Abstract
Haploidentical hematopoietic stem cell transplantation provides an option for patients with advanced hematologic malignancies lacking a compatible donor. In this prospective phase 1/2 trial, we evaluated the role of reduced-intensity conditioning (RIC) followed by early add-backs of CD8-depleted donor lymphocyte infusions (DLIs). The RIC regimen consisted of thiotepa, fludarabine, cyclophosphamide, and 2 Gy total body irradiation. Twenty-eight patients with advanced lymphoproliferative diseases (n = 24) or acute myeloid leukemia (n = 4) were enrolled. Ex vivo and in vivo T-cell depletion was carried out by CD34+ cell selection and alemtuzumab treatment. The 2-year cumulative incidence of nonrelapse mortality was 26% and the 2-year overall survival (OS) was 44%, with a better outcome for patients with chemosensitive disease (OS, 75%). Overall, 54 CD8-depleted DLIs were administered to 23 patients (82%) at 3 different dose levels without loss of engraftment or acute toxicities. Overall, 6 of 23 patients (26%) developed grade II-IV graft-versus-host disease, mainly at dose level 2. In conclusion, our RIC regimen allowed a stable engraftment with a rather low nonrelapse mortality in poor-risk patients; OS is encouraging with some long-term remissions in lymphoid malignancies. CD8-depleted DLIs are feasible and promote the immune reconstitution.
Introduction
Allogeneic stem cell transplantation (SCT) is a curative option for several hematologic malignancies. However, its application is frequently limited by donor availability. Other sources of stem cells include matched unrelated donors, cord blood units, and haploidentical family donors. Haploidentical SCT was unsuccessful for many years because of graft rejection and high incidence of acute graft-versus-host disease (GVHD). The use of megadoses of highly purified CD34+, pioneered by the Perugia group, allowed a high engraftment rate with a limited incidence of acute GVHD.1,2 Sustained engraftment and rapid natural killer (NK)–cell reconstitution were also achieved using CD3/CD19-depleted grafts.3
In adults, the relevant nonrelapse mortality (NRM) and disease recurrence remain the major obstacles to the routine use of haploidentical SCT. NRM is mainly caused by infections resulting from delayed immune reconstitution in the first 12 months after SCT. Disease recurrence may be partly explained by the use of extensive T-cell depletion, which impairs the graft-versus-tumor effect.
Donor-versus-recipient NK alloreactivity has emerged as a crucial factor for the outcome of haploidentical SCT. Ruggeri et al showed a low relapse risk for patients with acute myeloid leukemia transplanted from NK-alloreactive donors.4 Because the chance of finding an alloreactive donor is approximately 40% to 50%, new strategies are required for patients lacking an alloreactive donor or affected by hematologic malignancies that are resistant to NK–cell cytotoxic activity.
Adoptive immunotherapy, using unmanipulated donor T cells from haploidentical family donors, is probably not feasible because of the extremely high risk of severe acute GVHD. Infusions of donor T cells, depleted of alloreactive lymphocytes by anti-CD25 immunotoxin or transduced with suicide genes, have been recently investigated to improve immune reconstitution.5,6
In human leukocyte antigen (HLA)–matched sibling transplantations, CD8 depletion of donor lymphocytes was effective to reduce the incidence and severity of acute GVHD while preserving the antitumor response.7-9 Nevertheless, contamination by residual CD8+ T cells was too high to allow its use in unrelated or haploidentical settings. In 2007, Meyer et al administered CD8-depleted donor lymphocyte infusions (DLIs) after unrelated SCT with an 18% incidence of GVHD. They obtained a very limited CD8+ T-cell contamination using the Miltenyi CliniMACS device (Miltenyi Biotec, Auburn, CA).10 In addition, Zheng et al have shown that CD4+ effector memory T cells could be added safely to T cell–depleted major histocompatibility complex–mismatched allograft in the murine model.11 These cells promoted both graft-versus-leukemia and immune reconstitution with far less GVHD than would be expected by unfractionated T cells or by naive CD4+ T cells.11
Here, we report the results of a phase 1/2 prospective trial in which haploidentical SCT using a reduced-intensity conditioning (RIC) regimen was followed by early add-backs of CD8-depleted DLIs.
Methods
Patient characteristics
Between January 2003 and January 2007, we conducted a prospective phase 1/2 trial. Twenty-eight patients received a transplantation from haploidentical family donors. Patients with advanced relapsed or refractory hematologic malignancies were eligible if they fulfilled the following criteria: (1) absence of HLA-related or unrelated donors and (2) a failed autologous SCT and/or a transplantation comorbidity score more than or equal to 2. Nineteen patients younger than 50 years came from a failed autologous SCT (n = 17) or high-dose chemotherapy (n = 2). Eleven had a SCT comorbidity index more than or equal to 2 according to Sorror et al.12
Family members were assessed for HLA compatibility by high-resolution molecular analysis (HLA-A,-B, -C, -DR, -DP, -DQ). Patients were screened for detection of anti-HLA antibodies by multiplex Luminex (BioSource International, Camarillo, CA) and excluded in case of positivity. Potential donor-versus-recipient NK-cell alloreactivity was analyzed according to missing expression of HLA-C groups 1 or 2 and of HLA-Bw4 alleles. The protocol was approved by the Institutional Review Board and Ethics Committee of the Fondazione Istituto di Ricovero e Cura a Carattere Scientifico Istituto Nazionale dei Tumori. All patients gave their written informed consent to participate in accordance with the Declaration of Helsinki. Patient and disease characteristics are summarized in Table 1. Median age was 38 years (range, 15-65 years). Only 13 patients were chemosensitive at the time of transplantation (n = 8 complete remission, n = 5 partial remission). The median time from diagnosis to transplantation was 29 months (range, 7-150 months). Twenty-one of 28 patients (75%) came from a failed autologous SCT. Twenty-three patients (82%) were at risk for cytomegalovirus (CMV) reactivation (CMV-seropositive recipient (R CMV+) receiving graft from CMV-seropositive [D CMV+] or -seronegative donors [D CMV−]).
Patient characteristics
| UPN . | Disease . | Age, y . | Previous therapy lines . | Previous auto-SCT . | Previous irradiation N/M . | Disease status at SCT . | KIR mismatch . | Donor . | HLA . |
|---|---|---|---|---|---|---|---|---|---|
| 001 | HL | 33 | 3 | 1 | Yes | PD | Sibling | haplo | |
| 002 | NHL/high-grade (DLCL-B) | 34 | 4 | 1 | Yes | PD | Sibling | haplo | |
| 003 | CLL/Richter | 56 | 5 | 0 | No | MR | Sibling | haplo | |
| 004 | AML | 57 | 1 | 0 | No | PD | Yes | Son | haplo |
| 005 | CLL/Richter | 35 | 1 | 1 | No | PR | Sibling | haplo | |
| 006 | NHL/high-grade (ALCL ALK+) | 21 | 3 | 1 | Yes | MR | Mother | Equal B and C | |
| 007 | NHL/high-grade (ALCL ALK+) | 15 | 5 | 1 | No | CR | Yes | Father | Equal A |
| 008 | NHL/high-grade (ALCL ALK−) | 44 | 3 | 1 | Yes | CR | Cousin | Equal B and C | |
| 009 | ALL | 53 | 3 | 0 | No | CR | Yes | Sibling | Equal A |
| 010 | HL | 29 | 2 | 1 | No | CR | Mother | Equal A | |
| 011 | MM (del cr 13) | 51 | 3 | 1 | No | PD | Sibling | haplo | |
| 012 | HL | 32 | 5 | 1 | Yes | MR | Father | haplo | |
| 013 | HL | 47 | 5 | 1 | Yes | PD | Mother | haplo | |
| 014 | AML t(8;21) | 41 | 3 | 0 | No | CR | Yes | Mother | haplo |
| 015 | HL | 19 | 6 | 1 | Yes | PD | Yes | Mother | haplo |
| 016 | CLL (del cr 17, unmutated) | 35 | 2 | 0 | No | PR | Sibling | Equal B and C | |
| 017 | CLL (del cr 17) | 62 | 2 | 1 | No | MR | Sibling | haplo | |
| 018 | NHL/high-grade (DLCL-B) | 31 | 4 | 1 | Yes | PD | Yes | Sibling | haplo |
| 019 | CLL (del cr17) | 65 | 5 | 1 | No | PD | Son | haplo | |
| 020 | HL | 19 | 3 | 1 | No | MR | Sibling | haplo | |
| 021 | HL | 24 | 5 | 1 | Yes | CR | Yes | Sibling | haplo |
| 022 | HL | 19 | 4 | 1 | Yes | PR | Yes | Father | haplo |
| 023 | MM (del cr 13) | 58 | 3 | 1 | No | CR | Son | haplo | |
| 024 | NHL/high-grade (AILD) | 48 | 3 | 1 | No | PR | Yes | Sibling | Equal A |
| 025 | HL | 31 | 3 | 1 | No | PR | Father | Equal C | |
| 026 | NHL/follicular lymphoma (grade III) | 49 | 4 | 1 | No | CR | Son | haplo | |
| 027 | NHL/high-grade (NK lymphoma) | 58 | 1 | 0 | No | CR | Yes | Cousin | haplo |
| 028 | AML | 63 | 2 | 0 | No | PD | Yes | Son | haplo |
| UPN . | Disease . | Age, y . | Previous therapy lines . | Previous auto-SCT . | Previous irradiation N/M . | Disease status at SCT . | KIR mismatch . | Donor . | HLA . |
|---|---|---|---|---|---|---|---|---|---|
| 001 | HL | 33 | 3 | 1 | Yes | PD | Sibling | haplo | |
| 002 | NHL/high-grade (DLCL-B) | 34 | 4 | 1 | Yes | PD | Sibling | haplo | |
| 003 | CLL/Richter | 56 | 5 | 0 | No | MR | Sibling | haplo | |
| 004 | AML | 57 | 1 | 0 | No | PD | Yes | Son | haplo |
| 005 | CLL/Richter | 35 | 1 | 1 | No | PR | Sibling | haplo | |
| 006 | NHL/high-grade (ALCL ALK+) | 21 | 3 | 1 | Yes | MR | Mother | Equal B and C | |
| 007 | NHL/high-grade (ALCL ALK+) | 15 | 5 | 1 | No | CR | Yes | Father | Equal A |
| 008 | NHL/high-grade (ALCL ALK−) | 44 | 3 | 1 | Yes | CR | Cousin | Equal B and C | |
| 009 | ALL | 53 | 3 | 0 | No | CR | Yes | Sibling | Equal A |
| 010 | HL | 29 | 2 | 1 | No | CR | Mother | Equal A | |
| 011 | MM (del cr 13) | 51 | 3 | 1 | No | PD | Sibling | haplo | |
| 012 | HL | 32 | 5 | 1 | Yes | MR | Father | haplo | |
| 013 | HL | 47 | 5 | 1 | Yes | PD | Mother | haplo | |
| 014 | AML t(8;21) | 41 | 3 | 0 | No | CR | Yes | Mother | haplo |
| 015 | HL | 19 | 6 | 1 | Yes | PD | Yes | Mother | haplo |
| 016 | CLL (del cr 17, unmutated) | 35 | 2 | 0 | No | PR | Sibling | Equal B and C | |
| 017 | CLL (del cr 17) | 62 | 2 | 1 | No | MR | Sibling | haplo | |
| 018 | NHL/high-grade (DLCL-B) | 31 | 4 | 1 | Yes | PD | Yes | Sibling | haplo |
| 019 | CLL (del cr17) | 65 | 5 | 1 | No | PD | Son | haplo | |
| 020 | HL | 19 | 3 | 1 | No | MR | Sibling | haplo | |
| 021 | HL | 24 | 5 | 1 | Yes | CR | Yes | Sibling | haplo |
| 022 | HL | 19 | 4 | 1 | Yes | PR | Yes | Father | haplo |
| 023 | MM (del cr 13) | 58 | 3 | 1 | No | CR | Son | haplo | |
| 024 | NHL/high-grade (AILD) | 48 | 3 | 1 | No | PR | Yes | Sibling | Equal A |
| 025 | HL | 31 | 3 | 1 | No | PR | Father | Equal C | |
| 026 | NHL/follicular lymphoma (grade III) | 49 | 4 | 1 | No | CR | Son | haplo | |
| 027 | NHL/high-grade (NK lymphoma) | 58 | 1 | 0 | No | CR | Yes | Cousin | haplo |
| 028 | AML | 63 | 2 | 0 | No | PD | Yes | Son | haplo |
SCT indicates stem cell transplantation; N/M, radiotherapy to neck and mediastinum; HL, Hodgkin lymphoma; NHL, non-Hodgkin lymphoma; DLCL-B, diffuse large cell lymphoma B; CLL, chronic lymphocytic leukemia; AML, acute myeloid leukemia; ALCL, anaplastic large cell lymphoma; ALK, anaplastic lymphoma kinase; ALL, acute lymphoblastic leukemia; MM, multiple myeloma; AILD, angioimmunoblastic T-cell lymphoma; PD, progressive disease; MR, minimal response; CR, complete remission; and PR, partial remission.
Conditioning regimen, stem cell mobilization, and supportive care
The preparative regimen included thiotepa (10 mg/kg) on day −7; cyclophosphamide (30 mg/kg) on days −6 and −5; and fludarabine (30 mg/ms) from day −6 to day −3, alemtuzumab (15 mg/ms) on day −2, and total body irradiation (2 Gy) on day −1. Donors received filgrastim 8 μg/kg twice daily beginning 4 days before leukapheresis. CD34+ cells were selected using the CliniMACS device (Miltenyi Biotec). The target doses of CD34+ and CD3+ T cells were 8 × 106/kg and 104/kg, respectively. No immunosuppressive therapy or posttransplantation granulocyte–colony stimulating factors were given. Patients were managed in laminar airflow rooms. Red cell and platelet transfusions were given according to institutional policy. Prophylaxis against viral and fungal infections consisted of high-dose acyclovir (500 mg/ms every 8 hours daily) and lyposomal amphotericine-B (1 mg/kg daily) from day −7 until the end of neutropenia. Prophylaxis after neutrophil recovery consisted in acyclovir, thrimethoprim-sulfamethoxazole, and itraconazole until recovery of CD4+ cells. Blood samples from patients were screened weekly for CMV and Epstein-Barr virus (EBV).
CD8-depleted DLIs
Donors collected lymphocytes 2 weeks before filgrastim mobilization. The depletion of CD8+ T cells was performed using CliniMACS CD8 MicroBeads and the CliniMACSplus device (Miltenyi Biotec) with a good manufacturing practice procedure. Patients received CD8-depleted DLIs after SCT if they had no signs of active GVHD or rapidly progressive disease. Dose level 1 consisted of 104/kg CD8-depleted T cells on days +45, +75, and +105. If one case of acute GVHD more than 2 occurred in this cohort, the DLI program would have been stopped. Dose level 2 consisted of 5 × 104/kg CD8-depleted T cells on days +45, +75, and +105. Dose reduction was required if 2 cases of acute GVHD more than 2 occurred at this level.
Chimerism analysis and immunophenotype analysis
Chimerism was assessed on DNA extracted from peripheral blood samples by multiplex fluorescent short-tandem repeat analysis (AmpFISTR Profiler Plus PCR kit; Applied Biosystems, Foster City, CA). Peripheral blood was collected in ethylenediaminetetraacetic acid for lymphocyte analysis at 1, 3, 4, 5, 6, 9, 12, and 18 months after haploidentical SCT. Direct 2- or 3-color flow cytometric immunophenotyping was performed to analyze lymphocyte subsets, including CD3−/CD19+, CD19+/IgD+, CD19+/IgD−, CD3−/CD16+/CD56+, CD3+/CD4+, CD3+/CD8+, CD4+/CD45RA+, CD4+/CD45RO+, and CD8+/CD11ahigh. CD45RA and CD45RO were purchased from BD Biosciences PharMingen (San Diego, CA); all remaining antibodies were obtained from BD Biosciences (San Jose, CA). Samples were analyzed using the FACSCalibur flow cytometer (BD Biosciences). Aliquots of CD8-depleted DLIs were used to examine CD25 expression with a phycoerythrin-conjugated murine anti–human CD25 monoclonal antibody (BD Biosciences). The frequency of CD25-expressing T cells was calculated by sequential gating of CD3+/CD4+ lymphocytes, then of CD4+/CD25+ (high), and finally of CD4+/CD25+ (intermediate) cells. Intracytoplasmatic staining for human FOX-P3 was performed using the anti-FOX-P3 staining kit (eBioscience, San Diego, CA) according to the manufacturer's instructions.
De novo cell-surface expression of the tumor necrosis factor receptor family member CD137 (4-1BB) identifies currently activated, but not resting, human alloreactive CD8+ T cells and can therefore be exploited to identify antigen-specific T cells.13 Peripheral blood mononuclear cells (PBMCs) isolated by centrifugation on a Ficoll density gradient were resuspended in RPMI 1640 (Sigma-Aldrich, St Louis, MO) supplemented with 5% human serum, penicillin/streptomycin, and 2 mM l-glutamine at a dilution of 107 cells/mL. For in vitro T-cell stimulation, 5 × 106 cells were plated in 48-well culture plate and left untreated or stimulated overnight with PepTivator CMV pp65, PepTivator EBV-BZLF1, or PepTivator EBV-EBNA-1 (peptide pools that consisting mainly of 15-mer peptides; Miltenyi Biotec), at a concentration of 0.6 nmol peptide/mL. For positive control, PBMCs were stimulated overnight with CytoStim (Miltenyi Biotec), an antibody-based reagent that acts similarly to a superantigen and causes activation of both CD4+ and CD8+ T cells. Subsequently, cells were stained with CD137-phycoerythrin, CD8-allophycocyanin, and CD4–peridinin chlorophyll protein. Comparison of immune-monitoring based on CD137 expression with the results based on interferon-γ production assay was performed and showed similar results (data not shown).
T-cell receptor and immunoglobulin heavy chain third complementarity-determining region spectratyping and T-cell receptor excision circle analysis
Heparinized blood samples were obtained from healthy controls and patients at various time points before and after SCT. PBMCs were isolated by Ficoll/Hypaque density gradient centrifugation, cryopreserved with 10% dimethyl sulfoxide, and stored until time of analysis. RNA was extracted from 10 × 106 PBMCs using the Trizol method (Invitrogen, Carlsbad, CA) and then reverse-transcribed into cDNA in a reaction primed with oligo(dT) 12-18 using the SuperScript III Reverse Transcriptase (Invitrogen). The polymerase chain reaction (PCR)–based method for analysis of the T-cell receptor (TCR) Vβ repertoire has been described previously.14,15 The fluorescence intensity of each band was depicted as a peak. TCR complexity score was assigned as described by Bomberger et al.16
Immunoglobulin heavy chain (IgH) third complementarity determining region (CDR3) genes were amplified by nested PCR. Briefly, PCR were set up using each of the consensus primers homologous to conserved regions within the variable gene segments (Vh1 consensus [5′-CAGGTGCAGCTGGTGCAGTCTG-3′] for amplification of VH1,VH3,VH5 and VH7 families; Vh2 consensus [5′-CAGGTCAGCTGCAGAGTCGG-3′] for amplification of VH4 and VH6 families; Vh2FS [5′-CAGTCACCTTGAAGGAGTCTG-3′] for amplification of the VH2 family) and a reverse primer designed on framework region 4 (FR4, JHD 5′-ACCTGGGAGACGGTGACCAGGGT-3′). In the second PCR, each of 7 primers located at 3′ of FR1 region (primers Vh1D to VH6D) was used in conjunction with a reverse primer labeled with a fluorescent dye (JHD nested FAM-5′-CATGGTCCCTTGGCCCCAG-3′). PCR products were analyzed as described for TCR spectratyping. Genomic DNA was extracted from 10 × 106 PBMCs with DNAzol BD reagent (Invitrogen) according to the manufacturer's instructions and used for the quantification of TCR excision circles (TRECs) by real-time quantitative PCR on an ABI7000 system (Applied Biosystems), as described previously.17
Endpoints of the study and statistical analysis
The primary endpoints of the study were engraftment and NRM at 1 year. The secondary endpoints were the incidence of grade II-IV acute GVHD before and after CD8-depleted DLIs; GVHD was assessed by consensus criteria.18,19 Overall survival (OS) and progression-free survival curves were estimated by the Kaplan-Meier method and compared using the log-rank test. Crude cumulative incidence curves of relapse and NRM were estimated in a competing risk framework.20 Comparative analysis between dose level 1 and dose level 3 was performed with Mann-Whitney test.
Results
Engraftment and chimerism
The stem cell target dose was achieved with a median of 2 leukophereses (range, 1-3). The median number of CD34+ and CD3+ cells infused was 10.5 × 106/kg (range, 1.5-15 × 106/kg) and 1 × 104/kg (range, 0.5-5 × 104/kg), respectively. Median CD34+ purity, using the Miltenyi Biotec device, was 95% (range, 81%–98%). The median number of CD19+ and CD56+ cells was 9.5 × 104/kg (range, 1-70 × 104/kg) and 1.1 × 104/kg (range, 0.2-32 × 104/kg), respectively. One donor was a poor mobilizer (final inoculum contained only 1.5 × 106/kg CD34+); then the recipient received a combination of peripheral blood and bone marrow cells achieving long-term engraftment. Potential donor-versus-recipient NK alloreactivity was detected in 11 patients (39%).
All patients had a sustained engraftment as defined by neutrophil counts more than 0.5 × 109/L and untransfused platelet counts more than 20 × 109/L for at least 3 consecutive days. The median time for an absolute neutrophil count of 0.5 × 109/L was 14 days (range, 8-18 days); median time for platelet count more than 20 × 109/L was 11 days (range, 8-17 days). One patient (4%) experienced secondary graft failure at day +150, but she did not receive a second SCT from a different donor because of rapidly progressive disease.
To avoid the binding of alemtuzumab to CD8-depleted DLIs, plasma levels were measured by enzyme-linked immunosorbent assay21 in the first 10 patients at day 30 after SCT: in only 2 patients was the drug detectable at a lympholytic level (≥ 0.1 μg/mL).
We assessed chimerism in peripheral blood for all patients at day +30: 26 (92%) had full donor hematopoiesis (> 95% donor cells) and 2 (8%) were mixed chimeras. All patients achieved full donor chimerism at 4 months. Lineage-specific analysis for chimerism was not performed because of the low number of circulating lymphocytes in the first months after SCT.
Toxicity, NRM, and infections
Six patients died of NRM at a median of 250 days after transplantation (range, 132-609 days). Causes of death were pneumonia (n = 3), posttransplantation lymphoproliferative disorder (PTLD) (n = 2), and intestinal chronic GVHD (n = 1). Five patients died in remission and were older than 45 years; 4 of them received the higher dose of CD8-depleted DLIs at day +45 (5 × 104/kg) and 1 received the lower dose. One patient died of pneumonia and never received CD8-depleted DLIs. The 1-year and 2-year NRM cumulative incidences were 15% and 26%, respectively (Figure 1). Only 2 of 6 patients died more than 1 year after transplantation.
Crude cumulative incidence curves of relapse and NRM for all the patients (n = 28), estimated in a competing risk framework.
Crude cumulative incidence curves of relapse and NRM for all the patients (n = 28), estimated in a competing risk framework.
Before CD8-depleted DLIs, only 2 of 28 patients (7%) developed grade II-IV de novo acute GVHD: one grade II and one grade III. Both these patients evolved to extensive GVHD: one died of pneumonia in complete remission and the other of progressive disease.
All patients experienced some infectious complications, which are summarized in Table 2. Despite the high incidence of CMV reactivation, no patients developed CMV disease. Five patients had EBV reactivation with a high viral load: 2 died of PTLD while receiving GVHD treatment (these 2 patients received CD8-depleted DLIs and in their donors the number of CD19+, achieved after CD8 depletion, were 12.3 × 106/kg and 8.3 × 106/kg, respectively, which was slightly higher than the median value).
Main infections after engraftment
| Infections . | No. (%) of patients . |
|---|---|
| Viral infections | |
| CMV reactivation | 23 (82) |
| CMV disease | 0 (0) |
| Herpes zoster | 5 (18) |
| EBV reactivation | 5 (18) |
| PTLD | 2 (7) |
| Parvovirus | 1 (4) |
| Fungal infections | |
| Lung–Aspergillosis | 1 (4) |
| Fungemia–Candida | 2 (7) |
| Bacterial infections | |
| Gram-positive infections | 3 (22) |
| Gram-negative infections | 12 (43) |
| Infections . | No. (%) of patients . |
|---|---|
| Viral infections | |
| CMV reactivation | 23 (82) |
| CMV disease | 0 (0) |
| Herpes zoster | 5 (18) |
| EBV reactivation | 5 (18) |
| PTLD | 2 (7) |
| Parvovirus | 1 (4) |
| Fungal infections | |
| Lung–Aspergillosis | 1 (4) |
| Fungemia–Candida | 2 (7) |
| Bacterial infections | |
| Gram-positive infections | 3 (22) |
| Gram-negative infections | 12 (43) |
CMV indicates cytomegalovirus; EBV, Epstein-Barr virus; and PTLD, posttransplant lymphoproliferative disorder.
Feasibility of CD8-depleted DLIs
Processing with CliniMACS CD8 MicroBeads was performed on 28 lymphophereses. The median CD4+ T-cell recovery was 84% (range, 31%-130%). CD8-depletion reduced the content of CD8+/CD3+ T cells by a median value of at least 3.3 log (range, 1.7-4). The median CD3+, CD56+/CD16+, and CD19+ cell recovery was 55% (range, 25%-91%), 50% (range, 17%-82%), and 68% (range, 23%-128%), respectively (Table 3). The median number of total CD3+, CD3+/CD4+, CD56+/CD16+, and CD19+ achieved after CD8 depletion was 18.6 × 106/kg, 17.4 × 106/kg, 3.2 × 106/kg, and 2.8 × 106/kg, respectively. We also analyzed the total amount of CD4+CD25+high T cells infused into each patient with CD8-depleted DLIs. Recipients experiencing GVHD did not receive a statistically different amount of CD4+CD25+high T cells (median, 0.26 × 104/kg; range, 0.01-1.13 × 104/kg vs 0.08 × 104/kg, range, 0.001-1.21 × 104/kg; P = .4). These CD4+CD25+high T cells were confirmed Fox P3–positive.
CD8 depletion: values before and after processing of mononuclear cells and lymphocyte subsets
| . | MNC × 106/kg . | CD3+ × 106/kg . | CD4+/CD3+ × 106/kg . | CD8+/CD3+ × 106/kg . | CD19+ × 106/kg . | CD56+CD16+ × 106/kg . |
|---|---|---|---|---|---|---|
| Median before (range) | 50.6 (28-141) | 31.4 (16-118) | 22.5 (11-83) | 11 (6-31) | 4.5 (2.5-11) | 5.8 (2-21) |
| Median after (range) | 25.5 (11-111) | 18.6 (8-61) | 17.4 (8-69) | 0.003 (0.001-0.200) | 2.8 (1.4-12.5) | 3.2 (0.5-12) |
| P | < .001 | < .001 | .01 | < .001 | .001 | < .001 |
| . | MNC × 106/kg . | CD3+ × 106/kg . | CD4+/CD3+ × 106/kg . | CD8+/CD3+ × 106/kg . | CD19+ × 106/kg . | CD56+CD16+ × 106/kg . |
|---|---|---|---|---|---|---|
| Median before (range) | 50.6 (28-141) | 31.4 (16-118) | 22.5 (11-83) | 11 (6-31) | 4.5 (2.5-11) | 5.8 (2-21) |
| Median after (range) | 25.5 (11-111) | 18.6 (8-61) | 17.4 (8-69) | 0.003 (0.001-0.200) | 2.8 (1.4-12.5) | 3.2 (0.5-12) |
| P | < .001 | < .001 | .01 | < .001 | .001 | < .001 |
MNC indicates mononuclear cells.
Twenty-three of 28 patients (82%) received CD8-depleted DLIs. The total number of infusions was 54, with a median of 2 per patient (range, 1-3). The median dose per patient was 6 × 104/kg (range, 2-15 × 104/kg). Five patients did not receive the scheduled DLIs because of acute GVHD (n = 2), early death for progressive disease (n = 2), and rapid immune reconstitution with T cells more than 1000/μL (n = 1). Interestingly, no systemic acute toxicity or loss of engraftment was observed after CD8-depleted DLIs.
No GVHD was observed in the first group of 4 patients (dose level 1) receiving 3 doses of 1 × 104/kg CD8-depleted T cells on days +45, +75, and +105. Because of the absence of major complications, dose escalation was started. Only 5 of 11 patients (45%) of the second group (dose level 2) completed the planned 3 doses of 5 × 104/kg CD8-depleted T cells because of GVHD (n = 4) or disease progression (n = 2). A patient developed GVHD after all the 3 planned doses. Because this dose level was found to be associated with an unacceptably high incidence of GVHD, the remaining patients (n = 8) received a deescalated schedule (104/kg day +45, followed by 5 × 104/kg on days +75 and +105; dose level 3): 6 patients completed all the infusions without any GVHD; one experienced skin grade 2 acute GVHD; one died of pneumonia and did not receive the day +105 infusion.
Overall, 6 of 23 patients (26%) developed acute GVHD (n = 4 grade II, n = 2 grade III/IV) after DLIs, requiring systemic immunosuppressive treatment. Acute GVHD resolved after a brief course of therapy in 2 of them. Chronic GVHD was observed in 4 patients (limited, n = 1; extensive, n = 3) and was de novo in only one patient; the limited form resolved after topical steroids.
Evaluation of T- and B-cell immune reconstitution
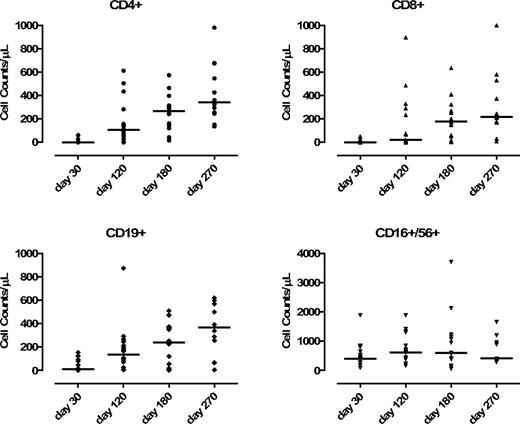
We observed a statistically significant increase of circulating CD3+/CD4+ T cells (median, 107/μL; range, 1-600/μL) at day +120 post-DLI, compared with day +30 (P = .002, unpaired t test) with a higher proportion of CD4+/CD45RO+/CD45RA− versus CD4+/CD45RO−/CD45RA+ T cells (88% vs 12%). At day +120, the expansion of CD3+/CD8+ was not significant (compared with the day +30 value) with a median value of 23/μL (range, 2-490/μL), which expressed the CD11ahigh memory/effector phenotype (P = .07). NK-cell levels did not change significantly before and after DLIs. CD19+ B cells increased from a median of 10 cells/μL (range, 2-100 cells/μL) at day +30 to 135 cells/μL at day +120 (range, 6-300 cells/μL) (P = .01; Figure 2). The median numbers of CD3+/CD4+, CD3+/CD8+, CD19+, and CD56+ cells were 187/μL, 120/μL, 186/μL, and 813/μL at day 150, respectively.
Kinetics of recovery of CD4+, CD8+, NK+, and CD19+ in the patients receiving CD8-depleted DLI (n = 23). Evaluation was performed before DLI (day −30) and after DLI (days +120, +180, and +270).
Kinetics of recovery of CD4+, CD8+, NK+, and CD19+ in the patients receiving CD8-depleted DLI (n = 23). Evaluation was performed before DLI (day −30) and after DLI (days +120, +180, and +270).
We performed a comparative analysis between the dose level 1 and 3 groups: the median value of circulating CD4+ T cells at day +120 was 57/μL versus 136/μL, respectively (P = .03). No statistically significant difference was observed in the subsequent time points. We observed a trend for a better immune reconstitution at dose level 3 also for CD19+ cells: the median value of circulating CD19+ at day +120 was 37/μL (dose level 1) versus 105/μL (dose level 3; P = .08). Of note, circulating T cells in the dose level 2 group were very low because of the immunosuppressive therapy with corticosteroids (5 patients had acute GVHD, thus preventing any comparison with the other dose levels).
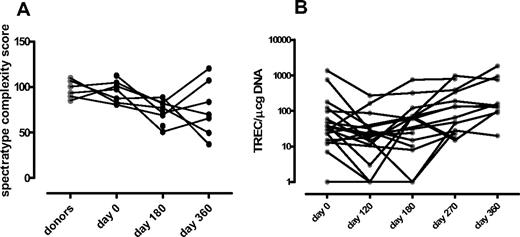
In 20 patients (72%), TREC counts per microgram (TRECs/μg) of DNA were determined by quantitative PCR in PB lymphocytes, with a median observation time of 360 days (range, 180-990 days). The median value of TREC/μg of DNA was 2.2 × 103 (range, 0.4-14 × 103) in 15 normal donors with the same median age. TREC counts were very low before SCT in the majority of patients (75%). Median baseline TREC values were low also in patients younger than 50 years (median value, 0.4 × 102). Ten of 20 (50%) patients showed measurable TREC at 1 year with a median value of 1.3 × 102 TREC/μg of DNA (range, 0.6-18 × 102; Figure 3). Nine of 10 patients with measurable TREC were younger than 50 years and are alive.
T-cell receptor spectratyping analysis and TREC evaluation. (A) T-cell receptor Vβ spectratyping (n = 10): median value of TCR Vβ spectratyping complexity score. (B) TREC evaluation in peripheral blood lymphocytes (n = 20): the short solid line in each group of data points represents the median value.
T-cell receptor spectratyping analysis and TREC evaluation. (A) T-cell receptor Vβ spectratyping (n = 10): median value of TCR Vβ spectratyping complexity score. (B) TREC evaluation in peripheral blood lymphocytes (n = 20): the short solid line in each group of data points represents the median value.
In 10 patients, TCR spectratyping was performed before and at days +180 and +360 after SCT. The TCR complexity score was evaluated as previously reported by Bomberger et al16 ; in a control group of 10 normal donors, the median TCR complexity was 98% (range, 85%-109%). Before conditioning, most patients had a Gaussian-like TCR-β repertoire pattern (median, 97% TCR complexity; range, 82%-112%). At 1 year after SCT, median TCR complexity was 70% (range, 49%–120%; Figure 3). All the patients showing posttransplantation thymopoiesis had a high TCR complexity score at 1 year after SCT.
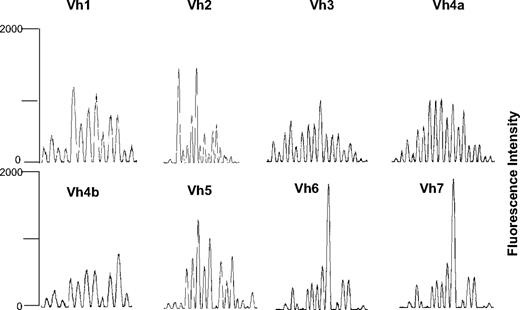
IgH CDR3 spectratyping has been analyzed in 9 patients: a partial return to a polyclonal repertoire at 1 year after SCT was observed. Median number peaks at day 360 in Vh1, Vh2, Vh3, Vh4a, Vh4b, Vh5, and Vh6 were 21, 16.5, 17, 15, 15.5, 17, 16.5, and 20 in normal donors and 11.5, 10.5, 12.5, 10.5, 8.5, 13, 7.5, and 16.5 in patients (Figures 4 and S1, available on the Blood website; see the Supplemental Materials link at the top of the online article). The majority of the patients achieved normal level of IgM at 6 months after SCT (median, 94 mg/dL; range, 27-230 mg/dL); only at 1 year after SCT, the majority of the patients had normal values of IgG and IgA: median, 746 mg/dL (range, 440-1500 mg/dL), median 78 mg/dL (range, 50-161 mg/dL).
Representative spectratyping of IgH CDR3 in a patient affected by multiple myeloma at day 360 after haploidentical SCT.
Representative spectratyping of IgH CDR3 in a patient affected by multiple myeloma at day 360 after haploidentical SCT.
CMV- and EBV-specific T cells after CD8-depleted lymphocyte infusions
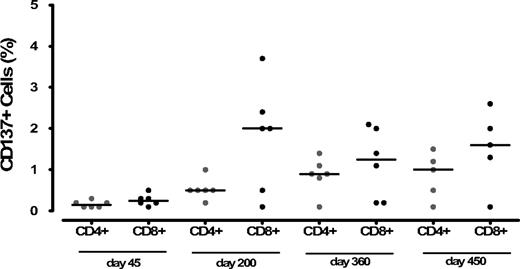
After SCT, we examined functional immune recovery in 6 patients of dose level 3, longitudinally from day +45 until day +450 (Figures 5, S2). To confirm the specificity of antigen-induced CD137 expression, we first analyzed PBMCs from CMV-negative (n = 5) and CMV-positive healthy donors (n = 3) stimulated with CMV peptides: the median frequencies of CMV peptide-reactive CD137+/CD8+ and CD137+/CD4+ T cells were 0.2% (range, 0.1%-0.4%) and 0.3% (range, 0.1%-0.6%), and 2.5% (range, 2.1%-3.5%) and 1.45% (range, 1%-2%), respectively. The median frequencies of CD137+/CD8+ and CD137/CD4+ T cells in donors after activation with CytoStim (Miltenyi Biotec), were 10.2% (range, 4%-15.2%) and 14% (range, 9%-15.8%), respectively. Responses to CytoStim (Miltenyi Biotec) were detected for both CD8+ and CD4+ T cells in all the patients analyzed (median CD137+/CD8+ and CD137+/CD4+ T cells were 4% [range, 1%-7%] and 4.5% [range, 1%-8.5%], respectively).
Percentage value of CD4+/CD137+ and CD8+/CD137+ after stimulation of patients' PBMCs with a CMVpp65 peptide pool at different time points after haploidentical stem cell transplantation. Positive and negative control ranges are given in the text.
Percentage value of CD4+/CD137+ and CD8+/CD137+ after stimulation of patients' PBMCs with a CMVpp65 peptide pool at different time points after haploidentical stem cell transplantation. Positive and negative control ranges are given in the text.
Before CD8-depleted DLIs, we did not observe any CMV response in all 6 patients analyzed. Only 2 of 3 CMV+ patients were allografted from CMV+ donors, and CD8 T-cell responses were observed after CD8-depleted DLIs: the frequencies were 2.4% and 2.0%, respectively, at day +200 after SCT. In 2 other patients (R CMV+/D CMV−), the frequency of CMV-specific CD8+ T cells was 3.7% and 2% at day +200. All the aforementioned patients did not experience further episodes of CMV reactivation. In the last patients analyzed (R CMV+/D CMV−) not experiencing CMV reactivation, CD137+/CD8+ and CD137+/CD4+ T cells were less than 0.5%. The expansion of CMV-specific CD8+ T cells in 4 of 6 patients exceeded the one of CD4+ T cells (median frequencies CD4+/CD137+, 0.9%, range, 0.1%-1.4%), although frequencies of CD4+/CD137+ T cells tended to increase progressively starting from day +200.
EBV-specific T cells (CD8+ and CD4+) against BZLF1 and EBNA1 antigens were evaluated in the same 6 patients from day +100 to day +450. All patients received allografts from EBV+ donors. EBV-specific responses were detected in 4 of 6 of them: the median frequencies of BZLF1- and EBNA1-specific CD137+/CD8+ T cells were 2.1% (range, 0.5%-5%) and 1.5% (range, 0.3%-2.5%), respectively. Only 2 patients showed an early response to BZLF1 at day +200: 1 patient showed a concomitant expansion of CD4+ and CD8+, the other one showed an expansion of CD4+ only (Figure S3).
OS, progression-free survival, and disease response to CD8-depleted lymphocytes
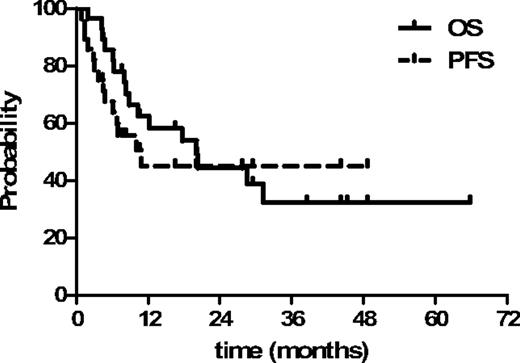
At a median follow-up of 28 months (range, 6-65 months), 12 patients (43%) were alive and 16 died of disease (n = 10) or NRM (n = 6). Nine of 12 patients were in complete remission (n = 1 chronic lymphocytic leukemia [CLL], n = 2 HL, n = 3 aggressive lymphomas of T phenotype, n = 1 follicular cell lymphoma, n = 1 multiple myeloma, and n = 1 acute myeloid leukemia; Table 4). The estimated 2-year OS and progression-free survival were 44% (95% confidence interval, 26%-62%) and 45% (95% confidence interval, 25%-63%), respectively (Figure 6). Patients with chemosensitive disease had a better outcome: 2-year OS 75% versus 20% of the chemorefractory group (P < .004). Disease relapse or progression occurred in 14 of 28 (50%) patients at median time of 4 months after SCT (range, 1-11 months), causing death in 10 of them (n = 4 aggressive NHL, n = 1 CLL/Richter, n = 4 HL, n = 1 multiple myeloma). Four patients with refractory disease (n = 1 acute myeloid leukemia, n = 2 HL, n = 1 CLL) responded to CD8-depleted DLIs: all these patients achieved complete remission concomitant to a GVHD flare (median duration of response, 14 months; range, 7-19 months), but unfortunately they died because of disease progression (n = 1) or toxicity (n = 3).
Patient outcomes
| UPN . | Status at SCT . | Day of relapse . | OS . | Day* . | Cause of death . |
|---|---|---|---|---|---|
| 001 | PD | +114 | Alive/PD | 1973 | — |
| 002 | PD | +60 | Dead | 189 | NHL |
| 003 | MR | +205 | Dead | 856 | CLL |
| 004 | PD | No | Dead | 609 | PTLD |
| 005 | PR | No | Alive/CR | 1462 | — |
| 006 | MR | +42 | Dead | 264 | NHL |
| 007 | CR | +209 | Alive/CR | 1359 | — |
| 008 | CR | No | Alive/CR | 1325 | — |
| 009 | CR | No | Dead | 187 | PTLD |
| 010 | CR | +87 | Dead | 367 | HL |
| 011 | PD | +92 | Dead | 144 | MM |
| 012 | MR | +187 | Dead | 530 | HL |
| 013 | PD | No | Dead | 241 | Pneumonia |
| 014 | CR | No | Alive/CR | 884 | — |
| 015 | PD | +144 | Dead | 310 | HL |
| 016 | PR | +302 | Alive/PR | 1158 | — |
| 017 | MR | No | Dead | 253 | Pneumonia |
| 018 | PD | +28 | Dead | 130 | NHL |
| 019 | PD | No | Dead | 603 | GVHD |
| 020 | MR | +326 | Dead | 936 | HL |
| 021 | CR | No | Alive/CR | 833 | — |
| 022 | MR | No | Dead | 132 | Pneumonia |
| 023 | CR | No | Alive/CR | 607 | — |
| 024 | PR | +38 | Dead | 60 | NHL |
| 025 | PR | No | Alive/CR | 495 | — |
| 026 | CR | No | Alive/CR | 370 | — |
| 027 | PR | No | Alive/CR | 280 | — |
| 028 | PD | +137 | Alive/PD | 145 | — |
| UPN . | Status at SCT . | Day of relapse . | OS . | Day* . | Cause of death . |
|---|---|---|---|---|---|
| 001 | PD | +114 | Alive/PD | 1973 | — |
| 002 | PD | +60 | Dead | 189 | NHL |
| 003 | MR | +205 | Dead | 856 | CLL |
| 004 | PD | No | Dead | 609 | PTLD |
| 005 | PR | No | Alive/CR | 1462 | — |
| 006 | MR | +42 | Dead | 264 | NHL |
| 007 | CR | +209 | Alive/CR | 1359 | — |
| 008 | CR | No | Alive/CR | 1325 | — |
| 009 | CR | No | Dead | 187 | PTLD |
| 010 | CR | +87 | Dead | 367 | HL |
| 011 | PD | +92 | Dead | 144 | MM |
| 012 | MR | +187 | Dead | 530 | HL |
| 013 | PD | No | Dead | 241 | Pneumonia |
| 014 | CR | No | Alive/CR | 884 | — |
| 015 | PD | +144 | Dead | 310 | HL |
| 016 | PR | +302 | Alive/PR | 1158 | — |
| 017 | MR | No | Dead | 253 | Pneumonia |
| 018 | PD | +28 | Dead | 130 | NHL |
| 019 | PD | No | Dead | 603 | GVHD |
| 020 | MR | +326 | Dead | 936 | HL |
| 021 | CR | No | Alive/CR | 833 | — |
| 022 | MR | No | Dead | 132 | Pneumonia |
| 023 | CR | No | Alive/CR | 607 | — |
| 024 | PR | +38 | Dead | 60 | NHL |
| 025 | PR | No | Alive/CR | 495 | — |
| 026 | CR | No | Alive/CR | 370 | — |
| 027 | PR | No | Alive/CR | 280 | — |
| 028 | PD | +137 | Alive/PD | 145 | — |
SCT indicates stem cell transplantation; PD, progressive disease; MR, minimal response; CR, complete remission; PR, partial remission; NHL, non-Hodgkin lymphoma; CLL, chronic lymphocytic leukemia; PTLD, posttransplantation lymphoproliferative disorder; HL, Hodgkin lymphoma; MM, multiple myeloma; GVHD, graft-versus-host disease; and —, not applicable.
Day of last follow-up.
Kaplan-Meier plot of estimated progression-free survival and overall survival for all the patients.
Kaplan-Meier plot of estimated progression-free survival and overall survival for all the patients.
Discussion
In this prospective phase 1/2 trial, we showed that (1) RIC regimen allows a stable engraftment of haploidentical stem cells with a limited incidence of acute GVHD; (2) younger patients with chemosensitive lymphoid malignancies can attain a long-term disease control; and (3) CD8-depleted DLIs are feasible and promote immune reconstitution.
Myeloablative haploidentical SCT with megadoses of CD34+ cells and no GVHD prophylaxis, pioneered by the Perugia group,1,2 had some limitations, including graft failures, an NRM rate ranging from 25% to 40%, and the occurrence of organ toxicity related to high-dose conditioning in poor-risk patients. For such reasons, the use of RIC regimens and the new methods for graft manipulation became an appealing area of investigation. Rizzieri et al22 used a RIC regimen, unmanipulated grafts, and GVHD prophylaxis, reporting a 63% 1-year OS in standard-risk patients. More recently, a strategy including a RIC regimen followed by CD3/CD19-depleted grafts has been proposed3,23 as an alternative option for haploidentical transplantations. Despite the rapid and sustained engraftment and the accelerated immune reconstitution, a relevant incidence of acute GVHD was reported.
Although the escalation of the stem cell dose played a crucial role to favor a high engraftment rate, graft failures ranging from 5% to 14% have been frequently reported after haploidentical SCT.1,2,22 In our study, only one secondary graft failure was observed; probably the pretransplantation screening for anti-HLA antibodies has limited this complication. Indeed, the detection of donor-specific HLA class I and II antibodies was associated with engraftment failure in unrelated transplantations.24 Despite the inclusion of poor-risk advanced malignancies, an encouraging 26% NRM rate and 44% OS at 2 years were observed in our study. Interestingly, late NRM was restricted only to 2 patients dying 1 year after SCT. Possible explanations for the promising clinical results are (1) RIC could have reduced acute organ toxicity and thymic damage; (2) drugs used in the conditioning regimen as well as alemtuzumab had a significant antitumor activity; and (3) low-dose alemtuzumab allowed the use of early DLI add-backs.
The occurrence of defective immune recovery after haploidentical SCT was associated with a high risk of severe infections, which heavily affected morbidity and mortality. Nonetheless, adoptive immunotherapy was investigated mostly in children who have a functional thymus and lower incidence of GVHD compared with adults. Amrolia et al demonstrated that the infusion of low-dose donor T cells, depleted of alloreactive lymphocytes, significantly increased CD4+ and CD8+ T cells, accelerating CMV- and EBV-specific immunity in the first 4 months after SCT.5 In adults, data concerning the use of T-cell add-backs are very limited. Perruccio et al described the immune recovery in myeloablative haploidentical SCT, indicating that (1) CD4+ cell counts were inferior to 100/μL and 200/μL at 3 and 6 months, respectively; and (2) pathogen specific T-cell responses occurred after 9 months.25 They also evaluated the infusion of nonalloreactive clones specific for CMV and Aspergillus and demonstrated a rapid development of T-cell responses against these pathogens. Such an approach was effective but expensive and labor intensive, and the effect on global immune reconstitution was unclear. Recently, Rizzieri et al investigated immune reconstitution in adults receiving RIC and alemtuzumab followed by haploidentical SCT.22 Only a minority of patients received adoptive immunotherapy, and the time to achieve more than 200 CD3+/CD4+ T cells/μL was 6 months.22
We report the first experience on the use of CD8-depleted DLIs after RIC haploidentical SCT. First, CD8-depleted DLIs did not induce loss of engraftment or other acute organ toxicities. Overall, a 26% incidence of acute GVHD was observed. However, our trial was designed to explore different dose levels of DLIs; and in dose level 3, only 1 of 8 patients had GVHD. Although the overall incidence of GVHD is higher than in the Perugia studies, our NRM is similar.
After CD8-depleted DLIs, we observed (1) an early and significant increase of CD4+ T cells and CD19+ B cells; and (2) a significant increase of CD4+ T cells at dose level 3 compared with dose level 1. At dose level 2, 5 of 11 patients developed acute GVHD, thus precluding any comparative analysis of immune recovery with the other 2 cohorts.
Dose level 3 was giving the best results in terms of immune reconstitution and toxicity; thus, functional analyses were performed and demonstrated a recovery of CMV- and EBV-specific CD4+ and CD8+ T cells starting at +200 days after SCT. The unexpected expansion of CD19+ cells prompted us to analyze the B-lymphocyte repertoire. A partial recovery of B-cell repertoire at 1 year after SCT was observed. In addition, the majority of patients achieved normal values of IgG and IgA within 1 year.
Five patients experienced EBV reactivations, and 2 died of PTLD while on treatment for GVHD. Both had low levels of circulating CD8+ T cells at the time of infection. Such an incidence of EBV reactivation might be the result of the use of low-dose alemtuzumab unable to kill all the B cells in the graft. In addition, no association between the number of CD19+ cells infused with DLIs and occurrence of EBV reactivation was found.
In the clinical setting, haploidentical SCT has been usually employed for salvage of acute myeloid leukemias, and few data on its use in lymphomas are available. Sykes et al26 reported a very small study in which 2 of 5 lymphoma patients with refractory disease had a clinical response. In addition, Burroughs et al,27 in patients with Hodgkin lymphoma, obtained an encouraging 60% progression-free survival. Our study reports the data on a larger number of lymphoma patients and shows that those with chemosensitive disease had a better outcome and could experience long-term remissions. Larger prospective trials are required before considering this promising therapeutic option as a routine salvage strategy for advanced hematologic malignancies.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported in part by grants from Associazione Italiana Ricerca sul Cancro (Milano, Italy), FIRST University of Milano, Ministero dell'Università e della Ricerca Scientifica (Rome, Italy), Ministero della Salute (Rome, Italy), and Michelangelo Foundation for Advances in Cancer Research and Treatment (Milano, Italy).
Authorship
Contribution: A.D. analyzed and interpreted the data and wrote the paper; C.C. performed experiments, analyzed data, and revised the paper; A.R., A.V., and S.D.T. performed experiments; L.F., F.S., and C.C.-S. included data of patients treated; M.M. and P.L. performed cell separation and immunophenotype analysis; L.G. performed radiotherapy; C.L. performed HLA analysis; and P.C. conceived the study, interpreted the data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Paolo Corradini, Division of Hematology and Bone Marrow Transplantation, Fondazione Istituto di Ricovero e Cura a Carattere Scientifico Istituto Nazionale dei Tumori, Via Venezian 1, 20133 Milano, Italy; e-mail: paolo.corradini@unimi.it.
References
Author notes
*A.D. and C.C. contributed equally to the final version of the paper.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal