Currently, there is a major need in hematopoietic stem cell (HSC) transplantation to develop reduced-intensity regimens that do not cause DNA damage and associated toxicities and that allow a wider range of patients to receive therapy. Cytokine receptor signals through c-Kit and c-Mpl can modulate HSC quiescence and engraftment, but the intracellular signals and transcription factors that mediate these effects during transplantation have not been defined. Here we show that loss of one allele of signal transducer and activator of transcription 5 (STAT5) in nonablated adult mutant mice permitted engraftment with wild-type HSC. Conditional deletion of STAT5 using Mx1-Cre caused maximal reduction in STAT5 mRNA (> 97%) and rapidly decreased quiescence-associated c-Mpl downstream targets (Tie-2, p57), increased HSC cycling, and gradually reduced survival and depleted the long-term HSC pool. Host deletion of STAT5 was persistent and permitted efficient donor long-term HSC engraftment in primary and secondary hosts in the absence of ablative conditioning. Overall, these studies establish proof of principle for targeting of STAT5 as novel transplantation conditioning and demonstrate, for the first time, that STAT5, a mitogenic factor in most cell types, including hematopoietic progenitors, is a key transcriptional regulator that maintains quiescence of HSC during steady-state hematopoiesis.
Introduction
The hematopoietic stem cell (HSC) niche has a direct role in supporting hematopoietic engraftment and the potential for maintenance of normal hematopoiesis. Because of the critical nature of the hematopoietic microenvironment for the long-term HSC (LT-HSC) activity in the host, the ability to get HSCs into the niche efficiently has been the goal of clinical bone marrow (BM) transplantation since its first inception. With the aim of lifelong disease correction, this has classically been achieved through general nonspecific destruction of the BM cells and, along with them, some of the niche components, including stroma and vasculature. Common approaches rely on high-dose radiation or DNA alkylating agents, both of which are stem cell toxic because they target dividing and nondividing cells and have nonhematopoietic toxicities, especially of the gastrointestinal tract. The DNA damage ultimately kills quiescent host HSCs when they reenter the cell cycle and allows for donor engraftment. Defects in the repair of DNA damage by nonhomologous end joining in DNA ligase IV-deficient mice permits high levels of donor HSC engraftment in nonablated hosts1 by mimicking the effects of traditional conditioning. In contrast, drugs, such as 5-fluorouracil, which spare quiescent HSCs,2 do not effectively promote donor engraftment.
Murine preclinical studies as early as 1968 demonstrated that engraftment could be achieved without myeloablation using large cell doses.3,4 A series of studies using single or cumulative doses up to 2 × 108 cells in wild-type adult Balb/c recipients showed significant donor chimerism without ablation.5,,,,–10 Other groups also reported similar levels of engraftment.11 Studies in nonablated newborn mice with intravenous injections with a cumulative dose of 3 × 107 adult BM cells were able to show donor engraftment.12 However, fetal and newborn mice are generally more receptive to engraftment than adults. In adult mice, either local irradiation13 or intrafemoral injection14 of donor BM cells improved engraftment in the entire BM space and showed that homing was essentially independent of irradiation.
Although increasing the cell dose can overcome some barriers to HSC engraftment in experimental models, in the clinical setting repeated injections with large numbers of stem cells might not be feasible without safe and efficient methods for HSC expansion ex vivo. Although myeloablative conditioning is generally required for donor engraftment of BM HSCs, some genetically altered mice provide clues toward targeted approaches with less DNA damaging treatment. Mice with mutations at the W locus are very receptive to donor engraftment without the need for myeloablative conditioning. Recent studies have shown important roles for the cell surface receptor tyrosine kinases stem cell factor/c-Kit15 or thrombopoietin (TPO)/c-Mpl16,17 in maintaining HSC quiescence. Furthermore, proof of principle has been obtained that targeting these receptors can be manipulated to support donor HSC engraftment. Although cytokine/receptor signaling is now known to be important for maintaining HSC survival and occupancy within the BM niche, much less is known about downstream effectors of cytokines and integrins during HSC engraftment. In our studies of the JAK/STAT signaling pathway, neonatal Janus kinase 3 (JAK3)−/− mice were unable to be engrafted with HSCs in the absence of myeloablation.18 Similar to reports with neonatal severe combined immune deficient mice,19 we found only lymphoid engraftment but not myeloid. Our prior work in RAG2−/−20 and STAT6−/−21 mice showed that immune defects alone do not alter HSC repopulating potential.
Our previous studies have reported that one injection of 5 × 106 wild-type BM cells into unconditioned newborn signal transducer and activator of transcription 5 (STAT5)abΔN/ΔN mice resulted in high levels of donor engraftment.22 However, adult STAT5abΔN/ΔN mice were complicated by the development of an autoimmune disorder because of the residual T cells allowed by STAT5ΔN expression. Development of conditional knockout mice with floxed STAT5a/STAT5b alleles has provided a unique tool for examining the role of STAT5 in adult steady-state hematopoiesis without expression of complicating STAT5 ΔN isoforms. Here we used null alleles and conditional floxed alleles to test novel nonmyeloablative conditioning in adult hosts based solely on suppression of STAT5 in vivo and injection of 5 × 106 donor BM cells. STAT5 deletion in the host permitted high levels of donor engraftment of all hematopoietic lineages. Furthermore, conditional deletion of STAT5 permitted characterization of enriched HSC populations for cellular and molecular changes associated with reduced niche competition.
Methods
Mice
The C57BL/6 (CD45.2) mice and the congenic strains B6.SJL-PtprcaPep3b/BoyJ (BoyJ:CD45.1) were obtained from The Jackson Laboratory (Bar Harbor, ME) and housed in a specific pathogen-free environment. We have used 3 genetic models for STAT5 deficiency: STAT5abΔN/ΔN mice,23 true null STAT5abnull/null,24 and conditional knockout mice.24 STAT5abflox/+ mice were obtained from Lothar Hennighausen (National Institutes of Health, Bethesda, MD). Mx1-Cre mice were obtained from The Jackson Laboratory, and knockout mice included either Mx1-Cre/+STAT5abflox/flox or Mx1-Cre/+STAT5abflox/null genotypes. Gab2−/− mice were obtained from Toshio Hirano (Osaka University, Osaka, Japan). Wild-type control mice used for conditional deletion studies included Mx1-Cre/+, STAT5abflox/+, or STAT5abflox/flox genotypes. All mouse studies were approved by the institutional animal care and use committee at Case Western Reserve University (Cleveland, OH).
Treatment of mice and analysis of deletion efficiency
Adult 4- to 6-week-old mice were treated with 16 mg/kg poly(I)-poly(C) (pI:pC) on days 1, 3, and 5. On day 7, the treated mice were killed by cervical dislocation and c-Kit+Lin−Sca-1+ (KLS) cells were sorted from lineage-depleted whole BM. For some experiments as indicated, mice were treated every other day with 7 doses of pI:pC over 14 days and analyzed 1 to 5 months later. Deletion efficiency was determined by real-time polymerase chain reaction (PCR) either using DNA purified from sorted Gr-1+ cells or using total mRNA purified from sorted KLS cells.
Hematology and flow cytometry
Mouse hematology was determined using a HEMAVET 950 (Drew Scientific, Dallas, TX) and as described.22 Flow cytometry was performed on a BD LSRI or LSRII (BD Biosciences, San Jose, CA), and data were analyzed using WinList 5.0 software (Verity Software House, Topsham, ME) or FlowJo software (TreeStar, Ashland, OR).
Nonablated transplantation and measurement of engraftment
A total of 5 × 106 BM cells were injected via the lateral tail vein into nonconditioned recipients. Engraftment of the donor cells was determined by staining peripheral blood leukocytes with either fluorescein isothiocyanate (FITC)– or allophycocyanin (APC)–labeled antibody to CD45.2 or FITC- or phycoerythrin (PE)–labeled antibody to CD45.1. For the multilineage analyses, cells were stained with PE- or APC-conjugated Ly-6G/Gr-1, CD45R/B220, Ter119/Ly76, or CD4 (L3T4) antibodies. Mice receiving secondary transplantation were conditioned with 1100 cGy from a 137Cs source.
Analysis of HSC and progenitor populations
BM cells were harvested from the femurs and tibias. Red blood cells were lysed using a 0.3-M ammonium chloride solution. For the analysis of HSCs, BM cells were stained with PE-conjugated lineage markers (B220, Gr-1, Ter119, Mac-1, CD8, CD4, and NK1.1), anti–c-Kit-APC, anti–Sca-1-PE-Cy7, anti–Flk2-biotin, and subsequently with streptavidin (SAv)–peridinin chlorophyll protein. For some experiments, anti–CD34-FITC was used. For progenitor populations, BM cells were stained with biotin-conjugated lineage markers (B220, Gr-1, Ter119, Mac-1, CD5), anti–c-Kit-APC, anti–Sca-1-PE-Cy7, anti–IL7R-PE, anti–CD16/CD32-Alexa700, and anti–CD34-FITC. Fluorescence minus one was used for setting the gating on control samples.
BrdU staining and cell-cycle analysis of HSC
Two weeks after pI:pC treatment of age-matched wild-type and STAT5 knockout mice (10-12 weeks old), mice were given an intraperitoneal injection of 1 mg bromodeoxyuridine (BrdU; Sigma-Aldrich, St Louis, MO) per 6 g body weight and 1 mg/mL BrdU was added to the drinking water for 60 hours before isolation of BM for analysis. BrdU incorporation within gated KLS cells was evaluated using a BrdU intracellular staining kit (BD Biosciences PharMingen, San Diego, CA). Cell-cycle status within gated KLS cells was determined by Pyronin Y and Hoechst 33342 staining as described25 2 days or 4 months after pI:pC treatment. Anti–c-Kit-APC, anti–Sca-1-PE-Cy7, and biotin-conjugated antibodies to lineage markers subsequently stained with SAv-APC-Cy7 were used for KLS staining or biotin-conjugated CD34 subsequently stained with SAv-FITC or SAv-APC-Cy7 to detect CD34− KLS.
Apoptosis
BM cells were lineage depleted using a kit (Miltenyi Biotec, Auburn, CA) and stained with PE-labeled lineage antibodies (Gr-1, Mac-1, B220, Ter119, CD3, CD4, CD8), anti–c-Kit-APC or anti–c-Kit-APC-Cy7, anti–Sca-1-PE-Cy7, anti–CD34-APC, and anti–Flk2-biotin subsequently stained with SAv-peridinin chlorophyll protein. Apoptosis within defined Flk2− or CD34− KLS was determined by staining with anti–annexin V-FITC (BD Biosciences, San Jose, CA) and 4,6-diamino-2-phenylindole (DAPI; Invitrogen, Carlsbad, CA) as described26 2 days and 1 or 5 months after pI:pC treatment.
Quantitative PCR
Lineage-depleted BM cells from 2 days or 1 month after pI:pC treatment were stained with PE-conjugated lineage markers, anti–c-Kit-APC, anti–Sca-1-PE-Cy7, and sorted directly into 1 mL Trizol. Purified total RNA was treated with RNase-free DNase I for less than 10 minutes, and cDNA was prepared using SuperScript III First Strand Synthesis system for reverse-transcription (RT)-PCR (Invitrogen) and analyzed in triplicate with FastStart Universal SYBR Green Master (Roche Diagnostics, Indianapolis, IN). Real-time RT-PCR was performed using a 7500 FAST Real-time PCR system (Applied Biosystems, Foster City, CA). Specificity of products was confirmed by melting curve analysis and assessing band size in 2.5% agarose gels. Primers specific for STAT5, Tie-2, p57, p21, p27, Cyclin D1, Gfi-1, Bmi-1, CXCR4, CD44, and Myc were used and can be provided on request. Data were analyzed by 7500 Fast System Sequence Detection Software, version 1.3.1 (Applied Biosystems, Foster City, CA) (relative quantification [RQ] ΔΔCt study with analysis settings: WT samples as the calibrator sample, GAPDH as the endogenous control detector, and RQ minimum/maximum confidence set at 95%). Means are the average of RQ from 3 or 4 independent experiments, and SD is calculated by averaging the variances where n is the number of independent experiments.
Statistical analyses
Significance tests among groups was performed by 2-tailed Student t test or the Wilcoxon 2-sample test as indicated in the text (SPSS 16.0, SPSS, Chicago, IL).
Results
Host STAT5 dosage in adult mice controls donor HSC engraftment during nonablative transplantation
To determine whether STAT5 dosage impacts on the function of primitive hematopoietic cells in vivo, BM cells from mutant mice with one deleted or one hypomorphic STAT5abΔN allele were used as test cells in a 1:1 competitive repopulating assay. Both STAT5ab+/ΔN and STAT5ab+/null BM had a significant competitive disadvantage, resulting in a decreased contribution (2.3- and 2.8-fold) to peripheral blood leukocyte chimerism (Figure S1A, available on the Blood website; see the Supplemental Materials link at the top of the online article) and a similar decreased contribution to multilineage hematopoiesis (Figure S1B). These changes in repopulating activity were observed in the absence of a significant reduction in the LT-HSC number, survival, or cell-cycle status (Figure S1C-E). To determine whether decreased competitive repopulation in STAT5 mutant mice was sufficient to allow donor HSC engraftment without myeloablation, viable STAT5 mutant adult mice (CD45.2) as recipients were injected with 5 × 106 CD45.1+ or green fluorescent protein (GFP)–transgenic mouse BM cells, and donor engraftment was determined more than 16 weeks later. As expected, engraftment of control adult wild-type mice was only at background levels (Figure 1A). However, STAT5ab+/ΔN and STAT5ab+/null mice had low but very consistent engraftment with donor HSCs. The level of engraftment in STAT5ab+/null mice was significantly higher than undetectable levels in wild-type control mice (P = .01). Interestingly, greater depletion of STAT5 in STAT5abΔN/ΔN mice led to intermediate levels of engraftment. All STAT5abΔN/ΔN mice that survived (9 of 29) did not develop autoimmunity and were healthy. The 20 mice not shown all died before first analysis, resulting from the autoimmune perinatal lethality previously described.27 Rare STAT5abΔN/null mice were also obtained from the cross of STAT5ab+/ΔN X STAT5ab+/null mice for some experiments. The further deletions of STAT5 were associated with dramatic ability to be engrafted 16 weeks later (4 of 4) with HSC from 5 × 106 donor BM cells, achieving levels of 77% plus or minus 14% in the myeloid lineage (Figure 1B). The wild-type HSC engraftment was sufficient to permit long-term survival. These mice had profound declines in T and B lymphocytes, which were normalized after transplantation (Figure 1C), and the engraftment was maintained across multiple hematopoietic lineages in irradiated secondary transplanted hosts (Figure 1D).
Host STAT5 dosage in adult mice controls HSC engraftment during nonablative transplantation. Recipient mice (CD45.2) were transplanted with either 5 × 106 GFP-transgenic or CD45.1 BM cells under nonablative conditions. (A) Percentage of donor chimerism in each recipient mouse 16 weeks after transplantation. From 2 separate injection dates, wild-type (n = 10), STAT5ab+/ΔN (n = 8), STAT5ab+/null (n = 6), and from 10 separate injection dates (29 mice total), STAT5abΔN/ΔN (n = 9 surviving mice). (B) Percentage of donor-derived overall and Gr-1+, B220+, or CD4+ cells in each recipient mouse 16 to 24 weeks after transplantation. From 4 separate injection dates, wild-type (n = 4), STAT5abΔN/null (n = 4). (C) Peripheral blood hematology of each STAT5abΔN/null mouse before and 16 to 24 weeks after injection with donor BM cells. (D) Percentage of donor-derived Gr-1, B220, Ter119, or CD4 cells obtained in lethally irradiated secondary recipients. Two wild-type and 2 STAT5abΔN/null engrafted mice from panel B were used as donors for the secondary transplantation. Each donor was transplanted into 5 recipients. The representative dot plot from one secondary recipient is shown. Mean plus or minus SD values for all recipients are indicated above each plot. (E) E14.5 fetal liver cells from wild-type, STAT5abΔN/ΔN, or STAT5abnull/null CD45.2+ donors were transplanted into lethally irradiated CD45.1 recipients. Sixteen weeks later, the transplanted BM chimeras were challenged with 5 × 106 GFP-transgenic BM cells. The percentage of donor-derived (GFP+) overall and Gr-1, B220, and CD4 cells in each mouse was determined by flow cytometry 16 weeks later. The number of chimeras challenged from 2 separate injection dates were wild-type (n = 9), STAT5abΔN/ΔN (n = 7), and STAT5abnull/null (n = 5). For t tests relative to wild-type, P < .001.
Host STAT5 dosage in adult mice controls HSC engraftment during nonablative transplantation. Recipient mice (CD45.2) were transplanted with either 5 × 106 GFP-transgenic or CD45.1 BM cells under nonablative conditions. (A) Percentage of donor chimerism in each recipient mouse 16 weeks after transplantation. From 2 separate injection dates, wild-type (n = 10), STAT5ab+/ΔN (n = 8), STAT5ab+/null (n = 6), and from 10 separate injection dates (29 mice total), STAT5abΔN/ΔN (n = 9 surviving mice). (B) Percentage of donor-derived overall and Gr-1+, B220+, or CD4+ cells in each recipient mouse 16 to 24 weeks after transplantation. From 4 separate injection dates, wild-type (n = 4), STAT5abΔN/null (n = 4). (C) Peripheral blood hematology of each STAT5abΔN/null mouse before and 16 to 24 weeks after injection with donor BM cells. (D) Percentage of donor-derived Gr-1, B220, Ter119, or CD4 cells obtained in lethally irradiated secondary recipients. Two wild-type and 2 STAT5abΔN/null engrafted mice from panel B were used as donors for the secondary transplantation. Each donor was transplanted into 5 recipients. The representative dot plot from one secondary recipient is shown. Mean plus or minus SD values for all recipients are indicated above each plot. (E) E14.5 fetal liver cells from wild-type, STAT5abΔN/ΔN, or STAT5abnull/null CD45.2+ donors were transplanted into lethally irradiated CD45.1 recipients. Sixteen weeks later, the transplanted BM chimeras were challenged with 5 × 106 GFP-transgenic BM cells. The percentage of donor-derived (GFP+) overall and Gr-1, B220, and CD4 cells in each mouse was determined by flow cytometry 16 weeks later. The number of chimeras challenged from 2 separate injection dates were wild-type (n = 9), STAT5abΔN/ΔN (n = 7), and STAT5abnull/null (n = 5). For t tests relative to wild-type, P < .001.
To address whether the high level of donor engraftment was the result of a cell-intrinsic effect of STAT5 deletion or perhaps microenvironmental changes, further studies were performed after transplantation of STAT5abΔN/ΔN or STAT5abnull/null fetal liver (FL) cells into lethally irradiated hosts to generate FL chimeras. We have previously reported no change in HSC numbers for STAT5abΔN/ΔN FL,27 and we also found no change in absolute HSC numbers (c-Kit+Lin−Mac-1+Sca-1+CD48−CD150+) from STAT5abnull/null FL (+/+ 1260 ± 260, n = 3; Null/null 1290 ± 400, n = 6). Donor chimerism was high in the chimeras, except in lymphocytes as previously described20,28 and as shown in Figure S2. Therefore, FL chimeras were injected with GFP-transgenic BM cells and then analyzed to test whether the engraftment defects were cell intrinsic. Both STAT5abΔN/ΔN and STAT5abnull/null FL chimeras could be equivalently engrafted with wild-type donor GFP-transgenic HSC (Figure 1E), demonstrating that the HSC level defects were hematopoietic cell intrinsic and not mediated by the microenvironment. The residual T- and B-lymphoid (STAT5abΔN/ΔN) and T-lymphoid (STAT5abnull/null) chimerism in the primary hosts, as previously described,27,28 reduced the donor chimerism slightly in these mice relative to the Gr-1 population.
Improved engraftment in STAT5 heterozygote host mice by Gab2 deletion or immunologic tolerance to CD45 antigen
Because engraftment was still very low at the lower levels of STAT5 deletion, we next generated and characterized mice with combined deficiency in Grb2-associated binding protein-2 (Gab2) based on our observations that STAT5 and Gab2 genetically interact in hematopoiesis (G.L., K.D.B., manuscript in preparation). Using wild-type, STAT5ab+/null, Gab2−/−, and STAT5ab+/nullGab2−/− mice as recipients (CD45.2), 5 × 106 BoyJ (CD45.1) BM cells were injected to test whether there would be a benefit to the combined STAT5/Gab2 mutations. Because of the small sample size for STAT5ab+/null in this experiment, engraftment was not significantly different from wild-type. However, it should be noted that it was highly significant when all STAT5ab+/null mice injected from Figures 1A and 2A were considered together (P = .001; wild-type, n = 9; STAT5ab+null, n = 14; Wilcoxon test). In this very stringent nonablated model, STAT5ab+/nullGab2−/− mice (6 of 14 mice engrafted at 3%–54%) were more highly engrafted than STAT5ab+/nullGab2+/+ mice (3 of 9 mice engrafted at 2.7%-3.3%; Figure 2A). The higher levels of engraftment obtained in the double-mutant mice were consistent across multiple hematopoietic lineages and in secondary hosts (Figure 2B).
Improved engraftment in STAT5ab+/null host mice by Gab2 deletion during nonablative transplantation. Recipient mice (CD45.2) were transplanted with 5 × 106 CD45.1+ BM cells. (A) Percentage of donor chimerism (% CD45.1+ cells) in each recipient mouse 16 weeks after BM injection. From 2 separate injection dates, wild-type (n = 9), STAT5ab+/null (n = 8), Gab2−/− (n = 9), and from 5 separate injection dates STAT5ab+/nullGab2−/− (n = 14). The Wilcoxon 2 sample test was used for statistical analysis. Numbers 1 to 3 are mice that were further analyzed in panel B. (B) Representative dot plots of primary recipient mice as well as secondary transplanted recipients 16 weeks after BM injection and the percentage of donor-derived Gr-1, B220, Ter119, or CD4 cells are shown. In panel A, numbers 2 and 3 denote specific mice that were killed and BM analyzed by secondary transplantation (one donor into 5 recipients for Gab2−/−STAT5ab+/null), and average plus or minus SD values are indicated above each plot.
Improved engraftment in STAT5ab+/null host mice by Gab2 deletion during nonablative transplantation. Recipient mice (CD45.2) were transplanted with 5 × 106 CD45.1+ BM cells. (A) Percentage of donor chimerism (% CD45.1+ cells) in each recipient mouse 16 weeks after BM injection. From 2 separate injection dates, wild-type (n = 9), STAT5ab+/null (n = 8), Gab2−/− (n = 9), and from 5 separate injection dates STAT5ab+/nullGab2−/− (n = 14). The Wilcoxon 2 sample test was used for statistical analysis. Numbers 1 to 3 are mice that were further analyzed in panel B. (B) Representative dot plots of primary recipient mice as well as secondary transplanted recipients 16 weeks after BM injection and the percentage of donor-derived Gr-1, B220, Ter119, or CD4 cells are shown. In panel A, numbers 2 and 3 denote specific mice that were killed and BM analyzed by secondary transplantation (one donor into 5 recipients for Gab2−/−STAT5ab+/null), and average plus or minus SD values are indicated above each plot.
Because prior work has implicated CD45 as a minor antigen in nonablative HSC transplantation,29,30 we set out to determine whether this was impacting on engraftment in our system. We determined whether engraftment could be observed when the transplantation was performed using the opposite donor/host combination. CD45.1 background recipient mutant mice were generated and injected using the same approach. On the CD45.1 isogenic background, engraftment levels were again above the wild-type control at any time point examined up to 18 weeks later (Figure 3A). Interestingly, the levels of engraftment were higher than observed using CD45.2 C57BL/6 recipients (Figures 1A, 2A; P < .05), indicating that BoyJ mice were more receptive to C57BL/6 donor BM than vice versa. The engraftment was obtained in multiple hematopoietic lineages (Figure 3B) and maintained in lethally irradiated secondary hosts (Figure 3C). To test the immunologic issue further, we also generated STAT5ab+/null genotype on the F1 background by crossing STAT5ab+/null C57BL/6 (CD45.2) with BoyJ (CD45.1). These mice would be tolerant to either CD45.2 or CD45.1 grafts and thus immunologically unbiased toward the donor type in the engraftment readout. STAT5ab+/null F1 mice were engrafted to the highest levels relative to either the full C57BL/6 (P < .001) or the BoyJ (P < .001) background up to 25 weeks later (Figure 3D), and this engraftment was multilineage in primary (Figure 3E) and secondary hosts (Figure 3F).
Improved engraftment of STAT5ab+/null recipients on either the BoyJ (CD45.1) or F1 (CD45.1/CD45.2) background. (A,B) Wild-type or STAT5ab+/null mice on the CD45.1 background were injected with 5 × 106 CD45.2 BM cells. The numbers of mice injected from 3 separate injection dates were wild type (n = 8) and STAT5ab+/null (n = 8). The percentage of donor chimerism (% CD45.2+ cells) in each recipient mouse was determined 4, 8, and 18 weeks after transplantation. Multilineage analysis in Gr-1, B220, and CD4 cells was determined 18 weeks after transplantation on 4 of the wild-type and all of the mutant recipients. (C) A representative dot plot from the secondary transplantation is shown (one donor into 5 recipients). Data are from a pool of 3 wild-type or 3 STAT5ab+/null engrafted mice used as donors. (D,E) Recipient mice on the F1 background were transplanted with 5 × 106 CD45.1 BM cells on a single injection date. The percentage of donor chimerism (% CD45.1+CD45.2− cells) in each recipient mouse was determined 7, 19, and 25 weeks after transplantation for wild-type (n = 8) and STAT5ab+/null (n = 7) mice. One mouse died between 19 and 25 weeks. For multilineage analysis, the percentage of CD45.1+CD45.2− cells was determined for Gr-1, B220, or CD4 cells in each recipient mouse 25 weeks after transplantation. (F) Representative dot plot (gated first on Gr-1, B220, Ter119, or CD4) after secondary transplantation with a pool of 3 wild-type and 3 STAT5ab+/null engrafted mice (one donor into 5 recipients). Above each plot in panels C and F are mean plus or minus SD values of the percentage of donor chimerism from 5 recipients.
Improved engraftment of STAT5ab+/null recipients on either the BoyJ (CD45.1) or F1 (CD45.1/CD45.2) background. (A,B) Wild-type or STAT5ab+/null mice on the CD45.1 background were injected with 5 × 106 CD45.2 BM cells. The numbers of mice injected from 3 separate injection dates were wild type (n = 8) and STAT5ab+/null (n = 8). The percentage of donor chimerism (% CD45.2+ cells) in each recipient mouse was determined 4, 8, and 18 weeks after transplantation. Multilineage analysis in Gr-1, B220, and CD4 cells was determined 18 weeks after transplantation on 4 of the wild-type and all of the mutant recipients. (C) A representative dot plot from the secondary transplantation is shown (one donor into 5 recipients). Data are from a pool of 3 wild-type or 3 STAT5ab+/null engrafted mice used as donors. (D,E) Recipient mice on the F1 background were transplanted with 5 × 106 CD45.1 BM cells on a single injection date. The percentage of donor chimerism (% CD45.1+CD45.2− cells) in each recipient mouse was determined 7, 19, and 25 weeks after transplantation for wild-type (n = 8) and STAT5ab+/null (n = 7) mice. One mouse died between 19 and 25 weeks. For multilineage analysis, the percentage of CD45.1+CD45.2− cells was determined for Gr-1, B220, or CD4 cells in each recipient mouse 25 weeks after transplantation. (F) Representative dot plot (gated first on Gr-1, B220, Ter119, or CD4) after secondary transplantation with a pool of 3 wild-type and 3 STAT5ab+/null engrafted mice (one donor into 5 recipients). Above each plot in panels C and F are mean plus or minus SD values of the percentage of donor chimerism from 5 recipients.
Conditional deletion of STAT5 in adult host HSC permits donor engraftment
Because the STAT5 heterozygote mice could be modestly engrafted with donor HSCs, we moved on to conditional deletion of STAT5 to determine the effects of de novo loss of STAT5 in adult mice. Mx1-Cre/+STAT5abflox/+ mice were generated and crossed with STAT5ab+/null mice to yield Mx1-Cre/+STAT5abflox/null mice (referred to as knockout [KO]). Three doses of pI:pC treatment (days 1, 3, and 5) did not present toxicity and resulted in a high level deletion of the floxed STAT5ab locus in circulating Gr-1+ leukocytes either at day 7 or 4 months after the treatment determined by quantitative real-time PCR. The deletion efficiency in the HSC-enriched c-Kit+Lin−Sca-1+(KLS) fraction was also very high (Figure 4A). Two weeks after pI:pC treatment, loss of STAT5 caused a decline in peripheral blood hematology (Table S1). Four months after pI:pC treatment, circulating B220+, Ter119+, and NK1.1+ cells (Figure S3A) and Gr-1+ and B220+ cells were decreased in the BM (Figure S3B). High level deletion of STAT5 was compatible with long-term survival and was generally well tolerated. Notably, the CD4+ T-lineage remained approximately 50% undeleted, indicating a strong selection for the undeleted T-cell progenitors.
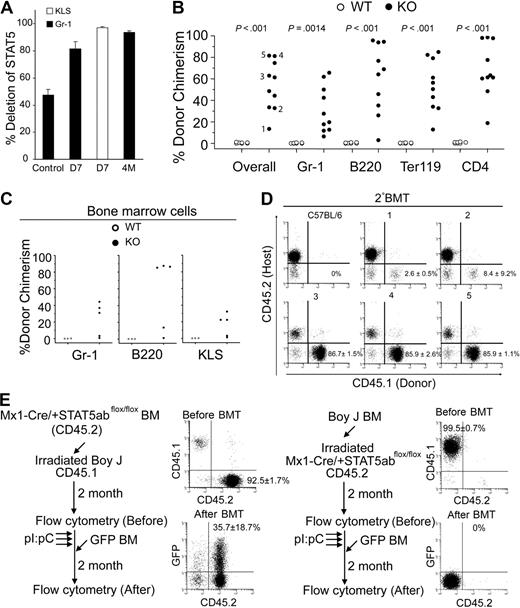
Conditional deletion of STAT5 in adult host HSCs, but not stroma, permits efficient stem cell replacement. (A) The percentage of deletion of STAT5 in Gr-1+ cells 7 days (D7) or 4 months (4M) after pI:pC treatment (3 doses, 16 mg/kg, every other day) was determined by real-time quantitative PCR (n = 5) normalized to wild-type STAT5 (■). The control is STAT5abflox/null mice, which should yield 50% the amount of wild-type STAT5. Deletion in the KLS pool on day 7 is also shown (□). (B) Percentage of CD45.1-derived overall and Gr-1, B220, Ter119, or CD4 cells in the peripheral blood of each recipient 22 to 40 weeks after transplantation into wild-type (n = 7) or STAT5 KO (n = 10) from 2 separate injection dates. (C) Percentage of CD45.1-derived Gr-1, B220, or c-Kit+Lin−Sca-1+ cells in BM from each recipient (1-5 in Figure 4B) 40 weeks after transplantation. (D) A representative dot plot from each secondary recipient mouse (nos. 1-5) 16 weeks after transplantation with one primary donor equivalent per 5 secondary recipients. Each engrafted mouse in panel C was transplanted into 3 lethally irradiated CD45.2 recipient, and the numbers shown are mean plus or minus SD values of the percentage of donor chimerism from all 3 recipients. (E) Experimental outline, representative fluorescence-activated cell sorting, and average donor chimerism (mean ± SD) from chimeric mice before and after transplantation with 5 × 106 GFP-transgenic BM cells.
Conditional deletion of STAT5 in adult host HSCs, but not stroma, permits efficient stem cell replacement. (A) The percentage of deletion of STAT5 in Gr-1+ cells 7 days (D7) or 4 months (4M) after pI:pC treatment (3 doses, 16 mg/kg, every other day) was determined by real-time quantitative PCR (n = 5) normalized to wild-type STAT5 (■). The control is STAT5abflox/null mice, which should yield 50% the amount of wild-type STAT5. Deletion in the KLS pool on day 7 is also shown (□). (B) Percentage of CD45.1-derived overall and Gr-1, B220, Ter119, or CD4 cells in the peripheral blood of each recipient 22 to 40 weeks after transplantation into wild-type (n = 7) or STAT5 KO (n = 10) from 2 separate injection dates. (C) Percentage of CD45.1-derived Gr-1, B220, or c-Kit+Lin−Sca-1+ cells in BM from each recipient (1-5 in Figure 4B) 40 weeks after transplantation. (D) A representative dot plot from each secondary recipient mouse (nos. 1-5) 16 weeks after transplantation with one primary donor equivalent per 5 secondary recipients. Each engrafted mouse in panel C was transplanted into 3 lethally irradiated CD45.2 recipient, and the numbers shown are mean plus or minus SD values of the percentage of donor chimerism from all 3 recipients. (E) Experimental outline, representative fluorescence-activated cell sorting, and average donor chimerism (mean ± SD) from chimeric mice before and after transplantation with 5 × 106 GFP-transgenic BM cells.
Because STAT5 was deleted efficiently in HSC-enriched BM fractions, we next investigated whether challenge of these mice with wild-type donor HSCs would achieve engraftment. When mice treated with pI:pC (days 1, 3, and 5) were injected on day 7, we found that treatment led to high level sustained multilineage engraftment of primary (22-40 weeks) and secondary hosts (16 weeks) after injection (Figure 4B-D). Mice were engrafted with donor Gr-1+, B220+, Ter119+, and CD4+ cells between 7% and 66%, 3% and 96%, 13% and 85%, or 18% and 99%, respectively. Interestingly, sorted Gr-1+ cells from host mice at day 7 or remaining at week 40 showed maintenance of deletion (Figure S4). Gr-1 chimerism paralleled BM chimerism as shown in Figure 4C where lymphoid and myeloid lineages were donor derived in primary as well as secondary C57BL/6 (CD45.2) hosts (Figure S5). The donor chimerism was also observed within the KLS fraction and at levels comparable with chimerism in Gr-1+ cells. These data demonstrate that STAT5 can be conditionally knocked out in transplant recipients, and this can permit sustained donor HSC reconstitution in the absence of myeloablative conditioning.
Because conditional deletion of STAT5 occurs in hematopoietic and nonhematopoietic cells, reciprocal chimera challenge experiments were performed. BoyJ BM cells (CD45.1) were transplanted into lethally irradiated Mx1-Cre/+STAT5abflox/flox mice or vice versa. Two months after transplantation, STAT5 was conditionally deleted and the BM chimeras were challenged with 5 × 106 GFP-transgenic BM cells. No evidence was observed for the nonhematopoietic STAT5 knockout host in the engraftment defects (Figure 4E). To determine whether the order of deletion/donor cell injection was critical for HSC engraftment in this model, experiments were also performed where the order was reversed. Importantly, injection of donor BM 1 day before the pI:pC regimen (and STAT5 deletion) did not permit donor HSC engraftment in knockout mice. Importantly, 12 weeks after the pI:pC treatment, these same mice could be engrafted with GFP-transgenic donor cells (CD45.2), which could be distinguished from the BoyJ (CD45.1)–positive cells that were injected before pI:pC treatment (Figure S6).
Conditional deletion of STAT5 gradually increases host HSC apoptosis and reduces pool size
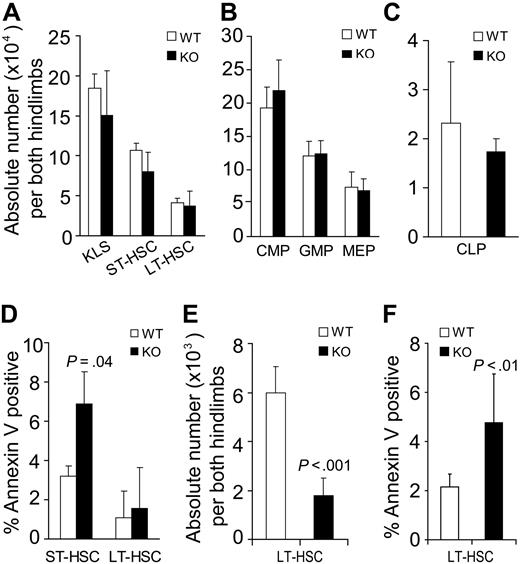
The defective steady-state engraftment ability of STAT5-deficient host HSCs suggested that HSC functional competition for the niche was impaired or that HSC number was being depleted. Flow cytometry analysis of the BM from STAT5 knockout mice was performed on day 7 after pI:pC treatment. ST-HSC (c-Kit+Lin−Sca-1+Flk2+) and LT-HSC (c-Kit+Lin−Sca-1+Flk2−) were present in the BM at normal number on day 7 after conditional deletion of STAT5 (Figure 5A). Further analysis of progenitor populations revealed that common lymphoid progenitor (CLP), common myeloid progenitor (CMP), granulocyte-macrophage progenitor (GMP), and megakaryocyte-erythroid progenitor (MEP) levels were normal (Figure 5B,C). Separate analyses for annexin V–positive cells within the short-term (ST)–HSC and LT-HSC fraction were performed after isolation of Lin− cells. Differences in survival of LT-HSC fraction were minimal, but moderately increased apoptosis was detected within the ST-HSC fraction (Figure 5D). Because the short-term effects of STAT5 deletion did not result in changes in LT-HSC survival or pool size, the long-term effects were examined. Three to 4 months later, for n = 11 in each group, the total white blood cell count per microliter had declined to 3252 plus or minus 738 relative to wild-type (8266 ± 1004). Similarly, the hematocrit declined to 38 plus or minus 2 relative to wild-type (50 ± 2). In the BM, the absolute number of LT-HSC (CD34−Flk2−KLS) was reduced 3.4-fold (Figure 5E), and the percentage of annexin V–positive cells within the LT-HSC (CD34−KLS) pool was increased 2.2-fold relative to wild-type control (Figure 5F).
Conditional deletion of STAT5 increases host HSC apoptosis and decreases HSC pool size. BM from STAT5 KO and littermates was assayed by multiparameter flow cytometry to quantitate the number of primitive BM fractions. BM was stained with antibodies against lineage markers, c-Kit, Sca-1, and Flk2 (A) or lineage markers, c-Kit, Sca-1, CD34, CD16/32, and IL-7R (B,C). Three separate experiments were performed with 3 to 5 mice per genotype compared. (A) The absolute number of primitive HSC populations in BM cells from both hind limbs. KLS cells were defined as c-Kit+Lin−Sca-1+ cells. ST-HSCs are identified as Flk2+KLS cells and LT-HSCs as identified as Flk2−KLS cells. (B) The absolute number of common myeloid progenitor (CD34+/lowCD16/32intLin−c-Kit+Sca-1−), granulocyte-macrophage progenitor (CD34+CD16/32+Lin−c-Kit+Sca-1−), and megakaryocyte-erythroid progenitor (CD34−CD16/32−Lin−c-Kit+Sca-1−) cells per both hind limbs are shown. (C) The absolute number of CLP was defined by IL-7R+Lin−Sca-1lowc-Kitlow phenotype and is shown per both hind limbs. (D) The proportion of annexin V–positive (DAPI-negative) cells within the ST-HSC and LT-HSC fractions is shown (n = 3). (E) Three to 4 months after pI:pC treatment (7 doses), the absolute number of LT-HSC defined both as CD34− and Flk2− KLS were analyzed from both hind limbs (n = 7). (F) One or 5 months after pI:pC treatment (7 doses), the percentage of annexin V–positive/DAPI-negative LT-HSCs (CD34− KLS) was analyzed for wild-type (n = 6) and KO (n = 7) mice.
Conditional deletion of STAT5 increases host HSC apoptosis and decreases HSC pool size. BM from STAT5 KO and littermates was assayed by multiparameter flow cytometry to quantitate the number of primitive BM fractions. BM was stained with antibodies against lineage markers, c-Kit, Sca-1, and Flk2 (A) or lineage markers, c-Kit, Sca-1, CD34, CD16/32, and IL-7R (B,C). Three separate experiments were performed with 3 to 5 mice per genotype compared. (A) The absolute number of primitive HSC populations in BM cells from both hind limbs. KLS cells were defined as c-Kit+Lin−Sca-1+ cells. ST-HSCs are identified as Flk2+KLS cells and LT-HSCs as identified as Flk2−KLS cells. (B) The absolute number of common myeloid progenitor (CD34+/lowCD16/32intLin−c-Kit+Sca-1−), granulocyte-macrophage progenitor (CD34+CD16/32+Lin−c-Kit+Sca-1−), and megakaryocyte-erythroid progenitor (CD34−CD16/32−Lin−c-Kit+Sca-1−) cells per both hind limbs are shown. (C) The absolute number of CLP was defined by IL-7R+Lin−Sca-1lowc-Kitlow phenotype and is shown per both hind limbs. (D) The proportion of annexin V–positive (DAPI-negative) cells within the ST-HSC and LT-HSC fractions is shown (n = 3). (E) Three to 4 months after pI:pC treatment (7 doses), the absolute number of LT-HSC defined both as CD34− and Flk2− KLS were analyzed from both hind limbs (n = 7). (F) One or 5 months after pI:pC treatment (7 doses), the percentage of annexin V–positive/DAPI-negative LT-HSCs (CD34− KLS) was analyzed for wild-type (n = 6) and KO (n = 7) mice.
Conditional deletion of STAT5 rapidly and persistently decreases the quiescent HSC fraction and expression of Tie-2 and p57
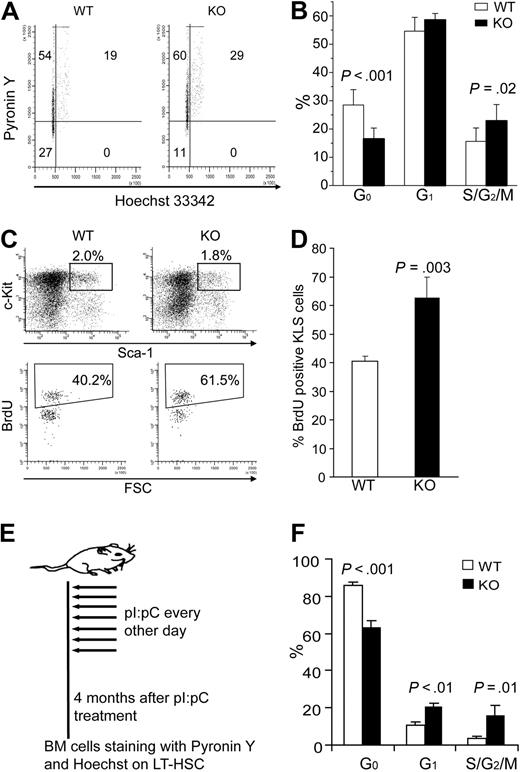
We next set out to determine whether the HSC quiescence or cell- cycle status was altered by the rapid loss of STAT5 from the HSC compartment. We used multiparameter flow cytometry on whole BM cells stained with Pyronin Y and Hoechst 33342 (Ho) to monitor RNA and DNA content, respectively. Quiescent HSCs have low Pyronin Y staining within the Holow population, corresponding to the G0 phase of cell cycle. Interestingly, STAT5 KO mice KLS cells had 16.5% plus or minus 3.8% of G0 phase, whereas wild-type control mice had 28.5% plus or minus 5.4% G0 (Figure 6A,B). Additional experiments using BrdU labeling confirmed that KLS cells from knockout mice were more actively cycling than wild-type controls (62.8% ± 7.0% vs 40.6% ± 1.9%; Figure 6C,D). To demonstrate that these effects were persistent, mice were analyzed 4 months after pI:pC treatment as shown (Figure 6E). In these mice, the CD34− KLS fraction was examined to analyze LT-HSC function; importantly, similar loss of quiescence was observed as well as significant increases in the G1 and S/G2/M fractions (Figure 6F).
Conditional deletion of STAT5 causes rapid and sustained loss of HSC quiescence. (A) Representative flow cytometry analysis of Pyronin Y and Hoechst 33342 staining on KLS cells. (B) The percentage of KLS cells in G0, G1, and S/G2/M phase is shown as mean plus or minus SD values from wild-type (n = 9) and KO (n = 6) analyses. (C) Wild-type or KO mice were injected intraperitoneally with a single dose of BrdU (as described in “BrdU staining and cell-cycle analysis of HSC”) and killed 3 days later. Representative flow cytometric analysis is shown for BrdU incorporation into gated KLS cells. (D) Bars represent the percentage of BrdU+ cells per KLS cells expressed as mean plus or minus SD values for n = 3 per group. P values are indicated when significant differences were observed between WT and KO groups. (E) The 7-dose pI:pC treatment regimen with treatments on alternating days for 2 weeks. These mice were analyzed 4 months later in LT-HSC subsets defined as CD34− KLS. (F) Mice that received 7 doses of pI:pC were analyzed for cell-cycle status by Hoechst/Pyronin Y staining. Shown are values for G0, G1, and S/G2/M fractions for both wild-type and KO mice (n = 3).
Conditional deletion of STAT5 causes rapid and sustained loss of HSC quiescence. (A) Representative flow cytometry analysis of Pyronin Y and Hoechst 33342 staining on KLS cells. (B) The percentage of KLS cells in G0, G1, and S/G2/M phase is shown as mean plus or minus SD values from wild-type (n = 9) and KO (n = 6) analyses. (C) Wild-type or KO mice were injected intraperitoneally with a single dose of BrdU (as described in “BrdU staining and cell-cycle analysis of HSC”) and killed 3 days later. Representative flow cytometric analysis is shown for BrdU incorporation into gated KLS cells. (D) Bars represent the percentage of BrdU+ cells per KLS cells expressed as mean plus or minus SD values for n = 3 per group. P values are indicated when significant differences were observed between WT and KO groups. (E) The 7-dose pI:pC treatment regimen with treatments on alternating days for 2 weeks. These mice were analyzed 4 months later in LT-HSC subsets defined as CD34− KLS. (F) Mice that received 7 doses of pI:pC were analyzed for cell-cycle status by Hoechst/Pyronin Y staining. Shown are values for G0, G1, and S/G2/M fractions for both wild-type and KO mice (n = 3).
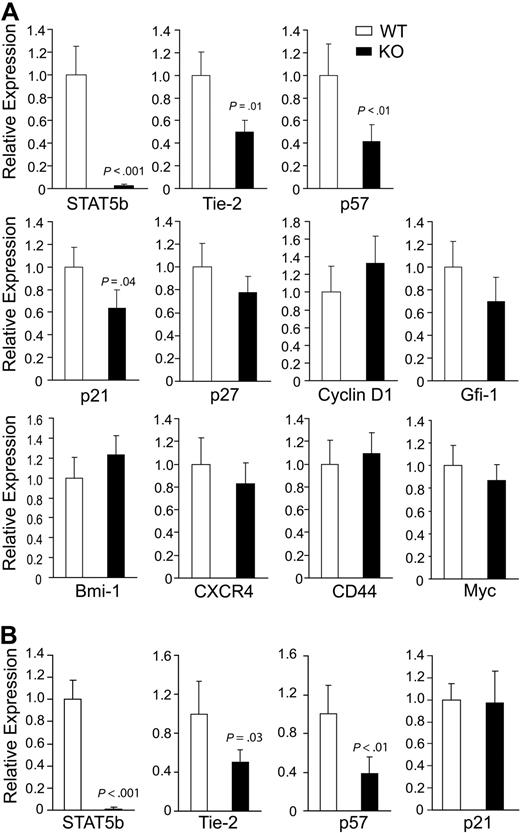
Because loss of quiescence was an observed phenotype of HSC after deletion of STAT5, we next examined KLS cells for expression of several HSC-related genes (Figure 7A). As a positive control, deletion of STAT5b was found to be reduced 35-fold. Interestingly, we found that 2 genes (Tie-2 and p57) previously described as TPO/c-Mpl regulated were down-regulated 2.0- and 2.4-fold, respectively, in STAT5-deficient KLS cells, indicating that STAT5 can be a key downstream transcription factor responsible for promoting TPO/c-Mpl–mediated HSC quiescence. Whereas p57 was found to lack conserved STAT5 binding sites (mouse/human), Tie-2 contains 2 conserved STAT5 binding sites that remain to be validated by chromatin immunoprecipitation in HSCs. Moderate down-regulation of the quiescence regulator p21 was observed. Importantly, deletion of STAT5b 100-fold was associated with reduction in Tie-2 and p57 mRNA, which was still observed 1 month after pI:pC treatment (Figure 7B). However, reduction in p21 expression was not maintained.
Conditional deletion of STAT5 causes rapid and sustained reduction in expression of quiescence-associated genes in HSCs. (A) BM was isolated from 4 independent pairs of wild-type and STAT5 KO mice, and KLS cells were sorted before mRNA isolation. Deletion of STAT5b is shown as a control. The relative mRNA expression levels of Tie-2, p57, p21, p27, Cyclin D1, Gfi-1, Bmi-1, CXCR4, CD44, and Myc were evaluated by quantitative real-time RT-PCR. Means from 3 or 4 independent experiments are shown with SD. (B) BM was isolated 1 month after 7 doses of pI:pC, and means from 3 independent experiments are shown for analysis of STAT5b, Tie-2, p57, and p21. From panels A and B, each batch of sorted KLS cells from WT and KO mice was analyzed by quantitative real-time PCR performed with the gene of interest and endogenous control GAPDH in triplicate.
Conditional deletion of STAT5 causes rapid and sustained reduction in expression of quiescence-associated genes in HSCs. (A) BM was isolated from 4 independent pairs of wild-type and STAT5 KO mice, and KLS cells were sorted before mRNA isolation. Deletion of STAT5b is shown as a control. The relative mRNA expression levels of Tie-2, p57, p21, p27, Cyclin D1, Gfi-1, Bmi-1, CXCR4, CD44, and Myc were evaluated by quantitative real-time RT-PCR. Means from 3 or 4 independent experiments are shown with SD. (B) BM was isolated 1 month after 7 doses of pI:pC, and means from 3 independent experiments are shown for analysis of STAT5b, Tie-2, p57, and p21. From panels A and B, each batch of sorted KLS cells from WT and KO mice was analyzed by quantitative real-time PCR performed with the gene of interest and endogenous control GAPDH in triplicate.
Discussion
Cytokines and their receptors control many aspects of HSC biology, especially their proliferation and survival within the supportive BM niche. Using modifications of the original technique by Ladd et al31 and application to mouse HSCs, recent studies have uncovered major roles for cell surface molecules, such as Tie-2,32 c-Kit,15 c-Mpl,16,17 and CXCR4,33 in promoting the quiescence of HSCs and their interaction with the niche. Quiescent HSCs are predominately the most active in hematopoietic repopulating activity, and maintenance of quiescence is important for self-renewal.34 Several intracellular signaling molecules have also been reported to influence HSC quiescence and long-term repopulating activity but not in the context of steady-state HSC engraftment in the nonablated setting. Although cyclin-dependent kinases and their inhibitors are associated with quiescent HSCs, the upstream regulation has not been clear. We specifically focused on STAT5 based on our prior studies of STAT5abΔN/ΔN mice.20 We report here that STAT5 is a major downstream signaling molecule capable of promoting maintenance of the quiescent HSC fraction and steady-state hematopoiesis.
The role of early-acting cytokine signaling in physiologic HSC quiescence is paradoxical, considering that cytokines promote proliferative responses in progenitor pools. STAT5-mediated gene expression is highly context dependent, requiring cell type-specific cofactors or receptors. In addition, the dosage of STAT5 activation dictates expression of certain targets,35 and hyperphosphorylation of STAT5 in leukemic cells results in Myc expression and cycling HSCs.36 Analyses of gene expression in STAT5-deleted HSCs revealed reduction in 2 important genes associated with HSC quiescence, Tie-237 and p57,38 and no changes in other selected genes associated with HSC niche interactions. Reduction in p21 expression was not maintained, which is consistent with no major role for p21 in steady-state hematopoiesis39 but a requirement during stress hematopoiesis.25 It is interesting that Tie-2 and p57 have been identified in HSCs as important for c-Mpl16,17 signaling. p57 is associated with quiescent BM side population cells38 and dormant HSCs before Mpl-mediated lipid raft reorganization and signaling.40 Because we have not yet been able to examine whether Tie-2 is a direct or indirect target gene regulated by STAT5, future chromatin immunoprecipitation studies on HSC fractions will be needed. Our prior studies showed comparable HSC level defects between STAT5abΔN/ΔN and c-Mpl−/− mice, which led to the conclusion that STAT5 and c-Mpl share overlapping activities in the HSC self-renewal processes.41 This report now extends the association to include HSC quiescence and gene expression at the molecular level. A recent report has also implicated the adapter protein Lnk in down-modulation of c-Mpl in HSC quiescence through interaction with JAK2.42 Therefore, the c-Mpl/JAK2/STAT5 signaling axis appears to be especially important for controlling HSC quiescence.
Depending on the degree of STAT5 deletion in our experimental models, injection of 5 × 106 BM cells could routinely lead to engraftment to very high levels in the nonablated host. This role parallels the known major role for STAT5 in competitive repopulating ability in lethally irradiated hosts and, in this study, the role in proliferation and survival of the HSC pool. In comparison, antibody-mediated depletion of c-Mpl required 5-fluorouracil treatment and achieved relatively small levels of engraftment (5%-6% overall donor chimerism using 10 000 KLS cells).16 However, deletion of all c-Mpl signaling affects multiple signaling pathways that may have antagonistic effects. Antibody targeting of c-Kit was very efficient at inducing HSC apoptosis, effectively clearing the niche for LT-HSC engraftment using high HSC doses.43 c-Kit targeting allowed 5000 HSCs to give 16% Gr-1+ donor chimerism using Rag2−/−CD45.1 mice and 35 000 HSCs to give an average of 78% Gr-1+ donor chimerism using Rag2−/−γC−/− mice. Other alternative conditioning regimens have been reported that involve mobilization using AMD3100,44 which was sufficient for chimerism at levels of 5% overall chimerism using 4 × 107 BM cells. Rac2−/− mice could be modestly engrafted45 at less than 5% overall using 2.5 × 107 cells, but this was associated with mobilization that was most profound when both Rac1/2 were deleted.46
An important observation of these studies is that STAT5 dosage regulates HSC competition. Even a 50% reduction in STAT5 was sufficient to allow significantly enhanced engraftment relative to wild-type control mice, suggesting that partial reduction in STAT5 activation in HSCs might be an effective adjuvant for improved HSC transplantation under nonmyeloablative conditions. In STAT5ab+/null hosts, engraftment occurred without changes in cell cycle status or LT-HSC number but with a selective advantage in multilineage development. Therefore, a dosage threshold effect occurs where 50% STAT5 is sufficient for maintenance of HSC quiescence. In contrast, a study using common γ-chain–deficient mice showed selection restricted to the CLP stage.47 Therefore, some engraftment can be achieved to fill vacated niches at any given time, but subsequent competition at the HSC or CLP level determines long-term donor chimerism. HSCs are capable of extensive transit and migration during the steady state. Therefore, it is still conceivable that HSC engraftment of donor HSCs could occur in alternative niches and then filter into the BM niche slowly over time, especially in a model with an inherent HSC defect. Engraftment of donor hematopoiesis was very rapid and detectable at 4 weeks (initially ∼ 20%; Figure S4B), which was the earliest time point examined; and when donor HSCs were injected 1 day before the pI:pC regimen, we were unable to obtain long-term repopulation of the STAT5-deficient hosts. Therefore, a rapid change in HSC competition permits wild-type HSC advantage followed by progressive vacancy of niches resulting from HSC apoptosis and filling of those niches with donor HSCs.
Because the STAT5 model used was based on deletion, it was also interesting to examine deletion efficiency before and after transplantation and correlate this with an engraftment time course (Figure S4). Host deletion was maintained 40 weeks after transplantation in the Gr-1+ host population, but yet the donor chimerism at this time point varied. Lower deletion efficiency at day 7 was more predictive of low engraftment, but higher deletion did not guarantee high engraftment. Similarly, in secondary recipients with transplantation, the lowest engrafted primary recipients showed Gr-1+ donor chimerism at approximately 1% when using typical stringent gates for high intensity Gr-1+ cells (neutrophils). However, it can be noted that approximately 13% engraftment was observed when gating on the intermediate intensity Gr-1+ cells (Figure S5) in secondary hosts, which probably reflects other myeloid cell types. Overall, these data are consistent with engraftment at limiting dilution of HSCs.
The goal of obtaining stable mixed chimerism is achievable in mouse allogeneic transplant models.48 Immune deficiency combined with a stem cell competition defect in conditional STAT5 knockout mice can be contrasted with stem cell–defective severe combined immune deficiency mice that have radiation sensitivity and thymic lymphomas. The main advantage of signal transduction molecules as targets for transplantation conditioning would be the expected lack of DNA damaging events. The CD45 congenic model has been reported to induce a minor allogeneic response in nonablated hosts.29,30 The potential for allograft response resulting from minor antigenic response to CD45.1 was addressed using F1 recipients, and we found that immune response was limiting to engraftment levels. More aggressive allogeneic transplantations have not yet been tested. Because STAT5abΔN/ΔN mice also develop autoimmune disease, it is probable that this inhibited higher level engraftment in mice where donor HSCs were engrafted before a critical stage of autoimmunity was established. These data provide insight toward transplantation for treating autoimmune diseases.
In conclusion, these studies demonstrate proof of principle that knockdown of STAT5 might be considered as an adjuvant for transplantation conditioning. The impact of STAT5 deletion was rapid and sustained, leading to changes in mRNA expression and quiescence that were related to HSC competitive engraftment ability. Because the short-term effects were not overly toxic and did not lead to rapid BM ablation, STAT5 deletion may permit donor HSC engraftment and recovery of blood counts with minimal side effects. A major conceptual advance of these studies is the demonstration that STAT5 maintains HSC quiescence during physiologic conditions. Further studies to address HSC localization and to define precisely how the balance between pro-proliferative and antiproliferative gene expression is modulated by STAT5 could provide key insight into the biology of HSC self-renewal.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Mike Sramkoski from the Case Comprehensive Cancer Center for assistance with flow cytometry using Hoechst 33342/Pyronin Y-stained bone marrow and Kristy Miskimen for assistance with identifying conserved STAT5-binding sites.
This work was supported by National Institutes of Health (R01HL073738 and R01DK059380) (K.D.B.), Center for Stem Cell and Regenerative Medicine, and the Flow Cytometry and Radiation Resources Core Facilities of the Case Comprehensive Cancer Center (P30CA43703).
National Institutes of Health
Authorship
Contribution: G.L. performed research and analyzed data; W.T. designed research; and Z.W. and K.D.B. performed research, designed research, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Kevin D. Bunting, Division of Hematology/Oncology, Department of Medicine, Case Western Reserve University, Cleveland, OH 44106; e-mail: kdb10@case.edu.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal