Abstract
Although implicated in antagonistic functions, both regulatory T cells (Tregs) and Th17 effector cells play an important role in controlling autoimmune pathogenesis. Paradoxically, recent studies indicate that Tregs have the capacity to produce interleukin-17 (IL-17), although the ability of these cells to retain their suppressive function remains unknown. Here we report that human Tregs within the CD4+CD45RA−CD25highCCR6+HLA-DR−FoxP3+ population produce IL-17 when activated in the presence of the proinflammatory cytokines IL-1β and IL-6, whereas IL-17 secretion was inhibited by TGFβ. To assess the ability of a single Treg to secrete IL-17 and to suppress in vitro immune function, we isolated clones from this population. We found that IL-17+/FoxP3+ Treg clones retain suppressive function and exhibit the plasticity to secrete IL-17 or suppress depending on the nature of the stimulus provided. IL-17 production by these Treg clones was accompanied by sustained FoxP3 expression and concomitant, but reversible, loss of suppressive activity. Our data demonstrate that at the single cell level a subset of in vitro suppressive FoxP3+ cells can be driven to secrete IL-17 under inflammatory conditions. These findings suggest a new mechanism by which inflammation can drive Tregs to secrete IL-17, thereby dampening suppression and promoting an inflammatory milieu.
Introduction
Regulatory T cells (Tregs), characterized by high expression of FoxP3, are potent immunomodulators of T-cell activation that suppress proliferation and cytokine secretion by effector T cells.1-5 Loss of Tregs by either gene deletion of FoxP3 in mice or mutations in humans results in autoimmune disease and the inability to effectively regulate T-cell activation.6,7 While Tregs play a fundamental role in protection of autoimmunity, their differentiation is tightly linked to the development of IL-17–producing (Th17) cells, a highly pathogenic effector T-cell subset involved in inducing inflammation and autoimmune tissue injury.8,9 Both loss of Treg function and induction of Th17 cells have been implicated in the pathogenesis of human autoimmune diseases, such as multiple sclerosis, rheumatoid arthritis, Crohn disease, and psoriasis.10-14
Although Tregs appear to be central in regulating immune responses to self-antigens, mechanisms must be present to rapidly block their suppressive activity and enable immune activation during an acute microbial infection. In such circumstances, antigen-presenting cells (APC) secrete inflammatory cytokines, such as interleukin-1β (IL-1β) and IL-6, which induce the potent effector responses necessary to clear the infection. In this regard, our data and the work of others have demonstrated that IL-1β and IL-6 can drive memory CD4+ T cells to secrete IL-17.15,16 IL-6, which has been shown to abrogate suppression by Treg cells,17 also drives Th17 differentiation in naive cells when paired with transforming growth factor β (TGFβ) in mouse18 or with IL-1β, interleukin-23 (IL-23), and TGFβ in human,19 although the minimal requirements for Th17 differentiation in human are still being defined.15,20-22 The role of TGFβ in Th17 development is surprising, given that TGFβ promotes adaptive Treg differentiation in murine models and enhances FoxP3 expression in the human system.23 Yet recent studies in mouse have indicated that TGFβ and IL-6 can induce IL-17 production in Tregs.24,25 Despite the apparent duality in Treg/Th17 function, it is becoming increasingly clear that these cell subsets share common cytokine signaling pathways.
The memory CD4+CD25high human Treg lineage can be subdivided into 2 functionally distinct subsets classified by expression of the major histocompatibility complex (MHC) class II dimer human leukocyte antigen–DR (HLA-DR).26 DR+ Tregs do not enter into cell cycle with T-cell receptor (TCR) cross-linking and exhibit immediate contact-dependent suppressor function. In contrast, DR− Tregs can enter into cell cycle after activation, secrete the inhibitory cytokine interleukin-10 (IL-10) but not the effector cytokine interferon-γ (IFNγ), and demonstrate delayed kinetics of suppression, requiring 4 to 5 days for maximal inhibition of proliferation of responder CD4+ T cells.26
We have previously reported that, in cocultures of human Tregs and responder T cells (Tresp) provided with strong TCR stimulation, the Tresp are refractive to suppression.27 At the time, we hypothesized that this strong signal strength could be provided by foreign antigen but not self-antigen, allowing discrimination between pathologic microbial infections and physiologic responses to autoantigens. The mechanism for loss of suppression in this model remains unknown. It is likely that not only Tresp resistance, but also impairment of Treg function, contributes to the observed lack of suppression. This led us to hypothesize that Tregs may be capable of assuming Th17 effector function under the same microenvironmental conditions that drive CD4+ cells to secrete IL-17.
Here, we demonstrate that DR− Tregs, but not DR+ Tregs, are able to secrete IL-17 ex vivo and in vitro. IL-17 production by DR− Tregs was favored by the proinflammatory cytokines IL-1β and IL-6, but was inhibited by TGFβ. Moreover, only Tregs expressing the chemokine receptor CCR6 ex vivo were able to produce IL-17. DR− Tregs stimulated under conditions that promote Tresp suppression did not secrete IL-17; whereas in conditions where strong TCR signals and APCs were present, DR− Tregs were no longer suppressive and gained the capacity to secrete IL-17. We confirmed the dual function of DR− Tregs with single-cell cloning, in which we isolated individual Tregs and assayed for both suppressive and effector functions. We observed that a subset of FoxP3+DR− clones suppressed only in response to weak TCR stimulation and that these clones transiently lost regulatory function and secreted IL-17 in response to strong TCR signals and APCs. These data suggest a mechanism of immune regulation by which inflammation can drive DR− Tregs to affect an effector Th17 phenotype, allowing the rapid shut down of suppression and induction of proinflammatory responses.
Methods
Cell-culture reagents and antibodies
Cells were cultured in 96-well U-bottom plates (Costar; Corning, Lowell, MA) in serum-free X-Vivo 15 medium, or in RPMI 1640 medium (both from BioWhittaker, Walkersville, MD) supplemented as described previously27 with 5% human AB serum (Cellgro; Mediatech, Manassas, VA). The monoclonal antibodies (mAbs) αCD3 (clone UCHT1) and αCD28 (clone 28.2) were purchased from BD Biosciences (San Jose, CA). To generate αCD3/αCD2 beads, tosyl-activated beads (Dynal Biotech-Invitrogen, Carlsbad, CA) were covalently bound with αCD3 (UCHT1) and αCD2 (BMA 0111; Dade Behring, Deerfield, IL) mAbs at 1 μg/107 beads per the manufacturer's instructions. Recombinant human interleukin-2 (IL-2) was obtained through the AIDS Research and Reference Reagent Program, Division of AIDS, National Institute of Allergy and Infectious Diseases (NIAID), National Institutes of Health (NIH): human rIL-2 from Dr Maurice Gately, Hoffmann-La Roche (Nutley, NJ). This study was approved by the Institutional Review Board at the Brigham and Women's Hospital.
Cell purification and sorting
Whole mononuclear cells were isolated from human blood drawn from healthy control volunteers, after informed consent was obtained in accordance with the Declaration of Helsinki, in green-capped, lithium-heparinized tubes by Ficoll-Hypaque (GE Healthcare, Little Chalfont, United Kingdom) gradient centrifugation. This work was approved by the Institutional Review Board at the Brigham and Women's Hospital. Total CD4 T cells were isolated by negative selection via the CD4+ T-cell isolation kit II (Miltenyi Biotec, Bergisch Gladbach, Germany) and stained for fluorescence-activated cell sorting (FACS) with αCD45RA (HI100), αCD25 (M-A251) and αHLA-DR (L-243) mAbs. All antibodies were purchased from BD Biosciences and used after they had been dialyzed to remove azide. The specific DR− Treg (CD45RA−CD25highDR−), DR+ Treg (CD45RA−CD25highDR+), memory Tresp (CD45RA−CD25med) and naive Tresp (CD45RA+CD25−) populations were isolated by sorting with a FACSAria (BD Biosciences) to typically greater than 98% purity in postsort analysis. CCR6+ and CCR6− populations of DR− Tregs and memory Tresp were isolated as described above, with an additional αCCR6 mAb (53103, R&D Systems, Minneapolis, MN). In coculture assays, total CD4 Tresp (sorted as CD25med/low), including naive and memory cells, were used. T-depleted APCs were isolated from peripheral blood mononuclear cells (PBMCs) by negative selection with αCD2 magnetic beads (Dynal) and irradiated with 3000 rad.
T-cell stimulation
Tresp and Treg cell populations (104/well) were stimulated in serum-free X-Vivo medium with phorbal myristate acetate (PMA; 50 ng/mL) and ionomycin (250 ng/mL; both from Sigma-Aldrich, St Louis, MO) for 4 to 20 hours, or for 5 days with plate-bound αCD3 (1.5 μg/mL), soluble αCD28 (1.5 μg/mL) and IL-2 (50 U/mL). Where indicated, recombinant human IL-1β (12.5 ng/mL), IL-6 (25 ng/mL) or TGFβ (5 ng/mL; all from R&D Systems) were added at the start of the cultures.
Suppression assays
In suppression assays, carboxyfluorescein succinimidyl ester (CFSE)–labeled Tresp cells and unlabeled Treg cells (104/well) were cultured in RPMI 1640 medium containing 5% human serum, either alone or in coculture (1:1 ratio) in the absence of IL-2. Cells were stimulated with αCD3/αCD2 beads (4 × 104/well), or with irradiated T-depleted APCs (4 × 104/well) and plate-bound αCD3 (0.1 μg/mL or 0.5 μg/mL). To determine proliferation, fluorescence was assessed on day 4 of stimulation on a FACSCalibur (BD Biosciences) and analyzed using FlowJo software (TresStar, Ashland, OR).
Generating single-cell clones
Tresp and Treg cells were sorted at one cell per well in RPMI 1640 medium containing 5% human serum and stimulated with soluble αCD3 (clone Hit3a, BD Biosciences) and αCD28 (both at 1 μg/mL), irradiated APCs (1-5 × 104/well) and IL-2 (50 U/mL). Half of the medium was replaced with fresh medium containing IL-2 (50 U/mL) starting at day 10 and every 3 to 4 days thereafter. After 5 weeks of expansion, each clone was tested for FoxP3 expression, IL-17 production and suppressive activity. To assess suppressive function, a portion of each clone was intensively washed to remove IL-2 and stimulated (1250/well) alone or in coculture at 1:1/2 (Tresp:clone) ratios with freshly isolated Tresp (2500/well). Two different stimuli were used: αCD3/αCD2 beads (104/well) or plate-bound αCD3 (0.5 μg/mL) and irradiated APCs (104/well). To determine proliferation, the culture supernatant (100 μL) was replaced on day 4 with 1 μCi [3H]thymidine (NEN) for a 16-hour pulse.
Enzyme-linked immunosorbent assay and intracellular cytokine staining
Cytokine secretion in culture supernatants was analyzed by enzyme-linked immunosorbent assay (ELISA; IL-17A, DuoSet ELISA; R&D Systems) or by cytometric bead arrays (Human Th1/Th2 cytokine kit II; BD Biosciences). For intracellular staining, cells were stimulated for 4 hours with PMA (50 ng/mL) and ionomycin (250 ng/mL) in the presence of GolgiStop (BD Biosciences). Cells were fixed and made permeable (Fix/Perm; eBioscience, San Diego, CA) according to the manufacturer's instructions, and incubated at room temperature with αFoxP3 (206D; Biolegend, San Diego, CA), αIL-17 (eBio64-DEC17; eBioscience), αIFNγ (B27, BD Biosciences) and αIL-2 (MQ1-17H12, BD Biosciences) mAbs. When APCs were used in the stimulation, staining with αCD2 (RPA-2.10; BD Biosciences) was performed before fixation to allow gating on CD2+ T cells.
Real-time polymerase chain reaction analysis
RNA was isolated via the RNeasy Micro, RNase-free DNase procedure (Qiagen, Germantown, MD), quantified using a spectrophotometer (ND-1000; Nanodrop, Wilmington, DE), and converted to cDNA (0.2 μg RNA/reaction) via reverse transcription (RT) by random hexamers and Multiscribe RT (TaqMan Gold RT-PCR kit; Applied Biosystems, Foster City, CA). The primers used for this study were purchased from Applied Biosystems: β2m (4326319), IL-17A (HS00174383_M1), and retinoic acid–related orphan receptor C (RORC) (HS01076112_M1). The values are represented as the difference in Ct values normalized to β2-microglobulin for each sample as per the following formula: Relative RNA expression = (2−dCt) × 1000.
Statistics
Bar graphs are represented as mean plus or minus standard error of the mean (SEM). A standard 2-tailed t test was used for statistical analysis; P values of .05 or less were considered significant.
Results
Sorted DR− Tregs, but not DR+ Tregs, produce IL-17 in response to short-term mitogenic activation
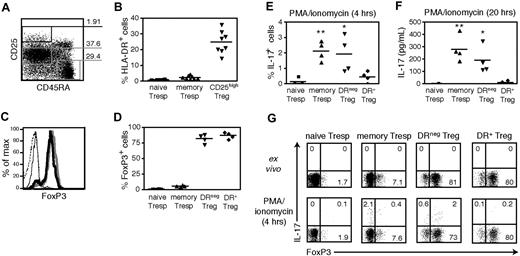
Our group has identified 2 functionally distinct subsets of human peripheral Tregs, which are defined by ex vivo MHC class II (HLA-DR) expression.26 In light of recent findings on IL-17 expression in murine Tregs, we examined the ability of the human DR+ and DR− Treg subsets to produce IL-17. Peripheral blood CD4 T cells were FACS-sorted into populations of naive Tresp (CD45RA+CD25−), memory Tresp (CD45RA−CD25int), DR− Tregs (CD45RA−CD25highDR−) and DR+ Tregs (CD45RA−CD25highDR+), the latter representing approximately 25% of CD4+CD25high Treg cells (Figure 1A,B). The FACS-sorted CD25high populations were greatly enriched in FoxP3+ Tregs, as 82.2% (±7%) of DR− Tregs and 86.9% (± 5.3%) of DR+ Tregs expressed FoxP3 ex vivo (n = 4; Figure 1C,D). As we have previously reported,26 both Treg subsets expressed CD62L and CD45RO and functionally suppressed proliferation of CD4 cells.
Sorted DR− Tregs, but not DR+ Tregs, produce IL-17 in response to short-term mitogenic activation. (A) Gating strategy for FACS sorting of CD4+ peripheral blood T cells into populations of Tregs (CD45RA−CD25high), memory Tresps (CD45RA−CD25med), and naive Tresps (CD45RA+CD25−). (B) Percentages of HLA-DR+ cells within the 3 different gates (n = 9). (C) FoxP3 intracellular staining of FACS-sorted naive Tresps (dashed line), memory Tresps (thin solid line), DR− Tregs (bold line), and DR+ Tregs (gray line). (D) The percentages of FoxP3+ cells is shown (n = 4). (E) FACS-sorted naive Tresps, memory Tresps, DR− Tregs, and DR+ Tregs (104 cells/well) were stimulated in serum-free X-Vivo medium for 4 hours with PMA/ionomycin and GolgiStop, and stained for intracellular IL-17 (n = 4). (F) IL-17 levels measured by ELISA in the same samples after 20 hours of stimulation with PMA/ionomycin. (G) One representative staining of intracellular FoxP3 and IL-17 in the 4 populations ex vivo or after 4 hours of stimulation with PMA/ionomycin and GolgiStop. **P < .01; *P < .05.
Sorted DR− Tregs, but not DR+ Tregs, produce IL-17 in response to short-term mitogenic activation. (A) Gating strategy for FACS sorting of CD4+ peripheral blood T cells into populations of Tregs (CD45RA−CD25high), memory Tresps (CD45RA−CD25med), and naive Tresps (CD45RA+CD25−). (B) Percentages of HLA-DR+ cells within the 3 different gates (n = 9). (C) FoxP3 intracellular staining of FACS-sorted naive Tresps (dashed line), memory Tresps (thin solid line), DR− Tregs (bold line), and DR+ Tregs (gray line). (D) The percentages of FoxP3+ cells is shown (n = 4). (E) FACS-sorted naive Tresps, memory Tresps, DR− Tregs, and DR+ Tregs (104 cells/well) were stimulated in serum-free X-Vivo medium for 4 hours with PMA/ionomycin and GolgiStop, and stained for intracellular IL-17 (n = 4). (F) IL-17 levels measured by ELISA in the same samples after 20 hours of stimulation with PMA/ionomycin. (G) One representative staining of intracellular FoxP3 and IL-17 in the 4 populations ex vivo or after 4 hours of stimulation with PMA/ionomycin and GolgiStop. **P < .01; *P < .05.
We stimulated the 4 different Treg and Tresp populations directly ex vivo with PMA and ionomycin to examine their ability to produce IL-17 by intracellular staining (4 hours) or ELISA (20 hours). As shown in Figure 1E, a low but significant fraction of memory Tresp produced IL-17 after only 4 hours of activation, as compared with similarly stimulated naive Tresp (P = .001, n = 4). Interestingly, DR−, but not DR+ Tregs, also produced IL-17 (P = .025 vs naive Tresp). After 20 hours of activation, both memory Tresp and DR− Tregs secreted significantly greater amounts of IL-17 than the DR+ Treg population (P = .002 and P = .017, respectively, n = 4; Figure 1F). While the DR−CD25high cells are enriched for Tregs, they also contain 15 to 20% FoxP3− nonregulatory cells. To confirm that IL-17 secretion within the DR− Treg population was derived from FoxP3+ cells, we costained these cells for FoxP3 and IL-17 (Figure 1G). We observed that more than 75% of the IL-17 producing cells expressed high levels of FoxP3. The slight reduction in the frequency of FoxP3-expressing cells observed in these strongly activated DR− Tregs was primarily the result of cell death (data not shown). Short-term mitogenic activation was sufficient to induce IL-17 secretion in ex vivo DR− Tregs, indicating that FoxP3+IL-17+ cells are fully differentiated IL-17 competent cells at the time of isolation. These data demonstrate that a small subset within the memory CD25highDR−FoxP3+ population has the capacity to secrete IL-17.
IL-17 secretion by DR− Tregs is induced by IL-1β and IL-6 and inhibited by TGFβ
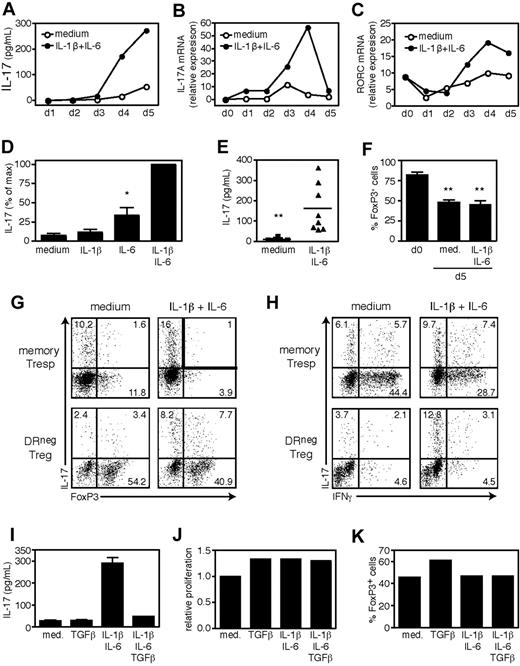
Cytokine production by memory T cells is influenced by both TCR stimulation and the cytokine milieu. IL-1β and IL-6 have been shown to promote IL-17 secretion by memory Th17 cells.15,16 Our preliminary data suggested that stimulation of DR− Tregs in the presence of APCs and strong TCR signals induced IL-17, an effect that was strongly reduced by blocking the activity of IL-1β and IL-6 (data not shown). To determine whether IL-1β and IL-6 drove IL-17 secretion by Tregs, we stimulated FACS-sorted DR− Tregs for 5 days in serum-free X-Vivo medium with αCD3/αCD28 and IL-2 in the presence of exogenous IL-1β and IL-6. As shown in Figure 2A, the combination of IL-1β and IL-6 strongly induced IL-17 production by DR− Tregs, which was detected in culture supernatants by day 4 of stimulation, or at the level of IL-17A mRNA 24 hours earlier (Figure 2B). IL-17 secretion by IL-1β/IL-6 stimulated DR− Tregs was preceded by increased RORC mRNA expression, the human homologue for the Th17-associated transcription factor RORγt28 (Figure 2C). Although we found that IL-6 had greater effects than IL-1β, the combination of both cytokines had synergistic effects (Figure 2D) and consistently increased the amount of IL-17 secreted by DR− Tregs (P = .003, n = 8; Figure 2E). Similarly stimulated DR+ Tregs failed to secrete IL-17 in response to IL-1β/IL-6 (data not shown).
IL-17 secretion by DR− Tregs is induced by IL-1β and IL-6 and inhibited by TGFβ. FACS-sorted DR− Tregs (104 cells/well) were stimulated in serum-free X-Vivo medium for 5 days with plate-bound αCD3, soluble αCD28, and IL-2 in the presence of exogenous IL-1β and IL-6. Supernatants and cells were harvested at 24-hour intervals and analyzed for IL-17 content by ELISA (A) or RNA expression of IL-17A (B) and RORC (C) by real-time polymerase chain reaction (PCR). Data are representative of 2 independent experiments. (D) IL-17 secretion measured by ELISA in day 5 supernatants and represented as percentage of max (n = 5). (E) The IL-17 levels in day 5 supernatants are shown (n = 8). (F) Percentages of FoxP3+ cells ex vivo (n = 4) or on day 5 of stimulation (n = 8). At the end of the culture, cells were pulsed for 4 hours with PMA/ionomycin and GolgiStop, and stained for intracellular IL-17 versus FoxP3 (G) or IFNγ (H). Data are representative of 4 independent experiments. (I) DR− Tregs were stimulated for 5 days in the presence of exogenous IL-1β/IL-6 or TGFβ and tested for IL-17 secretion by ELISA. (J) Although the cultures were established at identical cell numbers, the relative cell numbers at the end of culture were determined for each condition by flow cytometry and represented as relative proliferation versus untreated cells. (K) Percentages of FoxP3+ cells on day 5 of stimulation. Data are representative of 5 independent experiments. **P < .01; *P < .05.
IL-17 secretion by DR− Tregs is induced by IL-1β and IL-6 and inhibited by TGFβ. FACS-sorted DR− Tregs (104 cells/well) were stimulated in serum-free X-Vivo medium for 5 days with plate-bound αCD3, soluble αCD28, and IL-2 in the presence of exogenous IL-1β and IL-6. Supernatants and cells were harvested at 24-hour intervals and analyzed for IL-17 content by ELISA (A) or RNA expression of IL-17A (B) and RORC (C) by real-time polymerase chain reaction (PCR). Data are representative of 2 independent experiments. (D) IL-17 secretion measured by ELISA in day 5 supernatants and represented as percentage of max (n = 5). (E) The IL-17 levels in day 5 supernatants are shown (n = 8). (F) Percentages of FoxP3+ cells ex vivo (n = 4) or on day 5 of stimulation (n = 8). At the end of the culture, cells were pulsed for 4 hours with PMA/ionomycin and GolgiStop, and stained for intracellular IL-17 versus FoxP3 (G) or IFNγ (H). Data are representative of 4 independent experiments. (I) DR− Tregs were stimulated for 5 days in the presence of exogenous IL-1β/IL-6 or TGFβ and tested for IL-17 secretion by ELISA. (J) Although the cultures were established at identical cell numbers, the relative cell numbers at the end of culture were determined for each condition by flow cytometry and represented as relative proliferation versus untreated cells. (K) Percentages of FoxP3+ cells on day 5 of stimulation. Data are representative of 5 independent experiments. **P < .01; *P < .05.
We observed that in vitro–stimulated Tregs have reduced FoxP3 expression, even in the presence of exogenous IL-2. Indeed, the 5-day stimulation of DR− Tregs typically resulted in a decrease in the frequency of cells expressing FoxP3 as compared with ex vivo frequencies, regardless of the presence of IL-1β/IL-6 (FoxP3+ cells: 48.1% ± 9.4% and 45.2% ± 16.1% vs 82.2% ± 7%, respectively, n = 8; Figure 2F). To determine whether or not the secreted IL-17 was derived from the FoxP3+ or the FoxP3− population, we stained memory Tresp and DR− Tregs for intracellular expression of FoxP3 and IL-17 on day 5 of stimulation. As shown in Figure 2G, stimulation of DR− Tregs in the presence of IL-1β/IL-6 resulted in an increased frequency of IL-17 secreting cells, both in the FoxP3+ and the FoxP3− populations. These proinflammatory cytokines failed to induce other effector cytokines in DR− Tregs, such as IFNγ (Figure 2H).
It is likely that the FoxP3− cells (IL-17+ and IL-17−) observed in the 5-day stimulated DR− cultures represent expanded Tresp contaminants, although the possibility remains that some of these cells initially expressed FoxP3 and subsequently lost FoxP3 expression upon activation. Yet regardless of the presence of FoxP3−/IL-17+ cells, it is clear that stimulation of DR− Tregs in the presence of IL-1β/IL-6 results in the induction of a double-positive FoxP3highIL-17high population.
Given that TGFβ plays a fundamental role in the induction and maintenance of FoxP3 expressing Tregs,29 but can also drive naive human CD4 T cells to differentiate into Th17 cells when provided in concert with proinflammatory cytokines,15,19,20 we investigated whether exogenous TGFβ would alter IL-17 production by DR− Tregs. We cultured these cells with αCD3/αCD28 and IL-2 in the presence of IL-1β/IL-6 and TGFβ, and assayed them for IL-17 secretion, proliferation and FoxP3 expression. Strikingly, the addition of TGFβ to DR− Tregs treated with IL-1β/IL-6 significantly abrogated IL-17 secretion (inhibition 82.5% ± 4.1%, n = 5; Figure 2I). This inhibition was not due to impaired proliferation or up-regulation of FoxP3, as TGFβ did not affect the proliferative capacity (Figure 2J) or FoxP3 expression (Figure 2K) of IL-1β/IL-6 treated DR− Tregs. We concluded that IL-17 secretion by DR− Tregs was promoted by the combination of IL-1β and IL-6, but negatively controlled by TGFβ.
The DR− Treg that produce IL-17 express CCR6 ex vivo
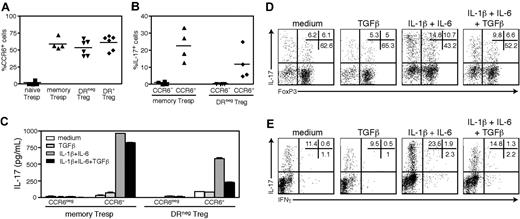
We next investigated whether the IL-17 secreting cells found within the DR− Treg population expressed the Th17-associated chemokine receptor CCR6.30 As shown in Figure 3A, CCR6 was expressed on approximately 60% of the ex vivo memory Tresp, DR− Treg cells and DR+ Tregs and, as expected, was not detected on naive responder cells. To determine whether IL-17+FoxP3+ cells were exclusively derived from the CCR6+ population, we FACS-isolated CCR6− and CCR6+ cells of the memory Tresp and DR− Treg populations and stimulated them for 5 days with αCD3/αCD28 and IL-2 in the presence of IL-1β/IL-6. As shown in Figure 3B, only the cells derived from the CCR6+ subsets secreted IL-17 (P = .03, memory Tresp and P = .005, DR− Tregs vs CCR6−, n = 4), indicating that ex vivo expression of CCR6 discriminates DR− Tregs with the potential to secrete IL-17. Yet, as more than half of the DR− Tregs and the IL-17−DR+ Tregs expressed CCR6, expression of CCR6 alone was insufficient to identify IL-17+ Tregs. Further separation of DR− Tregs with CCR4, which together with CCR6 identifies Th17 cells secreting IL-17 but not IFNγ,30 did not enrich for IL-17+ cells, as virtually all the CD25highCCR6+ cells coexpressed CCR4 (data not shown).
The DR− Tregs that produce IL-17 express CCR6 ex vivo. (A) Percentages of CCR6+ cells in gated ex vivo naive Tresps (n = 4), memory Tresps (n = 4), DR− Tregs (n = 6) and DR+ Tregs (n = 6). (B) CCR6− and CCR6+ fractions of DR− Tregs or memory Tresps were FACS-sorted and stimulated for 5 days with αCD3/αCD28 and IL-2 in the presence of IL-1β/IL-6. The percentages of IL-17+ cells at the end of the culture is shown (n = 4). (C) Cells were stimulated in the presence of IL-1β/IL-6 or TGFβ, and analyzed for IL-17 secretion by ELISA. At the end of the culture, CCR6+DR− Tregs were stained for intracellular IL-17 versus FoxP3 (D) or IFNγ (E). Data are representative of 4 independent experiments. **P < .01; *P < .05.
The DR− Tregs that produce IL-17 express CCR6 ex vivo. (A) Percentages of CCR6+ cells in gated ex vivo naive Tresps (n = 4), memory Tresps (n = 4), DR− Tregs (n = 6) and DR+ Tregs (n = 6). (B) CCR6− and CCR6+ fractions of DR− Tregs or memory Tresps were FACS-sorted and stimulated for 5 days with αCD3/αCD28 and IL-2 in the presence of IL-1β/IL-6. The percentages of IL-17+ cells at the end of the culture is shown (n = 4). (C) Cells were stimulated in the presence of IL-1β/IL-6 or TGFβ, and analyzed for IL-17 secretion by ELISA. At the end of the culture, CCR6+DR− Tregs were stained for intracellular IL-17 versus FoxP3 (D) or IFNγ (E). Data are representative of 4 independent experiments. **P < .01; *P < .05.
Having made this observation, we used CCR6+CD25highDR− cells to verify the inhibitory effects of TGFβ on IL-17 secretion. We found that although TGFβ had little effect on IL-17 secretion by CCR6+ memory Tresp cells, it strongly dampened IL-17 secretion in both the FoxP3+ and FoxP3− cells derived from the CCR6+DR− Tregs (Figure 3C,D). Further confirming our previous observations, the effector cytokine IFNγ was expressed only at very low levels in the CCR6+DR− Treg cultures (Figure 3E).
IL-17 secretion upon coculture of DR− Tregs with Tresp cells inversely correlates with suppressive activity
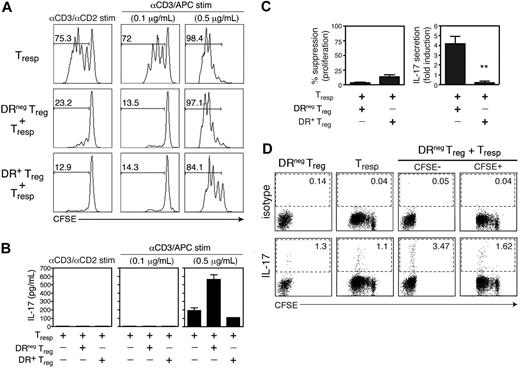
We next examined whether IL-17 production by Tregs was associated with changes in suppressive activity. As we have previously reported, the suppressive function of Tregs is reduced in response to increased TCR signal strength.27 We wanted to know whether this loss of suppression was associated with induction of IL-17 secretion as well. To answer this question, we stimulated FACS-sorted DR− Tregs, DR+ Tregs, and CFSE-labeled CD25− Tresp cells for 4 days alone or together in 1:1 Treg:Tresp cocultures stimulated with αCD3/αCD2 beads or with increasing concentrations of plate-bound αCD3 and APCs. As shown in Figure 4A, stimulation with αCD3/αCD2 beads or a low dose of αCD3 (0.1 μg/mL) and APCs permitted complete suppression of responder proliferation. This suppression was accompanied by the absence of detectable IL-17 (Figure 4B). In contrast, stimulation of these cocultures with a high dose of αCD3 (0.5 μg/mL) and APCs only allowed partial suppression by the DR+ Tregs, and no suppression at all by the DR− Tregs. This stronger stimulation in the presence of APCs resulted in the striking induction of IL-17 in the DR− but not in the DR+ Treg cocultures (fold induction vs Tresp-only cultures: 4.16 ± 1.54 and 0.22 ± 0.29, respectively, P = .002, n = 4; Figure 4B,C). These data indicate that the loss of suppression that occurs with strong TCR signals in the presence of APCs correlates with increased IL-17 secretion in the DR− Treg cocultures.
IL-17 secretion upon coculture of DR− Tregs with Tresp cells inversely correlates with suppression. (A) CFSE-labeled CD25− Tresp cells were cultured in RPMI medium containing 5% human AB serum, alone or with DR− Treg or DR+ Treg (1:1 ratio) under 3 different stimulatory conditions: αCD3/αCD2 beads (left), or irradiated T-cell depleted APCs and plate-bound αCD3 at 0.1 μg/mL (middle) or 0.5 μg/mL (right). On day 4 of stimulation, proliferation of Tresp cells was analyzed by assessing CFSE dilution by FACS analysis. The numbers represent the percentage of Tresp cells that divided (CFSE dilution). (B) IL-17 production in these differentially stimulated Tresp cultures or DR− or DR+ Treg cocultures was analyzed by ELISA from day 4 supernatants. Data shown are representative of 4 independent experiments. (C) The results of the same assay performed on 4 independent donors and represented as percentage suppression of proliferation and fold induction of IL-17 in DR− and DR+ Treg cocultures stimulated with αCD3 (0.5 μg/mL) and APCs (mean + SEM). (D) Cultures containing CFSE-labeled Tresp cells, DR− Tregs, or both cell types (1:1 ratio) were stimulated with high dose αCD3 and APCs. On day 4, PMA/ionomycin and GolgiStop were added to the cultures for a 4-hour pulse before the cells were harvested, surface stained with αCD2 to distinguish the CD4 T cell populations from the irradiated, T-depleted APCs, and then permeabilized and stained for IL-17. Data shown are representative of 3 independent experiments. **P < .01.
IL-17 secretion upon coculture of DR− Tregs with Tresp cells inversely correlates with suppression. (A) CFSE-labeled CD25− Tresp cells were cultured in RPMI medium containing 5% human AB serum, alone or with DR− Treg or DR+ Treg (1:1 ratio) under 3 different stimulatory conditions: αCD3/αCD2 beads (left), or irradiated T-cell depleted APCs and plate-bound αCD3 at 0.1 μg/mL (middle) or 0.5 μg/mL (right). On day 4 of stimulation, proliferation of Tresp cells was analyzed by assessing CFSE dilution by FACS analysis. The numbers represent the percentage of Tresp cells that divided (CFSE dilution). (B) IL-17 production in these differentially stimulated Tresp cultures or DR− or DR+ Treg cocultures was analyzed by ELISA from day 4 supernatants. Data shown are representative of 4 independent experiments. (C) The results of the same assay performed on 4 independent donors and represented as percentage suppression of proliferation and fold induction of IL-17 in DR− and DR+ Treg cocultures stimulated with αCD3 (0.5 μg/mL) and APCs (mean + SEM). (D) Cultures containing CFSE-labeled Tresp cells, DR− Tregs, or both cell types (1:1 ratio) were stimulated with high dose αCD3 and APCs. On day 4, PMA/ionomycin and GolgiStop were added to the cultures for a 4-hour pulse before the cells were harvested, surface stained with αCD2 to distinguish the CD4 T cell populations from the irradiated, T-depleted APCs, and then permeabilized and stained for IL-17. Data shown are representative of 3 independent experiments. **P < .01.
To determine whether the responder T cell or the Treg population produced IL-17, we stimulated cocultures of CFSE-labeled Tresp and unlabeled DR− Tregs with high dose αCD3 and APCs for 4 days, followed by intracellular staining for IL-17 (Figure 4D). Although both the Tresp and the DR− Tregs produced IL-17 when stimulated alone, approximately 3-fold more DR− Tregs produced IL-17 when stimulated in the coculture (the frequency of IL-17 producing Tresp cells did not change). These data are consistent with the hypothesis that increasing the strength of stimulation in the presence of APCs causes DR− Tregs to shift to a nonsuppressive phenotype that is accompanied by IL-17 secretion.
FoxP3+/IL-17+ clones derived from the DR− Treg population exhibit in vitro suppressive function
To definitively demonstrate the dual capacity of a single Treg cell to both suppress and produce IL-17, we performed single cell cloning of DR− Tregs, DR+ Tregs and CD25− Tresp cells. Each population was seeded at one cell per well with FACS-sorting to ensure that each clone was derived from a single cell. From 60 wells, we generated 22 independent T-cell clones from the DR− Treg population and 34 from the Tresp population (36.6% and 56.6% cloning efficiency, respectively), while, as previously reported,26 the DR+ Tregs exhibited poor cloning efficiency (1.7%). After expansion, 16 clones from the DR− Treg population (the others were too low in cell number) and 9 from the Tresp population were examined in detail for their ability to express FoxP3, produce IL-17, and suppress Tresp proliferation. Suppressive activity was assessed in cocultures with freshly isolated Tresp cells stimulated with αCD3/αCD2 beads, a stimulus that permitted suppression, but not IL-17 secretion, with ex vivo DR− Tregs.
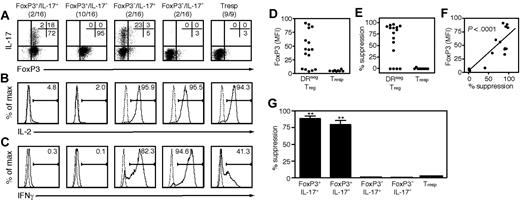
We partitioned the clones into 4 subgroupings based on FoxP3 and IL-17 expression (Figure 5A): FoxP3+/IL-17+ clones (2/16, 12.5%), FoxP3+/IL-17− (10/16, 62.5%), FoxP3−/IL-17+ (2/16, 12.5%) and FoxP3−/IL-17− (2/16, 12.5%). Our control population, T-cell clones derived from CD25− Tresp cells (9/9), lacked both FoxP3 and IL-17. Both FoxP3+/IL-17+ and FoxP3+/IL-17− clones exhibited phenotypic Treg features, as they lacked IL-2 (Figure 5B) and IFNγ (Figure 5C), whereas FoxP3− and Tresp clones strongly expressed IL-2, and typically produced IFNγ. Remarkably, FoxP3 levels highly correlated with suppression (r2 = 0.6788, P < .001; Figure 5B-D) and FoxP3+/IL-17+ clones demonstrated suppressive activity similar to that of FoxP3+/IL-17− clones (Figure 5E). These data demonstrate that the FoxP3+/IL-17+ cells are potent suppressors. From replicate cloning studies, the frequency of these dual suppressor/IL-17–producer clones was estimated to be 8.9% (±3.6%) of the CD25highDR− population.
One subset of DR− Treg clones can suppress or secrete IL-17 in response to different stimuli. The clones that grew from wells seeded at 1 DR− Treg per well were analyzed for several features and compared with clones derived from Tresp cells. After 5 weeks of expansion, a portion of each clone was stained for FoxP3 and IL-17 expression and tested for ability to suppress the proliferation of freshly isolated Tresp cells in cocultures stimulated with αCD3/αCD2 beads. On day 4, half the media in each well replaced with 3H thymidine to monitor proliferation. Data are representative of 2 independent cloning experiments. Intracellular expression of FoxP3/IL-17 (A), IL-2 (B), and IFNγ (C) of DR− Treg clones after 4 hours of stimulation with PMA/ionomyicin and GolgiStop. One representative clone from each pattern is shown. (D) Mean FoxP3 expression by each clone. (E) The suppressive capacity of each clone represented as percentage suppression. (F) Mean FoxP3 expression by each clone is shown relative to its suppressive ability. (G) Suppression by each pattern of clone.
One subset of DR− Treg clones can suppress or secrete IL-17 in response to different stimuli. The clones that grew from wells seeded at 1 DR− Treg per well were analyzed for several features and compared with clones derived from Tresp cells. After 5 weeks of expansion, a portion of each clone was stained for FoxP3 and IL-17 expression and tested for ability to suppress the proliferation of freshly isolated Tresp cells in cocultures stimulated with αCD3/αCD2 beads. On day 4, half the media in each well replaced with 3H thymidine to monitor proliferation. Data are representative of 2 independent cloning experiments. Intracellular expression of FoxP3/IL-17 (A), IL-2 (B), and IFNγ (C) of DR− Treg clones after 4 hours of stimulation with PMA/ionomyicin and GolgiStop. One representative clone from each pattern is shown. (D) Mean FoxP3 expression by each clone. (E) The suppressive capacity of each clone represented as percentage suppression. (F) Mean FoxP3 expression by each clone is shown relative to its suppressive ability. (G) Suppression by each pattern of clone.
IL-17 production by FoxP3+/IL-17+ clones is associated with concomitant loss of suppressive function
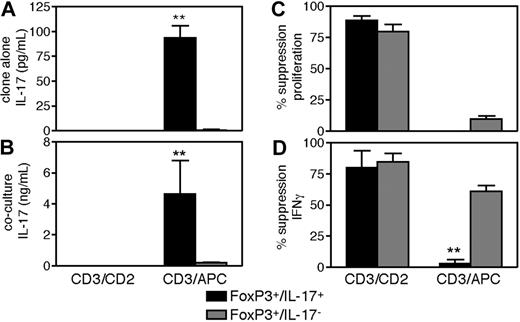
To determine whether IL-17 production by FoxP3+/IL-17+ clones modified their suppressive activity, these clones were assayed in cocultures established with αCD3/αCD2 beads or with high dose αCD3 and APCs, a stimulus that permitted IL-17 production in DR− cocultures. As shown in Figure 6, FoxP3+/IL-17+ but not FoxP3+/IL-17− clones secreted IL-17 with strong αCD3/APC stimulation when stimulated alone (P < .001; Figure 6A) or in coculture (P < .001; Figure 6B), while stimulation with αCD3/αCD2 failed to induce IL-17 secretion as we previously observed with the ex vivo Treg population. Although stimulation with αCD3 and APCs was too strong a signal to enable suppression of responder T-cell proliferation (Figure 6C), the FoxP3+/IL-17− clones efficiently suppressed IFNγ production, while the FoxP3+/IL-17+ clones did not (P = .001; Figure 6D). Both types of clones, however, suppressed IFNγ production with αCD3/αCD2 stimulation. The failure of FoxP3+/IL-17+ clones to suppress IFNγ production when they produce IL-17 suggests that maturation into IL-17–producers results in a concomitant loss of suppressive function, although the possibility remains that these 2 mechanisms are unrelated consequences of strong TCR stimulation. Stimulation with αCD3/αCD2 beads and APCs failed to induce IL-17 and allowed complete suppression of proliferation and IFNγ production (data not shown), indicating that not only APC-derived factors, but also strong TCR signals were required for induction of IL-17 and loss of suppressive function, while with the strongest αCD3/αCD28/IL-1β/IL-6 stimulation, none of the clones demonstrated suppressive capacity (data not shown).
IL-17 production by FoxP3+/IL-17+ clones results in concomitant loss of suppressive function. The suppressive and effector functions of the clones were assessed in cocultures stimulated with αCD3/αCD2 beads or with 0.5 μg/mL αCD3 and APCs. On day 4, half the media in each well was removed, interrogated for content of IL-17 and IFNγ by ELISA, and replaced with 3H thymidine to monitor proliferation. Two representative clones for each pattern are shown. Data are representative of 2 independent cloning experiments. Panels show the ability of the clones to secrete IL-17 in response to the different stimuli (A), the levels of IL-17 induced in the respective clone cocultures (B), the ability of the clones to suppress Tresp proliferation (C), and the ability of the clones to suppress IFNγ production (D). **P < .01.
IL-17 production by FoxP3+/IL-17+ clones results in concomitant loss of suppressive function. The suppressive and effector functions of the clones were assessed in cocultures stimulated with αCD3/αCD2 beads or with 0.5 μg/mL αCD3 and APCs. On day 4, half the media in each well was removed, interrogated for content of IL-17 and IFNγ by ELISA, and replaced with 3H thymidine to monitor proliferation. Two representative clones for each pattern are shown. Data are representative of 2 independent cloning experiments. Panels show the ability of the clones to secrete IL-17 in response to the different stimuli (A), the levels of IL-17 induced in the respective clone cocultures (B), the ability of the clones to suppress Tresp proliferation (C), and the ability of the clones to suppress IFNγ production (D). **P < .01.
The loss of suppressive function in FoxP3+/IL-17+ clones that have been induced to secrete IL-17 is reversible
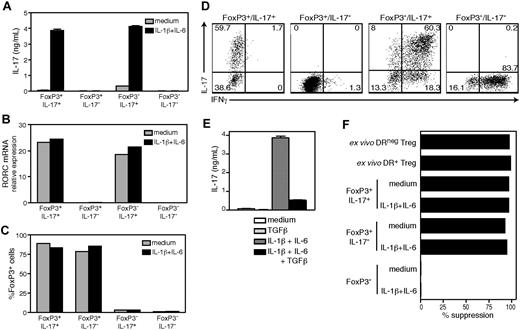
To determine whether the loss of suppression that occurs in FoxP3+/IL-17+ clones when they secrete IL-17 was reversible, we first induced IL-17 secretion, then allowed the clones to return to a resting, non–IL-17–producing state before testing them in suppression assays. Stimulation with αCD3/αCD28/IL-2 in the presence of IL-1β/IL-6 strongly induced IL-17 in FoxP3+/IL-17+ clones and FoxP3−/IL-17+ clones (Figure 7A). These conditions, however, failed to induce IL-17 production by FoxP3+/IL-17− and FoxP3−/IL-17− clones, which also lacked RORC mRNA (Figure 7B). Stimulation of FoxP3+/IL-17+ clones did not result in down-modulation of FoxP3 expression (Figure 7C), nor did it induce IFNγ secretion (Figure 7D), in contrast to FoxP3− clones, which typically produced IFNγ. Moreover, similar to our observations in the bulk DR− Treg population, the addition of exogenous TGFβ abrogated the IL-1β/IL-6 induction of IL-17 secretion in FoxP3+/IL-17+ clones (Figure 7E).
FoxP3+/IL-17+ clones remain suppressive after they have been induced to secrete IL-17. The clones derived from the DR− Treg population were stimulated for 5 days with αCD3/αCD28 and IL-2 in the presence of IL-1β/IL-6, and analyzed for IL-17 secretion by ELISA (A), RORC mRNA expression by real-time PCR (B), and FoxP3 expression by intracellular staining (C). (D) Intracellular IL-17/IFNγ staining of IL-1β/IL-6 treated clones. (E) FoxP3+/IL-17+ clones were stimulated in the presence of IL-1β/IL-6 or TGFβ, and analyzed for IL-17 secretion by ELISA. (F) IL-1β/IL-6 treated clones were expanded for 2 weeks and tested for suppressive function in cocultures stimulated with αCD3/αCD2 beads, as compared with freshly isolated DR− and DR+ Tregs. One representative clone from each pattern is shown.
FoxP3+/IL-17+ clones remain suppressive after they have been induced to secrete IL-17. The clones derived from the DR− Treg population were stimulated for 5 days with αCD3/αCD28 and IL-2 in the presence of IL-1β/IL-6, and analyzed for IL-17 secretion by ELISA (A), RORC mRNA expression by real-time PCR (B), and FoxP3 expression by intracellular staining (C). (D) Intracellular IL-17/IFNγ staining of IL-1β/IL-6 treated clones. (E) FoxP3+/IL-17+ clones were stimulated in the presence of IL-1β/IL-6 or TGFβ, and analyzed for IL-17 secretion by ELISA. (F) IL-1β/IL-6 treated clones were expanded for 2 weeks and tested for suppressive function in cocultures stimulated with αCD3/αCD2 beads, as compared with freshly isolated DR− and DR+ Tregs. One representative clone from each pattern is shown.
After having induced these clones to secrete IL-17, we expanded them for 2 weeks in IL-2 and then tested them for suppressive activity in cocultures stimulated with αCD3/αCD2 beads. The coculture supernatants were negative for IL-17 (data not shown), indicating that the clones no longer produced IL-17. We observed that the FoxP3+/IL-17+ clones that had previously been stimulated with IL-1β/IL-6 were as suppressive as their untreated counterparts or the FoxP3+/IL-17− clones (Figure 7F). Altogether, these data demonstrate that a subset of highly suppressive FoxP3+DR− Tregs can be induced to secrete IL-17 with a simultaneous but reversible loss of suppressive activity.
Discussion
Regulatory T cells are potent suppressors of autoimmune responses, but how these cells are controlled during an acute infection requiring immune activation is still unclear. Here, we demonstrate that a subset of suppressive human Tregs retains the plasticity to secrete IL-17 and lose suppressor function under inflammatory conditions. We found that a small, but reproducible percentage of CD25highFoxP3+DR−CCR6+ Tregs express IL-17 both directly ex vivo and in short term cultures. This IL-17 production was potentiated by IL-2 and IL-6, enhanced by cotreatment with IL-1β and blocked by TGFβ. Our data reveal that both ex vivo and in vitro a subset of human Tregs can produce the effector cytokine IL-17.
To address several limitations related to the examination of in vitro human Treg cell function, we were concerned with contamination of the CD25high population with nonregulatory memory cells (approximately 15%-20%), which have the potential to both secrete IL-17 and overgrow the hypoproliferative Tregs upon stimulation. We addressed this concern in 2 ways: first, by assessing IL-17 production in FoxP3+ cells directly ex vivo, after only 4 hours of mitogenic stimulation, a time frame that precludes FoxP3 induction and cell division in activated memory T cells; and second, by isolating and cloning individual Tregs.
Single-cell cloning from the CD25highDR− Treg population enabled us to verify that an individual Treg can both suppress and secrete IL-17. By generating clones, which retain their ex vivo suppressive function, we were able to clearly demonstrate that FoxP3+/IL-17+ clones exhibit in vitro suppressive function similar to that of IL-17− Tregs in conditions that permit suppression. Our data suggested that this type of Treg transiently loses suppressive activity when secreting IL-17, which was illustrated by both loss of suppression and the inability of these clones to inhibit IFNγ production under inflammatory conditions. This loss of inhibition was reversible, as regulatory function was regained in FoxP3+/IL-17+ clones that had previously been induced to secrete IL-17.
This FoxP3+/IL-17+ phenotype was restricted to a fraction of DR− Treg clones, which, unlike memory contaminants, were functionally suppressive and expressed high levels of FoxP3. Our data indicate that cells cloned from the CD25highDR− Treg population are heterogeneous in both phenotype and function, recapitulating the ex vivo DR− Treg population. FoxP3+/IL-17+ cells comprise only a small fraction of both the ex vivo and clonal CD25highDR− Treg populations.
The FoxP3+/IL-17+ cells represent a relatively minor population in the periphery of healthy subjects. From intracellular cytokine staining and cloning data, we estimate the frequency of these cells to be approximately 4 to 8% of total CD4+CD25high Tregs. It is yet unknown whether all IL-17–secreting Tregs reside within the CD4+CD25high population, which is merely enriched for FoxP3+ cells. In addition, IL-17+ Tregs express both CCR6 and CCR4; the possibility remains that these cells may be better represented in tissue rather than in the blood. Expression of chemokine receptors and adhesion molecules has been shown to confer specific migratory capacities that can reduce the number of circulating cells. CCR6, for example, was recently shown to mediate the migration of Th17 cells to inflamed tissue.14,31 Furthermore, as we have observed in vitro, IL-17+ Tregs can proliferate under inflammatory conditions. Migration and proliferation of FoxP3+ cells has been observed in murine models of experimental autoimmune encephalomyelitis, where FoxP3+ cells infiltrating the central nervous system secrete IL-17 at the peak of disease progression (T. Korn, V.K.K., unpublished data, 2007). Analysis of tissue-resident Tregs in both healthy subjects and patients with autoimmune disease will be required to confirm the role of FoxP3+/IL-17+ Tregs in human disease progression.
Our findings in ex vivo Tregs are similar to those of Koenen and colleagues,32 who observed IL-17 secretion in Tregs stimulated with allogenic antigen presenting cells. Unlike Koenen et al, we chose to isolate our purified Tregs using HLA-DR as a biomarker because the CD25highDR− population is functionally distinct from DR+ Tregs.26 Furthermore, it was critical to separately examine DR− Tregs, as DR+ Tregs do not express IL-17. Other cell-surface determinants such as loss of CD127 have been similarly used, in addition to expression of CD4 and the IL-2 receptor, in the identification of Tregs.33,34 In this regard, preliminary data suggest that CD127 may be important in reducing the heterogeneity of the DR− Treg population (C.B.-A., manuscript in preparation).
We demonstrate here that human FoxP3+ Treg cells can be induced to secrete IL-17 in an inflammatory environment. In this study, IL-17 secretion by FoxP3+/IL-17+ cells was induced by proinflammatory cytokines (IL-1β/IL-6) and inhibited by anti-inflammatory cytokines (TGFβ). TGFβ is known to promote the generation and function of Treg cells.29 Our findings point toward an additional mechanism by which TGFβ can promote Treg function: TGFβ inhibits IL-17 secretion by FoxP3+ cells. Both ex vivo and in vitro expression of FoxP3 was maintained with IL-17 production, leading to coexpression of FoxP3 and IL-17. These data are potentially of interest in light of recent studies that suggest that FoxP3 interacts with RORγt to inhibit Th17 differentiation in naive CD4+ T cells.35 How these transcription factors interact in IL-17+ Tregs is unknown. While suppression is lost under conditions that lead to IL-17 production, this is true of all Tregs, not just those that secrete IL-17. What is clear, however, is that IL-17+ Tregs are memory cells that have acquired the capacity to produce IL-17 before isolation. These cells are likely to contain epigenetic modifications that predispose toward IL-17 phenotype32 ; and in these cells, the same factors that drive transcription of suppression mediators may inhibit IL-17 expression.
As human Tregs will soon be used in clinical trials as an immunotherapy, it is essential that we understand the behavior of these cells in inflammatory environments. Here, we demonstrate the plasticity of a subset of peripheral Tregs, which are influenced by environmental factors to exhibit dual Treg and Th17 function. Further studies will be required to determine the pathogenic potential, as well as the origin, of this population in vivo.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors would like to thank D. Kozoriz, A. Iglesias, and A. Vogelgesang for technical assistance.
This work was supported by the National Multiple Sclerosis Society grants FG1744A1 (G.B.) and RG3825A1 (C.B.A.) and by the National Institutes of Health grants U19AI070352, R01NS024247, P01AI03971, and P01NS038037 (D.A.H.). D.A.H. is a Jacob Javits Scholar of the National Institute of Neurological Disorders and Stroke.
National Institutes of Health
Authorship
Contribution: G.B., C.M.C., C.W.A., L.Y., and C.B.-A. performed experiments; G.B., C.M.C, and C.B.-A. analyzed results and made the figures; G.B., V.K.K., C.B.-A., and D.A.H. designed the research, G.B., C.M.C., C.B.-A., and D.A.H. wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Clare Baecher-Allan, NRB 641, 77 Ave Louis Pasteur, Boston, MA 02115; e-mail: callan@rics.bwh.harvard.edu.
References
Author notes
*C.B.-A. and D.A.H. contributed equally to this study.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal