Abstract
We investigated the impact of rhesus macaque (RM) B-cell depletion before inoculation with the isolate SIVsmmD215. Seven RMs were treated every 3 weeks with 50 mg/kg of an anti-CD20 antibody (rituximab) starting 7 days before inoculation for 2 (n = 4) and 5 (n = 3) months. Four control animals received no antibody. Three animals were completely depleted of CD20+ B cells, but 4 were only partially depleted of CD20 cells in the LNs and intestine. The decrease in antibody production was consistent with the efficacy of tissue CD20 depletion. Seroconversion and neutralizing antibody production was significantly delayed in animals showing complete tissue CD20 depletion and remained at low titers in all CD20-depleted RMs. Surprisingly, there was no significant difference in acute or chronic viral loads between CD20-depleted and control animal groups. There was a tendency for lower viral set points in CD20-depleted animals. At 6 weeks after inoculation, cellular immune responses were significantly stronger in CD20-depleted animals than in controls. There was no significant difference in survival between CD20-depleted and control animals. Our data suggest that a deficiency of Ab responses did not markedly affect viral replication or disease progression and that they may be compensated by more robust cellular responses.
Introduction
Despite 25 years of effort, an effective anti-HIV vaccine remains elusive. One contributing factor in this failure is that the correlates of immune protection against HIV infection are still incompletely understood.1,2 Studies in HIV-infected patients and SIV-infected rhesus macaques (RMs) have demonstrated a key role for cellular immune responses in controlling viral replication and disease progression.3-7 However, vaccines aimed at developing sustained cellular immune responses for SIV have not been able to prevent infection or disease progression in RMs inoculated with pathogenic SIVmac.8 Moreover, the recent failure of a vaccine designed to elicit cellular immunity in humans suggests that renewed efforts in understanding immune correlates are badly needed.9,10
Humoral immune responses are likely to be a crucial component of an effective anti-HIV vaccine. Animal studies have been instrumental in understanding the efficacy of antibodies. Intravenously administered antibodies have been shown to protect macaques against intravenous or mucosal SHIV challenge.11-14 Topically applied antibodies can also protect macaques against vaginal SHIV challenge.12,15 Antibody protection is achieved mainly through neutralization (ie, antibody ability to inhibit viral entry into target cells, thus preventing infection), but also, as recently shown, by other antiviral effects (ie, effector functions mediated by the crystalizable fragment of antibody molecule, such as complement activation and antibody-dependent cellular cytotoxicity, thus clearing the viral particles).16 Considering their prime role in many successful vaccines in the past, antibody-based vaccines were the first choice in the initial stages of vaccine development.17 However, the resistance of primary HIV isolates to neutralization has been a major hurdle.17 Hope for a solution to this challenge was provided by the observation that neutralizing antibodies effective against primary patient isolates develop after many infections.18,19 However, the neutralization activity tends to be rather type-specific and the high sequence variability in Env means that the virus can easily escape.19-21 Nevertheless, a fraction of patients go on to develop broadly HIV-neutralizing antibodies, providing a paradigm for what we would like to achieve with a vaccine.22
CD8 depletion studies in vivo provided clear indications of the role of cellular immunity in SIV infection.23-25 Similar experiments to investigate the role of B-cell responses need to consider that it is the antibodies produced by the cells and not the cells themselves that provide immunity. Because antibodies persist in the circulation for long periods, B-cell depletion has a delayed rather than immediate effect on nascent or established antibody titers that should be considered. Therefore, the role of humoral immune responses in controlling SIV replication can be assessed only by depleting B cells before SIV inoculation to prevent antibody development. Only 2 such experiments have been reported thus far and their results did not permit a clear conclusion on whether antibodies play an essential role in the control of post-acute and chronic viral replication.26,27 In the first study, a short-term depletion of CD20 cells by infusion of an anti-CD20 commercial monoclonal antibody (rituximab; Genentech, San Francisco, CA) had no significant impact on the resolution of peak viremia in SIVmac251-infected RMs.27 However, the authors reported a temporal inverse correlation between the emergence of anti-SIV–neutralizing antibodies and the control of viral replication during the post-acute phase of infection, suggesting that humoral immune responses may contribute to the control of chronic SIV replication. However, because both humoral and cellular immune responses emerged simultaneously, the role of neutralizing antibodies in the control of viremia remains unclear. A second study, in which rituximab was administered continuously at a higher dosage until AIDS developed, reported a significantly higher rate of disease progression in animals exhibiting complete B-cell depletion compared with those that exhibited incomplete depletion.26 However, as the animals were infected with SIVmac239, a virus that is very difficult to neutralize, the role of neutralizing antibodies in this case remains unclear.26 Here we reassessed the role of humoral responses in SIV infection by pretreating RMs with an anti-CD20 monoclonal antibody (Rituxan, rituximab; gift from Genentech) and inoculating with the strain SIVsmmD215 directly derived from a naturally infected sooty mangabey (SM) and that is highly susceptible to neutralization. We report that humoral immune responses have a limited ability to control SIV replication.
Methods
Virus characterization and stock preparation
During our previous survey of SIVsmm diversity in SMs from US Primate Centers,28 we identified a neutralization-sensitive SIVsmm strain (D215; see Document S1, available on the Blood website; see the Supplemental Materials link at the top of the online article). An SIVsmmD215 stock virus was grown in SM peripheral blood mononuclear cells (PBMCs) and frozen at −80°C for future studies.29 Plasma samples from the naturally infected SM D215 were also collected.
Animals
Eleven male Indian RMs (Macaca mulatta) aged 4 to 7 years old were housed at the Tulane National Primate Research Center (TNPRC), an Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC) International–accredited facility, in accordance with the Guide for the Care and Use of Laboratory Animals (US Public Health Service)30 and the Animal Welfare Act. All protocols and procedures were reviewed and approved by the Tulane University Institutional Animal Care and Use Committee. All RMs were MHC genotyped at the AIDS Vaccine Research Laboratory of the University of Wisconsin (Madison, WI), as described.31
Rituximab treatments and virus inoculation
All 11 RMs were infected with plasma originating from the naturally infected SM D215 at an infectious dose equivalent to 100 000 copies of SIVsmmD215.
One week before infection, 7 RMs were treated intravenously with 50 mg/kg rituximab (Genentech), a human-mouse chimeric, monoclonal anti-CD20 antibody. Additional administrations of rituximab were given as follows: 4 RMs (DG04, CA16, CF03, and BT49) received 3 administrations (50 mg/kg) for up to 2 months (days 14, 35, and 56 postinfection [pi]) and 3 RMs (EL55, EI09, and EI74) received additional rituximab infusions every 3 weeks up to 5 months pi (days 77, 98, 119, 140, and 161 pi). Four RMs (DG34, DH44, DD93, and BV85) served as controls and were inoculated with SIVsmmD215 only.
Follow-up was more than 2 years for control RMs and SIV-infected RMs that received rituximab infusions for 2 months, and for 450 days for the 3 RMs that received rituximab for 5 months.
Sampling of blood, LNs, and intestine
For all animals, 4.9 mL EDTA-blood was collected twice per week during the first 2 weeks of infection, weekly during the first month, bimonthly for the first 3 months, and then every 2 months during the follow-up. Plasma was separated by centrifugation (583g for 10 minutes), and PBMCs were extracted as described.32
Superficial LN (axillary and inguinal) biopsies were removed from animals using sterile surgical procedures on days −7, 0, 7, 10, 14, 28, 42, 120, 180, 240, 300, and 360 pi LN mononuclear cells were isolated as described.32
Intestinal samples (proximal jejunum) consisting of approximately 10 to 15, 1- to 2-mm2 pieces were obtained by endoscopic-guided biopsy on days −7, 0, 10, 14, 21, 28, 42, and 56 pi. Subsequent intestinal biopsies were collected at the same time points as blood samples. In addition, 2- to 3-inch intestinal resections were removed from the animals surgically: before rituximab treatment and virus inoculation, at the end of acute infection, and during the chronic infection (days −7, 28, and 300 pi). Lymphocytes were separated from pinch biopsies and resections as described.33-35 Cells from blood, LNs, and intestine were stained for flow cytometry or frozen at −80°C in freezing media.
Flow cytometric analysis of lymphocyte populations
Immunophenotyping of lymphocytes isolated from blood, LNs, and intestine was performed using fluorescently conjugated monoclonal antibodies and a 4-color staining technique. The samples were run using a FACSCalibur flow cytometer and the data were analyzed using Cell Quest software (both from Becton Dickinson, San Jose, CA). The monoclonal antibody panels used were as follows: CD3-fluorescein isothiocyanate (FITC), CD8 phycoerythrin (PE), CD4-peridinin chlorophyll protein (PerCP), CD20 allophycocyanin (APC); CD95 FITC, CCR5 PE, CD4 PerCP, CD28 APC; CD3 FITC, CD25 PE, CD4 PerCP, CD69 APC; CD3 FITC, CD8 beta PE (Beckman Coulter), HLADR PerCP, CD4 APC; CD3 FITC, CD8 PE, CD4 PerCP, CD79A APC; Ki67 FITC, CD8 PE, CD3 PerCP, CD4 APC. All MAbs were from BD Biosciences (San Jose, CA) unless otherwise indicated. Whole blood and mononuclear cells from LNs and intestine were stained as described.33-35
Plasma viral loads
Plasma viral loads were determined by branched DNA assay (bDNA; Bayer Diagnostics, Tarrytown, NY).
Immunohistochemistry
Tissues were formalin-fixed and paraffin-embedded (LNs and intestine from CD20-depleted and control RMs) and probed using an avidin-biotin complex HRP technique (Vectastain Elite ABC kit; Vector Laboratories, Burlingame, CA) and anti-CD79a (Dako, Carpinteria, CA), anti-CD20 (Dako), and P28 SIV (Mardex Diagnostics, Carlsbad, CA) as primary antibodies. Sections were visualized with DAB (Dako) and counterstained with hematoxylin.
Analysis of anti-SIV antibody responses
Production of specific anti-SIV IgG was determined using 4 serologic methods on serum or plasma samples from all animals at different time points pi. Anti-gp41 and anti-V3 antibody titers were determined by a specific SIVsmm primate immunodeficiency virus enzyme immunoassay (PIV-EIA), as described.36 SIVmac Western blots (WBs; Zeptometrix, Buffalo, NY) were performed on serial plasma or serum samples to investigate the dynamics of anti-SIVsmm seroconversion. Viral neutralization was measured as reductions in luciferase reporter gene expression in either 5.25.EGFP.Luc.M7 cells (for TCLA-SIVmac251) or TZM-bl cells (for SIVsmmD215), as described.37
Cellular immune responses
Interferon γ (IFN-γ) enzyme-linked immunosorbent spot (ELISPOT) assays were performed at 2- to 6-week intervals during the first 6 months pi and every2 to 3 months thereafter, as described.38 Intracellular cytokine staining (ICS) assays for IL-2, IFN-γ, TNF-α, and CD107a were performed at selected time points using cryopreserved PBMCs, as described.38,39 The 9-color polychromatic flow cytometry panel consisted of CD3 APC-Cy7, CD4 PerCP-Cy5.5, CD8 Alexa 700, CD69 PE-Texas Red, CD95 biotin-streptavidin Qdot655, IFN-γ PE-Cy7, TNF-α FITC, IL-2 APC, and CD107a PE. Samples were run on a LSR II flow cytometer (BD Biosciences) and at least 200 000 total events were acquired. The frequency of responding memory (CD95+) CD4+ and CD8+ T lymphocytes for each effector function was ascertained by coexpression of CD69 and the respective cytokine or surface CD107a positivity. The frequency of SIV-specific responses after stimulation with peptides representing single SIV proteins was calculated after subtraction of background responses obtained in unstimulated cells. Median background responses for IFN-γ, IL-2, and TNF-α secretion were less than 0.5% for memory CD8+ T lymphocytes and less than 0.1% for memory CD4+ T lymphocytes. Background responses for degranulation tended to be high in memory CD8+ T lymphocytes (median, 1.8%).
Statistical analysis of data
To compare values of measured variables at a given time point between rituximab-treated and control animals, we used the nonparametric Mann-Whitney test. To analyze changes over time, such as those for HLA-DR, we used linear mixed-effects models.40 Significance was assessed at the α equals .05 level.
Results
During our survey of SIVsmm diversity in naturally infected SMs housed in different Primate Centers in the United States,28 we identified a strain (SIVsmmD215) that is highly sensitive to neutralization by both autologous and heterologous sera (Figure S1). Here, we have used this strain to identify the role of humoral responses in the control of SIVsmm replication in RMs.
Anti-CD20 mAb treatment depletes B cells in blood and variably depletes tissue B cells
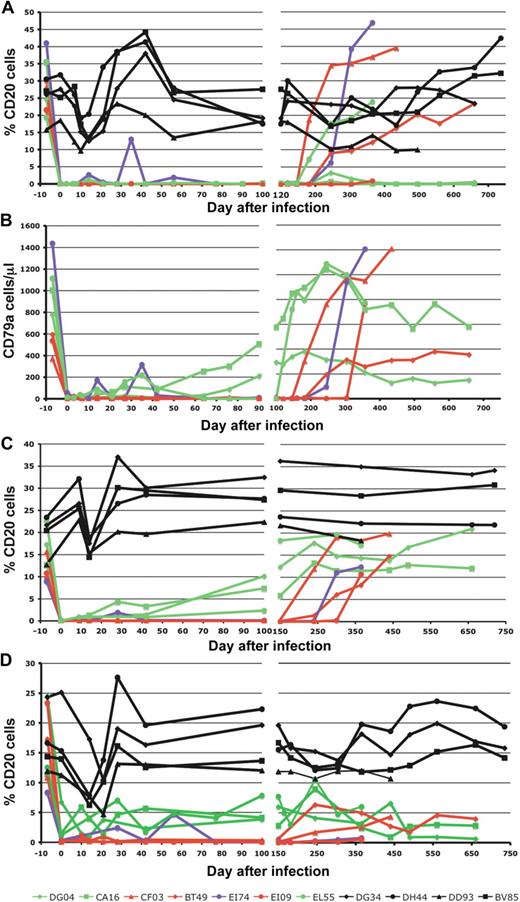
Seven RMs were repeatedly infused with rituximab, as described in “Methods.” At the time of SIV infection, 7 days after the first rituximab administration, peripheral blood levels of CD20+ B cells were undetectable in all but one RM (Figure 1A). In the remaining RM (EI74-illustrated in violet throughout the figures), detectable CD20 levels were observed at the time of the rituximab administration (days 14, 35, and 56 after SIV infection), suggesting a rapid rebound of CD20+ cells. Interestingly, the efficacy of CD20+ B-cell depletion was independent of the duration of rituximab administration. Indeed, after the interruption of rituximab treatment, a major rebound of CD20+ B cells occurred between days 180 to 200 pi, irrespective of the duration of treatment (Figure 1A). At the end of the follow-up, in 2 animals from the first group (DG04 and CA16) and 1 from the second group (EI09) peripheral CD20+ cells were still significantly depleted (Figure 1A).
Effect of rituximab infusion on concentrations of CD20+ B cells. Changes to CD20+ B cells are shown in blood (A), lymph nodes (C), and intestine (D). Rituximab infusion induced a depletion of B cells and did not mask the CD20 molecule, as shown by the CD79a staining (B). Black symbols and lines denote the control monkeys (DG34, DH44, DD93, and BV85). Red symbols and lines denote monkeys in which rituximab administration induced complete B-cell depletion in both peripheral blood and tissues (CF03, BT49, and EI09). Green symbols and lines indicate monkeys in which rituximab induced complete peripheral but incomplete tissue B-cell depletion (DG04, CA16, and EL55). The violet dots and line indicate a RM in which B-cell depletion was achieved after every rituximab administration but B cells rebound before the following treatment. Day 0 is the day of SIV inoculation. Rituximab was infused every 21 days beginning 1 week before SIV inoculation. RMs DG04, CA16, CF03, and BT49 received 4 rituximab administrations. RMs EI74, EI09, and EL55 received rituximab for up to 160 days pi.
Effect of rituximab infusion on concentrations of CD20+ B cells. Changes to CD20+ B cells are shown in blood (A), lymph nodes (C), and intestine (D). Rituximab infusion induced a depletion of B cells and did not mask the CD20 molecule, as shown by the CD79a staining (B). Black symbols and lines denote the control monkeys (DG34, DH44, DD93, and BV85). Red symbols and lines denote monkeys in which rituximab administration induced complete B-cell depletion in both peripheral blood and tissues (CF03, BT49, and EI09). Green symbols and lines indicate monkeys in which rituximab induced complete peripheral but incomplete tissue B-cell depletion (DG04, CA16, and EL55). The violet dots and line indicate a RM in which B-cell depletion was achieved after every rituximab administration but B cells rebound before the following treatment. Day 0 is the day of SIV inoculation. Rituximab was infused every 21 days beginning 1 week before SIV inoculation. RMs DG04, CA16, CF03, and BT49 received 4 rituximab administrations. RMs EI74, EI09, and EL55 received rituximab for up to 160 days pi.
As rituximab can cross-block anti-CD20 MAbs used for the detection of B cells, CD79a was included in the flow cytometry panel as a marker for B cells. Staining with CD79a showed slightly different dynamics compared with CD20 staining (compare Figure 1A,B). Three animals demonstrated undetectable levels of CD79a+ cells up to day 150 after infection, similar to what was observed using the CD20 marker. However, the other 3 RMs demonstrated incomplete depletion of CD79a+ B cells (Figure 1B). RM EI74 showed the same CD79a+ dynamic pattern as observed for the CD20 marker, with repeated rebounds before rituximab administration (Figure 1B).
The flow cytometric analysis of CD20 expression in LNs showed complete depletion of CD20 cells in 3 RMs (CF03, BT49, and EI09: illustrated in red throughout the figures) starting from day 0 pi onward (Figure 1C), whereas in the remaining 3 RMs (DG04, CA16, and EL55: illustrated in green throughout the figures) only a partial CD20 depletion was observed (Figure 1C). The same dynamic pattern was observed in the intestine, where depletion was complete in 3 RMs (CF03, BT49, and EI09) and incomplete in 3 RMs (DG04, CA16, and EL55; Figure 1D). The efficacy of CD20 cell depletion after rituximab administration was not predicted by the FCRγIIIA polymorphisms (Figure S2). The duration of tissue depletion of CD20 cells in animals receiving short-term treatments was similar to that observed in RMs that received long-term treatments (Figure 1C,D), and similar to that observed in peripheral blood. Notably, RM EI74 exhibited repeated rebounds of peripheral CD20+ and CD79a+ B cells between consecutive rituximab administrations and showed similar depletion pattern in tissues, which was different from those observed in RMs with incomplete tissue CD20 depletion. In control RMs (illustrated in black throughout the figures), there was no significant variation in the dynamics of CD20 cells during the follow-up (Figure 1A,C,D).
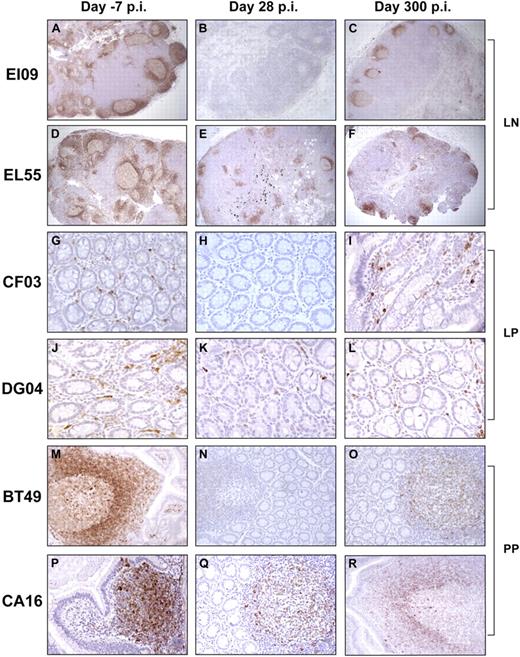
Immunohistochemical staining for CD79a (Figure 2) confirmed the flow cytometry data. Representative data are shown in Figure 2. Complete B-cell depletion was observed in 3 RMs at 5 weeks after the first administration of rituximab (day 28 pi) in the LNs (Figure 2B) and effector (Figure 2H) and inductive (Figure 2N) sites in the intestine, compared with baseline levels (Figure 2A,G,M). Furthermore, B-cell depletion was incomplete in 3 RMs in the LNs (Figure 2E) and intestinal effector (Figure 2K) and inductive (Figure 2Q) sites, compared with baseline levels (Figure 2D,J,P). At day 300 pi, a significant but partial restoration of LN and intestinal CD79a+ cells was observed (Figure 2C,F,I,L,O,R). CD20+ B-cell depletion resulted in a significant reduction in the size of the germinal centers compared with undepleted control RMs (Figures 2, S3).
Effect of rituximab infusion on the frequencies of CD79a+ B cells in tissues. Panels A through F demonstrate the rituximab effect on LN CD79a+ B cells in a RM with complete B-cell depletion in tissues (A-C) versus a RM with incomplete B-cell depletion in tissues (D-F). LNs were collected before (day −7 pi; A,D) at 28 days pi (B,E) and at day 300 pi (C,F). Panels G through R demonstrate the rituximab effect on intestinal CD79a+ B cells from both lamina propria (LP; G-L) and Peyer patches (PP; M-R). Slides were examined with a Leica DMLB microscope (Leica Microsystems, Wetzlar, Germany) using the following objective lenses and numeric apertures: N PLAN 5×/0.12 (panels A-F); N PLAN 10×/0.25 (panels M-R); and N PLAN 20×/0.4 (panels G-L). Images were acquired with Spot Insight color 3.2.0 software (Diagnostic Instruments, Sterling Heights, MI) and processed with Adobe Photoshop Elements version 3.0 (Adobe Systems, San Jose, CA).
Effect of rituximab infusion on the frequencies of CD79a+ B cells in tissues. Panels A through F demonstrate the rituximab effect on LN CD79a+ B cells in a RM with complete B-cell depletion in tissues (A-C) versus a RM with incomplete B-cell depletion in tissues (D-F). LNs were collected before (day −7 pi; A,D) at 28 days pi (B,E) and at day 300 pi (C,F). Panels G through R demonstrate the rituximab effect on intestinal CD79a+ B cells from both lamina propria (LP; G-L) and Peyer patches (PP; M-R). Slides were examined with a Leica DMLB microscope (Leica Microsystems, Wetzlar, Germany) using the following objective lenses and numeric apertures: N PLAN 5×/0.12 (panels A-F); N PLAN 10×/0.25 (panels M-R); and N PLAN 20×/0.4 (panels G-L). Images were acquired with Spot Insight color 3.2.0 software (Diagnostic Instruments, Sterling Heights, MI) and processed with Adobe Photoshop Elements version 3.0 (Adobe Systems, San Jose, CA).
Impact of B-cell depletion on anti-SIV dynamics
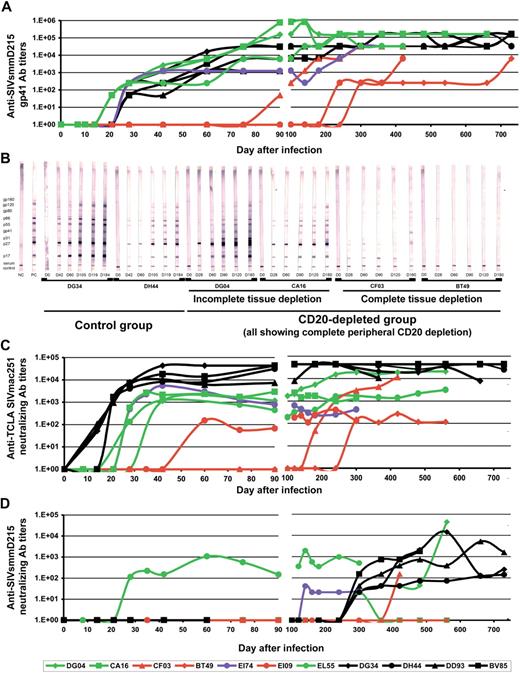
To investigate the impact of rituximab-induced B-cell depletion on humoral immune responses, we compared the dynamics of anti-SIV Ab in both CD20-depleted and undepleted RMs in various serologic assays. We first investigated the dynamics of anti-SIVsmm gp41 seroconversion by enzyme-linked immunosorbent assay (ELISA). As shown in Figure 3A, delays in anti-gp41 seroconversion were observed in RMs (CF03, BT49 and EI09) in which tissue CD20 depletion was complete. For RMs showing an incomplete tissue CD20 depletion (DG04, CA16, and EL55), as well as for the RM showing a rapid rebound in CD20+ B cells, there was no difference in the seroconversion patterns or antibody titers, compared with undepleted RM controls (Figure 3A). Testing the dynamics of antiSIVsmm-V3 antibodies36 revealed no significant difference in the seroconversion patterns in CD20-depleted and control RMs, irrespective of the efficiency of CD20 depletion (data not shown). Anti-V3 seroconversion in RMs occurred later than anti-gp41 seroconversion, similar to what has been previously reported,36 which perhaps explains the similar patterns observed between different groups.
Impact of B-cell depletion after rituximab administration on anti-SIVsmm humoral immune responses. (A) ELISA testing of anti-SIVsmm gp41 antibodies showed significant delay in the seroconversion patterns in RMs with complete tissue CD20+ B-cell depletion (red symbols and lines) and no significant difference between control RMs (black symbols and lines) and rituximab-treated RMs with incomplete tissue CD20+ B-cell depletion (green symbols and lines). (B) Western blot testing on serial samples confirmed the trend observed by ELISA, with no significant difference in the seroconversion patterns between control RMs (illustrated by RMs DG34 and DH44) and rituximab-infused RMs with incomplete tissue depletion (illustrated by RMs DG04 and CA16). Significant delays in the WB seroconversion were observed in RMs with complete CD20+ B-cell depletion after rituximab infusion (illustrated by RMs CF03 and BT49). Neutralizing antibody testing against both highly susceptible TCLA-SIVmac251 strain (M7-Luc assay; C) and SIVsmmD215 strain (TZM-bl assay; D) showed delayed seroconversion and lower titers in RMs infused with rituximab. RMs showing complete tissue CD20+ B-cell depletion showed the lowest Nab titers. Day 0 corresponds to SIV inoculation. Rituximab was infused every 21 days beginning 1 week before SIV inoculation. RMs DG04, CA16, CF03, and BT49 received 4 rituximab administrations. RMs EI74, EI09, and EL55 received rituximab for up to 160 days pi.
Impact of B-cell depletion after rituximab administration on anti-SIVsmm humoral immune responses. (A) ELISA testing of anti-SIVsmm gp41 antibodies showed significant delay in the seroconversion patterns in RMs with complete tissue CD20+ B-cell depletion (red symbols and lines) and no significant difference between control RMs (black symbols and lines) and rituximab-treated RMs with incomplete tissue CD20+ B-cell depletion (green symbols and lines). (B) Western blot testing on serial samples confirmed the trend observed by ELISA, with no significant difference in the seroconversion patterns between control RMs (illustrated by RMs DG34 and DH44) and rituximab-infused RMs with incomplete tissue depletion (illustrated by RMs DG04 and CA16). Significant delays in the WB seroconversion were observed in RMs with complete CD20+ B-cell depletion after rituximab infusion (illustrated by RMs CF03 and BT49). Neutralizing antibody testing against both highly susceptible TCLA-SIVmac251 strain (M7-Luc assay; C) and SIVsmmD215 strain (TZM-bl assay; D) showed delayed seroconversion and lower titers in RMs infused with rituximab. RMs showing complete tissue CD20+ B-cell depletion showed the lowest Nab titers. Day 0 corresponds to SIV inoculation. Rituximab was infused every 21 days beginning 1 week before SIV inoculation. RMs DG04, CA16, CF03, and BT49 received 4 rituximab administrations. RMs EI74, EI09, and EL55 received rituximab for up to 160 days pi.
Western blots confirmed no difference in seroconversion patterns between those animals in which only partial depletion of tissue CD20 cells occurred (Figure 3B) and control animals (Figure 3B). Conversely, significant delays in seroconversion patterns were observed in RMs for which tissue CD20 depletion was complete (Figure 3B).
Furthermore, the dynamics of SIV-neutralizing antibodies against both heterologous TCLA SIVmac251-6 strain (Figure 3A) and autologous SIVsmmD215 strain (Figure 3D) were significantly delayed and were retained at lower titers in all the CD20-depleted RMs, irrespective of the efficacy of CD20 depletion.
In the rituximab-infused RMs, the virus was dispersed throughout the tissue rather than congregating at the germinal centers (Figure S3A,C) in contrast to significant viral trapping observed in control RMs (Figure S3B,D). As follicular dendritic cells trap virions in complex with IgG and complement via complement CR2 receptors and possibly Fcγ receptors,41,42 these results offer an additional, albeit indirect, indication that rituximab successfully ablated anti-SIV humoral immune responses at inductive tissue sites.
Moreover, the defect in the formation of germinal centers (where somatic hypermutation and affinity maturation of neutralizing antibodies occur) explains why neutralizing Abs were detected later and at lower titers in all CD20-depleted RMs, whereas binding antibodies (likely low affinity and produced before affinity maturation) were delayed only in the tissue CD20-depleted RMs.
Impact CD20 depletion on the control of SIVsmmD215 replication
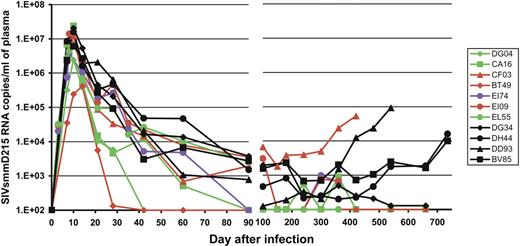
No significant difference in the viral loads (VLs) between those RMs with undetectable levels of B cells in the blood and tissues and those animals with incomplete depletion in these compartments was observed (Figure 4). Moreover, there was no statistically significant difference in the SIVsmm RNA copies per milliliter between rituximab-infused RMs and control RMs at either the viral peak (P = .11) or viral set point (P = .11) for the average VL between days 90 and 240 (Figure 4), although these P values could indicate a trend toward lower VLs in the rituximab-treated group compared with the control RMs. Six of 7 rituximab-infused RMs showed undetectable VLs during the follow-up, compared with detectable VLs in all control RMs (Figure 4). The control of virus replication was not due to any particular MHC profiles, as all but one RM in this study were negative for the MaMu-A*01, -B*17, and -B*08 alleles that are associated with better control of SIV infection (data not shown). The exception was one RM in the rituximab-treated group (CF03) that was positive for the MaMu-B*17 MHC allele that has been associated with “elite controller” status.6 However, CF03 replicated the virus at the highest levels in comparison with all other rituximab-treated animals (Figure 4).
Dynamics of SIVsmm D215 plasma vRNA loads in rituximab-infused RMs and control monkeys. Black symbols and lines denote the control monkeys (DG34, DH44, DD93, and BV85). Red symbols and lines denote monkeys in which rituximab administration induced complete CD20 depletion in both peripheral blood and tissues (CF03, BT49, and EI09). Green symbols and lines indicate monkeys in which rituximab induced complete peripheral but incomplete tissue CD20+ B-cell depletion (DG04, CA16, and EL55). The violet dots and line indicate a RM in which CD20+ B-cell depletion was achieved after every rituximab administration but CD20 cells rebounded before the following treatment. Day 0 is the day of SIV inoculation. Rituximab was infused every 21 days beginning 1 week before SIV inoculation. RMs DG04, CA16, CF03, and BT49 received 4 rituximab administrations. RMs EI74, EI09, and EL55 received rituximab for up to 160 days pi.
Dynamics of SIVsmm D215 plasma vRNA loads in rituximab-infused RMs and control monkeys. Black symbols and lines denote the control monkeys (DG34, DH44, DD93, and BV85). Red symbols and lines denote monkeys in which rituximab administration induced complete CD20 depletion in both peripheral blood and tissues (CF03, BT49, and EI09). Green symbols and lines indicate monkeys in which rituximab induced complete peripheral but incomplete tissue CD20+ B-cell depletion (DG04, CA16, and EL55). The violet dots and line indicate a RM in which CD20+ B-cell depletion was achieved after every rituximab administration but CD20 cells rebounded before the following treatment. Day 0 is the day of SIV inoculation. Rituximab was infused every 21 days beginning 1 week before SIV inoculation. RMs DG04, CA16, CF03, and BT49 received 4 rituximab administrations. RMs EI74, EI09, and EL55 received rituximab for up to 160 days pi.
Overall, our results showed no significant difference in post-acute viral replication between CD20-depleted and undepleted RMs infected with a neutralization-sensitive SIVsmm strain, strongly suggesting that humoral immune responses are not controlling SIV replication.
Comparative changes in immune cells from different compartments in CD20+ B cell–depleted and undepleted monkeys
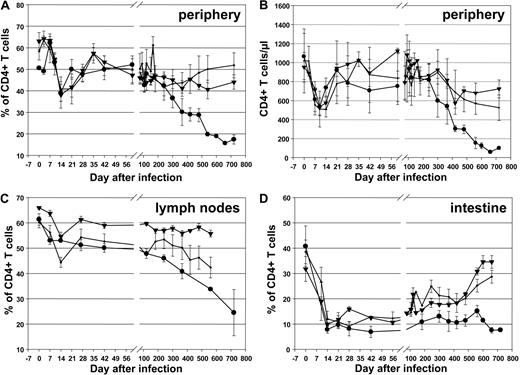
We next investigated the effect of rituximab treatment on other lymphocyte subsets in the blood, LNs, and intestine using flow cytometry. There were no significant differences in CD4+ T-cell depletion in blood (Figure 5A,B; P > .4 at 6 weeks and 8 months), LNs (Figure 5C; P = .11), and intestine (Figure 5D; P = .06), indicating that rituximab had little or no impact on SIV pathogenicity. However, at later time points, control animals tended to have lower CD4+ T-cell counts compared with rituximab-infused RMs (Figure 5), consistent with the slightly higher levels of viral replication (Figure 4). Phenotyping the CD4+ T-cell subsets did not reveal any differences in the dynamics of naive, central memory, and effector memory CD4+ T cells in the blood (Figure S4A,C,E) and intestine (Figure S4B,D,F) between treated or untreated groups (Figure S4).
CD4+ T cells in rituximab-infused RMs and controls. Changes in CD4+ T cells in blood (A, percentages; B, absolute counts), lymph nodes (C), and intestine (D) in rituximab-infused RMs with complete CD20 depletion (■), incomplete tissue CD20 depletion (▾), and in control RMs (●). Plots represent the average expression for the animals in each study group. Vertical lines represent the SEM.
CD4+ T cells in rituximab-infused RMs and controls. Changes in CD4+ T cells in blood (A, percentages; B, absolute counts), lymph nodes (C), and intestine (D) in rituximab-infused RMs with complete CD20 depletion (■), incomplete tissue CD20 depletion (▾), and in control RMs (●). Plots represent the average expression for the animals in each study group. Vertical lines represent the SEM.
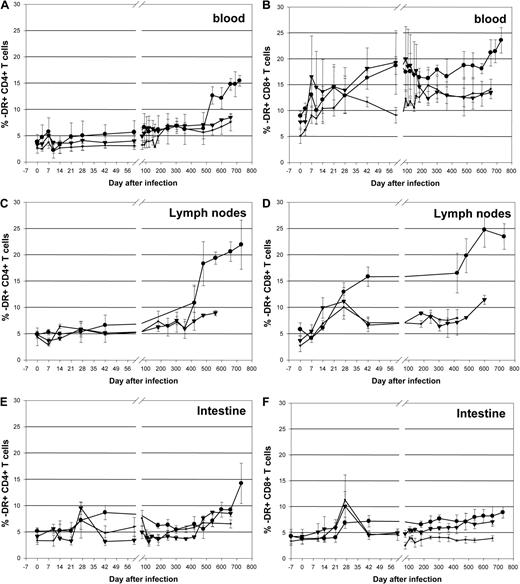
Different from previous results reported during infection with highly pathogenic SIVmac strains,43,44 RMs infected with SIVsmmD215 did not experience significant increases in the levels of CD4+ T-cell activation. A minimal, transient increase in -DR expression on CD4+ T cells was observed during acute SIVsmmD215 infection in periphery (Figure 6A) and no change was observed in the LNs (Figure 6C). Even in the intestine, where the activation levels of CD4+ T cells are higher than in periphery, there was no major increase in CD4+ T-cell immune activation after SIVsmmD215 infection (Figure 6E). More significant increases in the levels of -DR expression were observed on CD8+ T cells from peripheral blood (Figure 6B), LNs (Figure 6D), and intestine (Figure 6F). Note that during the initial stages of SIVsmmD215 infection, no difference was observed in the levels of immune activation in CD20+ B cell–depleted RMs and control RMs (P > .9 and P = .2 for CD4+ and CD8+ T cells in blood, respectively) or between monkeys with complete or incomplete CD20+ B cell depletion (Figure 6). During the follow-up, higher levels of immune activation of CD4+ and CD8+ T cells were observed in control RMs, probably as a consequence of higher viral replication (Figure 6). Analysis of another T-cell activation marker (CD69) showed a similar pattern of changes in blood, LNs, and intestine (data not shown). The levels of CD4+ and CD8+ T-cell proliferation, as assessed using Ki-67, were not significantly different between the different groups of RMs during the initial stages of the follow-up from baseline levels (data not shown). These results show that rituximab infusion had no significant effect on proliferation and activation of CD4+ and CD8+ T cells in SIVsmmD215-infected RMs.
Dynamics of CD4+ and CD8+ T-cell immune activation in rituximab-infused RMs and controls. Immune activation (as defined by changes in the expression of -DR markers) in blood (A,B), lymph nodes (C,D), and intestine (E,F) in rituximab-infused RMs with complete CD20 depletion (■), incomplete tissue CD20 depletion (▾), and in control RMs (●). Plots represent the average expression for the animals in each study group. Vertical lines represent the SEM.
Dynamics of CD4+ and CD8+ T-cell immune activation in rituximab-infused RMs and controls. Immune activation (as defined by changes in the expression of -DR markers) in blood (A,B), lymph nodes (C,D), and intestine (E,F) in rituximab-infused RMs with complete CD20 depletion (■), incomplete tissue CD20 depletion (▾), and in control RMs (●). Plots represent the average expression for the animals in each study group. Vertical lines represent the SEM.
Cellular immune responses
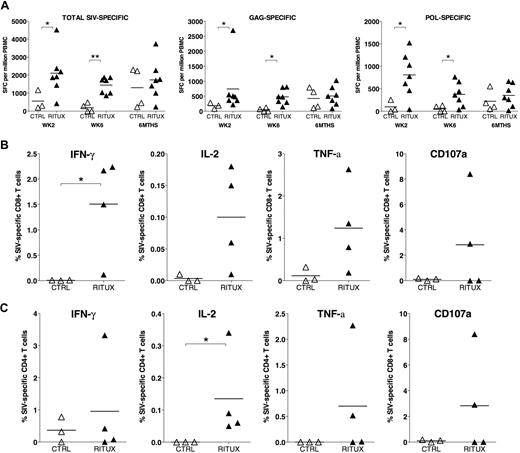
To determine the effect that depletion of CD20 cells has on cellular immune responses, we longitudinally tested PBMCs from rituximab-treated and control animals for SIV-specific T-cell responses in an IFN-γ ELISPOT assay. Virus-specific cellular immune responses were detected in both groups by 2 weeks after SIV infection (Figure S5). In the first 6 weeks, the magnitude of the SIV-specific IFN-γ ELISPOT response was significantly higher in the rituximab-treated animals compared with the control animals (Figures S5, 7A). The increased magnitude was largely due to the anti-Gag and anti-Pol T-cell responses (Figures 7A, S5). By 6 months, these differences were no longer detected (Figure 7A). ELISPOT responses against 1 to 8 SIV proteins were detected in RMs over the course of SIV infection; the breadth of the response was not statistically different between the rituximab-treated and the control RMs at any time point (data not shown). To determine whether the increase in cellular immune responses was mediated by CD4+ or CD8+ T lymphocytes, ICS assays were performed on cryopreserved cells at early (week 6) and late (≥ 6 months) time points. The increase in IFN-γ ELISPOT responses in rituximab-treated compared with control RMs at 6 weeks pi was due to an increase in the frequency of IFN-γ–secreting SIV-specific CD8+ but not CD4+ T lymphocytes (Figure 7B,C). This difference was not detected at later time points more than 6 months pi (data not shown). A trend for increased frequency of SIV-specific CD8+ T lymphocytes secreting IL-2 and TNF-α, and undergoing degranulation in rituximab-infused RMs at 6 weeks pi did not reach statistical significance (Figure 7B). In addition, rituximab-infused RMs showed a significant persistent increase in the frequency of IL-2–secreting SIV-specific CD4+ T lymphocytes at early and late time points pi (Figure 7C and data not shown). Overall, rituximab-treated RMs demonstrated quantitative and qualitative differences in the SIV-specific T-lymphocyte response compared with control RMs, and these differences were more apparent early in SIV infection during the period of B-cell depletion.
Comparison of the magnitude of SIV-specific cellular immune responses in control and rituximab-treated rhesus macaques at early and late time points after SIV infection. (A) SIV-specific IFN-γ ELISPOT responses in control (CTRL) and rituximab-treated (RITUX) RMs at weeks 2 and 6, and 6 months after SIV infection shown. ELISPOT responses against SIV Gag and Pol proteins (Gag-specific and Pol-specific) as well as the sum of responses against individual SIV proteins representing the entire SIV proteome (total SIV-specific) shown. (B,C) Intracellular cytokine staining assay in control and rituximab-treated RMs at 6 weeks after SIV infection showing the frequency of (B) total SIV-specific CD8+ T lymphocytes and (C) total SIV-specific CD4+ T lymphocytes secreting IFN-γ, IL-2, TNF-α, and undergoing degranulation (surface CD107a+) after 16 hours of in vitro stimulation with SIV peptides in the presence of brefeldin A and monensin. Stimulation with SIVmac239 sequence-based peptide pool. Asterisks denote P values less than .05 (*) and less than .01 (**) for differences between and control and rituximab-treated RMs using the Mann-Whitney U test.
Comparison of the magnitude of SIV-specific cellular immune responses in control and rituximab-treated rhesus macaques at early and late time points after SIV infection. (A) SIV-specific IFN-γ ELISPOT responses in control (CTRL) and rituximab-treated (RITUX) RMs at weeks 2 and 6, and 6 months after SIV infection shown. ELISPOT responses against SIV Gag and Pol proteins (Gag-specific and Pol-specific) as well as the sum of responses against individual SIV proteins representing the entire SIV proteome (total SIV-specific) shown. (B,C) Intracellular cytokine staining assay in control and rituximab-treated RMs at 6 weeks after SIV infection showing the frequency of (B) total SIV-specific CD8+ T lymphocytes and (C) total SIV-specific CD4+ T lymphocytes secreting IFN-γ, IL-2, TNF-α, and undergoing degranulation (surface CD107a+) after 16 hours of in vitro stimulation with SIV peptides in the presence of brefeldin A and monensin. Stimulation with SIVmac239 sequence-based peptide pool. Asterisks denote P values less than .05 (*) and less than .01 (**) for differences between and control and rituximab-treated RMs using the Mann-Whitney U test.
The mechanism for the increased magnitude of IFN-γ–positive SIV-specific CD8+ T-lymphocyte responses in the rituximab-treated monkeys is not clear. Its presence at early but not later time points suggests that it may be an indicator of a compensatory mechanism in the absence of humoral immunity and could be a contributory factor to the trend for lower VLs in the rituximab-treated group. The persistent increase in IL-2 responses in the rituximab-treated group is interesting. However, whether it is causally related to the lower VL or a result of better control of viremia in the rituximab group cannot be ascertained.
Discussion
Several studies in macaque models have shown that antibodies are capable of preventing SHIV infection,12-14 raising some optimism for the potential protective efficacy of antibody-based vaccines. However, it is unlikely that antibodies play a major role in controlling virus replication in the chronic phase of infection, as demonstrated by passive infusion of immune serum experiments in HIV-1–infected patients45,46 and SIV-infected macaques.47,48 Although it can be argued that the mechanism of action of infused antibodies in these studies45-48 is inconsistent with virus neutralization, but may be virus mediated, infusion of a combination of the most potent neutralizing antibodies had very little effect on postinfection VL in HIV-1–infected human peripheral blood leukocyte (hu-PBL) severe combined immunodeficient (SCID) mice.49 Antibody failure to control viral replication is also due to delays in antibody production in the face of a very rapidly evolving virus, such that the swift appearance of escape mutants far outpaces adaptation of the humoral responses to recognize them.50
Nevertheless, CD20 B-cell depletion studies in SIV-infected RMs have reported a possible role for antibodies in controlling SIVmac251 replication and disease progression during SIVmac239 infection.26,27 However, these studies both had the caveat that they used highly pathogenic SIV strains that are characterized by rapid progression to simian AIDS. Moreover, SIVmac strains (SIVmac251 and SIVmac239) are very difficult to neutralize,51,52 so humoral immune responses may be expected to have only a limited impact on the outcome of infection. To circumvent these potential problems, we performed CD20+ B-cell depletions in RMs subsequently infected with SIVsmmD215, an isolate that we recently identified in a naturally infected SM during our surveys of SIVsmm diversity in Primate Centers.28 This strain has multiple advantages over the highly pathogenic SIVmac strains for assessing the impact of humoral immune responses on SIV pathogenicity: (1) high susceptibility to neutralization by homologous and heterologous sera; (2) better reproduction in RMs of the HIV-1 pathogenesis, with lower set points, progressive depletion of CD4+ T cells, and longer disease progression. Chronic SIVsmmD215 VLs in RMs range from 102 to 104 SIVsmmD215 RNA copies per milliliter of plasma, significantly lower (3-4 logs) than those observed during SIVmac infections,53 and are stable for long periods of time. Thus, changes in viral replication resulting from CD20 depletion can be easily observed. Moreover, the impact of SIVsmmD215 infection on CD4+ T cells can be evaluated over a long period of time, as opposed to pathogenic infections where catastrophic CD4+ T-cell depletion occurs.53 (3) Finally, SIVsmmD215-infected RMs show significantly slower disease progression. Thus, differences in disease progression between CD20-depleted RMs and controls can be readily observed.
We report that chronic administration of anti-CD20 mAb infusion in RMs resulted in complete depletion in 3 of 7 monkeys and incomplete depletion in 3 of 7, whereas in the remaining RM the depletion, although complete, was shorter than the time interval between 2 administrations (Figure 1). As a result, anti-SIV IgG antibody production varied, being completely abolished in those RMs showing complete CD20 depletion, and detectable in the remaining ones. Although the levels of CD20 cells were significantly lower in RMs with incomplete CD20 depletion compared with the controls, there was no significant difference in seroconversion timing or antibody titers between animals in the 2 groups, suggesting that the low levels of residual tissue B cells are sufficient to produce an anti-SIV humoral immune response that is not quantitatively different from those observed in control animals. The incomplete CD20 depletion could be defined based only on the levels observed in tissues, as CD20 levels in peripheral blood were undetectable in all rituximab-infused animals. We investigated both the dynamics of neutralizing antibodies, as well as that of nonneutralizing antibodies reported to be able to inhibit HIV-1 replication in macrophages and immature dendritic cells, namely those detected against the V3 loop and the principal immunodominant domain of the gp41.54
We found no significant differences in the dynamics of VLs or disease progression between rituximab-infused RMs with complete or incomplete CD20 depletion, suggesting that anti-SIV antibodies had no significant impact on viral replication. Although there was also no significant difference in VLs between rituximab-infused and control RMs, in Figure 4 there was a tendency for lower VLs in rituximab-infused RMs compared with controls, which was associated with less depletion of CD4+ T cells of various phenotypes and stronger cellular immune responses.
There are several potential mechanisms that can explain the lower VLs observed in rituximab-infused RMs compared with controls: first, there were significantly higher levels of cellular immune responses in these animals at 6 weeks after infection (Figure 7). As previous studies have reported a significant involvement of cell-mediated immunity in controlling viral replication,3-7 we may assume that these higher levels of cellular responses may indeed be responsible for the observed differences in viral replication. Note, however, that previous studies have been unable to identify a clear correlation between the magnitude of cellular immune responses and the levels of viral replication during chronic HIV/SIV infection.3-7 More effective CD8+ T-cell responses could have been due to the depletion of CD20+ B cells that results in less competition for dendritic cells, more “space” for CD8 expansion in the LNs where the germinal centers are reduced generating an enlargement of the interfollicular (T cell) areas, or to a larger availability of cytokines. It is possible that the more effective T-cell responses are observed in CD20 depletion RMs as a result of the depletion of certain B-cell subpopulations, such as the recently described B-regulatory cells that were reported to dampen T-cell responses or Th2 polarize.55
Alternatively, the lower VLs in the monkeys with CD20 B-cell depletion may result from impairment of viral antigen presentation to CD4+ T cells. Our experiments may have acted both directly, by depleting CD20 cells that have an antigen-presenting function, or by ablating the antibody production, thus impairing the contact with dendritic cells, which are also antigen-presenting cells. The lack of viral trapping in germinal centers supports this hypothesis.
Our results showing no increase in viral replication after CD20+ B-cell depletion are vastly different from the effect of experimental CD8 depletion that resulted in significant increases of viral replication during both acute and chronic SIV infection.23-25 They suggest that cellular immune responses may be essential for controlling viral replication, but humoral immune responses have only a limited impact.
Finally, with regard to the observation that B-cell depletion had no effect on a neutralization-sensitive challenge virus, it is possible that rapid virus escape may be responsible for these results. The alternative explanation, that the challenge stock quasispecies contains sensitive and resistant variants and that only a resistant variant was transmitted (eg, sensitive virus might be less fit for transmission), seems unlikely because we detected neutralizing antibodies in chronically infected RMs. Cloning of multiple functional Env genes from SIVsmmD215 to test as pseudoviruses in neutralization assays should help us to understand persistent in vivo replication of neutralization-sensitive strains.
No difference in survival was observed between treated and untreated groups in this study. At 2 years after infection, survival in the control group was 75%, whereas in the CD20-depleted group survival was 85%. This follow-up is significantly longer than any survival reported thus far for CD20-depletion studies.26,27 As most of the studies were carried out with highly pathogenic SIVmac strains, the shorter duration of disease progression during such highly pathogenic infections did not allow a long-term assessment of the impact of CD20 B-cell depletion on survival.
We conclude that humoral immune responses have only a limited impact on the control of viral replication or disease progression during the chronic infection with the neutralization-sensitive SIVsmmD215 strain. We stress, however, that this observation does not preclude a role for antibodies in preventing SIV/HIV transmission and therefore the possibility of developing an effective anti-HIV vaccine based on stimulating specific humoral immune responses.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Dina Washington and Genentech for the generous gift of Rituxan; James Binley, Andrew A. Lackner, Preston A. Marx, Christopher J. Miller, Louis Picker, James Robinson, and Guido Silvestri for helpful discussions; the Division of Veterinary Medicine of the TNPRC for animal care; and Robin Rodriguez for help in preparing figures.
This work was supported by grants R01 AI065325 and P20 RR020159 (C.A.), RO1 AI064066 and R21AI069935 (I.P.), AI30034 (D.M.), and P51 RR000164 (TNPRC) from the National Institute of Allergy and Infectious Diseases and from the National Center for Research Resources, Bethesda, MD.
National Institutes of Health
Authorship
Contribution: C.A., I.P., and T.G. designed the research; C.A., T.G., R.G., and E.R. performed the animal studies; T.G., M.K., M.P., and J.M. performed cell separation and flow cytometry studies; M.B. and D.M. performed the serologic investigations; M.K. and A.K. performed the cell-mediated immunity studies; I.P., C.T., and M.P. performed the immunohistochemistry studies; C.A. and R.R. performed data analyses; and C.A., I.P., A.K., R.R., and T.G. wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Cristian Apetrei, Division of Microbiology, Tulane National Primate Research Center, 18703 Three Rivers Rd, Covington, LA 70433; e-mail: capetrei@tulane.edu.
References
Author notes
*T.G. and R.G. contributed equally to this work.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal