cross-linking platelet Fc receptors,8 with resulting platelet activation9 and an associated procoagulant response.10 HIT is characterized by an increased risk of venous and arterial thromboses.11
Antibodies against PF4/heparin can be detected in virtually every patient with HIT, indicating that the disorder is antibody mediated.1,,,,–6 However, HIT has several atypical features that differ from the classically described antigen-induced immune response. For example, patients often develop HIT as early as 5 days after their first exposure to heparin.12,13 In addition, these rapidly forming antibodies include relatively high levels of IgG, which is more typical of a secondary immune response. Second, and unlike antibodies formed during exposure to common viral and vaccine antigens,14 heparin-dependent antibodies remain detectable for only a few weeks or months after an episode of HIT.12 Third, an anamnestic (immune memory) response generally does not occur in patients with a previous history of HIT, because these patients can receive heparin months or years later without either regenerating antibodies quickly or developing recurrence of the syndrome.12,15,16 Fourth, a significant proportion of patients who form antibodies against PF4/heparin complexes do not develop a fall in the platelet count and remain well without any clinical features of HIT.
We participated in a series of prospective trials of heparin thromboprophylaxis in which patients were documented to have serologically proven HIT. Many of these patients had serial blood samples collected during the evolution of their anti-PF4/heparin immune response, allowing us to address the following questions:
When do anti-PF4/heparin antibodies form in relation to the development of HIT?
When do the immunoglobulin classes IgG, IgA, and IgM form in patients with HIT, and is there evidence that IgM formation precedes the other immunoglobulin classes?
Is there a difference in the serologic characteristics of the patients who develop HIT compared with those patients who develop anti-PF4/heparin antibodies but who do not develop HIT?
Is there a difference in the type and timing of antibody formation among patients with differing histories of previous heparin exposure?
In this report, we summarize our data indicating the atypical nature of the HIT immune response.
Methods
Patients
Patients had participated in 1 of 2 randomized controlled trials involving patients after orthopedic surgery.17,18 All patients provided signed informed consent in accordance with the Declaration of Helsinki for enrollment into these studies, including permission for blood testing. Approval for this study was obtained from the McMaster Research Ethics Board. Previous studies of HIT involving these trials did not investigate the questions addressed in this report.5,11,19,20
From these 2 clinical trials, we tested serial plasma samples from 12 patients with HIT and 36 control patients who developed anti-PF4/heparin antibody seroconversion but who did not develop HIT (“seropositive non-HIT controls”). HIT was defined as a 50% or greater decrease in the platelet count that occurred between day 5 and 14 of heparin therapy, with or without symptomatic thrombosis, plus a positive platelet activation assay (platelet serotonin-release assay21 ). In general, these 12 patients with HIT tested strongly positive in the serotonin-release assay: the median serotonin release at 0.1 IU/mL unfractionated heparin (UFH) was 97.5% (interquartile range [IQR], 93.8%-99.3%). All patients with HIT also tested positive in the anti-PF4/heparin enzyme immunoassay (EIA). Eight (66.7%) of the 12 patients had one or more clinically evident manifestations, including venography-documented deep vein thrombosis (n = 6), arterial thrombosis (n = 1), postbolus anaphylactoid reaction (n = 1), or skin lesions at the heparin injection sites (n = 2; 2 patients had 2 clinical manifestations). All patients with HIT scored high probability (6-8 points) in the 4T scoring system.22
The 36 seropositive non-HIT controls met the following criteria. First, they developed a strong positive result (> 1.00 units of optical density [OD405]) in a commercial anti-PF4/polyanion EIA (described subsequently). Second, they did not develop HIT-related thrombocytopenia, as defined previously. Third, they did not develop thrombosis, including at venogram assessment. Despite positive antibody results, all 36 control patients scored low probability (≤ 2 points) for HIT in the 4T scoring system. The presence of a positive platelet serotonin-release assay did not exclude a patient from being classified as a seropositive non-HIT control, provided that neither thrombocytopenia (as defined above) nor thrombosis occurred. Eight (22.2%) of the 36 controls had a positive serotonin-release assay (median, 84.5% release; [IQR, 79.0%-91.0%] for these 8 patients who tested positive). Overall, for the 36 controls, the median serotonin release at 0.1 IU/mL UFH was only 6.0% (IQR, 2.0%-18.3%).
For each of the 12 patients with HIT and the 36 seropositive non-HIT controls, at least 7 serial blood samples were available that documented seroconversion (total, 502 samples tested, giving a mean of 10.5 samples per patient).
Timing and magnitude of the anti-PF4/heparin seroconversion response
For the 48 patients (12 with HIT and 36 seropositive non-HIT controls), we tested for anti-PF4/heparin antibodies using an EIA (PF4 Enhanced X-HAT 45; Genetic Testing Institute [GTI] Diagnostics, Waukesha, WI),23 and also our own immunoassay that detects anti-PF4/heparin antibodies using alkaline phosphatase–conjugated goat anti–human antibodies (anti-IgG, Fc specific; anti-IgA, α chain specific; or anti-IgM, H and L chain specific; Jackson ImmunoResearch Laboratories, West Grove, PA).5,19,24 For the commercial assay, the cutoff defining a positive test result was 0.40 OD units (as per the manufacturer); for each of the in-house assays, the cutoff was 0.45 OD units established using 100 healthy controls for each assay, as described.5,19,24 The day of onset of seroconversion by immunoassay was defined as the first day a positive test result was obtained. For both patient groups, the daily mean (± SEM) OD values were also determined.
For the 12 patients with HIT, individual patient results, as well as the median and IQR, were determined for 3 time points: (1) the early postoperative platelet count nadir, that is, the day of the lowest platelet count value observed between postoperative days 0 to 3 (because of perioperative platelet consumption and/or hemodilution); (2) the earliest onset of HIT, that is, the first day that the platelet count began to fall because of HIT; and (3) the day the platelet count fell by 50% or greater because of HIT. In addition, we determined (4) the highest OD value in each immunoassay observed for each patient.
Determination of a schematic HIT timeline
For the 12 patients with HIT, we determined the median day, as well as the IQR and range, for each of the following parameters: (1) first day of UFH or low-molecular-weight heparin administration (day of surgery = day 0), (2) first day of detection of anti-PF4/heparin antibodies by commercial EIA, (3) day of onset of the platelet count fall associated with anti-PF4/heparin antibody formation, (4) day that the platelet count fall exceeded 50%, and (5) the first day that thrombosis or another clinical event associated with HIT first occurred (if applicable).
Day of onset of the anti-PF4/heparin immune response: comparison of immunoglobulin classes
We determined the first day of a positive test result for each of the 3 immunoglobulin classes for the 12 patients with HIT and for the 36 seropositive non-HIT control patients. We also compared the timing of onset in relation to the history of previous heparin exposure, classified as unlikely, possible, or definite.12
Statistical methods
Analysis of variance (ANOVA) was used to compare the seroconversion times for anti-PF4/heparin antibodies of IgG, IgA, and IgM classes, as well as those detected by the commercial assay, across all 3 groups classified as per history of previous heparin exposure. Times of seroconversion were measured both from the day of starting heparin (primary analysis) and from the day of surgery. The Student t test was used to compare OD values of the immunoassays between patients with HIT and the seropositive non-HIT controls, as well as between controls testing positive and negative in the serotonin-release assay. For the 12 patients with HIT, timeline diagrams25 were used to plot (in relation to the day of surgery) the beginning of postoperative heparin prophylaxis, the first detection of anti-PF4/heparin antibodies (by commercial EIA), the course of the HIT-associated platelet count fall (assessed at 2 points, namely the day that the HIT-associated platelet count fall began, as well as the day that the platelet count fall was ≥ 50%), as well as the first day that a thrombotic event occurred (if applicable). From these timeline diagrams, summary data (median, IQR, range) were tabulated. All P values are 2-tailed. Plots were created in Microsoft PowerPoint (Redmond, WA), and statistical analyses were carried out using S-Plus (TIBCO Software, Palo Alto, CA) and Microsoft Excel 2003.
Results
Timing and magnitude of the anti-PF4/heparin immune response
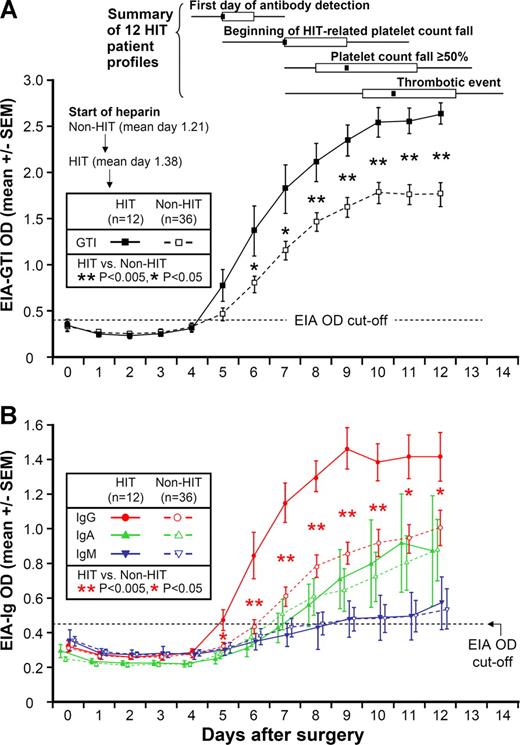
Figure 1A shows the mean (±SEM) OD values on each postoperative day for anti-PF4/heparin antibodies by the commercial EIA, both for the 12 patients with HIT, as well as for the 36 seropositive non-HIT controls. For both patient groups, mean OD values in the commercial EIA tested negative at the time of the early postoperative platelet count nadir, which occurred at approximately day 2. Subsequently, the 12 patients with HIT developed a positive test, which occurred at a median of 4 days after starting heparin, ie, most frequently on postoperative day 5. From postoperative day 6 onward, there was a significant difference in the mean values between the 2 patient groups (P < .05).
Anti-PF4/heparin antibodies (by EIA) per postoperative day in 12 patients with HIT and 36 seropositive non-HIT control patients. (A) Mean (±SEM) optical density (OD) of anti-PF4/heparin antibodies detected using commercial immunoassay (EIA-GTI) that detects antibodies of all 3 immunoglobulin classes (IgG, IgA, IgM). HIT patients are indicated by ■, and seropositive non-HIT controls by □. On each day beginning on postoperative day 6, there is a significant difference in the mean of the OD levels between the patients with HIT and the seropositive non-HIT controls (P < .05 by nonpaired t test). At the top of the figure, summary data for 12 HIT patient profiles are shown for 4 key events (first day of antibody detection, beginning of HIT-related platelet count fall, platelet count fall ≥ 50%, and thrombotic event), summarized as median (small black squares within rectangles), IQR (open rectangles), and range (ends of thin black lines). (B) Mean (±SEM) OD values of anti-PF4/heparin antibodies detected using an in-house immunoassay (EIA-Ig) that detects antibodies of the individual immunoglobulin classes, IgG (red circles), IgA (green triangles), and IgM (blue inverted triangles) for HIT (solid symbols) and non-HIT (open symbols). On each postoperative day beginning on day 5, there is a significant difference in the mean of the OD units for the IgG immunoassay between the patients with HIT and the seropositive non-HIT controls (**P < .005 for days 6-10; *P < .05 for days 5, 11, and 12). In addition, among the 34 non-HIT controls who tested positive for IgG antibodies, mean (±SEM) maximum OD values for the EIA-IgG were significantly greater in the 8 patients who tested positive in the serotonin-release assay compared with the 26 patients who tested negative in the serotonin-release assay (1.30 ± 0.15 vs 0.96 ± 0.07 units; P = .025). Among the 20 patients who tested positive in the serotonin-release assay, mean (±SEM) maximum OD values for the EIA-IgG showed a trend to higher levels in the 12 patients with clinical HIT, compared with the 8 seropositive non-HIT controls (1.63 ± 0.09 vs 1.30 ± 0.15 units; P = .059). EIA indicates enzyme immunoassay; and HIT, heparin-induced thrombocytopenia.
Anti-PF4/heparin antibodies (by EIA) per postoperative day in 12 patients with HIT and 36 seropositive non-HIT control patients. (A) Mean (±SEM) optical density (OD) of anti-PF4/heparin antibodies detected using commercial immunoassay (EIA-GTI) that detects antibodies of all 3 immunoglobulin classes (IgG, IgA, IgM). HIT patients are indicated by ■, and seropositive non-HIT controls by □. On each day beginning on postoperative day 6, there is a significant difference in the mean of the OD levels between the patients with HIT and the seropositive non-HIT controls (P < .05 by nonpaired t test). At the top of the figure, summary data for 12 HIT patient profiles are shown for 4 key events (first day of antibody detection, beginning of HIT-related platelet count fall, platelet count fall ≥ 50%, and thrombotic event), summarized as median (small black squares within rectangles), IQR (open rectangles), and range (ends of thin black lines). (B) Mean (±SEM) OD values of anti-PF4/heparin antibodies detected using an in-house immunoassay (EIA-Ig) that detects antibodies of the individual immunoglobulin classes, IgG (red circles), IgA (green triangles), and IgM (blue inverted triangles) for HIT (solid symbols) and non-HIT (open symbols). On each postoperative day beginning on day 5, there is a significant difference in the mean of the OD units for the IgG immunoassay between the patients with HIT and the seropositive non-HIT controls (**P < .005 for days 6-10; *P < .05 for days 5, 11, and 12). In addition, among the 34 non-HIT controls who tested positive for IgG antibodies, mean (±SEM) maximum OD values for the EIA-IgG were significantly greater in the 8 patients who tested positive in the serotonin-release assay compared with the 26 patients who tested negative in the serotonin-release assay (1.30 ± 0.15 vs 0.96 ± 0.07 units; P = .025). Among the 20 patients who tested positive in the serotonin-release assay, mean (±SEM) maximum OD values for the EIA-IgG showed a trend to higher levels in the 12 patients with clinical HIT, compared with the 8 seropositive non-HIT controls (1.63 ± 0.09 vs 1.30 ± 0.15 units; P = .059). EIA indicates enzyme immunoassay; and HIT, heparin-induced thrombocytopenia.
Schematic HIT timeline
Figure 1A also shows summary data for the 12 patient timelines (see “Summary of 12 HIT patient profiles”). The median day of start of heparin prophylaxis was day 1. The median day of the first positive test (by commercial EIA) for anti-PF4/heparin antibodies was postoperative day 5. Thus, the median interval between start of heparin prophylaxis and the first positive test for antibodies was only 4 days.
The median day of onset of the platelet count fall indicating HIT was postoperative day 7, which represented a median of 2 days (range, 1-5 days) from the first day of antibody detection. Platelet count decline criteria indicating HIT (≥ 50% platelet count fall) were first met on a median of postoperative day 9 (range, 7-13). There was a median interval of 2 days (range, 1-4 days) between the beginning of the platelet count fall and the day that the platelet count decline criteria for HIT were met.
The median day of onset of a thrombotic event occurred on day 10.5. Thus, a summary of the typical schematic timeline of HIT can be summarized as follows: (1) seroconversion beginning at 4 days (median) after starting postoperative heparin thromboprophylaxis, followed (2) approximately 2 days later by the beginning of the HIT-associated platelet count fall, followed (3) approximately 2 days later by the platelet count criteria (> 50% platelet count decline) for HIT being met. Whereas the first events—seroconversion followed by progressive thrombocytopenia—were consistent in their order, the thrombotic events occurred unpredictably. For 5 of the 8 patients who developed HIT-associated thrombosis, the thrombosis occurred early during the time of progressive platelet count decline, either before the platelet count fell by 50% or more (n = 3) or on the same day that that platelet count decline threshold was reached (n = 2). For the remaining 3 patients, HIT-associated thrombosis occurred a few days after the platelet count criteria for HIT were met.
Anti-PF4/heparin antibodies of different immunoglobulin classes: comparison of HIT patients versus seropositive non-HIT controls
Figure 1B shows the mean (±SEM) OD values on each postoperative day for anti-PF4/heparin antibodies of the individual immunoglobulin classes, IgG, IgA, and IgM, both for the 12 patients with HIT, as well as for the 36 seropositive non-HIT controls. As with the commercial EIA, for both patient groups, mean OD values in the EIAs tested negative at the time of the early postoperative platelet count nadir. For the patients with HIT, the mean OD value of the IgG class of antibodies first became positive on postoperative day 5 (ie, a mean of 4 days after beginning heparin). For the seropositive non-HIT control patients, the mean OD values increased approximately 1 to 2 days later. From postoperative day 5 onward (by IgG immunoassay), the mean OD values between the patients with and without HIT differed significantly (Figure 1B).
Despite significantly greater mean levels of anti-PF4/heparin antibodies of IgG class among the 12 patients with HIT compared with the seropositive non-HIT control patients, no differences were seen in the mean levels of anti-PF4/heparin antibodies of the IgA and IgM classes (Figure 1B).
Anti-PF4/heparin EIAs are positive at the beginning of the HIT-associated platelet count fall
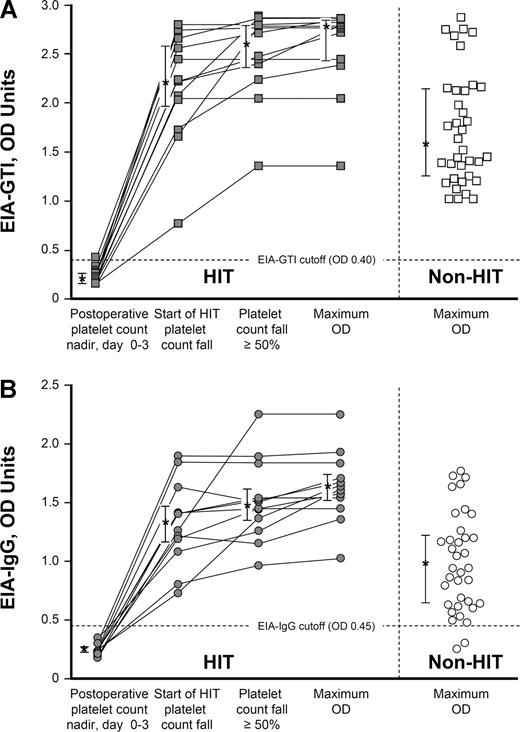
Figure 2A shows the individual OD values in the commercial immunoassay (EIA-GTI) for the 12 patients with HIT at 3 sequential time points during their episode of HIT (early postoperative platelet count nadir, start of platelet count fall indicating HIT, and day that a 50% or greater decline in the platelet count occurred), as well as the maximum OD values for the 12 patients with HIT and the 36 seropositive non-HIT controls. Figure 2B shows the corresponding data for the IgG immunoassay (EIA-IgG).
Anti-PF4/heparin antibodies (by EIA) per significant events in the evolution of HIT: comparison of 12 patients with HIT and 36 seropositive non-HIT controls. (A) Individual results of anti-PF4/heparin antibodies detected using the commercial immunoassay (EIA-GTI) at 3 sequential time points for 12 patients with HIT: (1) early postoperative platelet count nadir (ie, preceding the platelet count fall indicating HIT), (2) earliest platelet count decrease associated with HIT, and (3) platelet count fall of 50% or greater. (4) Maximum OD value for each patient is also shown.  connected by lines indicate data from the 12 patients with HIT; □ represent 36 seropositive non-HIT controls (maximum OD values). Asterisks (*) indicate the median OD values, with the bars representing the 25% and 75% IQR values. For the 12 patients with HIT, the maximum OD values were significantly greater than the maximum OD results observed for the 36 seropositive non-HIT control patients (median, 2.77 vs 1.59, respectively; P < .001). (B) Individual results of anti-PF4/heparin antibodies detected using an in-house immunoassay that detects IgG class antibodies (EIA-IgG) at 3 time points for 12 patients with HIT (
connected by lines indicate data from the 12 patients with HIT; □ represent 36 seropositive non-HIT controls (maximum OD values). Asterisks (*) indicate the median OD values, with the bars representing the 25% and 75% IQR values. For the 12 patients with HIT, the maximum OD values were significantly greater than the maximum OD results observed for the 36 seropositive non-HIT control patients (median, 2.77 vs 1.59, respectively; P < .001). (B) Individual results of anti-PF4/heparin antibodies detected using an in-house immunoassay that detects IgG class antibodies (EIA-IgG) at 3 time points for 12 patients with HIT ( indicate patients with HIT; ◯ indicate seropositive non-HIT controls; time point designations are as in panel A). For the patients with HIT, the maximum OD values were significantly greater than for the maximum OD results observed for the 36 seropositive non-HIT controls (median, 1.63 vs 0.94; P < .001). EIA indicates enzyme immunoassay; and HIT, heparin-induced thrombocytopenia.
indicate patients with HIT; ◯ indicate seropositive non-HIT controls; time point designations are as in panel A). For the patients with HIT, the maximum OD values were significantly greater than for the maximum OD results observed for the 36 seropositive non-HIT controls (median, 1.63 vs 0.94; P < .001). EIA indicates enzyme immunoassay; and HIT, heparin-induced thrombocytopenia.
Anti-PF4/heparin antibodies (by EIA) per significant events in the evolution of HIT: comparison of 12 patients with HIT and 36 seropositive non-HIT controls. (A) Individual results of anti-PF4/heparin antibodies detected using the commercial immunoassay (EIA-GTI) at 3 sequential time points for 12 patients with HIT: (1) early postoperative platelet count nadir (ie, preceding the platelet count fall indicating HIT), (2) earliest platelet count decrease associated with HIT, and (3) platelet count fall of 50% or greater. (4) Maximum OD value for each patient is also shown.  connected by lines indicate data from the 12 patients with HIT; □ represent 36 seropositive non-HIT controls (maximum OD values). Asterisks (*) indicate the median OD values, with the bars representing the 25% and 75% IQR values. For the 12 patients with HIT, the maximum OD values were significantly greater than the maximum OD results observed for the 36 seropositive non-HIT control patients (median, 2.77 vs 1.59, respectively; P < .001). (B) Individual results of anti-PF4/heparin antibodies detected using an in-house immunoassay that detects IgG class antibodies (EIA-IgG) at 3 time points for 12 patients with HIT (
connected by lines indicate data from the 12 patients with HIT; □ represent 36 seropositive non-HIT controls (maximum OD values). Asterisks (*) indicate the median OD values, with the bars representing the 25% and 75% IQR values. For the 12 patients with HIT, the maximum OD values were significantly greater than the maximum OD results observed for the 36 seropositive non-HIT control patients (median, 2.77 vs 1.59, respectively; P < .001). (B) Individual results of anti-PF4/heparin antibodies detected using an in-house immunoassay that detects IgG class antibodies (EIA-IgG) at 3 time points for 12 patients with HIT ( indicate patients with HIT; ◯ indicate seropositive non-HIT controls; time point designations are as in panel A). For the patients with HIT, the maximum OD values were significantly greater than for the maximum OD results observed for the 36 seropositive non-HIT controls (median, 1.63 vs 0.94; P < .001). EIA indicates enzyme immunoassay; and HIT, heparin-induced thrombocytopenia.
indicate patients with HIT; ◯ indicate seropositive non-HIT controls; time point designations are as in panel A). For the patients with HIT, the maximum OD values were significantly greater than for the maximum OD results observed for the 36 seropositive non-HIT controls (median, 1.63 vs 0.94; P < .001). EIA indicates enzyme immunoassay; and HIT, heparin-induced thrombocytopenia.
For both EIAs, the OD values were above the cutoff that defines a positive test for all 12 patients on the first day that the platelet count began to decline in association with HIT. Even at this early time point during the evolution of HIT, the median OD value for the commercial EIA was markedly elevated at 2.22 units (IQR, 1.97-2.58 units), with the lowest value observed being 0.76 OD units. On the day that the platelet count first fell by at least 50%, the corresponding median OD values were 2.61 (IQR, 2.37-2.79). The median maximum OD value for the 12 patients with HIT was 2.77 (IQR, 2.43-2.83), which represented a significantly greater value than seen for the 36 seropositive control patients without HIT (median, 2.77 vs 1.59, respectively; P < .001). For the IgG immunoassay, on the first day that the platelet count began to decline because of HIT, the median OD level was 1.34 (IQR, 1.17-1.47), with the lowest value observed being 0.74. On the day that the platelet count first fell by at least 50%, the corresponding median OD value was 1.48 (IQR, 1.35-1.61). The median maximum OD value for the 12 patients with HIT was 1.63 (IQR, 1.51-1.74). The maximum OD values for the 12 patients with HIT were significantly greater than the maximum OD values for the 36 seropositive non-HIT controls (median, 1.63 vs 0.94, respectively; P < .001).
Variability of immunoglobulin class response profiles
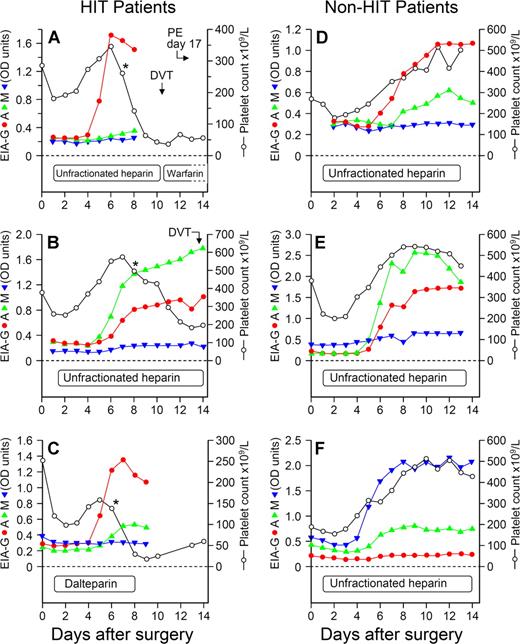
Despite anti-PF4/heparin antibodies of IgG class being detectable among all 12 patients with HIT (Figure 2B), the individual immunoglobulin responses otherwise could differ considerably. For example, patients could develop antibodies of 1, 2, or even all 3 immunoglobulin classes (Figure 3). Overall, among the 48 patients, an IgG response occurred most often (n = 46 patients, 95.8%), followed by IgA (n = 23, 47.9%) and IgM (n = 16, 33.3%).
Individual seroconversion profiles from 6 representative patients, 3 with HIT and 3 exhibiting seroconversion without HIT, shown in relation to the platelet count profile. For the 3 patients with HIT, the profiles shown include (A) isolated IgG response (red), (B) predominant IgA (green) with IgG response, and (C) predominant IgG with IgA immune responses. For the 3 antibody-positive patients without HIT, the profiles shown include (D) predominant IgG with IgA immune response, (E) predominant IgA with IgG and IgM (blue) responses, and (F) predominant IgM with IgA response. “Predominant” immunoglobulin responses refers only to relative OD values and should not be taken to infer differences in relative concentrations of antibodies. For patients A, B, and C, the asterisks (*) designate the beginning of the platelet count decline indicating HIT. DVT indicates deep vein thrombosis; and PE, pulmonary embolism.
Individual seroconversion profiles from 6 representative patients, 3 with HIT and 3 exhibiting seroconversion without HIT, shown in relation to the platelet count profile. For the 3 patients with HIT, the profiles shown include (A) isolated IgG response (red), (B) predominant IgA (green) with IgG response, and (C) predominant IgG with IgA immune responses. For the 3 antibody-positive patients without HIT, the profiles shown include (D) predominant IgG with IgA immune response, (E) predominant IgA with IgG and IgM (blue) responses, and (F) predominant IgM with IgA response. “Predominant” immunoglobulin responses refers only to relative OD values and should not be taken to infer differences in relative concentrations of antibodies. For patients A, B, and C, the asterisks (*) designate the beginning of the platelet count decline indicating HIT. DVT indicates deep vein thrombosis; and PE, pulmonary embolism.
Lack of systematic IgM class precedence in the anti-PF4/heparin immune response
Among the 48 patients (12 with HIT) who formed an anti-PF4/heparin immune response, we found that the median day of onset for the 3 individual immunoglobulin classes was identical, at day 6 (Table 1). No evidence for consistent IgM precedence was observed, nor was there a tendency to any transient appearance of IgM antibodies before IgA or IgG antibody formation. However, for the 12 patients with HIT, all of whom developed IgG class antibodies, there was a significantly shorter time to formation of anti-PF4/heparin antibodies of IgG class, compared with the 34 non-HIT controls who formed anti-PF4/heparin antibodies of IgG class, whether the analysis was performed with respect to day of first heparin exposure (P × .0018) or in relation to day of surgery (P × .0017; Table 1).
Comparison of the distribution of the times to seroconversion between HIT and antibody-positive non-HIT control patients
| Type of antibody . | Patients with HIT . | Non-HIT antibody-positive controls . | P . | ||
|---|---|---|---|---|---|
| n . | Days to seroconversion, mean (SD) . | n . | Days to seroconversion, mean (SD) . | ||
| From day of beginning postoperative heparin | |||||
| IgG | 12 | 4.75 (0.93) | 34 | 6.03 (2.09) | .0018 |
| IgA | 7 | 6.57 (5.29) | 16 | 6.37 (5.72) | .855 |
| IgM | 4 | 7.50 (9.00) | 12 | 6.00 (6.00) | .412 |
| IgG/A/M | 12 | 4.25 (1.11) | 36 | 4.47 (2.14) | .574 |
| From day of surgery | |||||
| IgG | 12 | 5.92 (0.81) | 34 | 7.12 (1.80) | .0017 |
| IgA | 7 | 7.86 (5.48) | 16 | 7.50 (5.07) | .740 |
| IgM | 4 | 8.75 (10.92) | 12 | 7.08 (6.81) | .407 |
| IgG/A/M | 12 | 5.42 (0.81) | 36 | 5.55 (1.85) | .690 |
| Type of antibody . | Patients with HIT . | Non-HIT antibody-positive controls . | P . | ||
|---|---|---|---|---|---|
| n . | Days to seroconversion, mean (SD) . | n . | Days to seroconversion, mean (SD) . | ||
| From day of beginning postoperative heparin | |||||
| IgG | 12 | 4.75 (0.93) | 34 | 6.03 (2.09) | .0018 |
| IgA | 7 | 6.57 (5.29) | 16 | 6.37 (5.72) | .855 |
| IgM | 4 | 7.50 (9.00) | 12 | 6.00 (6.00) | .412 |
| IgG/A/M | 12 | 4.25 (1.11) | 36 | 4.47 (2.14) | .574 |
| From day of surgery | |||||
| IgG | 12 | 5.92 (0.81) | 34 | 7.12 (1.80) | .0017 |
| IgA | 7 | 7.86 (5.48) | 16 | 7.50 (5.07) | .740 |
| IgM | 4 | 8.75 (10.92) | 12 | 7.08 (6.81) | .407 |
| IgG/A/M | 12 | 5.42 (0.81) | 36 | 5.55 (1.85) | .690 |
Data are shown separately for the commercial EIA (which detects antibodies of all 3 classes [IgG/A/M] against PF4/polyanion) and EIAs that detect antibodies of the 3 major immunoglobulin classes individually. Combining the data for the 48 patients (12 with HIT, 36 seropositive non-HIT controls), the median day of onset for the 3 immunoglobulin classes (with respect to the day of beginning heparin therapy) are as follows: IgG, day 6 (IQR, day 5-7; n = 46 seroconversions); IgA, day 6 (IQR, day 5-8; n = 23 seroconversions); and IgM, day 6 (IQR, day 5-9; n = 16 seroconversions).
HIT indicates heparin-induced thrombocytopenia; EIA, enzyme-immunoassay; and IQR, interquartile range.
No influence of previous heparin exposure
The distribution of histories of previous heparin exposure (unlikely, possible, definite) did not differ between the patients with HIT and the antibody-positive patients without HIT (P = .913; Table S1, available on the Blood website; see the Supplemental Materials link at the top of the online article). We also did not find significant differences in the probabilities of formation of the specific classes of immunoglobulins in relation to the distribution of previous heparin exposure (P = .506 for IgA, P = .331 for IgG, P = .797 for IgM; Table S2). Furthermore, we did not find any differences in the timing of onset of forming antibodies of the different immunoglobulin classes, in relation to the history of previous heparin exposure (Table S3).
Discussion
HIT is an important adverse drug reaction. Unlike other drugs, such as quinine and vancomycin, that cause severe but isolated thrombocytopenia and bleeding, HIT is noted for its high risk of venous or arterial thromboses, as well as its potential for life- and limb-threatening thrombotic complications, which include ischemic limb necrosis (often resulting in limb amputation), fatal pulmonary embolism, and bilateral adrenal necrosis.1,2 Moreover, HIT has atypical clinical features, including the onset of thrombocytopenia as early as 5 days after even a first exposure to heparin,12,13 antibody transience,12,24 and lack of immune memory, based on the usual lack of recurrence of an immune response on subsequent heparin exposure.12,15 Indeed, even “rapid-onset” HIT that occurs within 24 hours of heparin administration—once regarded as evidence of immune memory—is now recognized as being caused by giving heparin to a patient with already-circulating antibodies that resulted from a recent exposure to heparin.12
The current report represents one approach to study the serologic basis for HIT. We studied the time of formation, the immunoglobulin class, and immunoglobulin levels in a group of patients who had serologically confirmed HIT. We compared these results to a group of seropositive non-HIT control patients who did not develop HIT despite having detectable anti-PF4/heparin antibodies. The blood samples allowing these serologic investigations were obtained from previous prospective studies. The availability of a relatively large number of serial blood samples (mean of 10.5 samples per patient) coinciding with seroconversion provided the opportunity to study the immune response that underlies HIT.
This study shows that HIT does not conform to the classic paradigm of a primary immune response. According to classic immunology,26 a primary immune response is characterized by the early generation of antibodies of IgM class, followed several days later by production of IgG class antibodies. Even for patients who had a first exposure to heparin, we did not observe any tendency for 1 of the 3 immunoglobulin classes to precede the others. Rather, we saw that all 3 immunoglobulin classes became detectable at a median of 6 days after starting heparin. We also found that patients with HIT had significantly higher mean levels of anti-PF4/heparin antibodies than the patients who underwent seroconversion, but who evinced no clinical evidence of HIT. When we examined the individual immunoglobulin classes, we found that this difference in antibody levels primarily reflected differences in the IgG class of antibodies, rather than in IgA or IgM. Although there was overlap in anti-PF4/heparin IgG assay reactivity among the patients with HIT and seropositive non-HIT controls (ie, not all patients with relatively high IgG levels developed HIT), this might be explained by differences in other patient-dependent susceptibility factors, including differences in platelet Fc receptor numbers,21 platelet PF4 levels,27 among others.
Although comparisons between the 2 patient groups clearly showed higher mean levels of anti-PF4/heparin antibodies of IgG class among the patients with HIT, no inferences can be drawn regarding the levels of antibodies of the 3 immunoglobulin classes relative to one another, because the methods do not permit quantitative interassay comparisons. Accordingly, the data in Figure 1B should not be interpreted to indicate that mean levels (by OD values) of the 3 antibody classes reflect relative HIT antibody concentrations as IgG > IgA > IgM (although this may be true), or that any such relationship holds for the individual patients depicted in Figure 3.
Our study also provides new information regarding the relationships between heparin administration, antibody formation, and the ensuing fall in the platelet count. Patients with HIT developed detectable anti-PF4/heparin antibodies at a median of only 4 days after starting heparin. This was followed by a subsequent lag of 2 days (median) until the initial fall in platelet count began, with an additional lag of 2 days between the beginning of the platelet count fall and the decline reaching levels that would typically lead to the clinical suspicion of HIT, such as a platelet count drop of 50% or greater from the postoperative peak. Further, we found that the antibodies are readily detectable by immunoassay, with OD values approximately 80% of maximal at the beginning of the platelet count fall associated with HIT (Figure 2). These findings are consistent with the concept that the PF4-dependent EIAs are sensitive for the diagnosis of HIT,3,,–6 and that a negative assay essentially rules out the diagnosis of HIT as the explanation for the platelet count fall (or other clinical event) that led to the test being performed.16
This study provides further insights into the anti-PF4/heparin immune response associated with HIT. For as yet unexplained reasons, exposure to heparin results in the relatively rapid formation of antibodies of any or all of the 3 major immunoglobulin classes, without evidence of IgM class precedence. HIT is characterized by formation of relatively high levels of IgG class antibodies that become detectable at 4 days after initiation of heparin exposure and that precede the onset of HIT. The time to formation of IgG class antibodies is somewhat shorter among those patients who develop HIT, compared with non-HIT controls who form IgG antibodies. Further, there is no association between the history of previous heparin exposure and the timing or antibody formation or in the class of antibodies generated. The biologic basis of these atypical immunologic features remains to be elucidated, but perhaps they reflect the autoimmune nature of the anti-PF4/heparin immune response, rather than a more conventional (classic) immune response to a foreign antigen.
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Ker-Ai Lee for assistance with the statistical analyses and James W. Smith for help in the preparation of the figures.
This work was supported by grants T6157 (T.E.W.) and T5542 (J.G.K. and J.C.M.) from the Heart and Stroke Foundation of Ontario (Toronto, ON).
Authorship
Contribution: T.E.W. designed and supervised the experiments, analyzed the data, interpreted the results, and was the primary author of the paper; J.-A.I.S. and J.C.M. performed the experiments and analyzed the data; R.J.C. performed biostatistical analyses and helped to write the paper; and J.G.K. interpreted the results and helped to write the paper. All authors reviewed and approved the final version of the manuscript.
Conflict-of-interest disclosure: T.E.W. has received lecture honoraria from Sanofi-Aventis, has provided consulting services to GTI, and has provided expert witness testimony relating to heparin-induced thrombocytopenia. The other authors declare no competing financial interests.
Correspondence: Theodore E. Warkentin, Hamilton Regional Laboratory Medicine Program, Rm 1-180A, Hamilton Health Sciences (General Site), 237 Barton St E, Hamilton, ON L8L2X2, Canada; e-mail: twarken@mcmaster.ca.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal