Abstract
The peculiar site of development of primary effusion lymphoma (PEL) highlights a specific role of body cavities in the pathogenesis of this neoplasia. We used a xenograft murine model of PEL to characterize the contribution of the host microenvironment to PEL growth. The activity of a murine (ie, host-specific) interferon-α1 (IFN-α1)–expressing lentiviral vector (mIFN-α1-LV) was compared with that of a human (h) IFN-α2b-LV. LVs efficiently delivered the transgene to PEL cells and conferred long-term transgene expression in vitro and in vivo. Treatment of PEL-injected severe combined immunodeficiency mice with hIFN-α2b-LV significantly prolonged mice survival and reduced ascites development. Interestingly, mIFN-α1-LV showed an antineoplastic activity comparable with that observed with hIFN-α2b-LV. As mIFN-α1 retained species-restricted activity in vitro, it probably acted in vivo on the intracavitary murine milieu. mIFN-α1–treated murine mesothelial cells were found to express tumor necrosis factor–related apoptosis-inducing ligand and to significantly trigger apoptosis of cocultured PEL cells in a tumor necrosis factor–related apoptosis-inducing ligand-dependent manner. These data suggest that the interaction between lymphomatous and mesothelial cells lining the body cavities may play a key role in PEL growth control and also indicate that the specific targeting of microenvironment may impair PEL development.
Introduction
Primary effusion lymphoma (PEL) is an aggressive human herpesvirus 8 (HHV8)–driven non-Hodgkin lymphoma that arises in serous body cavities with a peculiar proliferative pattern characterized by liquid-phase growth.1 PEL occurs in immunocompromised persons and more frequently in AIDS patients. All PEL patients feature poor survival; they rarely respond to conventional chemotherapy and have short life expectancy after diagnosis.2 PEL cells are usually monoclonal and of B-cell origin, as shown by immunoglobulin gene rearrangements; they express a “null” lymphocyte phenotype, as B-cell and T-cell markers are generally absent, while expressing several activation moieties and the characteristic CD138/syndecan-1. These features suggest that these cells probably represent a preterminal stage of B-cell differentiation close to that of plasma cells.2 HHV8 was also recently detected in lymphoma cases presenting as tissue masses in human immunodeficiency virus type 1–infected patients,2 as well as in polyclonal effusions in patients with multicentric Castleman disease and/or Kaposi sarcoma,3 thus indicating that the spectrum of HHV8-associated lymphoproliferative disorders may be wider than initially defined.
A status of chronic inflammation, common in many steps of tumorigenesis, appears to be involved in PEL initiation, with consequent increased levels of proinflammatory cytokines that may be responsible for activation of mesothelia, which in turn secrete chemokines and express adhesion molecules and inflammatory enzymes.4 This environment may support homing of HHV8-infected lymphocytes and their growth in liquid-phase within serous cavities. The interplay between proangiogenic molecules and autocrine and paracrine growth factors of cellular and viral origin might be then required to promote cell proliferation and the transition from a polyclonal to a monoclonal cell population. In this scenario, whereas the association between HHV8 and PEL development is widely accepted, mechanisms involved in the tropism of HHV8-infected lymphocytes to body cavities and in PEL progression, as well as the role of the host microenvironment, remain to be fully clarified.
Interferons (IFNs) are multifunctional regulatory cytokines that exert pleiotropic biologic activities, including antiproliferative, antiviral, immunomodulatory, and angiostatic effects.5,6 IFN-α up-regulates tumor necrosis factor–related apoptosis-inducing ligand (TRAIL), causing apoptosis in PEL-derived cell lines, and this phenomenon is markedly increased by the concomitant presence of azidothymidine, which sensitizes PEL cells to TRAIL-mediated apoptosis.7,8 This combination therapy was used with success in one patient8 and was found to increase mean survival time in a PEL preclinical model.9 Furthermore, the combined use of IFN-α and arsenic trioxide was found to induce caspase-dependent apoptosis in PEL cell lines.10
The intracavitary localization of PEL renders this tumor suitable for local treatment, such as gene therapy, which allows for the prolonged expression of a specific agent at high local concentration with minimal systemic side effects. This approach was used to examine the specific contribution of the host microenvironment in severe combined immunodeficiency (SCID) mice intraperitoneally injected with PEL-derived cells. This xenograft model was used to analyze the therapeutic activity of a lentiviral vector (LV) expressing the human IFN-α2b (hIFN-α2b-LV) and to specifically target the peritoneal host microenvironment using a murine (ie, host-specific) IFN-α1–expressing LV (mIFN-α1-LV).
Methods
Cell lines
The Epstein-Barr virus−/HHV8+ CRO-AP/3 PEL–derived cell line (kindly provided by A. Carbone, Department of Pathology, Istituto Nazionale Tumori, Milan, Italy),11 used to set up the preclinical model of PEL and for the in vitro transduction experiments, was grown in RPMI 1640 (Sigma-Aldrich, Munich, Germany) supplemented with 10% fetal calf serum (Life Technologies, Foster City, CA) and 2 mM l-glutamine (complete medium). The human embryonal kidney epithelial HEK 293A and 293T cell lines were used for titration experiments and as packaging cell line, respectively. 293T cells were also used to generate the native murine and human IFN-α. The murine MBL-2 leukemia cell line was used in in vitro experiments as control. These cell lines were grown in Dulbecco modified Eagle medium (Sigma-Aldrich) supplemented with 10% fetal calf serum and 2 mM l-glutamine. All cell lines were mycoplasma-free as resulting from periodical polymerase chain reaction (PCR) check.
Mice
Six-week-old female SCID/CB17 mice were purchased from Charles River Breeding Laboratories (Portage, MI), housed under specific pathogen–free conditions in the BL3 containment laboratory in our animal facility, and allowed to acclimate to local conditions for 1 week. Procedures involving animals and their care were in conformity with institutional guidelines that comply with national and international laws and policies (European Economic Community Council [EECC] Directive 86/609, OJ L 358, December 12, 1987; National Institutes of Health Guide for the Care and Use of Laboratory Animals, National Institutes of Health Publication 85-23, 1985). The project was evaluated and approved by the Italian Ministry of Health, Department of Food, Nutrition and Veterinary Public Health.
Isolation of peritoneal mesothelial cells
Mesothelial cells were isolated as reported previously.12 Briefly, the parietal peritoneum was excised under sterile conditions from normal 7-week-old SCID mice and from PEL-injected treated and untreated animals when they were culled. Fragments were washed with RPMI and incubated in 4 mL of 0.25% trypsin (Invitrogen) for 30 minutes. The cell suspension was then passed through nylon mesh and centrifuged for 5 minutes at 200g. Cells were washed twice, suspended in complete medium, and placed in flasks or 6-well culture plates. A monolayer of polygonal mesothelial cells was usually visible after 4 to 5 days of culture.
Primary mesothelial cells were exposed to 250 and 500 IU/mL “native” (ie, expressed from the transgene) mIFN-α1, in parallel with equivalent amounts of mock supernatants (from enhanced green fluorescence protein [EGFP]–LV–transduced cells) to assess the possible interference of cellular factors. CRO-AP/3 cells were exposed in parallel to the same amounts of native mIFN-α1. Cells were washed twice with RPMI medium 48 hours later, and RNA was extracted as previously reported13 to analyze the expression of IFN-stimulated genes.
To assess apoptosis induction in CRO-AP/3 cells, mesothelial cells were treated with 250 and 500 IU/mL native mIFN-α1 or with equivalent amounts of mock supernatants for 24 hours, then washed and cocultured with CRO-AP/3 cells. To evaluate TRAIL involvement in apoptosis induction, cocultures were performed by adding an anti–mouse TRAIL monoclonal antibody (mAb) or an isotype control (6 μg/mL, clone N2B2, low-endotoxin azide-free [LEAF]–purified anti–mouse TRAIL, or LEAF-purified isotype control; BioLegend, San Diego, CA). CRO-AP/3 cells were exposed in parallel to the same amounts of native murine cytokine or mock supernatants. Apoptosis was analyzed 12, 24, and 48 hours after coculture with mock- or mIFN-α1–treated mesothelial cells or after exposure to mIFN-α1.
LVs and in vitro transduction experiments
The self-inactivating, HIV-1–derived LVs expressing EGFP, human IFN-α2b, and murine IFN-α1 (denominated EGFP-LV, hIFN-α2b-LV, and mIFN-α1-LV, respectively) were generated, concentrated, and quantified for infectious particles and p24 vector equivalents as described previously.14,15 CRO-AP/3 cells (106 cells) were transduced with hIFN-α2b-LV or mIFN-α1-LV and, as control, EGFP-LV, at different (2, 10, and 25) multiplicities of infections (MOI) in 12-well culture plates for 12 hours, then washed twice, suspended in complete medium, and cultured in flasks. Mock-transduced CRO-AP/3 cells were set up in parallel in each transduction experiment. In vitro gene transfer efficiency and transgene expression were analyzed periodically for 30 days by assaying hIFN-α2b or mIFN-α1 release in culture supernatants by enzyme-linked immunosorbent assay (ELISA) and by measuring the percentage of EGFP+ cells in flow cytometry.
Quantitative assays for recombinant and native IFN-α and generation of the native cytokines
Recombinant IFN-α used for cell treatments were obtained from Schering Plough (Kenilworth, NJ; hIFN-α2b), and from PBL Biomedical Laboratories (PBL InterferonSource, Piscataway, NJ; mIFN-α1). To generate native hIFN-α2b or mIFN-α1, 293T cells were transduced with the respective IFN-expressing LVs using an MOI of 2, and amounts of each cytokine released in culture supernatants were assessed by ELISA. Mock supernatants (from EGFP-LV–transduced cells) were generated and used in parallel in subsequent experiments. ELISA assays (for hIFN-α2b: Human IFN-α ELISA kit, BioSource International, Camarillo, CA; mIFN-α1: Verikine Mouse Interferon Alpha, Mu-IFN-α, ELISA Kit, PBL Biomedical Laboratories) were used to quantify the amounts of human or murine IFN-α released in culture supernatants of LV-transduced CRO-AP/3 cells, of LV-transduced 293T cells, and of ex vivo cultures of CRO-AP/3 cells obtained from malignant ascites, peritoneal washings, and masses, and to measure the levels of human or murine IFN-α in ascitic fluids or peritoneal washings of treated animals. A total of 10 pg IFN-α is considered equivalent to 1 IU cytokine.
Assessment of engraftment and tumorigenesis of CRO-AP/3 cells in SCID mice
To characterize the ability of CRO-AP/3 cells to engraft, SCID mice were intraperitoneally injected with 2-fold increasing doses (from 6.25 × 106 to 50 × 106) of logarithmically growing CRO-AP/3 cells (3 mice/dose) and were examined periodically for the presence of ascites and/or solid tumors. In this pilot experiment, all mice injected with the highest dose developed consistent ascites in 9 to 12 days, and this dose (50 × 106 cells) was subsequently used in gene therapy experiments. At this dose, CRO-AP/3 cells efficiently engrafted in the peritoneal cavity of SCID mice after intraperitoneally inoculation, with the onset of liquid-phase tumor in usually 6 to 10 days from PEL cell inoculum, and the development of a consistent ascites generally within the same interval of days. The gross morphology of PEL/SCID mice was concomitant with a significant increase in body weight (20.58 ± 1.02 g vs 26.06 ± 1.6 g, P < .001) and abdominal distension. For ethical reasons, tumor-bearing animals were killed when presenting signs of suffering, and each mouse was considered as dead from tumor progression on this date.
In vivo gene therapy experiments
CRO-AP/3 cells were washed twice with RPMI medium, suspended at a final density of 108 cells/mL, and 0.5 mL of this cell suspension was injected intraperitoneally into each mouse. Fifteen or 16 mice were used in each in vivo experiment. Animals were divided into 3 groups: 2 control groups (receiving medium alone or EGFP-LV) and 1 receiving the “therapeutic” (hIFN-α2b-LV or mIFN-α1-LV) treatment. At 2, 4, and 7 days after intraperitoneal inoculation of PEL cells, each mouse received a total of 1 μg p24 vector equivalents in 0.9 mL RPMI medium (0.333 μg/300 μL per injection), which corresponded to 108 transducing units and to a theoretical MOI of 2. Control animals received an equivalent volume of medium or equivalent amounts of EGFP-LV. Sixteen mice were used to assess the antineoplastic activity of hIFN-α2b-LV, and this experiment was repeated twice. In this set of experiments, 4 animals received medium alone, 6 mice received control EGFP-LV, and 6 mice hIFN-α2b-LV. To examine the activity of mIFN-α1-LV, 15 mice were used (5 animals per group), and this experiment was repeated twice. Mice were monitored daily for increase in body mass, abdominal distension, and subcutaneous tumors, and survival studies were performed. At necroscopy, ascites were collected, measured as fluid volumes, and centrifuged for 10 minutes at 200g. Supernatants were centrifuged for 30 minutes at 2800g to eliminate cell debris, aliquoted, and stored at −80°C. Cell pellets were washed twice, used for flow cytometry analyses, and/or to set up ex vivo cultures. Peritoneal washings of ascites-negative animals were performed using 5 mL RPMI medium and processed as malignant effusions. Masses were minced, passed through nylon mesh, and the obtained cell suspension was used to measure ex vivo IFN-α production and/or perform flow cytometry analyses. When sufficient cells were available, 106 cells recovered from ascites, peritoneal washings, or masses were placed in 1 mL complete medium in a T12 plate and IFN-α measurement was done on supernatants collected after 72 hours.
Details about other techniques and reagents are reported in Document S1 (available on the Blood website; see the Supplemental Materials link at the top of the online article).
Results
Antiproliferative and proapoptotic effects of hIFN-α2b
The aim of this study was to compare the efficacy of human and murine IFN-α in a preclinical model of PEL to dissect the specific contribution of tumor cells from that of the host microenvironment. To this end, we preliminarily assessed whether hIFN-α2b expressed from the transgene retained proper functional activity in vitro in the PEL cell line used in the SCID mouse model. CRO-AP/3 cells were transduced with hIFN-α2b-LV, mIFN-α1-LV, or a control EGFP-LV at final MOIs of 2, 10, and 25. Reverse transcriptase (RT)–PCR analyses showed selective and robust expression of the 2′,5′-oligoadenylate synthetase (2′-5′ OAS) gene, a biomarker of IFN activity, only in hIFN-α2b-LV–transduced cells (Figure 1A), indicating that the transgene activated a classic IFN-inducible pathway. Proliferation of hIFN-α2b-LV–transduced cells, analyzed by [3H]-thymidine uptake, was reduced compared with EGFP-expressing cells (Figure 1B) or mIFN-α1-LV–transduced cells (data not shown), with a consistent, MOI-dependent impairment of cell viability, as assessed by trypan blue dye exclusion (not shown). Apoptosis was analyzed by flow cytometry after staining with annexin V/propidium iodide (PI). hIFN-α2b-LV–transduced cells showed a 2- to 3-fold increase in apoptotic cells compared with mock-, EGFP-LV–, and mIFN-α1-LV–transduced CRO-AP/3 cells (Figure 1C; and data not shown). Therefore, the human cytokine expressed from the transgene exerted a dose-dependent antiproliferative effect as well as a proapoptotic activity in CRO-AP/3 cells.
In vitro biologic activity of hIFN-α2b. (A) Activation of a classic IFN-inducible pathway. A multiplex RT-PCR analysis with primers specific for β-actin and 2′-5′oligoadenylate synthetase (2′-5′ OAS) performed using RNA extracted from hIFN-α2b-LV–transduced CRO-AP/3 cells at different MOI and from mock-transduced and EGFP-LV–transduced (MOI 25) cells 4 and 10 days after transduction showed the activation of 2′-5′ OAS expression only in hIFN-α2b-LV–transduced cells, indicating the specific activation of an IFN-inducible pathway. Lanes: M, 1-kb DNA ladder marker (Invitrogen); 1, water control; 2,7, RNA extracted from mock-transduced CRO-AP/3 cells 4 and 10 days after transduction; 3, RNA from EGFP-LV–transduced CRO-AP/3 cells (MOI 25) extracted 4 days after transduction; 4 to 6, RNA from CRO-AP/3 cells 4 days after transduction with the hIFN-α2b-LV using 3 MOIs (2, 10, and 25, respectively); 8 to 10, RNA from CRO-AP/3 cells 10 days after transduction with the hIFN-α2b-LV using 3 MOI. (B) Impaired proliferation of hIFN-α2b-LV–transduced CRO-AP/3 cells. [3H]-Thymidine incorporation was measured 10 days after transduction for 4 days, and a MOI-dependent reduction in cell proliferation was observed. Data are reported as ratio between the mean of triplicates of LV-transduced cells and the mean of triplicates of control EGFP-LV–transduced cells for each analyzed time point, and the SD of the ratio was calculated according to the theory of error propagation [σa/b = a/b√(σa/a)2 + (σb/b)2]. (C) Apoptosis induction in hIFN-α2b-LV–transduced CRO-AP/3 cells. Flow cytometric analyses after staining with annexin V/PI of hIFN-α2b-LV–transduced CRO-AP/3 cells showed an increase in apoptosis compared with control cells. A representative experiment is shown here, performed 18 days after transduction; density plot histograms of flow cytometric analysis show the percentage of total apoptosis (all annexin V–positive cells, reported in the circle) of the negative control (mock-transduced CRO-AP/3 cells) and hIFN-α2b-LV–transduced CRO-AP/3 cells.
In vitro biologic activity of hIFN-α2b. (A) Activation of a classic IFN-inducible pathway. A multiplex RT-PCR analysis with primers specific for β-actin and 2′-5′oligoadenylate synthetase (2′-5′ OAS) performed using RNA extracted from hIFN-α2b-LV–transduced CRO-AP/3 cells at different MOI and from mock-transduced and EGFP-LV–transduced (MOI 25) cells 4 and 10 days after transduction showed the activation of 2′-5′ OAS expression only in hIFN-α2b-LV–transduced cells, indicating the specific activation of an IFN-inducible pathway. Lanes: M, 1-kb DNA ladder marker (Invitrogen); 1, water control; 2,7, RNA extracted from mock-transduced CRO-AP/3 cells 4 and 10 days after transduction; 3, RNA from EGFP-LV–transduced CRO-AP/3 cells (MOI 25) extracted 4 days after transduction; 4 to 6, RNA from CRO-AP/3 cells 4 days after transduction with the hIFN-α2b-LV using 3 MOIs (2, 10, and 25, respectively); 8 to 10, RNA from CRO-AP/3 cells 10 days after transduction with the hIFN-α2b-LV using 3 MOI. (B) Impaired proliferation of hIFN-α2b-LV–transduced CRO-AP/3 cells. [3H]-Thymidine incorporation was measured 10 days after transduction for 4 days, and a MOI-dependent reduction in cell proliferation was observed. Data are reported as ratio between the mean of triplicates of LV-transduced cells and the mean of triplicates of control EGFP-LV–transduced cells for each analyzed time point, and the SD of the ratio was calculated according to the theory of error propagation [σa/b = a/b√(σa/a)2 + (σb/b)2]. (C) Apoptosis induction in hIFN-α2b-LV–transduced CRO-AP/3 cells. Flow cytometric analyses after staining with annexin V/PI of hIFN-α2b-LV–transduced CRO-AP/3 cells showed an increase in apoptosis compared with control cells. A representative experiment is shown here, performed 18 days after transduction; density plot histograms of flow cytometric analysis show the percentage of total apoptosis (all annexin V–positive cells, reported in the circle) of the negative control (mock-transduced CRO-AP/3 cells) and hIFN-α2b-LV–transduced CRO-AP/3 cells.
In vitro biologic activity and species specificity of recombinant and native mIFN-α1
We next examined the effects of mIFN-α1 on the proliferation of CRO-AP/3 cells. [3H]-Thymidine uptake was not impaired in human CRO-AP/3 cells after mIFN-α1 treatment (Figure 2A) but was reduced in murine MBL-2 cells (Figure 2C). Similarly, human IFN-α exerted a dose-dependent, species-specific reduction of [3H]-thymidine incorporation in CRO-AP/3 cells (Figure 2B), but not in MBL-2 cells (data not shown). In addition, mIFN-α1 did not exert proapoptotic effects on CRO-AP/3 cells (as shown below in Figure 6D). We next tested whether the murine cytokine expressed from the transgene (native mIFN-α1) retained proper functional activity in vitro. Murine MBL-2 cells were exposed to 100 and 1000 IU/mL of the native cytokine; this treatment efficiently inhibited [3H]-thymidine uptake (Figure 2D). RT-PCR analyses of murine interferon regulatory factor 7 (IRF-7) expression showed an IRF-7–specific transcript amplified from mIFN-α1–treated MBL-2 cells but not from mock-treated cells (Figure 2E). These data indicate that the murine cytokine expressed from the transgene exerted in vitro species-restricted antiproliferative effects and activated a classic IFN-stimulated pathway.
In vitro biologic activity and species specificity of mIFN-α1. Analysis of [3H]-thymidine uptake in CRO-AP/3 cells treated with 100 IU/mL or 1000 IU/mL recombinant mIFN-α1 (A), or hIFN-α2b (B), and in MBL-2 cells treated with 100 IU/mL or 1000 IU/mL recombinant (C) or native mIFN-α1 (D). Data are reported as ratio of values obtained in treated cells/untreated (A-C) or mock-treated (D) cells, and SDs are calculated as reported in the legend to Figure 1. Proliferation was not impaired in the PEL cell line after mIFN-α1 treatment, whereas both human and murine IFN-α induced a dose-dependent species-specific reduction in [3H]-thymidine incorporation. The native murine cytokine exerted an antiproliferative activity on MBL-2 cells similar to that observed with the recombinant one, but its effects were already evidenced 24 hours after treatment. (E) RT-PCR analyses performed on RNA extracted from MBL-2 cells exposed to 100 and 1000 IU/mL native mIFN-α1 for 72 hours showed the induction of murine interferon regulatory factor 7 (mIRF-7) expression in mIFN-α1–treated cells but not in mock-treated MBL-2 cells, indicating that the native cytokine retains the ability to activate a classic IFN-inducible transcript. Expression of murine β-actin (mβ-act) is shown as control. Lanes: M, 1-kb DNA ladder marker; 1, water control; 2, RNA extracted from mock-treated MBL-2 cells; 3, 4, RNA from MBL-2 cells treated with 100 or 1000 IU/mL native mIFN-α1; 5, positive control, RNA from MBL-2 cells exposed to 1000 IU/mL recombinant mIFN-α1.
In vitro biologic activity and species specificity of mIFN-α1. Analysis of [3H]-thymidine uptake in CRO-AP/3 cells treated with 100 IU/mL or 1000 IU/mL recombinant mIFN-α1 (A), or hIFN-α2b (B), and in MBL-2 cells treated with 100 IU/mL or 1000 IU/mL recombinant (C) or native mIFN-α1 (D). Data are reported as ratio of values obtained in treated cells/untreated (A-C) or mock-treated (D) cells, and SDs are calculated as reported in the legend to Figure 1. Proliferation was not impaired in the PEL cell line after mIFN-α1 treatment, whereas both human and murine IFN-α induced a dose-dependent species-specific reduction in [3H]-thymidine incorporation. The native murine cytokine exerted an antiproliferative activity on MBL-2 cells similar to that observed with the recombinant one, but its effects were already evidenced 24 hours after treatment. (E) RT-PCR analyses performed on RNA extracted from MBL-2 cells exposed to 100 and 1000 IU/mL native mIFN-α1 for 72 hours showed the induction of murine interferon regulatory factor 7 (mIRF-7) expression in mIFN-α1–treated cells but not in mock-treated MBL-2 cells, indicating that the native cytokine retains the ability to activate a classic IFN-inducible transcript. Expression of murine β-actin (mβ-act) is shown as control. Lanes: M, 1-kb DNA ladder marker; 1, water control; 2, RNA extracted from mock-treated MBL-2 cells; 3, 4, RNA from MBL-2 cells treated with 100 or 1000 IU/mL native mIFN-α1; 5, positive control, RNA from MBL-2 cells exposed to 1000 IU/mL recombinant mIFN-α1.
In vivo hIFN-α2b-LV treatment
Preliminary analyses indicated that EGFP- and IFN-α–expressing LVs efficiently delivered the transgene to CRO-AP/3 cells in vitro and conferred long-term transgene expression (Figure S1). We then evaluated the antineoplastic activity of hIFN-α2b-LV in a PEL xenograft model. Plots showing the survival curves obtained in 2 independent experiments are displayed in Figure 3A. All (8 of 8) untreated, medium-injected mice developed ascites and were killed by day 22 (median survival, 13 days). The majority of EGFP-LV–treated mice (11 of 12) developed ascites and were culled between days 15 and 21; 1 mouse developed an extraperitoneal mass and was killed on day 42 (median survival, 19 days). Figure 3B shows a macroscopic view of ascites developed in a mock-treated mouse (Figure 3B left panel) and in an EGFP-LV–treated animal (Figure 3B middle panel). At necroscopy, abundant (3-4 mL) clear opalescent fluid was found in the peritoneal cavity; at the liver hilum, a whitish highly friable neoplastic tissue was frequently detected. No lymph node, spleen, liver, or lung involvement was observed. Among the 12 animals receiving the hIFN-α2b-LV, 4 developed delayed ascites. In 2 of these 4 mice, ascites visually regressed in 10 to 12 days, and 200 to 300 μL of ascites was recovered when the animals had to be killed for the presence of an extraperitoneal mass, which consisted of white tissue infiltrating the abdominal and subcutaneous muscles surrounded by enlarged blood vessels. Microscopically, this neoplastic tissue was represented by lymphoma cells contained in a hemorrhagic, thin, and disorganized stroma; small necrotic areas were often evident. One of the mice killed for the presence of a mass is shown in Figure 3B (right panel). Five animals did not develop ascites, but peritoneal washings were obtained when the animals were culled for the presence of a solid neoplastic mass infiltrating the flank's soft tissues. The remaining 3 animals were disease-free throughout the experiment (ie, 90 days after PEL cell injection). Ascitic fluids and peritoneal washings contained lymphoma cells, as determined by microscopic evaluation and analysis of the expression of CD138/syndecan-1 by flow cytometry (not shown). The tissue found at the liver hilum was similarly composed of lymphoma cells packed into a tenuous fibrillar stroma.
In vivo antineoplastic activity of hIFN-α2b-LV. (A) Survival curves for CRO-AP/3–injected SCID mice treated with hIFN-α2b-LV. Data were obtained from 2 separate experiments with 16 animals each (6 mice per hIFN-α2b-LV group, 6 mice per EGFP-LV group, and 4 mice per medium group in each experiment). hIFN-α2b-LV–treated mice showed a statistically significant increase in the survival time compared with control mice. (B) Photograph of representative SCID mice after intraperitoneal injection of CRO-AP/3 cells and subsequent control or therapeutic treatments. Left and middle panels show ascites-bearing mice after control (medium or EGFP-LV) treatments; neoplastic ascites were characterized by a marked abdomen expansion and absence of subcutaneous lymph node involvement. Right panel shows a hIFN-α2b-LV–treated mouse bearing a lymphomatous subcutaneous mass on the left flank. (C) Proliferative capability of ex vivo PEL cells. Analysis of [3H]-thymidine uptake was performed on PEL cells recovered from an ascites (○) and 2 peritoneal washings (■ and ▿) of 3 hIFN-α2b-LV–treated animals; the CRO-AP/3 cell line (♦) was used as control. Data are expressed as counts per minute. A direct correlation between the expression of the therapeutic gene and the antiproliferative activity exerted on PEL cells recovered from mice was observed.
In vivo antineoplastic activity of hIFN-α2b-LV. (A) Survival curves for CRO-AP/3–injected SCID mice treated with hIFN-α2b-LV. Data were obtained from 2 separate experiments with 16 animals each (6 mice per hIFN-α2b-LV group, 6 mice per EGFP-LV group, and 4 mice per medium group in each experiment). hIFN-α2b-LV–treated mice showed a statistically significant increase in the survival time compared with control mice. (B) Photograph of representative SCID mice after intraperitoneal injection of CRO-AP/3 cells and subsequent control or therapeutic treatments. Left and middle panels show ascites-bearing mice after control (medium or EGFP-LV) treatments; neoplastic ascites were characterized by a marked abdomen expansion and absence of subcutaneous lymph node involvement. Right panel shows a hIFN-α2b-LV–treated mouse bearing a lymphomatous subcutaneous mass on the left flank. (C) Proliferative capability of ex vivo PEL cells. Analysis of [3H]-thymidine uptake was performed on PEL cells recovered from an ascites (○) and 2 peritoneal washings (■ and ▿) of 3 hIFN-α2b-LV–treated animals; the CRO-AP/3 cell line (♦) was used as control. Data are expressed as counts per minute. A direct correlation between the expression of the therapeutic gene and the antiproliferative activity exerted on PEL cells recovered from mice was observed.
The delivery of human IFN-α2b significantly increased the survival time (median survival, 51 days) of PEL-engrafted SCID mice by 2.7-fold compared with control EGFP-LV treatment (log-rank test, hIFN-α2b-LV vs EGFP-LV, P < .001). Moreover, longer survival was associated with a significant reduction in ascites development (Fisher exact test, P = .005).
PEL cells recovered from the peritoneal washings or ascites were found to release variable amounts of hIFN-α2b in the supernatant, whereas PEL cells recovered from the masses did not produce measurable amounts of the cytokine. As shown in Figure 3C, CRO-AP/3 cells recovered from 2 peritoneal washings produced 3.56 and 18.7 ng/106 cells/mL of hIFN-α2b and showed an impaired proliferative activity. In contrast, cells recovered from an ascites produced negligible amounts of hIFN-α2b and proliferated at levels similar to those of the control cell line. These data indicate a direct correlation between expression of the IFN-α gene and antiproliferative activity exerted on PEL cells and suggest that the development of a solid tumor in these mice might be related to the extraperitoneal escape of CRO-AP/3 cells, where they were not affected by the therapeutic hIFN-α2b-LV. Moreover, ex vivo cells recovered from ascites and producing undetectable or very low amounts of hIFN-α2b were found to maintain the in vitro responsiveness to the proapoptotic activity of IFN-α (data not shown), suggesting that ascites development in hIFN-α2b-LV–treated mice was not linked to the emergence of mechanisms of IFN-α resistance in tumor cells.
In vivo mIFN-α1-LV treatment
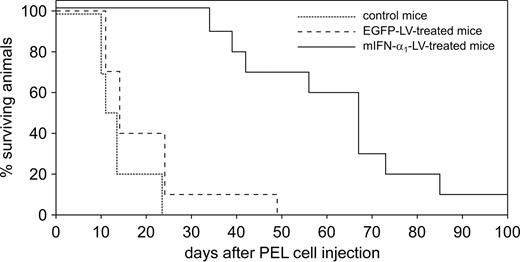
To evaluate the potential role of the host peritoneal microenvironment in PEL growth, CRO-AP/3–injected mice were treated with mIFN-α1-LV with the same protocol used for hIFN-α2b-LV–treated animals. Kaplan-Meier plots are shown in Figure 4. As expected, all untreated mice developed ascites and were killed between day 10 and day 24 (median survival, 12.5 days). Similarly, 9 of 10 EGFP-LV–treated mice developed ascites and were culled between days 11 and 24, whereas 1 mouse developed a delayed ascites and was killed on day 49 (median survival, 14 days). All 10 mIFN-α1-LV–treated mice remained tumor-free until day 31, when 1 developed ascites and was killed at day 34. Subsequently, 4 mice developed ascites, whereas 1 exhibited a very limited ascites with a solid tumor. Three others were killed for the presence of extraperitoneal solid tumors infiltrating the subcutaneous tissue on the flank. One animal remained disease-free throughout the experiment. The median survival time of mIFN-α1-LV–treated mice was 67 days.
In vivo antineoplastic activity of mIFN-α1-LV. Kaplan-Meier survival curves for CRO-AP/3–injected SCID mice treated with mIFN-α1-LV. Data were obtained from 2 separate experiments of 15 animals each (5 mice per group). mIFN-α1-LV–treated mice showed a statistically significant increase in survival time compared to control mice.
In vivo antineoplastic activity of mIFN-α1-LV. Kaplan-Meier survival curves for CRO-AP/3–injected SCID mice treated with mIFN-α1-LV. Data were obtained from 2 separate experiments of 15 animals each (5 mice per group). mIFN-α1-LV–treated mice showed a statistically significant increase in survival time compared to control mice.
Thus, mIFN-α1 treatment significantly delayed PEL development compared with control treatments, with a 4.8-fold increase in survival time of mIFN-α1-LV–treated mice (log-rank test, mIFN-α1-LV vs EGFP-LV, P = .001). Longer survival was associated with a significant reduction in ascites formation (Fisher exact test, P = .016), as only half of the mIFN-α1-LV–treated mice developed a malignant effusion.
PEL cells recovered from the ascites or peritoneal washings were found to produce measurable amounts of mIFN-α1 (2-8 ng/106 cells/mL), indicating long-term transgene expression in vivo. Prolonged expression of mIFN-α1 was confirmed by monitoring the expression of Ly-6C,16,17 a marker of IFN activity, by murine peripheral blood cells (Figure S2).
The significant increase in the survival time of animals treated with IFN-α–expressing LVs was also evident by assembling all in vivo experiments (Figure S3). Comparison of the 2 IFN-α treatments showed no significant differences in survival time or ascites accumulation. These results suggest that impairment of host microenvironment may efficiently interfere with PEL growth in vivo.
Analysis of the mechanisms involved in the antineoplastic activity of mIFN-α1
Cytokines, in particular interleukin 6 (IL-6) and IL-10, and angiogenic factors, such as vascular endothelial growth factor (VEGF), have been shown to play a critical role in the engraftment of PEL-derived cell lines in vivo.4 Culture supernatants of mIFN-α1-LV–transduced CRO-AP/3 cells contained similar levels of IL-6, IL-10, and VEGF compared with control cells (Figure 5), indicating that mIFN-α1 did not modulate the expression of these factors in PEL cells. Instead, a significant increase in IL-6 and IL-10 release (Student t test, P < .001) was observed in hIFN-α2b-LV–transduced cells (Figure 5A), indicating that PEL cells may counteract the antiproliferative effect of IFN-α through up-regulation of these factors. Moreover, VEGF secretion was found to be significantly reduced in hIFN-α2b-LV–transduced cells (P = .001; Figure 5B). These findings were confirmed in vivo as VEGF accumulated at similar levels in ascites fluids from control mice and in the ascites from hIFN-α2b-LV–treated animals, whereas regressed ascites showed a significant reduction in VEGF levels (P < .05; Figure 5B). Therefore, VEGF levels directly correlated with the volume of ascites in vivo, indicating that PEL-derived VEGF is required for expansive tumor growth by increasing fluid accumulation. VEGF (Figure 5B), IL-6, and IL-10 (not shown) levels in ascitic fluids developed in mIFN-α1-LV–treated SCID were similar to those found in control mice, further indicating that the murine cytokine did not directly affect secretion of these factors by PEL cells.
Analysis of factors involved in the antineoplastic activity of IFN-α. (A) Analysis of IL-6 and IL-10 release in culture supernatants of transduced CRO-AP/3 cells. Histograms resume data obtained by analyzing different time points in 2 independent transduction experiments using mIFN-α1-LV in parallel with EGFP-LV and in 2 independent experiments using hIFN-α2b-LV and EGFP-LV. Data refer to infections performed at MOI 2 and are reported as ratio between the mean of different time points of LV-transduced cells and the mean of control mock-transduced cells; SD of the ratio was calculated as stated in Figure 1. *Statistically significant differences between hIFN-α2b-LV–transduced cells and the other groups (Student t test). IL-6 and IL-10 secretion was significantly up-regulated in CRO-AP/3 cells expressing hIFN-α2b in vitro, whereas EGFP-LV– and mIFN-α1-LV–transduced cells released comparable levels of these cytokines. (B) Measurement of VEGF levels in supernatants from transduced CRO-AP/3 cells and ascites. Data are expressed in picograms per milliliter, and each histogram in the left panel represents the mean and SD of data obtained in the transduction experiments described above, at the time points shown below each column. Data in the in vivo panel report the mean and SD of VEGF measured in the cell-free fraction of ascites from 8 control mice (medium and EGFP-LV), 3 mIFN-α1-LV–treated mice, 2 hIFN-α2b-LV–treated mice with a fully developed effusion, and 2 hIFN-α2b-LV–treated animals with regressed ascites. *Statistically significant differences between hIFN-α2b-LV–transduced cells and the other groups, and between hIFN-α2b-LV–treated mice with regressed ascites and the other groups (Student t test). The murine cytokine did not affect VEGF secretion by PEL cells in vitro and in vivo, whereas human IFN-α significantly down-regulated VEGF release in vitro and was found to be consistently reduced in regressed ascites (volumes ≤ 0.5 mL).
Analysis of factors involved in the antineoplastic activity of IFN-α. (A) Analysis of IL-6 and IL-10 release in culture supernatants of transduced CRO-AP/3 cells. Histograms resume data obtained by analyzing different time points in 2 independent transduction experiments using mIFN-α1-LV in parallel with EGFP-LV and in 2 independent experiments using hIFN-α2b-LV and EGFP-LV. Data refer to infections performed at MOI 2 and are reported as ratio between the mean of different time points of LV-transduced cells and the mean of control mock-transduced cells; SD of the ratio was calculated as stated in Figure 1. *Statistically significant differences between hIFN-α2b-LV–transduced cells and the other groups (Student t test). IL-6 and IL-10 secretion was significantly up-regulated in CRO-AP/3 cells expressing hIFN-α2b in vitro, whereas EGFP-LV– and mIFN-α1-LV–transduced cells released comparable levels of these cytokines. (B) Measurement of VEGF levels in supernatants from transduced CRO-AP/3 cells and ascites. Data are expressed in picograms per milliliter, and each histogram in the left panel represents the mean and SD of data obtained in the transduction experiments described above, at the time points shown below each column. Data in the in vivo panel report the mean and SD of VEGF measured in the cell-free fraction of ascites from 8 control mice (medium and EGFP-LV), 3 mIFN-α1-LV–treated mice, 2 hIFN-α2b-LV–treated mice with a fully developed effusion, and 2 hIFN-α2b-LV–treated animals with regressed ascites. *Statistically significant differences between hIFN-α2b-LV–transduced cells and the other groups, and between hIFN-α2b-LV–treated mice with regressed ascites and the other groups (Student t test). The murine cytokine did not affect VEGF secretion by PEL cells in vitro and in vivo, whereas human IFN-α significantly down-regulated VEGF release in vitro and was found to be consistently reduced in regressed ascites (volumes ≤ 0.5 mL).
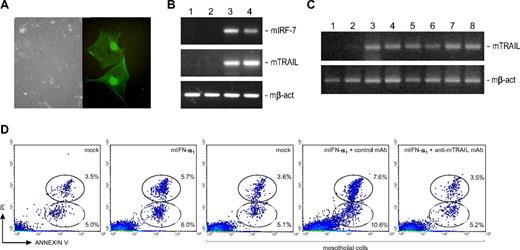
We also cultured murine mesothelial cells to study the effects of mIFN-α1 and to reproduce in vitro this aspect of the interaction of PEL cells with the peritoneum. We first isolated mesothelial cells from peritoneal fragments of EGFP-LV–treated SCID mice; 3% to 10% of these cells were EGFP-positive (Figure 6A), indicating that tissues surrounding PEL cells were also efficiently targeted by the LVs and expressed the transgene.
Analysis of the involvement of mesothelial cells in the antineoplastic activity of mIFN-α1. (A) Phase-contrast and immunofluorescence images of representative polygonal EGFP-positive mesothelial cells obtained from peritoneal fragments of EGFP-LV–treated SCID mice. EGFP-positive cells were visualized using a Carl Zeiss inverted fluorescence microscope (Axiovert 200; Carl Zeiss, Jena, Germany) with a 40×/0.55 NA objective, photographed using an Olympus camera (Camedia C3020; Olympus, Center Valley, PA), visualized and adjusted using the CorelDRAW Graphics Suite X3 (Corel Corporation, Ottawa, ON). Original magnifications ×40. (B) TRAIL up-regulation in mIFN-α1–treated murine mesothelial cells. RT-PCR analyses of murine IRF-7 (mIRF-7), TRAIL (mTRAIL), and β-actin (mβ-act, control) transcript expression were performed using RNA extracted from primary mesothelial cells 48 hours after native mIFN-α1 (250 IU/mL) or mock treatment. Up-regulation of mIRF-7 and mTRAIL was observed in mIFN-α1–treated but not in mock-exposed cells, suggesting that mesothelial cells may contribute to IFN-modulated antineoplastic activity. Lanes: 1, RNA extracted from untreated; 2, mock-treated; 3, mIFN-α1–treated mesothelial cells; 4, positive control, RNA from MBL-2 cells exposed to 500 IU/mL recombinant mIFN-α1. (C) In vivo TRAIL expression. RT-PCR analyses were performed using RNA extracted from peritoneal fragments of control and mIFN-α1-LV–treated PEL/SCID mice. Up-regulation of mTRAIL was observed in mIFN-α1-LV–treated but not in control mice, suggesting that host microenvironment may play a role in IFN-stimulated antineoplastic activity. Expression of murine β-actin is shown as control. Lanes: 1, RNA extracted from peritoneal fragments of medium-injected; 2, EGFP-LV–treated; 3 to 7, mIFN-α1–treated PEL/SCID mice; 8, positive control, RNA from MBL-2 cells exposed to 500 IU/mL recombinant mIFN-α1. (D) Role of TRAIL in IFN-induced apoptosis of CRO-AP/3 cells. Density plot histograms of flow cytometric analysis of a representative experiment show the percentage of early (annexin V–positive and PI-negative bottom circle) and late (annexin V– and PI-positive top circle) apoptosis in CRO-AP/3 cells 12 hours after mock or mIFN-α1 (250 IU/mL) treatments, or 12 hours after coculture with mock- or mIFN-α1 (250 IU/mL)–treated mesothelial cells in the presence of a control or a blocking anti-mTRAIL mAb. Treatment of CRO-AP/3 cells with mIFN-α1 did not substantially affect the level of apoptosis, whereas coculture with mIFN-α1–treated mesothelial cells significantly increased programmed cell death in CRO-AP/3 cells. CRO-AP/3 apoptosis was efficiently inhibited by the blocking anti-mTRAIL mAb, whereas an isotype-matched control mAb did not influence apoptosis induction, indicating that mesothelial cells activated by IFN-α induced apoptosis in CRO-AP/3 cells in a TRAIL-dependent manner.
Analysis of the involvement of mesothelial cells in the antineoplastic activity of mIFN-α1. (A) Phase-contrast and immunofluorescence images of representative polygonal EGFP-positive mesothelial cells obtained from peritoneal fragments of EGFP-LV–treated SCID mice. EGFP-positive cells were visualized using a Carl Zeiss inverted fluorescence microscope (Axiovert 200; Carl Zeiss, Jena, Germany) with a 40×/0.55 NA objective, photographed using an Olympus camera (Camedia C3020; Olympus, Center Valley, PA), visualized and adjusted using the CorelDRAW Graphics Suite X3 (Corel Corporation, Ottawa, ON). Original magnifications ×40. (B) TRAIL up-regulation in mIFN-α1–treated murine mesothelial cells. RT-PCR analyses of murine IRF-7 (mIRF-7), TRAIL (mTRAIL), and β-actin (mβ-act, control) transcript expression were performed using RNA extracted from primary mesothelial cells 48 hours after native mIFN-α1 (250 IU/mL) or mock treatment. Up-regulation of mIRF-7 and mTRAIL was observed in mIFN-α1–treated but not in mock-exposed cells, suggesting that mesothelial cells may contribute to IFN-modulated antineoplastic activity. Lanes: 1, RNA extracted from untreated; 2, mock-treated; 3, mIFN-α1–treated mesothelial cells; 4, positive control, RNA from MBL-2 cells exposed to 500 IU/mL recombinant mIFN-α1. (C) In vivo TRAIL expression. RT-PCR analyses were performed using RNA extracted from peritoneal fragments of control and mIFN-α1-LV–treated PEL/SCID mice. Up-regulation of mTRAIL was observed in mIFN-α1-LV–treated but not in control mice, suggesting that host microenvironment may play a role in IFN-stimulated antineoplastic activity. Expression of murine β-actin is shown as control. Lanes: 1, RNA extracted from peritoneal fragments of medium-injected; 2, EGFP-LV–treated; 3 to 7, mIFN-α1–treated PEL/SCID mice; 8, positive control, RNA from MBL-2 cells exposed to 500 IU/mL recombinant mIFN-α1. (D) Role of TRAIL in IFN-induced apoptosis of CRO-AP/3 cells. Density plot histograms of flow cytometric analysis of a representative experiment show the percentage of early (annexin V–positive and PI-negative bottom circle) and late (annexin V– and PI-positive top circle) apoptosis in CRO-AP/3 cells 12 hours after mock or mIFN-α1 (250 IU/mL) treatments, or 12 hours after coculture with mock- or mIFN-α1 (250 IU/mL)–treated mesothelial cells in the presence of a control or a blocking anti-mTRAIL mAb. Treatment of CRO-AP/3 cells with mIFN-α1 did not substantially affect the level of apoptosis, whereas coculture with mIFN-α1–treated mesothelial cells significantly increased programmed cell death in CRO-AP/3 cells. CRO-AP/3 apoptosis was efficiently inhibited by the blocking anti-mTRAIL mAb, whereas an isotype-matched control mAb did not influence apoptosis induction, indicating that mesothelial cells activated by IFN-α induced apoptosis in CRO-AP/3 cells in a TRAIL-dependent manner.
Among the IFN-stimulated genes, TRAIL selectively triggers apoptosis in tumor cells but not in most normal cells.18 To analyze the possible induction of TRAIL in murine cells, primary mesothelial cells were exposed to native mIFN-α1. As shown in Figure 6B, RT-PCR assays indicated that mIFN-α1 activated the classic IFN-inducible pathways in these cells, as mIFN-α1–exposed mesothelial cells, but not mock-treated or hIFN-α2b–exposed cells (not shown), selectively expressed mIRF-7. Moreover, murine TRAIL was found to be expressed in vitro in mIFN-α1–treated mesothelial cells (Figure 6B) and in vivo in peritoneal fragments from mIFN-α1-LV–treated mice (Figure 6C), raising the possibility that the host microenvironment might trigger TRAIL-mediated apoptosis in PEL cells. To test for this hypothesis, primary mesothelial cells were treated with native mIFN-α1 or with mock supernatants and subsequently cocultured with CRO-AP/3 cells, in the presence of a blocking anti-mTRAIL or an isotype-matched control mAb. Apoptosis was found to be significantly increased in CRO-AP/3 cells cocultured with mIFN-α1–exposed cells (2.1- to 3.5-fold increase vs all other groups; P ≤ .05 by Student t test), but not with mock-treated mesothelial cells (Figure 6D). CRO-AP/3 cell death induced by mIFN-α1–exposed mesothelial cells was significantly (Student t test, P ≤ .05) inhibited by the blocking anti-mTRAIL mAb, whereas an isotype-matched control mAb did not affect apoptosis induction (Figure 6D). Given the cross-species activity of TRAIL,19 these findings indicate that mesothelial cells activated by IFN-α induced apoptosis in CRO-AP/3 cells in a TRAIL-dependent manner. CRO-AP/3 cells exposed to the same amounts of mIFN-α1 or mock supernatants showed comparable lower levels of annexin V–positive cells, as previously observed, further confirming that mIFN-α1 does not act on human cells.
Discussion
The peculiar site of PEL growth implies a specific contribution of body cavities to the pathogenesis of this type of lymphoma. Soon after HHV8-associated PEL discovery, it was shown that HHV8-infected lymphomatous cells have full growth autonomy when injected into the peritoneal cavity of immunodeficient mice.20 To dissect the role of the host microenvironment in PEL development, we compared the therapeutic activity of hIFN-α2b with that of a host-specific mIFN-α1 in a xenograft mouse model mimicking the aggressive course of human PEL. We found that lentiviral delivery of hIFN-α2b exerted a remarkable antineoplastic activity by significantly prolonging mice survival (Figure 3) and by reducing ascites formation. Previous reports of the effects of IFN-α in the context of PEL showed that retroviral vector-mediated stable gene transfer of human type I consensus IFN (IFN-con1) in BCBL-1 cells abrogates their ability to grow as tumors after subcutaneous or intraperitoneal transplantation into SCID mice. Moreover, constitutive expression of IFN-con1 in BCBL-1 cells inhibited HHV8 lytic replication in vitro.21 Although these results reflect antitumor and antiviral activities of human IFN-α, the experimental approach did not test the potential therapeutic effect of IFN-con1 in mice that already harbor PEL tumors. A subsequent study showed that daily intraperitoneal injections of IFN-α and azidothymidine significantly prolonged the survival of PEL/SCID mice.9 We achieved PEL targeting by lentiviral delivery directly at the tumor site with minimal systemic spread of the vector. Indeed, analysis of lentivirus biodistribution after intraperitoneal injection showed that very limited amounts of virus escape the peritoneum (Table S1), indicating that transgene expression remained mainly confined to the peritoneal cavity. In patients with AIDS, the confined, intracavitary IFN-α expression may be important, not only to avoid adverse effects but also to restrict the systemic IFN-α–dependent loss of human immunodeficiency virus type 1–uninfected CD4+ T cells, as recently suggested.22
The pleiotropic activity of IFN-α gives the opportunity to dissect the contribution and interplay of different factors involved in PEL growth. In addition to the antiproliferative and proapoptotic activity of hIFN-α2b on PEL cells that we (Figure 1) and others have documented,7,21 this cytokine may modulate other processes involved in PEL pathogenesis. IFN-α exerts angiostatic activity by inhibiting VEGF transcription.23 In the context of PEL, VEGF production by lymphoma cells contributes to lymphoma growth mainly by accelerating vascular permeability, rather than tumor vascularization. Administration of a neutralizing antihuman VEGF antibody to BCBL-1–injected SCID/beige mice was found to inhibit ascites formation.24 Although all mice were killed on day 30 after cell inoculation, thus precluding long-term evaluation, this finding highlights the pathogenic role of this factor in effusion development. In line with these observations, we found that VEGF secretion was significantly reduced in in vitro hIFN-α2b-LV–transduced cells and in regressed ascites (Figure 5). This suggests that IFN-α may also efficiently work against expansive tumor growth by reducing VEGF-stimulated vascular leakage and fluid accumulation in this PEL model.
IL-6 was shown to be important for in vivo PEL cell proliferation,25 and IL-10 represents one of the most relevant autocrine growth factors for PEL cells. IL-10 is secreted by PEL cell lines at high levels in vitro and throughout tumor progression in a PEL murine model.25-27 In our study, IL-6 and IL-10 secretion (Figure 5) was found to be up-regulated in response to hIFN-α2b, thus indicating that PEL cells may antagonize the IFN-induced growth arrest through overexpression of these mitogenic cytokines. Whereas hIFN-α2b reduced HHV8 reactivation in CRO-AP/3 cells, we also found an MOI-correlated vIL-6 up-regulation in hIFN-α2b-LV–transduced CRO-AP/3 cells (in “Results” in Document S1 and Figure S4C). This suggests that these cells may respond to hIFN-α2b–induced down-modulation of the α chain (gp80) of the IL-6 receptor (IL-6Rα), as previously shown in PEL-derived BCP-1 cells,28 by overexpressing a virus-encoded proliferative factor that can act via direct interaction with the gp130 signal–transducing subunit.29 However, IL-6Rα was not detected by flow cytometry in CRO-AP/3 cells in either basal or IFN-exposed conditions (data not shown), suggesting that the significance of IL-6 up-regulation in response to hIFN-α2b in CRO-AP/3 cells may be restricted to indirect effects.30,31
We demonstrate here that mIFN-α1-LV exerted an antineoplastic activity comparable with that achieved with hIFN-α2b-LV (Figures 3,4). The murine cytokine did not exert a direct antiproliferative, proapoptotic, and antiviral effect on PEL cells in vitro but retained a species-restricted biologic activity (Figures 2, S4). These results indicate that in vitro and in vivo amounts of mIFN-α1 reached with an MOI of 2 respected the species specificity of IFN receptor–binding activity. Moreover, mIFN-α1 did not affect the secretion of VEGF, IL-6, and IL-10 by CRO-AP/3 cells (Figure 5), further suggesting that in vivo mIFN-α1 mainly acted on murine cells surrounding PEL cells. Thus, targeting the intracavitary milieu might have a therapeutic impact similar to that exerted by a therapy against PEL cells. The relevant role of the microenvironment in PEL development is also in line with previous results obtained with matrigel-embedded PEL cells xenografted into SCID mice.32
The PEL lymphoma microenvironment comprises a dynamic and interactive mixture of cell populations and cytokines. We found no differences in the levels of macrophages and natural killer cells present in lymphomatous effusions from control and mIFN-α1–treated mice (in “Results” in Document S1), suggesting that these 2 cell populations do not play a major role in affecting PEL cell growth in our experimental setting. On the other hand, PEL cells survive and proliferate within large serous body cavities lined by mesothelia, which may thus contribute both positive and negative signals to lymphoma cells. Ex vivo cultures of murine mesothelial cells showed that they were efficiently transduced and expressed the transgene (Figure 6A). We present here preliminary data suggesting that mesothelial cells may play an active role in PEL growth control. Not only do they contribute to transgene expression, they also may potentially exert a direct tumoricidal activity. IFN-α was previously shown to induce TRAIL in human epithelial and endothelial cells.33,34 Our findings indicate that mesothelial cells too can express TRAIL in response to mIFN-α1 in vitro (Figure 6B) and very probably in vivo (Figure 6C). Because TRAIL has a cross-species activity,19 we hypothesized that this mechanism may be involved in the antineoplastic activity of mIFN-α1. Indeed, mIFN-α1–treated mesothelial cells significantly triggered apoptosis in PEL cells in a TRAIL-dependent manner, as demonstrated by the inhibitory effect of the blocking anti-TRAIL monoclonal antibody (Figure 6D). It is interesting to note that human normal mesothelial cells were found to be TRAIL-resistant via the expression of decoy receptors,35 thus suggesting that mesothelial cells may contribute to the killing of target tumor cells while being resistant to TRAIL-triggered apoptosis.
In conclusion, the present findings provide evidence that the host microenvironment plays a relevant role in controlling PEL growth, and also indicate that the therapeutic targeting of the local milieu may impair PEL development. Moreover, our data suggest that the interplay between lymphomatous cells and mesothelium may be central to PEL pathogenesis.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Vincenzo Ciminale, Francis Dumont, and Giovanna Tosato for helpful discussion, Vito Barbieri, Elisa Bergamo, Giulia Chiapolino, Gianni Esposito, Antonella Facchinetti, Walter Habeler, Giulia Masserizzi, Lidia Moserle, Luca Persano, Veronica Tisato, and Valeria Tosello for their help in various aspects of this work, Pierantonio Gallo for artwork, and Colette Case for help in preparing the manuscript.
This work was supported by grants from the Associazione Italiana per la Ricerca sul Cancro (grants 1452 and 5045), Istituto Superiore di Sanità (grant 50G.3), and Ministry of Health, Oncology Program 2006 and Progetto Integrato Oncologia (RFPS-2006-2-342010). P.G. was the recipient of a fellowship from the Accademia Nazionale dei Lincei. I.M.D.G. was the recipient of a fellowship from Associazione Italiana per la Ricerca sul Cancro, and from Istituto Oncologico Veneto. M.B. was the recipient of a fellowship from the Associazione Italiana per la Lotta contro Linfomi, Leucemie e Mieloma.
Authorship
Contribution: M.L.C. and L.C.-B. designed and performed research, analyzed the results, and wrote the paper; P.G., I.M.D.G., and M.B. performed research and data analysis; S.I. contributed lentiviral vector expertise and discussed the results; and A.A. supervised the study and discussed the results.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Maria Luisa Calabrò, Immunology and Diagnostic Molecular Oncology, Istituto Oncologico Veneto, Via Gattamelata 64, Padova, I-35128 Italy; e-mail: lcalabro@unipd.it.
References
Author notes
*P.G. and I.M.D.G. contributed equally to this work.

![Figure 1. In vitro biologic activity of hIFN-α2b. (A) Activation of a classic IFN-inducible pathway. A multiplex RT-PCR analysis with primers specific for β-actin and 2′-5′oligoadenylate synthetase (2′-5′ OAS) performed using RNA extracted from hIFN-α2b-LV–transduced CRO-AP/3 cells at different MOI and from mock-transduced and EGFP-LV–transduced (MOI 25) cells 4 and 10 days after transduction showed the activation of 2′-5′ OAS expression only in hIFN-α2b-LV–transduced cells, indicating the specific activation of an IFN-inducible pathway. Lanes: M, 1-kb DNA ladder marker (Invitrogen); 1, water control; 2,7, RNA extracted from mock-transduced CRO-AP/3 cells 4 and 10 days after transduction; 3, RNA from EGFP-LV–transduced CRO-AP/3 cells (MOI 25) extracted 4 days after transduction; 4 to 6, RNA from CRO-AP/3 cells 4 days after transduction with the hIFN-α2b-LV using 3 MOIs (2, 10, and 25, respectively); 8 to 10, RNA from CRO-AP/3 cells 10 days after transduction with the hIFN-α2b-LV using 3 MOI. (B) Impaired proliferation of hIFN-α2b-LV–transduced CRO-AP/3 cells. [3H]-Thymidine incorporation was measured 10 days after transduction for 4 days, and a MOI-dependent reduction in cell proliferation was observed. Data are reported as ratio between the mean of triplicates of LV-transduced cells and the mean of triplicates of control EGFP-LV–transduced cells for each analyzed time point, and the SD of the ratio was calculated according to the theory of error propagation [σa/b = a/b√(σa/a)2 + (σb/b)2]. (C) Apoptosis induction in hIFN-α2b-LV–transduced CRO-AP/3 cells. Flow cytometric analyses after staining with annexin V/PI of hIFN-α2b-LV–transduced CRO-AP/3 cells showed an increase in apoptosis compared with control cells. A representative experiment is shown here, performed 18 days after transduction; density plot histograms of flow cytometric analysis show the percentage of total apoptosis (all annexin V–positive cells, reported in the circle) of the negative control (mock-transduced CRO-AP/3 cells) and hIFN-α2b-LV–transduced CRO-AP/3 cells.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/113/19/10.1182_blood-2008-09-180307/7/m_zh80170934190001.jpeg?Expires=1769263878&Signature=QRQcbA8PYQyJf6IWrPKN9uEDGy5-82RBDhcu1vG~HfeFY9uqAQNpBDjUdme9yte0I4k0Xhjr3HYx19L6wLR6PeAkFDJOdj~jnmKUfyp5YGV5H6fk~PVXYcj~XnKFnXG8am7TGZycysA8f8V04BbR5i4V2dV0n3OiynNteYxGrraPEHMpSoZuYRX0bPjQ5SI-6aswlN5~~u~tbqZ34-bAEASvP~vfxoXBNTGVwr-cwDnHgVvzGgbk3rUQxaPPTAgsFpxjlKXuF7KnNgaEwSCf-byaLgoCwkG5D1~BO6sXDi-G3NCAo2S3Atm~DLFG6a0EpCYXvGmnxR132z2t17YnEw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 2. In vitro biologic activity and species specificity of mIFN-α1. Analysis of [3H]-thymidine uptake in CRO-AP/3 cells treated with 100 IU/mL or 1000 IU/mL recombinant mIFN-α1 (A), or hIFN-α2b (B), and in MBL-2 cells treated with 100 IU/mL or 1000 IU/mL recombinant (C) or native mIFN-α1 (D). Data are reported as ratio of values obtained in treated cells/untreated (A-C) or mock-treated (D) cells, and SDs are calculated as reported in the legend to Figure 1. Proliferation was not impaired in the PEL cell line after mIFN-α1 treatment, whereas both human and murine IFN-α induced a dose-dependent species-specific reduction in [3H]-thymidine incorporation. The native murine cytokine exerted an antiproliferative activity on MBL-2 cells similar to that observed with the recombinant one, but its effects were already evidenced 24 hours after treatment. (E) RT-PCR analyses performed on RNA extracted from MBL-2 cells exposed to 100 and 1000 IU/mL native mIFN-α1 for 72 hours showed the induction of murine interferon regulatory factor 7 (mIRF-7) expression in mIFN-α1–treated cells but not in mock-treated MBL-2 cells, indicating that the native cytokine retains the ability to activate a classic IFN-inducible transcript. Expression of murine β-actin (mβ-act) is shown as control. Lanes: M, 1-kb DNA ladder marker; 1, water control; 2, RNA extracted from mock-treated MBL-2 cells; 3, 4, RNA from MBL-2 cells treated with 100 or 1000 IU/mL native mIFN-α1; 5, positive control, RNA from MBL-2 cells exposed to 1000 IU/mL recombinant mIFN-α1.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/113/19/10.1182_blood-2008-09-180307/7/m_zh80170934190002.jpeg?Expires=1769263878&Signature=Tk0kOC2R5JtqhkGWR~S-d0dODDBB0iQizZT7tLsLbhI9JJBbFzjBD1DYfA3eiUHn4b8oA7uF9hdvbCDtle-BfbsBbdlOwYbweJg5n37WdbWpbZJ4AcqxRKIGOGx2vPy0~E13zGmT02WcgrgkYW3dcjv52wyO1Aftb-tyA0XMDtiU2KImTVFQkXWDmP2GIAcNJb6~xb0FB2zrJ0m7j04pyIqaIrK6Iqry4kxrJNSW8QCsIYlm3zCC8CJQ3utCcnS4c2VoBTm-4W9iN5j-bDsSOjT5pv3olCMPVGTvjRpSEUSQ~334ACuNBMSULVx2lvDeeL995N-ER2FzJSPY-QDJkg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 3. In vivo antineoplastic activity of hIFN-α2b-LV. (A) Survival curves for CRO-AP/3–injected SCID mice treated with hIFN-α2b-LV. Data were obtained from 2 separate experiments with 16 animals each (6 mice per hIFN-α2b-LV group, 6 mice per EGFP-LV group, and 4 mice per medium group in each experiment). hIFN-α2b-LV–treated mice showed a statistically significant increase in the survival time compared with control mice. (B) Photograph of representative SCID mice after intraperitoneal injection of CRO-AP/3 cells and subsequent control or therapeutic treatments. Left and middle panels show ascites-bearing mice after control (medium or EGFP-LV) treatments; neoplastic ascites were characterized by a marked abdomen expansion and absence of subcutaneous lymph node involvement. Right panel shows a hIFN-α2b-LV–treated mouse bearing a lymphomatous subcutaneous mass on the left flank. (C) Proliferative capability of ex vivo PEL cells. Analysis of [3H]-thymidine uptake was performed on PEL cells recovered from an ascites (○) and 2 peritoneal washings (■ and ▿) of 3 hIFN-α2b-LV–treated animals; the CRO-AP/3 cell line (♦) was used as control. Data are expressed as counts per minute. A direct correlation between the expression of the therapeutic gene and the antiproliferative activity exerted on PEL cells recovered from mice was observed.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/113/19/10.1182_blood-2008-09-180307/7/m_zh80170934190003.jpeg?Expires=1769263878&Signature=XbmWLquJ8rZpzfuDEWtZ48zTE5-~GTgOaYluKyZ8m7Mf6jE~U22OS-j2ff5e9S1xzPsh20xuOAowSS6q6Dkl3BVDItNNZzVqs8aEq-gNpgYL-iLsgsIDzXZvad4XmU~KvpVX12eEgcvJO2MdkQLHTj3lsEJOlBN54vGOoXoPX-691C2sd5aanXQFD8Oahz-cUoN5p1oNNc7C0qHlK7U4qJDIJhe-TOxHpeHNl-LcgK8eF6jC56jgRlvbNrWbZRQVp6m0lG3PZu2jatNGmrO9C34U0xWh0ZNCz6gjzkgHkgtaAx5j~ZNqTO~h-UH~BQdU1tTS93A8VlD4EiuWzIZLdQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal