Relapsed B-cell lymphomas are currently incurable with conventional chemotherapy and radiation treatments. Radiolabeled antibodies directed against B-cell surface antigens have emerged as effective and safe therapies for relapsed lymphomas. We therefore investigated the potential utility of both directly radiolabeled 1F5 (anti-CD20), HD39 (anti-CD22), and Lym-1 (anti-DR) antibodies (Abs) and of pretargeted radioimmunotherapy (RIT) using Ab-streptavidin (SA) conjugates, followed by an N-acetylgalactosamine dendrimeric clearing agent and radiometal-labeled DOTA-biotin, for treatment of lymphomas in mouse models using Ramos, Raji, and FL-18 human lymphoma xenografts. This study demonstrates the marked superiority of pretargeted RIT for each of the antigenic targets with more complete tumor regressions and longer mouse survival compared with conventional one-step RIT. The Ab-SA conjugate yielding the best tumor regression and progression-free survival after pretargeted RIT varied depending upon the lymphoma cell line used, with 1F5 Ab-SA and Lym-1 Ab-SA conjugates yielding the most promising results overall. Contrary to expectations, the best rates of mouse survival were obtained using optimal single Ab-SA conjugates rather than combinations of conjugates targeting different antigens. We hypothesize that clinical implementation of pretargeted RIT methods will provide a meaningful prolongation of survival for patients with relapsed lymphomas compared with currently available treatment strategies.
Introduction
Radiolabeled monoclonal antibodies (Abs) have been evaluated as vehicles to selectively target radioactivity directly to tumor cells for more than a decade. Radioimmunotherapy (RIT) has proven to be particularly effective in non-Hodgkin lymphoma (NHL) because of the sensitivity of lymphocytes to radiation, the large number of target antigens on the surface of lymphocytes, and the vascular accessibility of the malignancies. Results have been reported by various investigators using single nonmyeloablative doses of radiolabeled Abs directed against a variety of lymphoid differentiation antigens, including HLA class II variant molecules, idiotypic immunoglobulins, the CD5, CD20, CD21, CD22, and CD37 antigens, and the IL2 receptor.1,2
Since targeting radiation to the CD20, CD22, and HLA class II DR antigens has proven to be the most effective in clinical NHL trials, we hypothesized that targeting the CD20, CD22, and HLA class II DR antigens simultaneously may provide an additive or synergistic benefit. We previously performed comparative assessments using conventional RIT targeting CD20, CD22, and HLA-DR on human Ramos, Raji, and FL-18 lymphoma xenografts in athymic mice to assess the potential for improving the efficacy of RIT by targeting other NHL cell surface antigens. Initial flow cytometric studies showed that the 3 Abs differed significantly in binding to these 3 lymphoma cell lines tested.3,4 Results of biodistribution studies demonstrated significant differences in tumor localization consistent with variable antigenic expression on the different lymphoma cell lines and thus the radioimmunoconjugate that yielded the best tumor-to-normal organ ratios differed in each tumor model.4 Administering all 3 radiolabeled Abs in combination, however, did not augment the localization of radioactivity to tumors compared with administration of the best single radiolabeled Ab alone. These data also suggested that the slow clearance of unbound radiolabeled Ab from the circulation and the resultant high levels of background radioactivity remained major obstacles to the optimal implementation of RIT in NHL.
In an attempt to improve the tumor-to-normal organ ratios of absorbed radiation that can be achieved, we and others have explored an alternative approach, pretargeted RIT, which uses the high affinity streptavidin (SA)–biotin system in which an Ab-SA conjugate and the radioactive biotin are administered separately.5,,,,,–11 After maximal accumulation of Ab-SA conjugate in targeted tissues, a clearing agent (CA) is administered to remove circulating Ab-SA conjugate from the circulation.12,13 Therapeutic radiobiotin is then administered and, due to its small size, penetrates tumor sites rapidly and attaches to the pretargeted Ab-SA conjugate bound specifically to tumor cells. If the tumor-bound Ab-SA does not capture this small radioactive molecule, the kidney rapidly excretes it. This pretargeted RIT approach has been shown to improve the ratios of radiation delivered to tumors compared with normal organs in both preclinical and clinical models.14,,,,,,,,,–24 We therefore investigated the biodistribution of radiation delivered to tumor and normal organs in 3 mouse xenograft models using pretargeted RIT with the CD20, CD22, and HLA-DR antigens as molecular targets. The pretargeting approach resulted in biodistributions of radioactivity that were far superior to those obtained with conventional RIT for all 3 antigenic targets.3,4 The Ab-SA conjugate yielding the best tumor uptake and tumor-to-normal organ ratios of radioactivity varied depending on the target antigen expression on the cell line used, with 1F5-SA and Lym-1-SA yielding the most promising biodistributions. The best tumor-to-normal organ ratios of absorbed radioactivity using the pretargeted RIT approach were also observed when single Ab-SA conjugates were used rather than combination therapy with all 3 conjugates.
Although these studies clearly proved that pretargeted RIT afforded superior biodistributions compared with conventional RIT, it was important to document that the improved targeting also translated into enhanced efficacy and diminished toxicity. As the next step toward potential human clinical trials, we report here RIT experiments in a murine system using antihuman CD20, CD22, and HLA-DR Ab to target human NHL. Results from these experiments demonstrate that a single optimal pretargeted conjugate yields more favorable objective tumor remissions and survival than using all 3 conjugates in combination and that the antigenic expression on NHL cells remains important to select the optimal reagent for RIT to achieve maximal therapeutic benefit.
Methods
Cell lines
The human Ramos and Raji Burkitt lymphoma cell lines were obtained from ATCC (Bethesda, MD). The transformed follicular FL-18 cell line was a gift from David Maloney (Fred Hutchinson Cancer Research Center [FHCRC], Seattle, WA). Cell lines were maintained as previously described.15 Cell viability exceeded 95% by trypan blue exclusion.
Antibodies, antibody conjugates, and pretargeting reagents
The 1F5 hybridoma cell line expressing the murine IgG2a anti–human CD20 Ab was a gift from Clay Siegall (Seattle Genetics, Seattle, WA). The HD39 hybridoma expressing the murine IgG1 anti–human CD22 Ab was a gift from Edward Clark (University of Washington, Seattle, WA). The Lym-1 hybridoma expressing murine IgG2a anti–HLA-DR Ab and a hybridoma expressing a nonspecific IgG2a control Ab, HB-8181, were obtained from ATCC. The HD39 Ab was produced by injecting the hybridoma into pristane-primed mice to generate ascites and purified as described previously.4 All other Abs were produced from the respective hybridomas using a hollow fiber bioreactor system in the monoclonal Ab production facility at the FHCRC. DOTA-Ab reagents were conjugated as described previously.15 Each Ab was also conjugated to SA to form covalent chemical conjugates using previously described methods.15,25
Radiolabeling
90Y (Nordion, Ottawa, ON) radiolabeling of intact DOTA-Ab for conventional RIT and DOTA-biotin for pretargeted RIT was performed as described.15 Radiochemical purity was typically greater than 99% as determined by high-performance liquid chromatography (HPLC), and labeling efficiencies were more than 90%. DOTA-biotin was synthesized as described.15
Mouse RIT and pretargeted RIT studies
Female BALB/c nu/nu mice, aged 6 to 8 weeks, were obtained from Harlan Sprague-Dawley (Indianapolis, IN) and Charles River Laboratories (Wilmington, MA) and housed under protocols approved by the FHCRC Institutional Animal Care and Use Committee. Ramos, Raji, or FL18 cells (1-1.2 × 107) were injected subcutaneously in the right flank approximately 10 days prior to therapy to obtain lymphoma xenografts. Mice with similar, palpable tumors were chosen for the studies. To compare the therapeutic efficacy of pretargeted and conventional radiolabeled Abs, groups of 10 Ramos, Raji, or FL18 lymphoma tumor–bearing mice were placed on biotin-free chow for 5 days and injected with either 1.4 nmol (215 μg) anti-CD20 1F5 Ab, anti-CD22 HD39 Ab, anti–HLA-DR Lym-1 Ab, or control HB8181 Ab each directly labeled with 90Y (200-300 μCi [7.4-11.1 MBq]) or equimolar amounts (250 μg) of anti-CD20 1F5 Ab-SA, anti-CD22 HD39 Ab-SA, anti–HLA-DR Lym-1 Ab-SA, or HB8181 Ab-SA conjugates, followed 22 hours later by 5.8 nmol (50 μg) CA and 2 hours later by 1.2 nmol (1 μg) 90Y-DOTA-biotin labeled with 400 μCi (14.8 MBq), 600 μCi (22.2 MBq), or 800 μCi (29.6 MBq) 90Y. For combination studies, mice were coinjected with an equimolar mix (1.4 nmol of each conjugate) of either all 3 conjugates (4.2 nmol of total conjugate in mice with Ramos xenografts) or the 2 Ab conjugates that had the most favorable biodistribution of radioactivity (1F5 and Lym-1 Ab-SA at 2.8 nmol of total conjugate in mice bearing FL-18 and Raji tumors). In each experiment, mice were also coinjected with 400 μg of an irrelevant IgG2a Ab (HB8181) to block nonspecific binding of the 1F5, HD39, and Lym-1 Abs to Fc receptors.26 Mice were monitored every other day for general appearance, tumor volume measurements, and body weight. Mice were killed if tumors grew large enough to cause obvious discomfort or impair ambulation.
Toxicity experiments assessing leukocyte, hemoglobin, and platelet counts, plus aspartate aminotransferase (AST), alanine aminotransferase (ALT), creatinine, and blood urea nitrogen (BUN) levels, were performed using mice that survived 120 days after pretargeted RIT, as described using methods previously defined.15 Tissues from surviving mice were also harvested, fixed in 10% buffered formalin, and stained with hematoxylin and eosin (H&E) for pathologic analysis as previously described to assess potential long-term lung, spleen, kidney, and liver toxicities.27
Results
RIT with either conventional or pretargeted anti-CD20, anti-CD22, or anti–HLA-DR Ab conjugates
To determine whether the superior pretargeted RIT biodistributions found in our prior comparative biodistribution studies3,4 would translate to enhanced efficacy compared with conventional one-step RIT, we synthesized and purified conjugates of SA with 1F5 anti-CD20 Ab, HD39 anti-CD22 Ab, Lym-1 anti–HLA-DR Ab, and HB8181 control Ab. Using standard cell binding assays, the immunoreactivities and avidities were determined for 1F5, 1F5-SA, Lym-1, Lym-1-SA, HD39, and HD39-SA using Ramos and Raji cells.3 No statistically significant differences in avidity were observed between the Ab-SA conjugates and the corresponding unconjugated Ab (paired t test, 2-tailed, P < .05). Flow cytometric studies were used to assess the relative densities of cell surface antigenic targets using the 3 whole Abs and their respective Ab-SA conjugates, as previously reported.3 As described in Table 1, these Ab conjugates differed significantly in binding to the 3 lymphoma cell lines tested (Ramos, Raji, and FL-18). The 1F5 Ab-SA conjugate showed the highest level of binding to Ramos cells, whereas Lym-1 Ab-SA demonstrated the highest binding to Raji cells, followed by 1F5 Ab-SA. Lym-1 Ab-SA and 1F5 Ab-SA showed similar high levels of binding to FL18 cells, whereas HD39 Ab-SA exhibited minimal binding.
Mean fluorescence index (MFI) from cell-binding studies
| Ab conjugate . | Ramos . | Raji . | FL-18 . |
|---|---|---|---|
| 1F5 | 700.6 | 489.6 | 744.1 |
| Lym-1 | 140.3 | 1484.4 | 802.4 |
| HD39 | 100.9 | 100.9 | 93.3 |
| HB8181 | 25.4 | 10.8 | 20.8 |
| Ab conjugate . | Ramos . | Raji . | FL-18 . |
|---|---|---|---|
| 1F5 | 700.6 | 489.6 | 744.1 |
| Lym-1 | 140.3 | 1484.4 | 802.4 |
| HD39 | 100.9 | 100.9 | 93.3 |
| HB8181 | 25.4 | 10.8 | 20.8 |
Therapy experiments targeting the 3 antigenic targets were assessed individually and in combination using these 3 types of lymphoma (Ramos, Raji, or FL18) xenografts. For pretargeted RIT, experimental groups of 10 mice bearing each human lymphoma xenograft were injected with either 1.4 nmol anti-CD20 (1F5), anti-CD22 (HD39), or anti–HLA-DR (Lym-1) Ab-SA conjugate, followed 22 hours later by 5.8 nmol CA, and then 2 hours after CA by 1.2 nmol 90Y-DOTA-biotin labeled with 400 μCi (14.8 MBq), 600 μCi (22.2 MBq), or 800 μCi (29.6 MBq) per mouse. Comparison groups were injected with 1.4 nmol directly radiolabeled 90Y-anti-CD20, anti-CD22, or anti–HLA-DR Ab (200 μCi [7.4 MBq] or 300 μCi [11.1 MBq]/mouse). Control groups were either untreated or injected with 1.4 nmol of the nonbinding HB8181 Ab labeled with 200 to 300 μCi (7.4-11.1 MBq) 90Y or 1.4 nmol HB8181 Ab-SA control conjugate followed by 5.8 nmol CA and then 800 μCi (29.6 MBq) 90Y-DOTA-biotin. In both conventional and pretargeted RIT studies for each lymphoma xenograft model, all mice in the control groups experienced exponential growth of their lymphoma xenografts requiring killing before day 16 (Figures 1,Figure 2,Figure 3,Figure 4,Figure 5–6, panel D in each). The rates of tumor growth in these control groups were indistinguishable from untreated xenograft-bearing mice.
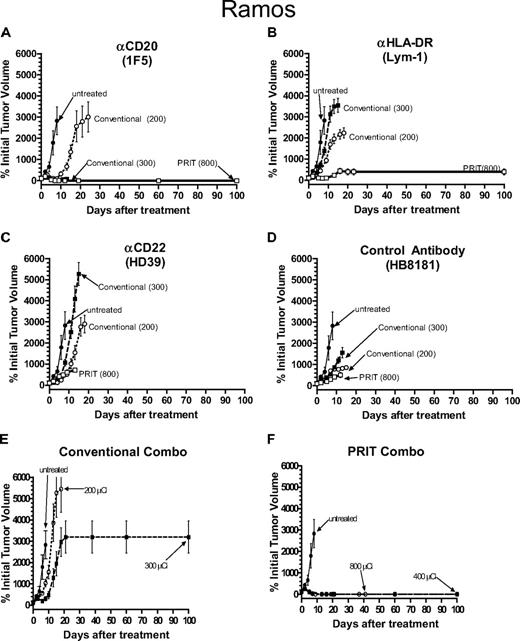
Therapeutic efficacy of pretargeted RIT (PRIT) compared with conventional RIT in athymic mice bearing Ramos Burkitt lymphoma xenografts. PRIT mice were injected intravenously via tail vein with 300 μg of either (A) anti-CD20 1F5-SA, (B) anti–HLA-DR Lym-1-SA, (C) anti-HD22 HD39-SA, (D) control HB8181-SA, or (F) a combination of the 3 Ab-SA conjugates followed 20 hours later with 50 μg CA, and 4 hours later with 800 μCi (29.6 MBq) 90Y-DOTA-biotin. Mice treated with conventional RIT received either 200 μCi (7.4 MBq) or 300 μCi (11.1 MBq) of either 90Y-labeled (A) 1F5-SA, (B) Lym-1-SA, (C) HD39-SA, (D) HB8181, or (E) a combination of the 3 directly labeled Abs injected intravenously via tail vein. Untreated mice did not receive any therapy. Tumor growth is reported as percentage of initial tumor volume (y-axis) measured over time (x-axis). When mice required killing due to tumor progression or severe toxicity, the final tumor volume was carried through until the last mouse in the group died or 100 days after treatment was reached. Error bars indicate SD.
Therapeutic efficacy of pretargeted RIT (PRIT) compared with conventional RIT in athymic mice bearing Ramos Burkitt lymphoma xenografts. PRIT mice were injected intravenously via tail vein with 300 μg of either (A) anti-CD20 1F5-SA, (B) anti–HLA-DR Lym-1-SA, (C) anti-HD22 HD39-SA, (D) control HB8181-SA, or (F) a combination of the 3 Ab-SA conjugates followed 20 hours later with 50 μg CA, and 4 hours later with 800 μCi (29.6 MBq) 90Y-DOTA-biotin. Mice treated with conventional RIT received either 200 μCi (7.4 MBq) or 300 μCi (11.1 MBq) of either 90Y-labeled (A) 1F5-SA, (B) Lym-1-SA, (C) HD39-SA, (D) HB8181, or (E) a combination of the 3 directly labeled Abs injected intravenously via tail vein. Untreated mice did not receive any therapy. Tumor growth is reported as percentage of initial tumor volume (y-axis) measured over time (x-axis). When mice required killing due to tumor progression or severe toxicity, the final tumor volume was carried through until the last mouse in the group died or 100 days after treatment was reached. Error bars indicate SD.
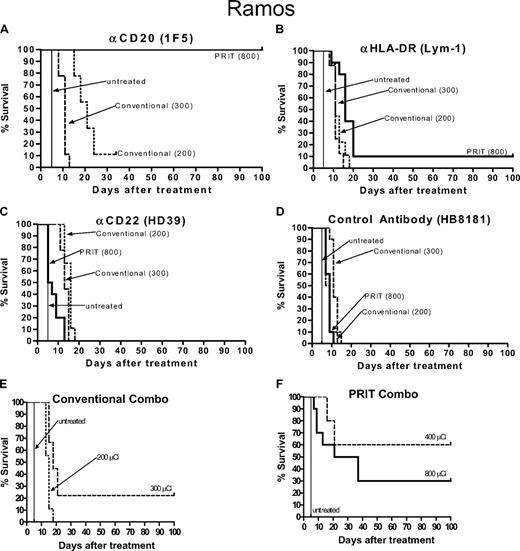
Kaplan-Meier cumulative survival plots for athymic mice treated with PRIT or conventional RIT following the establishment of palpable Ramos Burkitt lymphoma xenografts. Mice were treated as described in the legend of Figure 1 and analyzed for survival as a function of time. Treatment groups included mice receiving either (A) anti-CD20 1F5, (B) anti–HLA-DR Lym-1, (C) anti-CD22 HD39, (D) control HB8181 Ab conjugates, or (E,F) a combination of all 3 reagents.
Kaplan-Meier cumulative survival plots for athymic mice treated with PRIT or conventional RIT following the establishment of palpable Ramos Burkitt lymphoma xenografts. Mice were treated as described in the legend of Figure 1 and analyzed for survival as a function of time. Treatment groups included mice receiving either (A) anti-CD20 1F5, (B) anti–HLA-DR Lym-1, (C) anti-CD22 HD39, (D) control HB8181 Ab conjugates, or (E,F) a combination of all 3 reagents.
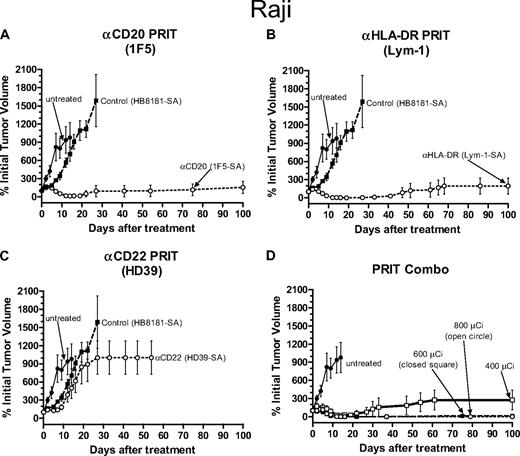
Therapeutic efficacy of PRIT in athymic mice bearing Raji Burkitt lymphoma xenografts. PRIT mice were injected intravenously via the tail vein with 300 μg of either (A) anti-CD20 1F5-SA, (B) anti–HLA-DR Lym-1-SA, (C) anti-HD22 HD39-SA, (A-C) control HB8181-SA, or (D) a combination of the 1F5-SA and Lym-1-SA conjugates followed 20 hours later with 50 μg CA, and 4 hours later with 800 μCi (29.6 MBq) 90Y-DOTA-biotin. Untreated mice did not receive any therapy. Tumor growth was measured as described in the legend to Figure 1. Error bars represent SD.
Therapeutic efficacy of PRIT in athymic mice bearing Raji Burkitt lymphoma xenografts. PRIT mice were injected intravenously via the tail vein with 300 μg of either (A) anti-CD20 1F5-SA, (B) anti–HLA-DR Lym-1-SA, (C) anti-HD22 HD39-SA, (A-C) control HB8181-SA, or (D) a combination of the 1F5-SA and Lym-1-SA conjugates followed 20 hours later with 50 μg CA, and 4 hours later with 800 μCi (29.6 MBq) 90Y-DOTA-biotin. Untreated mice did not receive any therapy. Tumor growth was measured as described in the legend to Figure 1. Error bars represent SD.
Kaplan-Meier cumulative survival plots for athymic mice treated with PRIT following the establishment of palpable Raji Burkitt lymphoma xenografts. Mice were treated as described in the legend of Figure 3 and analyzed for survival as a function of time. Treatment groups included mice receiving either anti-CD20 1F5, (B) anti–HLA-DR Lym-1, (C) anti-CD22 HD39, (A-C) control HB8181 Ab conjugates, or (D) a combination of 1F5-SA and Lym-1-SA conjugates.
Kaplan-Meier cumulative survival plots for athymic mice treated with PRIT following the establishment of palpable Raji Burkitt lymphoma xenografts. Mice were treated as described in the legend of Figure 3 and analyzed for survival as a function of time. Treatment groups included mice receiving either anti-CD20 1F5, (B) anti–HLA-DR Lym-1, (C) anti-CD22 HD39, (A-C) control HB8181 Ab conjugates, or (D) a combination of 1F5-SA and Lym-1-SA conjugates.
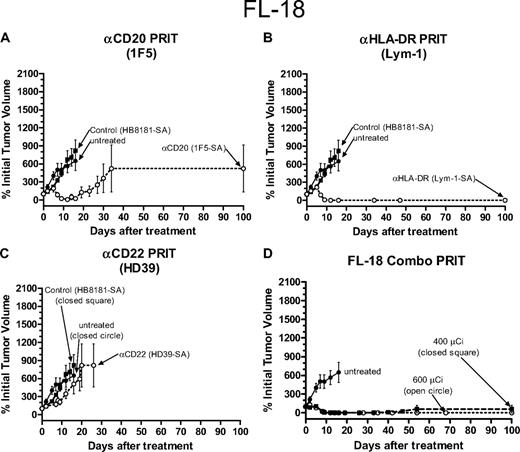
Therapeutic efficacy of PRIT in athymic mice bearing FL-18 transformed follicular lymphoma xenografts. PRIT mice were injected intravenously via tail vein with 300 μg of either (A) anti-CD20 1F5-SA, (B) anti–HLA-DR Lym-1-SA, (C) anti-HD22 HD39-SA, (A-C) control HB8181-SA, or (D) a combination of the 1F5-SA and Lym-1-SA conjugates followed 20 hours later with 50 μg CA, and 4 hours later with 800 μCi (29.6 MBq) 90Y-DOTA-biotin. Untreated mice did not receive any therapy. Tumor growth was measured as described in the legend to Figure 1. Error bars indicate SD.
Therapeutic efficacy of PRIT in athymic mice bearing FL-18 transformed follicular lymphoma xenografts. PRIT mice were injected intravenously via tail vein with 300 μg of either (A) anti-CD20 1F5-SA, (B) anti–HLA-DR Lym-1-SA, (C) anti-HD22 HD39-SA, (A-C) control HB8181-SA, or (D) a combination of the 1F5-SA and Lym-1-SA conjugates followed 20 hours later with 50 μg CA, and 4 hours later with 800 μCi (29.6 MBq) 90Y-DOTA-biotin. Untreated mice did not receive any therapy. Tumor growth was measured as described in the legend to Figure 1. Error bars indicate SD.
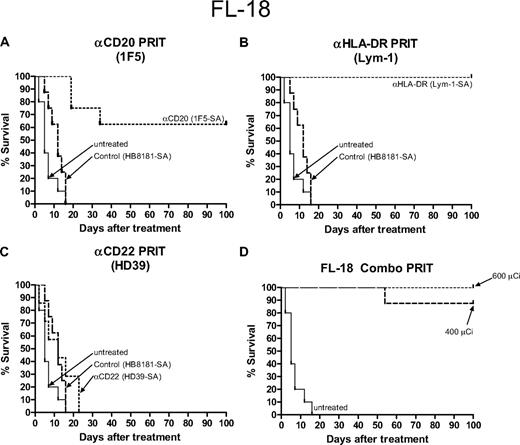
Kaplan-Meier cumulative survival plots for athymic mice treated with PRIT following the establishment of palpable FL-18 transformed follicular lymphoma xenografts. Mice were treated as described in the legend for Figure 5 and analyzed for survival as a function of time. Treatment groups included mice receiving either anti-CD20 1F5, (B) anti–HLA-DR Lym-1, (C) anti-CD22 HD39, (A-C) control HB8181 Ab conjugates, or (D) a combination of 1F5-SA and Lym-1-SA conjugates.
Kaplan-Meier cumulative survival plots for athymic mice treated with PRIT following the establishment of palpable FL-18 transformed follicular lymphoma xenografts. Mice were treated as described in the legend for Figure 5 and analyzed for survival as a function of time. Treatment groups included mice receiving either anti-CD20 1F5, (B) anti–HLA-DR Lym-1, (C) anti-CD22 HD39, (A-C) control HB8181 Ab conjugates, or (D) a combination of 1F5-SA and Lym-1-SA conjugates.
Conventional and pretargeted RIT of Ramos Burkitt lymphoma xenografts
Mice bearing Ramos human Burkitt lymphoma xenografts treated with conventional one-step RIT using 200 μCi (7.4 MBq) 90Y-anti-CD20, anti–HLA-DR, and anti-CD22 Ab experienced a slight tumor growth delay compared with untreated mice, but there were no complete regressions (Figure 1A-C) and all mice except one required killing by day 20 after delivery of the therapeutic radiolabeled Ab, with one mouse surviving to day 34 in this group before killing (Figure 2A-C). Mice treated with 300 μCi (11.1 MBq) conventional one-step 90Y-labeled anti–HLA-DR and anti-CD22 Abs experienced similar tumor growth to the mice that received 200 μCi (7.4 MBq) 90Y requiring killing by day 20 (Figures 1A-C, 2A-C). Mice that received 300 μCi (11.1 MBq) 90Y-anti-CD20 (1F5) Ab had more striking tumor regressions, with complete disappearance of 90% of the xenografts by day 7 after therapy (Figure 1A). However, all mice in this group experienced lethal toxicity, losing 21.9% plus or minus 9.0% of their initial weight and dying, likely from marrow suppression and infection, by day 12 (Figure 2A). Mice were not treated with doses of direct conjugates higher than 300 μCi (11.1 MBq), as doses higher than this were uniformly lethal using any of the 90Y-labeled Abs.
Experimental mice bearing Ramos xenografts pretargeted with anti-CD20 Ab-SA fared much better than the anti–HLA-DR and anti-CD22 groups in terms of toxicity, tumor responses, and survival (Figures 1A-C, 2A-C). Transient tumor responses were seen in mice receiving pretargeted anti-CD22 Ab-SA plus 800 μCi (29.6 MBq) 90Y-DOTA-biotin, however, all mice in this group exhibited subsequent progressive tumor growth and died by day 12 (Figure 2C). Based on the results of careful prior dose titration studies that found that 800 μCi (29.6 MBq) 90Y-DOTA-biotin yielded excellent outcomes with pretargeted 1F5 anti-CD20 Ab-SA conjugate and because of the large numbers of variables in these studies, it was not practical to repeat dose finding experiments, and thus as a compromise the 800 μCi (29.6 MBq) 90Y-DOTA-biotin dose was chosen for single-agent pretargeted RIT studies.3,4,15,28 Mice treated with pretargeted RIT using anti–HLA-DR Ab-SA and 800 μCi (29.6 MBq) 90Y-DOTA-biotin also experienced partial tumor remissions (∼ 50% of initial volume) by day 12 after treatment (Figure 1B). However, Ramos tumors regrew in 9 of the 10 mice, mandating killing by day 20 (Figure 2B). One mouse in the anti–HLA-DR Ab-SA pretargeted RIT group had complete regression of the Ramos xenograft and survived 100 days after treatment, and was likely cured (Figures 1B, 2B). In contrast, all mice receiving pretargeted anti-CD20 Ab-SA plus 800 μCi (29.6 MBq) 90Y-DOTA-biotin achieved complete remissions (CRs) before day 15 (Figures 1A, 2A). There was minimal toxicity in mice pretargeted with anti-CD20 Ab-SA followed by 800 μCi (29.6 MBq) 90Y-DOTA-biotin, with only 3.3% plus or minus 3.0% weight loss by day 2; all mice regained pretreatment weight by day 9.
Pretargeted RIT studies were also conducted using all 3 conjugates in combination to assess whether targeting multiple antigens yielded additive or synergistic therapeutic effects on Ramos xenografts (Figures 1F, 2F). Initial biodistribution experiments used a total amount of conjugate that was 3 times the total amount of protein used in the single-agent experiments (ie, 1.4 nmol of each Ab-SA was injected, or 4.2 nmol total).3 Conjugate injections in experimental groups of 10 were followed 22 hours later with 5.8 nmol CA and then 2 hours later with 1.2 nmol 90Y-DOTA-biotin. All mice bearing Ramos tumors that were treated with this combination schema achieved CR after delivery of CA and either 400 μCi (14.8 MBq) or 800 μCi (29.6 MBq) 90Y-DOTA-biotin by day 9 (Figure 1F). Four mice in the 400-μCi (14.8 MBq) 90Y-DOTA-biotin combination pretargeted RIT group died by day 16 from toxicity without tumor relapse and the remaining 60% of mice in this group survived to day 100 without recurrence of their Ramos tumors. Conversely, 9 of 10 mice that received each of the 3 Ab-SA conjugates followed by CA, and 800 μCi (29.6 MBq) 90Y-DOTA-biotin died of toxicity (8 mice died by day 16 and 1 died on day 56) without tumor relapse. One mouse in the combination group receiving 800 μCi (29.6 MBq) 90Y-DOTA-biotin survived in CR to day 100.
Although the exact determination of the lethal toxicities detected with the combination of the anti-CD20, anti-CD22, and anti–HLA-DR Ab remains unclear, it is likely that the increase in radioactivity delivered to normal organs, as demonstrated in the prior biodistribution studies, resulted in excessive normal organ toxicity.3 As suggested by prior biodistribution data, we believe that the simultaneous administration of all 3 Ab-SA conjugates in the combination pretargeted RIT schema exceeded the capacity of the liver to efficiently clear and metabolize conjugate-CA complexes prior to radiobiotin administration, leading to higher levels of circulating radiation and lethal marrow toxicity. Therefore, based on the high toxicity profile of the 3 Ab-SA conjugates initially delivered by combination pretargeted RIT to mice bearing Ramos tumors, subsequent conventional RIT combination Ab studies (and all subsequent combination RIT experiments in Raji and FL-18 xenograft bearing mice) used only the 2 most promising anti-CD20 (1F5) and anti–HLA-DR (Lym-1) Abs, and not the anti-CD22 (HD39) Ab, which was largely ineffective as a single therapeutic agent.
Mice bearing Ramos tumors were treated with a combination of conventional one-step RIT using 200 (7.4 MBq) and 300 μCi (11.1 MBq) 90Y-anti-1F5 and 90Y-anti-Lym-1 (Figures 1E, 2E). All mice that received 200 μCi (7.4 MBq) 90Y-labeled Ab experienced exponential growth of their Ramos xenografts requiring killing before day 20, whereas 2 of 10 mice in the 300-μCi (11.1 MBq) 90Y combination group had complete regression of tumor and survived 100 days after treatment. However, 80% of mice in the conventional RIT group that received 300 μCi (11.1 MBq) 90Y-labeled 1F5 and Lym-1 Ab had to be killed by day 20 due to progressive tumor growth.
Pretargeted RIT of Raji Burkitt lymphoma xenografts
Although these studies clearly proved that pretargeted anti-CD20 RIT of Ramos tumor xenografts afforded superior efficacy and diminished toxicity compared with either conventional or pretargeted RIT targeting the CD22 and HLA-DR antigens, it was important to explore the use of these radioimmunoconjugates using the pretargeted RIT schema in other lymphoma xenograft models. Therefore, in a similar manner we also compared pretargeted RIT using anti-CD20, anti-CD22, and anti–HLA-DR Ab-SA conjugates followed by CA and 800 μCi (29.6 MBq) 90Y-DOTA-biotin delivered to mice bearing Raji human lymphoma xenografts. Raji tumor–bearing mice pretargeted with either 1F5 Ab-SA or Lym-1-SA had similar excellent tumor responses, however, the 1F5 Ab-SA mice fared better than other groups in terms of toxicity and survival (Figures 3,4). Eight of 10 mice that received the 1F5-SA conjugate remained in compete remission for more than 100 days after therapy with one additional mouse having a stable, slowly progressing xenograft that eventually required killing on day 100 (Figures 3A, 4A). Three mice in the Lym-1 Ab-SA group slowly progressed with tumor after initial partial remissions, whereas the remaining 7 mice in this group survived to 100 days after therapy in CR (Figures 3B, 4B). Conversely, only 2 mice receiving pretargeted HD39-SA and 800 μCi (29.6 MBq) 90Y-DOTA-biotin achieved CR and were disease-free at day 100 after treatment, with the remaining 8 mice in this group requiring killing by day 22 (Figures 3C, 4C). There was minimal toxicity in mice pretargeted with each single-agent Ab-SA followed by CA and 800 μCi (29.6 MBq) 90Y-DOTA-biotin, with 4.9% plus or minus 2.4% for 1F5-SA and 5.8% plus or minus 2.5% for Lym-1 Ab-SA weight loss by days 2 and 5, respectively, however all mice regained pretreatment weight by day 9.
The inability of the liver to clear saturating complexes of all 3 CA-Ab-SA conjugates seen in the previous Ramos xenograft experiments convinced us to administer therapy in the Raji xenograft studies using only the 2 most promising Ab-SA conjugates, 1F5 Ab-SA and Lym-1 Ab-SA. Combinations of these 2 conjugates followed by 400 μCi (14.8 MBq) and 800 μCi (29.6 MBq) 90Y-DOTA-biotin resulted in more toxicity and less efficacy than treatment with either 1F5 Ab-SA or Lym-1 Ab-SA alone (Figures 3D, 4D). Although 90% of mice achieved a CR after receiving the combination of pretargeted 1F5 Ab-SA and Lym-1 Ab-SA followed by CA and 800 μCi (29.6 MBq) 90Y-DOTA-biotin, weight loss and toxicity resulted in the survival of only 3 mice 100 days after treatment. The combination group that received 400 μCi (14.8 MBq) 90Y-DOTA-biotin also exhibited significant efficacy, with the majority of mice achieving a CR, and 7 of the 10 mice treated remained alive without complications from therapeutic toxicity at day 100.
Pretargeted RIT of FL-18 transformed follicular lymphoma xenografts
Since in vivo biodistributions of the radioimmunoconjugates targeting the CD20, CD22, and HLA-DR antigens suggested that the optimal target may vary among different lymphoma cell lines, we also performed comparative therapy experiments using pretargeted RIT in athymic mice bearing transformed follicular (FL-18) lymphoma xenografts.3 In these studies, a slightly lower dose of 600 μCi (22.2 MBq) 90Y-DOTA-biotin was used since higher doses of radioactivity led to excessive toxicity in the FL-18 xenograft–bearing mice. The most promising results were seen in mice that received pretargeted Lym-1 Ab-SA followed by CA and 600 μCi (22.2 MBq) 90Y-DOTA-biotin where all 10 mice treated with this anti–HLA-DR Ab-SA conjugate had complete disappearance of their xenografts and survived to day 100 without significant toxicities (Figures 5B, 6B). Pretargeted anti-CD20 1F5 Ab-SA used in a similar manner with CA and subsequent delivery of 600 μCi (22.2 MBq) 90Y-DOTA-biotin produced CR in 75% of mice and 60% were tumor-free at day 100 (Figures 5A, 6A). Pretargeted anti-CD22 (HD39) Ab-SA conjugate followed by CA and 600 μCi (22.2 MBq) 90Y-DOTA-biotin had minimal effect on the ability to treat FL-18 tumor xenografts, as was also seen with mice bearing the Burkitt tumors (Figures 5C, 6C). FL-18 tumor–bearing mice receiving pretargeted RIT with HD39 Ab-SA all died by day 22 after experiencing exponential growth of their xenografts (Figures 5C, 6C). Combination pretargeted RIT experiments targeting the most promising 2 antigenic sites (CD20, HLA-DR) with 1F5 Ab-SA and Lym-1 Ab-SA conjugates, respectively, did not provide any additional benefit compared with targeting each antigen alone (Figures 5D, 6D).
Toxicity
Toxicities were assessed in selected groups of 5 mice evaluated 120 days after therapy with 400 μCi (14.8 MBq) to 800 μCi (29.6 MBq) 90Y-DOTA-biotin pretargeted with 1.4 nmol of either 1F5 Ab-SA, HD39 Ab-SA, or Lym-1 Ab-SA and cleared from blood with 5.8 nmol CA. At this time point, leukocyte, platelet, and hemoglobin values, as well as AST, ALT, creatinine, and BUN levels, were obtained using blood sampled from the retro-orbital venous plexus. Hemoglobin values and platelet levels did not significantly decrease in any long-term disease-free surviving pretargeted mice compared with normal control mice. Compared with normal athymic mice, mice treated with either 1F5 Ab-SA, HD39 Ab-SA, or Lym-1 Ab-SA had leukocyte counts that were approximately 300% plus or minus 50% of the control leukocyte value and platelet counts that were approximately 50% plus or minus 1.0% of the control baseline platelet value, respectively. In contrast, mild anemia was seen only in the mice treated with combination pretargeted Ab-SA conjugates and 800 μCi (29.6 MBq) 90Y-DOTA-biotin (eg, 9.2% ± 8.5% drop in hemoglobin for mice that received 1F5 Ab-SA and Lym-1 Ab-SA conjugates compared with normal control mice). In separate analyses, there was little evidence of hepatic toxicity in these groups of mice as there was no elevation of transaminases from baseline in all but one mouse in the pretargeted RIT combination group. The ALT in one mouse that was treated with combination pretargeted Ab-SA conjugates and 400 μCi (14.8 MBq) 90Y-DOTA-biotin was 311% higher than the normal expected level. We also obtained tissues from long-term pretargeted survivors to investigate any potential delayed radiation-induced injuries. Histologic sections from the kidneys, liver, and spleen in all pretargeted treatment groups at 120 days after therapy suggested the absence of renal, hepatic, or splenic toxicity, even in pretargeted animals receiving 800 μCi (29.6 MBq) 90Y-DOTA-biotin. Although no pathological changes were detected in any spleen and liver tissues, mild evidence of membranous glomerulonephritis was seen in all groups of mice, most notably in the pretargeted groups that received combinations of Ab-SA conjugates. However, there were no notable differences in BUN or creatinine values, with the most significant changes being 84% plus or minus 12% for BUN and 95% plus or minus 13% for creatinine levels in mice that received the combination of 1F5 Ab-SA and Lym-1 Ab-SA conjugates compared with the control value levels.
Discussion
The first RIT trials for B-cell lymphomas used 131I-labeled Lym-1 Ab.29 Lym-1 is a novel, murine, IgG2a monoclonal Ab that recognizes HLA-DR10, a 31- to 35-kDa membrane antigen expressed on most malignant B cells.30 Significant activity has been documented in relapsed and refractory NHL patients using Lym-1 Ab radiolabeled with a variety of therapeutic radionuclides including 90Y, 67Cu, and 131I.31,,,–35 Epratuzumab, a humanized IgG1Ab to CD22, has also been radiolabeled and studied in patients with NHL. CD22 is a B cell–restricted, lineage-dependent antigen that is rapidly internalized upon antibody binding.36,37 Data obtained after a single dose of 90Y-epratuzumab revealed that antitumor effects could be seen in both indolent and aggressive NHL, suggesting that this radiolabeled anti-CD22 Ab may be a promising agent for the treatment of NHL.38,39 Weekly administrations of 90Y-epratuzumab were also explored as a strategy to increase the amount of radionuclide administered to an individual patient.40 Weekly RIT with 90Y-epratuzumab was found to be safe with only minor toxicity and led to an encouraging objective response rate with some durable complete remissions in both indolent and aggressive disease. Anti-CD20 Ab conjugated with radioactivity for treatment of relapsed or refractory indolent NHL has yielded response rates varying from 50% to more than 80%.41,,,–45 Even better overall and CR rates (90%-100% overall response rate and 60%-90% CR) have been observed using CD20 RIT as first-line treatment in CD20+.46,,–49
Although targeting the CD20 antigen has been the main research focus for most investigators studying Ab-mediated immunotherapy of B-cell lymphomas, targeting alternative antigens such as CD22 and HLA-DR, singly and in combination, may further improve outcomes for lymphoma patients. We have performed experiments exploring the biodistribution of anti-CD20, anti-CD22, and anti–HLA-DR Ab conjugates for RIT using 3 different NHL xenografts in athymic mice.3,4 These studies demonstrated that high levels of radiation can be specifically localized to lymphoma xenografts targeting CD22, HLA-DR, and CD20; however, the amounts of the radiation accumulating in the 3 different B-lymphoma xenografts in in vivo biodistribution studies differed based on varying surface antigen expression levels on the targeted tumors. This is the first report to compare the therapeutic efficacies of Ab directed against the 3 antigenic targets using multiple different B-lymphoma cell lines with variable surface antigen expression levels and comparing conventional RIT and pretargeted RIT.
Multiple recent studies have documented the promise of pretargeted RIT as a method to improve target-to-nontarget organ ratios of absorbed radiation compared with ratios obtained using conventional RIT. Pretargeted RIT attempts to limit the radiation exposure of normal organs, and thus the attendant associated toxicities, while maintaining or amplifying the delivery of radiation therapy to tumor sites.50 Pretargeting approaches based on a bispecific Abs for localization of a radiolabeled hapten-peptide have been investigated by other investigators.51 More recently, a novel tri-Fab recombinant fusion protein for pretargeting NHL has been developed using 2 anti-CD20 Fabs and 1 antihapten Fab that together may further improve tumor uptake and the therapeutic index compared with a bispecific Ab conjugate or a directly anti-CD20 radiolabeled Ab, providing clinical promise for pretargeted RIT.52 Our group and others have studied a novel pretargeting approach using Ab constructs conjugated to SA that bind to radiolabeled biotin as a means to improve target-to-nontarget ratios of absorbed radiation doses. This is accomplished by reducing the relatively protracted circulating half-life of conventional radiolabeled Abs that results in nonspecific exposure of normal organs to radioactivity.13,15,16,18,26,28,53 This pretargeted RIT approach has been shown to improve the ratios of radiation delivered to tumors compared with normal organs in both preclinical and clinical models of solid tumors as well as hematologic malignancies. In particular we have demonstrated reduced toxicity and markedly enhanced efficacy using pretargeted anti-CD20 Ab-SA conjugate compared with directly labeled anti-CD20 Ab in mouse lymphoma xenograft studies.14,26
The results from this study confirm a clear therapeutic advantage for the pretargeted approach to RIT for NHL over conventional RIT and corroborates our prior studies demonstrating the effectiveness of anti-CD20 pretargeted RIT.3,14,15,26,28 Despite the continued promise of CD20 targeting, however, in some cases (eg, for FL-18 xenografts) targeting HLA-DR with a Lym-1 Ab-SA conjugate demonstrated a survival advantage over mice that received a 1F5 Ab-SA conjugate targeting CD20. The ability to eradicate all 3 lymphoma xenografts by targeting CD20 and HLA-DR antigens proved superior to targeting CD22. Although the reason for the suboptimal outcomes of animals treated with pretargeted anti-CD22 RIT remains unclear, these results are likely due to the rapid internalization of the CD22 antigen after Ab-SA conjugate binding,54 rendering it inaccessible for binding to the subsequently administered radiolabeled DOTA-biotin. Alternatively, it is conceivable that the specific anti-CD22 Ab selected for this study (HD39) may be less efficacious than other anti-CD22 Abs (eg, epratuzumab) that are proprietary and not available for these comparative studies.38,39 Nonetheless, it is likely that ongoing studies comparing combinations of directly radiolabeled Abs to PRIT combinations will help elucidate the exact cause of the toxicities seen using PRIT combinations in this preclinical model.
This study is the first to explore whether targeting multiple antigens simultaneously can augment the efficacy of pretargeted RIT. The results presented in this paper suggest that targeting CD20, HLA-DR, and/or CD22 in combination does not improve the outcome of the pretargeted RIT compared with targeting the optimal antigen alone because of enhanced toxicity and impaired efficacy of the CA, which is overwhelmed by the amount of Ab-SA conjugates administered. These findings are consistent with our prior biodistribution studies demonstrating that targeting these antigens in combination does not increase the amount of radiation that is specifically localized to each tumor site and thus does not improve tumor-to-normal organ ratios.3 Moreover, we found increased toxicity with combination therapies and this finding may have resulted from saturation of the liver and inability to clear complexes of conjugates associated with CA when combinations were used. These increased toxicities can be correlated with higher circulating levels of radioactivity detected in the blood and normal organs in animals receiving combinations and inferior tumor-to-normal organ ratios of absorbed radioactivity. It is tempting to speculate that avoidance of these toxicities might be overcome using each of these conjugates sequentially in a patient, who may have achieved only a partial remission after delivery of the first conjugate. These results, however, should be interpreted understanding the limitations of the murine models tested and may not represent the therapeutic outcome of RIT targeting multiple antigens in patients with NHL. It also remains to be determined whether RIT targeting CD20 alone would be superior to a combination of conjugates targeting 2 or 3 antigens in the era of rituximab therapy, where rituximab has been shown to block access to RIT targeting CD20.55 Given the long in vivo persistence of rituximab, targeting alternative lymphoma-associated antigens may circumvent the effects on binding and the associated efficacy of second anti-CD20 Abs used for RIT.
Several obstacles have limited the widespread acceptance of conventional RIT regimens such as 131I-tositumomab and 90Y-ibritumomab and despite the appeal and promise of pretargeted RIT, these obstacles have the potential to undermine the acceptance of this approach for lymphoma patients. A major concern has focused on the complexity of the pretargeted RIT approach, which requires multiple injections of reagents at specified time intervals. Importantly, the feasibility of anti-CD20 pretargeted RIT has already been established in 2 clinical trials for patients with advanced lymphoma.23,56 In addition, the complexity of the multistep pretargeted RIT approach explored in this paper may be considered less burdensome than complicated current multiagent chemotherapy regimens that hematologists and oncologists commonly administer to patients. Nonetheless, the widespread adoption of a potential clinically relevant pretargeted RIT modality will require that the approach significantly improve survival and cause less toxicity than currently available therapies. This goal may be achieved through the observations supported in this report where cell-binding information obtained on tumor biopsies in vitro may assist the selection of optimal in vivo targeting reagents for patients.
In conclusion, this study highlights the importance of antigenic expression on lymphomas to select the most advantageous target for RIT. These results further suggest that combinations of radiolabeled Abs may not be superior to a single radioimmunoconjugate. The amount of radiation that can be delivered to these single target antigens using a directly labeled Ab, however, continues to be limited by toxicities due to the suboptimal therapeutic index (target to nontarget ratio) currently achievable with conventional RIT methodologies. Therefore, in view of the compelling advantages for pretargeted RIT demonstrated in this paper, we have begun translating this approach in human studies targeting CD20 to diminish relapse rates and improve survival.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by National Institutes of Health (NIH, Bethesda, MD) grants R01-CA76287, P01-CA44991, and K08-CA095448; James and Sherry Raisbeck; Mary and Geary Britton-Simmons; the Wyner/Stokes Foundation; David and Patricia Giuliani; the Edson Foundation; and the Frederick Kullman Memorial Fund. J.M.P. is supported by a Career Development Award from the Lymphoma Research Foundation (New York, NY) and a Damon Runyon Career Development Award (New York, NY).
National Institutes of Health
Authorship
Contribution: J.M.P. contributed to the conception, design, analysis, and interpretation of the research and drafted the paper; N.O. designed and performed research, analyzed data, and revised the paper; D.K.H. contributed vital reagents; D.S.W. contributed vital reagents and contributed to the interpretation of data; T.A.G. performed all statistical analyses; S.I.P., D.J.G., Y.L., and A.K.G. contributed to the interpretation of data; and O.W.P. contributed to the conception, design, analysis, and interpretation of the research, revised the paper, and provided grant funding for experiments.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: John Pagel, Fred Hutchinson Cancer Research Center, 1100 Fairview Ave N, M/S D5-380, Seattle, WA 98109; e-mail: jpagel@fhcrc.org.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal