Abstract
The importance of T cells in the generation of antigen-specific B-cell immunity has been extensively described, but the role B cells play in shaping T-cell memory is uncertain. In healthy controls, exposure to Neisseria meningitidis in the upper respiratory tract is associated with the generation of memory T cells in the mucosal and systemic compartments. However, we demonstrate that in B cell–deficient subjects with X-linked agammaglobulinemia (XLA), naturally acquired T-cell memory responses to meningococcal antigens are reduced compared with healthy control patients. This difference is not found in T-cell memory to an obligate respiratory pathogen, influenza virus. Accordingly, we show that meningococcal antigens up-regulate major histocompatibility complex (MHC) class II, CD40, CD86/80 expression on mucosal and systemic associated B cells and that antigen presentation stimulates T-cell proliferation. A similar reduction in N meningitidis but not influenza antigen–specific T-cell memory was observed in subjects with X-linked hyper IgM syndrome (X-HIM), implicating the interaction of CD40-CD40L in this process. Together, these data implicate B cells in the induction and maintenance of T-cell memory to mucosal colonizing bacteria such as N meningitidis and highlight the importance of B cells beyond antibody production but as a target for immune reconstitution.
Introduction
The role of T cells in the generation and shaping of antigen-specific B-cell responses has been extensively researched. It is well established that cognate interactions between T and B cells induces germinal center formation, isotype switching, and affinity maturation. In addition, it is clear that reciprocal signaling from the B cell to the T cell through MHC–T-cell receptor (TCR) interactions, as well as following interactions between costimulatory receptors and CD40-CD40L binding, also influence the activation of the T cell. Although antigen presentation by B cells only weakly primes naive T cells, robust recall T-cell responses are effectively stimulated in vivo.1,2 However, the effects of these events on the development and maintenance of T-cell memory and their importance in the generation of immunity to infection in humans are unclear.
Neisseria meningitidis (Nm), a frequent colonizer of the upper respiratory tract (URT), is a leading cause of meningitis and septicemia in children and adolescents.3,4 Although Nm carriage occurs in 10% to 40% of the population in industrialized countries,3,5 invasive disease is infrequent. We and others have described the generation of naturally acquired T- and B-cell immunity to the meningococcus with age both in the systemic and mucosal compartments.6-9 Effector T cells provide help to B cells, resulting in the generation of circulating anti-Nm complement-fixing antibodies that are associated with protection from invasive disease.10 Deficiencies of B cells and loss of splenic function are associated with increased susceptibility to meningococcal disease. Patients who have undergone myeloablation before bone marrow transplantation or who have received anti–B-cell monoclonal antibodies therapeutically are at increased risk of infection by capsulated bacteria.11 We speculate that these susceptibilities are not simply attributable to an impairment of B-cell effector function. B cells are abundant in URT lymphoid tissue,6 and we have therefore investigated the possibility that B-cell interactions with CD4 T cells in the mucosal compartment are key to the development and maintenance of anti-Nm T-cell memory both at the mucosal surface and in the circulation.
A variety of B cell–deficient animal models have been used to investigate the role of B cells in the generation and maintenance of T-cell immunologic memory.12-16 Some studies have found impaired T-cell recall responses to the opportunistic pathogen Pneumocystis jiroveciii and vaccine antigens,14-16 whereas others show intact viral-specific CD4 and CD8 memory responses to live viral challenge.12,13 Disorders of B-cell maturation, such as X-linked agammaglobulinemia (XLA), offer a unique opportunity to investigate immunologic interactions involving T and B cells in humans. XLA is an X-linked recessive genetic disorder in which B-cell ontogeny is disrupted as the result of mutations within the Bruton tyrosine kinase (Btk) gene.17,18 Affected patients are susceptible to recurrent bacterial infections. Two reports in XLA patients have described long-term hepatitis B and tetanus toxoid T-cell recall responses after vaccination.19,20 In the present study, we have focused on immunity to Nm, an example of a bacterial colonizer of the URT where immune induction is thought to occur at the mucosal surface.21,22 We show that, in B cell–deficient subjects, naturally acquired T-cell recall responses to meningococcal antigens are reduced compared with healthy control patients and that this difference is not found in T-cell memory to influenza virus antigens. We show that meningococcal outer membrane vesicles (OMVs) up-regulate MHC class II, CD40, CD86/80 expression on mucosal associated tonsillar B cells and that B-cell antigen presentation results in the stimulation of meningococcal specific T-cell proliferation. These data implicate B cells in the induction of long-lasting T-cell memory within the mucosal compartment and highlight the importance of B-cell function beyond antibody production as a target for immune reconstitution.
Methods
Study subjects
Human peripheral blood samples were taken from 7 well-characterized subjects with XLA and 2 subjects with X-linked hyper IgM (XHIM) syndrome (ages 22-55 years, n = 9). All but XLA-6 and XHIM-1 had confirmation of recent influenza immunization. T-cell counts and function were generally normal in these patients except for a low total lymphocyte count for XLA-5 (Table 1). Peripheral blood mononuclear cells (PBMCs) from XLA-5 were used to study proliferative kinetics only. Healthy volunteers were used as control subjects (ages 25-45 years, n = 3). Palatine tonsils (PT) were obtained from normal male and female adults (age 22-50 years, n = 3) with no history of atopy or meningococcal disease who were undergoing tonsillectomy for recurrent tonsillitis. PT were collected in decontamination media comprising Hank balanced salt solution (HBSS) and antibiotics (1000 U/mL penicillin, 1 mg/mL streptomycin; Invitrogen, Carlsbad, CA). Peripheral blood was collected into citrate phosphate dextrose solution (Sigma-Aldrich, St Louis, MO) to prevent coagulation. Samples of human peripheral blood and PT were used fresh for the isolation of mononuclear cells (MNCs) as previously described.6 The collection of samples and the research described complies with relevant guidelines and institutional practices (United Bristol Healthcare Trust Local Research Ethics Committee E4388 and Southmead Local Research Ethics Committee 05/Q2002/29). This study is registered with the United Kingdom National Research Register (N0234161894), and informed consent was obtained in accordance with the Declaration of Helsinki.
Patient demographics, age, genetic mutation, immune status, and influenza vaccination
| Patient . | Age, y . | Mutation . | TLC . | % CD3 . | % CD4 . | % CD8 . | % CD19 . | TCF . | Year of flu vaccination . |
|---|---|---|---|---|---|---|---|---|---|
| XLA 1 | 23 | R28C | 2.0 | 93 | 56 | 32 | < 1 | NA | 2002 |
| XLA 2 | 27 | E636X | 2.2 | 95 | 64 | 28 | < 1 | Normal | 2005 |
| XLA 3 | 24 | E636X | 2.2 | 95 | 67 | 26 | < 1 | Normal | 2005 |
| XLA 4 | 23 | E636X | 2.1 | 95 | 69 | 22 | < 1 | Normal | 2005 |
| XLA 5 | 36 | R28C | 0.8 | 95 | 32 | 53 | < 1 | Normal | 2005 |
| XLA 6 | 24 | R28C | 1.5 | 92 | 50 | 26 | < 1 | NA | NA |
| XLA 8 | 38 | R28C | 2.0 | 70 | 55 | 19 | 2 | NA | 2005 |
| X-HIM 1 | 37 | 560delA | 0.9 | 74 | 48 | 23 | 2 | NA | NA |
| X-HIM 2 | 55 | 148T>C, 617T>A, and 618C>T | 1.6 | 75 | 47 | 23 | 11 | Normal | 2006 |
| Patient . | Age, y . | Mutation . | TLC . | % CD3 . | % CD4 . | % CD8 . | % CD19 . | TCF . | Year of flu vaccination . |
|---|---|---|---|---|---|---|---|---|---|
| XLA 1 | 23 | R28C | 2.0 | 93 | 56 | 32 | < 1 | NA | 2002 |
| XLA 2 | 27 | E636X | 2.2 | 95 | 64 | 28 | < 1 | Normal | 2005 |
| XLA 3 | 24 | E636X | 2.2 | 95 | 67 | 26 | < 1 | Normal | 2005 |
| XLA 4 | 23 | E636X | 2.1 | 95 | 69 | 22 | < 1 | Normal | 2005 |
| XLA 5 | 36 | R28C | 0.8 | 95 | 32 | 53 | < 1 | Normal | 2005 |
| XLA 6 | 24 | R28C | 1.5 | 92 | 50 | 26 | < 1 | NA | NA |
| XLA 8 | 38 | R28C | 2.0 | 70 | 55 | 19 | 2 | NA | 2005 |
| X-HIM 1 | 37 | 560delA | 0.9 | 74 | 48 | 23 | 2 | NA | NA |
| X-HIM 2 | 55 | 148T>C, 617T>A, and 618C>T | 1.6 | 75 | 47 | 23 | 11 | Normal | 2006 |
TCF indicates T-cell function in response to phytohemagglutinin; TLC, total lymphocyte count; and NA, not available.
Meningococcal, bacterial, and influenza antigens
Outer membrane vesicles (OMVs) from Neisseria meningitidis serogroup B H44/76 (B:15:P1.7,16;L3,7,9) and 2 isogenic mutants, TR4 (B:15:P1.7-8.4) and TR10 (B:15:P1.5-2,10), and a spontaneous PorA-negative mutant (B:15:P1.-,-) were kindly provided by Dr Jamie Findlow (Vaccine Evaluation Unit, Health Protection Agency North West, Manchester, United Kingdom). These differ only in the major outer membrane protein Porin A and have been selected to represent the most common colonizing strains in the United Kingdom. Membranes from the H44/76 lipopolysaccharide (LPS)–deficient strain LpxA− were prepared as previously described.6 LPSs were derived from Escherichia coli 0111:B4 (Sigma-Aldrich), DNA containing cytidine-phosphate-guanosine (CpG) was obtained from InvivoGen (San Diego, CA), Nm porin PorB was prepared as previously described by Dr Paola Massari,23 and the B-cell polyclonal stimulus heat-killed Staphylococcus aureus Pansorbin was obtained from Calbiochem-Behring (La Jolla, CA). Influenza antigens were derived from dialyzed inactive trivalent split virion influenza vaccine (Fluarix 2002/2003).24 The vaccine contained 15 μg of hemagglutinin (HA) of A/Moscow/10/99 (H3N2)-like strain, A/New Caledonia/20/99 (H1N1)–like strain, and B/Hong Kong/330/2001-like strain, but no adjuvant.
Flow cytometry
Fluorescence-activated cell sorting (FACS) analysis was performed by use of the FACSCalibur flow cytometer; at least 50 000 cells were acquired by CellQuest software (BD Biosciences), and the analysis was performed with FlowJo (TreeStar, Ashland, OR). CD19+ B cells were selected by use of a gate and expression of B-cell surface molecules was displayed on a histogram.
Isolation and culture of MNCs
Single-cell suspensions were isolated from PT as previously described.6 MNCs were isolated from blood and tonsil cell (TMNC) suspensions by 25 minutes' centrifugation at 400g on a density gradient (Histopaque; Sigma-Aldrich). MNCs were harvested, washed in Hank balanced salt solution (HBSS) at 400g for 10 minutes, and resuspended in complete RPMI (RPMI 1640 with 100 U/mL penicillin, 0.1 mg/mL streptomycin, 4 mmol/L l-glutamine, and 10 mmol/L HEPES buffer). MNCs were counted with 0.4% (wt/vol) trypan blue (Sigma-Aldrich), reconstituted in complete RPMI at a concentration of 0.3-1 × 106 cells/mL, and plated out as 2-mL cultures in 24-well plates (Corning, Corning, NY) with a final concentration of 1% to 5% (vol/vol) heat-inactivated human AB serum (National Blood Services, Southmead Hospital, Bristol, United Kingdom).
Positive and negative selection of cellular populations
CD45RA+ cells were depleted from isolated PBMCs by negative selection with the use of MACS microbeads coated with anti–human CD45RA and magnetic cell sorting according to the manufacturer's instructions on LD columns (Miltenyi Biotec, Bergisch Gladbach, Germany) and as previously described.6 CD3 were positively selected by the use of MACS microbeads coated with anti-human CD3 on LS columns. B cells were purified by negatively selecting non-B cells with the use of a human B-cell enrichment cocktail and magnetic nanoparticles for magnetic separation by EasySep separation method (StemCell Technologies, Vancouver, BC). The efficiency of depletion was determined with the use of phycoerythrin (PE)–labeled anti-CD45RA antibodies (BD Biosciences, San Jose, CA), PE-labeled anti-CD3 (Miltenyi Biotec) and fluorescein isothiocyanate (FITC)–labeled anti-CD19 (Caltag, Burlingame, CA), respectively. Cells were depleted with at least 98% purity, as determined by flow cytometry. The proliferative capacity of T cells was unaffected by MACS depletion because all fractions responded equally to anti-CD3 at 1 μg/mL and anti-CD28.8 at 2 μg/mL stimulation, and phytohemagglutinin PHA-M (Sigma-Aldrich) at 10 μg/mL (data not shown).
Cell culture and proliferation assay
All MNCs were cultured in complete RPMI at 37°C in 5% CO2. Whole and CD45RA-depleted PBMC were stimulated with 1 μg/mL OMVs (H44/76, TR4, TR10, PorA−, LpxA−) 90 ng/mL influenza vaccine, or cultured in complete RPMI as a negative control. PBMCs were cultured at 0.6 × 106 cells/mL, and CD45RA-depleted cells were cultured at an equivalent T-cell density. For CD3 proliferation assays, purified CD3 T cells (106/mL) were added to B cells (106/mL) at a 1:1 ratio, and background proliferation of antigen stimulated CD3 or B cell–only populations were deducted on analysis. As previously described,25 on days 2 to 9 of culture, triplicate 100-μL samples were transferred from culture assays into 96-well round-bottom plates. The cells were pulsed with 0.4 mCi 3H-thymidine (Amersham Pharmacia Biotech/GE Healthcare, Little Chalfont, United Kingdom) for 24 hours and then frozen at −20°C. On completion of the assay, all plates were thawed and harvested together (PeproTech, Rocky Hill, NJ). 3H-thymidine incorporation was quantified using a 1450 MicroBeta liquid scintillation counter (PerkinElmer, Waltham, MA), giving results in corrected counts per minute (ccpm).
B-cell activation
B cells (106/mL) purified from PBMCs and TMNCs were cultured in complete RPMI supplemented with 10% fetal calf serum, on 48-well plates (Corning). B cells were stimulated with H44/76 at 25 μg/mL, LpxA− at 25 μg/mL, and 50 μg/mL Pansorbin as a positive control.26 In addition, PBMC B cells were stimulated with purified bacterial antigens PorB (10 μg/mL), LPS (1.5 μg/mL), and DNA containing CpG motifs (0.2 μg/mL). After 48 hours, 106 viable B cells in 100 μL PBS were rinsed twice with PBS containing 2% FCS. B-cell FC receptors were blocked with 20 μL FC blocking reagent (Miltenyi Biotec) before staining. The cells were then stained for 30 minutes with 2 μg of the following appropriate Ab: FITC-conjugated CD19; PE-conjugated CD25 (Caltag); CD40, CD86, CD80, isotype control (Mouse IgG1, κ) PE-Cy5–conjugated CD69 (BD Biosciences); and Tricolor-conjugated human leukocyte antigen–DR (HLA-DR; Caltag). The stained cells were washed and fixed with 1% paraformaldehyde in phosphate-buffered saline (PBS). Isotype controls were used to evaluate nonspecific antibody binding but showed negligible differences to unstained cells (B.M.-A., unpublished data, December 2008).
Statistical analysis
Differences between healthy and XLA subjects were analyzed using analysis of variance with STATA software, version 8.0 (College Station, TX). A P value less than or equal to .05 was considered to be statistically significant.
Results
Alteration in PBMC proliferative kinetics to Nm antigens in subjects with XLA
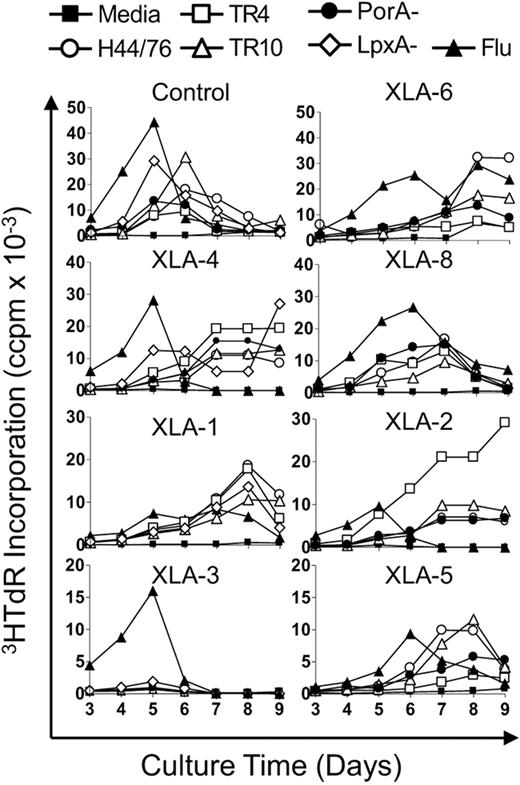
To investigate systemic T-cell responses to Nm serogroup B (MenB; influenza used as positive control), in XLA and healthy control subjects, PBMCs were stimulated with a variety of antigens and proliferation was measured over time. The antigens tested were Nm OMVs derived from a commonly used vaccine strain, H44/76 (B:15:P1.7,16;L3,7,9); 2 isogenic strains expressing different PorA types: TR4 (B15:P1.7-8,4) and TR10 (B:15:P1.5-2,10); and a spontaneous PorA-negative mutant (B:15:P1.-,-). The membranes were derived from a LPS-negative mutant of H44/76 (LpxA−) and influenza split virion vaccine. Our laboratory has previously shown that T cells display distinctive proliferative kinetics to Nm and influenza antigens.6,24 In healthy adults, CD45RO+ memory T cells typically proliferate between days 4 and 5 compared with CD45RA+-naive responses that peak at day 8. In this study, the peak of T-cell proliferation to the Nm and influenza antigens in healthy control patients was between days 4 and 6, indicative of a good ex vivo memory response, a representative control is shown in Figure 1. Interestingly, all of the XLA patients displayed early responses to influenza peaking between days 5 and 6; however, all but one had late responses to Nm, peaking on days 7 to 9 (Figure 1 XLA). The remaining subject (XLA no. 3) showed no T-cell proliferation in response to stimulation with any of the Nm antigen preparations.
PBMC proliferative kinetics to Nm but not influenza antigens are delayed in patients with XLA. PBMCs from XLA and healthy control patients were stimulated with Nm OMVs (H44/76, TR4, TR10, PorA−, LpxA−; 1 μg/mL) or influenza antigens (90 ng/mL). Proliferation was assessed by tritiated-thymidine (3HTdR) incorporation during days 3 to 9 of culture. Results are expressed as the mean number of triplicate corrected counts per minute (ccpm). The standard error of the mean cell counts was always less than 10% of the mean value shown. Data are representative of 10 separate experiments, control n = 3 and includes all XLA patients n = 7.
PBMC proliferative kinetics to Nm but not influenza antigens are delayed in patients with XLA. PBMCs from XLA and healthy control patients were stimulated with Nm OMVs (H44/76, TR4, TR10, PorA−, LpxA−; 1 μg/mL) or influenza antigens (90 ng/mL). Proliferation was assessed by tritiated-thymidine (3HTdR) incorporation during days 3 to 9 of culture. Results are expressed as the mean number of triplicate corrected counts per minute (ccpm). The standard error of the mean cell counts was always less than 10% of the mean value shown. Data are representative of 10 separate experiments, control n = 3 and includes all XLA patients n = 7.
Absence of CD4 CD45RO immune memory to Nm antigens in subjects with XLA
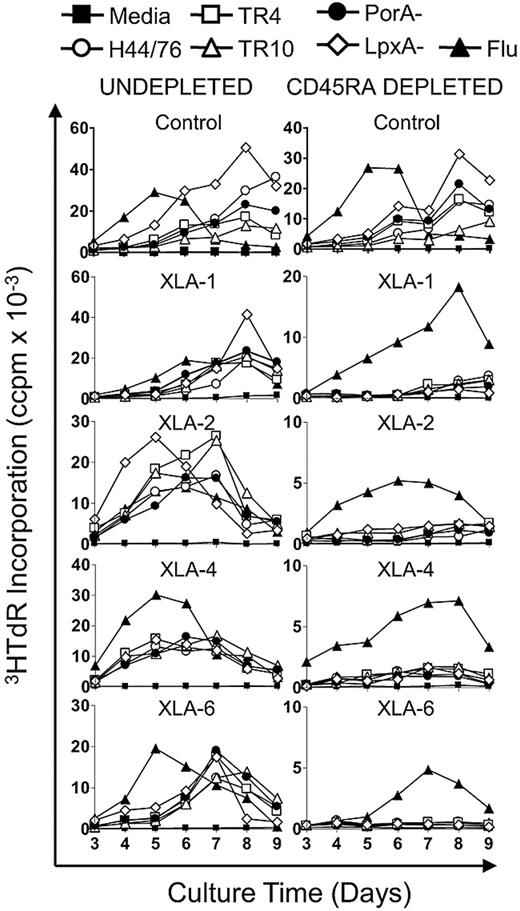
The alteration in anti-Nm proliferative kinetics in XLA patients implied a lack of memory responses. To investigate the frequency of systemic memory T cells in XLA and healthy subjects, PBMCs were depleted of the CD45RA+ naive cell pool with the use of MACS sorting. Undepleted and CD45RA-depleted PBMCs were stimulated with Nm OMVs (H44/76, TR4, TR10, PorA−, LpxA−) and influenza antigens. After CD45RA depletion, healthy control subjects continued to mount strong proliferative responses to Nm and influenza antigens (Figure 2 control), as reported previously.6,24 In contrast, removal of the naive T-cell pool through CD45RA depletion dramatically reduced proliferation to Nm OMVs in XLA patients, whereas influenza responses were preserved (Figure 2 XLA). To determine whether CD8 T cells were responsible for residual memory responses in XLA patients, undepleted and CD45RA+-depleted PBMCs were stained with carboxyfluorescein succinimidyl ester (CFSE) and stimulated with influenza antigens. This step showed that, after depleting CD45RA+ cells, CFSE-labeled T cells proliferating to influenza were all CD4 (data not shown).
CD4+CD45RO+ immune memory to Nm antigens is absent in subjects with XLA. PBMC from XLA and healthy controls were depleted of CD45RA+ naive T cells. Undepleted (0.6 × 106/mL) and CD45RA+-depleted (0.3 × 106/mL) fractions were stimulated with Nm OMVs (H44/76, TR4, TR10, PorA−, LpxA−; 1 μg/mL) and influenza antigens (90 ng/mL). Proliferation was assessed by tritiated-thymidine (3HTdR) incorporation during days 3 to 9 of culture. Results are expressed as the mean number of triplicate corrected counts per minute (ccpm). The standard error of the mean cell counts was always less than 10% of the mean value shown. Data are representative of control n = 3 and includes all XLA patients n = 4.
CD4+CD45RO+ immune memory to Nm antigens is absent in subjects with XLA. PBMC from XLA and healthy controls were depleted of CD45RA+ naive T cells. Undepleted (0.6 × 106/mL) and CD45RA+-depleted (0.3 × 106/mL) fractions were stimulated with Nm OMVs (H44/76, TR4, TR10, PorA−, LpxA−; 1 μg/mL) and influenza antigens (90 ng/mL). Proliferation was assessed by tritiated-thymidine (3HTdR) incorporation during days 3 to 9 of culture. Results are expressed as the mean number of triplicate corrected counts per minute (ccpm). The standard error of the mean cell counts was always less than 10% of the mean value shown. Data are representative of control n = 3 and includes all XLA patients n = 4.
Nm OMV, PorB, and other microbial products up-regulate molecules important for B-cell/T-cell interactions
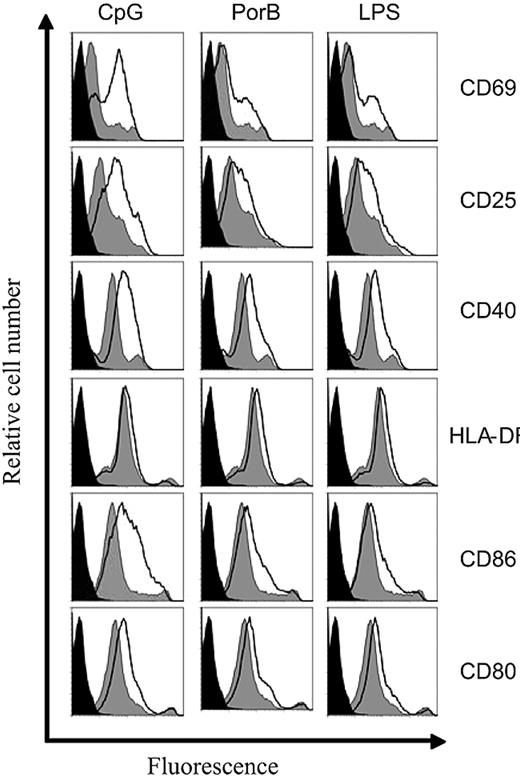
Because our data indicated that B cells are required for the generation of Nm-specific T-cell memory, we then investigated whether Nm antigens induce the expression of molecules associated with B-cell antigen presentation. B-cell activation results in the up-regulation of CD69, CD25 (the α chain of IL-2 receptor), MHC class II molecules, and costimulatory molecules CD40 and CD86/80.27-29 Nm OMVs contain both protein antigens and LPS that drive B-cell maturation in a T-dependent (TD) or T-independent (TI) manner, respectively.30,31 To evaluate the individual contribution of protein and LPS on B-cell activation, we used both the parent strain H44/76 OMV and the LPS-deficient LpxA− mutant. Peripheral blood B cells from healthy subjects were stimulated with H44/76, LpxA−, and a B-cell polyclonal stimulator, Pansorbin,26 as control. After 48 hours, the expression of CD69, CD25, MHC-DR, CD40, and CD86/80 was evaluated on CD19+ B cells. Both H44/76 and LpxA− OMVs were able to up-regulate all of the molecules tested (Figure 3). B-cell activation in response to LPS-deficient LpxA− was comparable with that of the parent strain H44/76, demonstrating that, as with other antigen-presenting cell (APC) types,32 activation is not induced solely by LPS. In addition, the stimulation of peripheral blood B cells with purified LPS, CpG DNA, and the meningococcal porin B (PorB) up-regulated CD69, CD25, CD40, CD86/80, but MHC class II was only weakly induced (Figure 4). These data indicate that B cells are susceptible to activation by a range of bacteria-derived stimuli carried by Nm, which are likely to be mediated through a variety of host receptors.
PBMC B-cell activation by Nm OMVs. Purified peripheral blood B cells (106/mL) from healthy controls were stimulated with Pansorbin, 50 μg/mL; H44/76, 25 μg/mL; and LpxA−, 25 μg/mL OMVs. After 48 hours, the expression of CD69, CD25, CD40, HLA-DR, and CD86/80 molecules was assessed on CD19+ B cells with the use of flow cytometry. The histogram black line represents antigen stimulated cells, gray shaded area is media only, and black fill is unstained cells. Data shown are normalized to the number of events. Results are representative of 5 separate experiments.
PBMC B-cell activation by Nm OMVs. Purified peripheral blood B cells (106/mL) from healthy controls were stimulated with Pansorbin, 50 μg/mL; H44/76, 25 μg/mL; and LpxA−, 25 μg/mL OMVs. After 48 hours, the expression of CD69, CD25, CD40, HLA-DR, and CD86/80 molecules was assessed on CD19+ B cells with the use of flow cytometry. The histogram black line represents antigen stimulated cells, gray shaded area is media only, and black fill is unstained cells. Data shown are normalized to the number of events. Results are representative of 5 separate experiments.
PBMC B-cell activation by LPS, CpG, and PorB. Purified peripheral blood B cells (106/mL) from healthy control patients were stimulated with LPS, 2.5 μg/mL; CpG, 1 μg/mL; and PorB, 10 μg/mL. After 48 hours, expression of CD69, CD25, CD40, HLA-DR, and CD86/80 molecules was assessed on CD19+ B cells with the use of flow cytometry. The histogram black line represents antigen stimulated cells, gray shaded area is media only, and black fill is unstained cells. Data shown are normalized to the number of events. Results are representative of 5 separate experiments.
PBMC B-cell activation by LPS, CpG, and PorB. Purified peripheral blood B cells (106/mL) from healthy control patients were stimulated with LPS, 2.5 μg/mL; CpG, 1 μg/mL; and PorB, 10 μg/mL. After 48 hours, expression of CD69, CD25, CD40, HLA-DR, and CD86/80 molecules was assessed on CD19+ B cells with the use of flow cytometry. The histogram black line represents antigen stimulated cells, gray shaded area is media only, and black fill is unstained cells. Data shown are normalized to the number of events. Results are representative of 5 separate experiments.
Mucosal B cells are activated by Nm OMV stimulation and subsequently induce T-cell proliferation
Because the initial site of interaction between Nm and immune cells is within the nasopharynx and associated B cell–rich mucosal lymphoid tissue, TMNCs were investigated. In view of the potential immunomodulatory effect of the Nm OMVs, LPS-sufficient and -deficient OMVs and purified LPS were used to ascertain the contribution of toll-like receptor (TLR)4-LPS ligation on B-cell activation and subsequent “bystander” T-cell proliferation. As demonstrated previously, H44/76 and LpxA− OMV up-regulated B-cell molecules involved in T- and B-cell interactions (Figure 5). In parallel, CD3 T cells were isolated from tonsil tissue and cocultured with stimulated B cells. Purified CD3 T cells proliferated in response to H44/76 activated B cells in a dose-dependent manner (Figure 5B) but did not proliferate in response to LPS treated B cells (data not shown). This finding suggests that cognate protein antigen presentation and not just LPS-mediated B-cell activation is responsible for this process.
Mucosal B cells are activated by Nm OMVs to induce T-cell proliferation. Purified tonsil B cells (106/mL) were stimulated with Pansorbin, 50 μg/mL; H44/76, 25 μg/mL; and LpxA−, 25 μg/mL OMVs. After 48 hours, the expression of CD25, CD40, and CD86 was evaluated on CD19+ B cells with the use of flow cytometry. The histogram black line represents antigen stimulated cells, gray shaded area is media only, and black fill is unstained cells. Data shown are normalized to the number of events (A). In parallel, purified CD3 T cells (106/mL) were added to unstimulated, H44/76 (15 μg/mL)- and H44/76 (25 μg/mL)-stimulated B cells (106/mL) at a 1:1 ratio. Proliferation was assessed by tritiated-thymidine (3HTdR) incorporation during days 4 to 9 of culture (B). Results are representative of at least 3 separate experiments.
Mucosal B cells are activated by Nm OMVs to induce T-cell proliferation. Purified tonsil B cells (106/mL) were stimulated with Pansorbin, 50 μg/mL; H44/76, 25 μg/mL; and LpxA−, 25 μg/mL OMVs. After 48 hours, the expression of CD25, CD40, and CD86 was evaluated on CD19+ B cells with the use of flow cytometry. The histogram black line represents antigen stimulated cells, gray shaded area is media only, and black fill is unstained cells. Data shown are normalized to the number of events (A). In parallel, purified CD3 T cells (106/mL) were added to unstimulated, H44/76 (15 μg/mL)- and H44/76 (25 μg/mL)-stimulated B cells (106/mL) at a 1:1 ratio. Proliferation was assessed by tritiated-thymidine (3HTdR) incorporation during days 4 to 9 of culture (B). Results are representative of at least 3 separate experiments.
CD40-CD40 ligand interactions are important to the proliferative response to Nm OMV stimulation
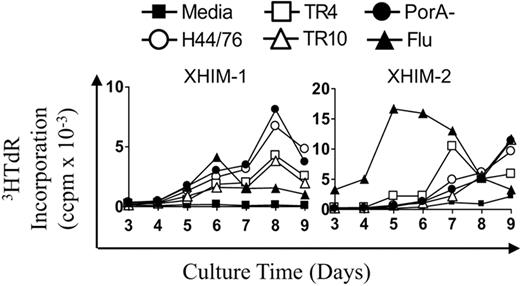
Because B cells up-regulate CD40 in response to Nm, the role of CD40-CD40L in the generation of T-cell memory was investigated in X-linked hyper IgM (X-HIM) patients. X-HIM is a genetic disorder characterized by the absence of CD40 ligand (CD40L) expression on CD4 T cells, leading to a failure of immunoglobulin isotype-switching.33,34 PBMCs from X-HIM patients were stimulated with Nm OMVs (H44/76, TR4, TR10, PorA−) and influenza. Both X-HIM subjects displayed late proliferative kinetics to Nm OMVs, peaking on days 7 to 9. X-HIM-2 responded with memory kinetics to the influenza antigen, peaking on day 5, mirroring previous findings in XLA patients. X-HIM-1 had no T-cell response to influenza but had no previous record of vaccination (Figure 6).
PBMC proliferative kinetics to Nm but not influenza are delayed in patients with X-HIM. X-HIM PBMC (0.6 × 106/mL) were stimulated with Nm OMVs (H44/76, TR4, TR10, PorA− 1 μg/mL) and influenza antigens (90 ng/mL). Proliferation was assessed by tritiated-thymidine (3HTdR) incorporation during days 3 to 9 of culture. Results are expressed as the mean number of triplicate corrected counts per minute (ccpm). The standard error of the mean cell counts was always less than 10% of the mean value shown.
PBMC proliferative kinetics to Nm but not influenza are delayed in patients with X-HIM. X-HIM PBMC (0.6 × 106/mL) were stimulated with Nm OMVs (H44/76, TR4, TR10, PorA− 1 μg/mL) and influenza antigens (90 ng/mL). Proliferation was assessed by tritiated-thymidine (3HTdR) incorporation during days 3 to 9 of culture. Results are expressed as the mean number of triplicate corrected counts per minute (ccpm). The standard error of the mean cell counts was always less than 10% of the mean value shown.
Discussion
How bacterial commensals of the URT induce long-lasting immunity is ill-defined. B-cell memory and maturation into antibody-producing plasma cells is widely held as a critical step in protection against N meningitidis.10 We now provide evidence implicating B cells in the generation/ maintenance of T-cell memory within the mucosal compartment. Although others have reported that T-cell immunity induced by systemic vaccination is not B cell–dependent,19,20 we show that B cells are required in the context of the generation of the natural immunity that follows exposure of the mucosa to the meningococcus. In comparison to healthy controls, robust T-cell responses to meningococcal antigens were not seen in the blood of human subjects with a defect in B-cell ontogeny (XLA) and subjects with a defect in CD40-CD40L interactions (X-HIM). Whether the reduced Nm memory T cells in B cell–deficient subjects has resulted from poor memory induction within the mucosa or T cells failing to exit the mucosa to recirculate is uncertain but we speculate that the maintenance of T-cell memory is impaired.
This lack of T-cell memory is not likely to be caused by a generalized defect affecting other non-B cell immune cells in these immunodeficient subjects as robust memory to influenza was seen in the same patients. This difference is not explained by the continued presence of CD8 memory cells to influenza as we showed that the T cells proliferating to influenza antigen were CD4 not CD8. Although influenza is also encountered in the URT mucosa, it is noteworthy that it is an obligate pathogen in its interactions with humans and that these immunodeficient patients are routinely given systemic flu vaccination. It is therefore not entirely clear which of these differences in the context of Nm and influenza exposure is responsible for their dependence on B cells for T-cell memory induction.
Induction of memory both at the mucosal surface and in the periphery requires antigen presentation by dendritic cells (DCs). The btk defect has effects on DC function that may hinder subsequent naive T-cell priming.35 A recent report in XLA subjects found intact DC maturation and cytokine production to the TLR4 ligand, bacterial LPS.36 This finding is supported by another description of effective DC TLR4 signaling and subsequent priming of naive allogeneic T cells.37 However, poor DC TNF-α and IL-6 production to viral ssRNA (a TLR8 ligand) has been demonstrated,36 which may explain an increased susceptibility to enteroviral infections in XLA patients. In our study, detectable naive responses pre-CD45RA+ depletion to N meningitidis, albeit slightly reduced to influenza antigens, suggest good DC function. There were no differences in priming of naive T-cell responses between LPS-sufficient and -deficient Nm OMVs (LpxA−). Therefore, it is unlikely that our observations are a consequence of impaired TLR-DC signaling, antigen presentation, and naive T-cell priming. In addition, T-cell memory responses to influenza were observed in XLA patients; therefore, influenza-specific T cells had been effectively primed by DC and differentiated into memory cells in those patients with the btk defect. We hypothesize that B cells are important for the generation or maintenance of memory T-cell responses in vivo but are not needed as APC for the restimulation of memory T cells.
Although primary Nm responses were similar in controls and XLA in vitro, antigen exposure may differ between the 2 groups in vivo. It is possible that because all XLA patients receive intravenous immunoglobulin therapy that contains antibody against Nm, this antibody may reduce the frequency of carriage. It is not known whether the levels of mucosal antibody achieved by IVIG therapy are sufficient to mediate mucosal clearance and how well mucosal immunoglobulins are maintained between infusions. However, a recent report by Trotter et al38 shows that, in healthy people, high titres of antimeningococcal serum bactericidal assay (SBA) in the blood occur at a time of increased carriage rates of N meningitidis at the mucosal surface. After human immunization with a MenB vaccine, we have been able to detect only a very modest increase in salivary IgG (not IgA) in the context of high levels of antibody in the blood.39 Mucosal IgA rather than IgG has been assumed to be the primary mediator of mucosal clearance,9 but this component of IVIG is extremely low. It is therefore unlikely that IVIG therapy would reduce meningococcal carriage.
During the generation of acquired immunity, B-cell peptide presentation to CD4 T cells results in the production of isotype switched complement fixing Abs, which have serum bactericidal activity.39,40 We speculate that this cognate interaction may be the point at which B cells influence the induction and maintenance of T-cell memory to Nm. The immunogenic protein PorB, derived from OMVs of Nm, was shown to up-regulate molecules associated with antigen presentation CD86 and MHC class II on murine B cells via TLR2 signaling.23 Other bacterial components, such as LPS and DNA containing CpG, are known to activate B cells via TLR428 and TLR9, respectively.27,29 We show that Nm OMVs and other purified bacterial TD and TI antigens up-regulate MHC class II, CD40 and CD86/CD80, on both peripheral and mucosal-associated B cells. These molecules are required for B-cell antigen presentation and could subsequently stimulate mucosal derived T cells. Tonsillar CD3 T cells proliferated in response to H44/76-activated B cells but not LPS-treated B cells, confirming that peptide presentation was needed to drive T-cell proliferation rather than via a B-cell TLR4-LPS “bystander” mechanism.
B-cell/T-cell interactions were therefore further investigated in X-HIM patients who lack CD40L. These patients have a variable clinical phenotype but have impaired B-cell immunoglobulin class switching and receive intravenous immunoglobulin therapy.34,40 Interestingly, the proliferative kinetic of Nm-specific T-cell responses also were altered in CD40L-deficient subjects. The combination of delayed proliferative kinetics from XLA and X-HIM patients suggests a role for CD40-CD40L interactions in the generation and maintenance of memory T-cell responses to bacteria at the mucosal surface.
We have shown that B cells are APC capable of presenting antigen and driving Nm-specific T-cell proliferation in vitro. Because B cells take up soluble antigens through their surface immunoglobulin, as well as by fluid-phase uptake, they are potentially required for restimulating memory responses to bacteria colonizing the URT. To ensure that the apparent lack of memory was not simply a result of the relative lack of APC in the cultures, the response of CD45RA-depleted PBMC from healthy donors was investigated after additional B-cell depletion. The removal of both B cells and naive T cells produced a negligible decrease in memory responses to all Nm antigens compared with depleting naive cells only (data not shown). Thus, we suggest that the defect does not result from a lack of APC able to restimulate memory T cells in vitro, but rather at the generation or maintenance of the memory response in vivo.
In conclusion, bacterial colonization of the URT does not lead to long-lasting Nm-specific memory T cells in B cell–deficient patients. In contrast, systemic influenza immunization generates systemic memory responses in the absence of B cells. These observations emphasize the impact of the site and the nature of the antigenic challenge on the immune response and emphasize the importance of B-cell function beyond antibody production but as a target for immune reconstitution. Whether systemic vaccination can boost T-cell immunologic memory to overcome residual B-cell defects remains to be determined. Furthermore, in immune-competent people the design of immunization strategies that target APC in the mucosal surface should take into account the role of B cells as well as DC in the maintenance of the immune response. To date vaccines against Nm serogroup B (MenB) have only been partially effective except in the context of the epidemic of a single strain.41,42 We have suggested that new vaccination approaches should attempt to mimic natural immunity both at the mucosa and in the circulation.43 Further focus of the role of B cells in the maintenance of mucosal T-cell memory may lead to novel treatment and vaccine strategies for the manipulation of antibacterial responses to protect the host from both carriage and disease.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Eleanor Groves for her technical assistance and Linda Hunt for her statistical advice. We thank Jamie Findlow and Paola Massari for the kind gift of Nm OMVs and PorB antigens, respectively. We are grateful to the study subjects for their participation.
This work was supported by a grant to R.S.H. and N.A.W. from the Meningitis Research Foundation (Bristol, United Kingdom), a grant to S.L.J. and R.S.H. from the North Bristol NHS Trust Research Foundation (Bristol, United Kingdom), and a Value in People Award to V.D. from The Wellcome Trust (London, United Kingdom).
Wellcome Trust
Authorship
Contribution: B.M.-A. performed the research and analyzed data; S.J.G. performed the research and wrote the paper; T.P.G. recruited study participants; R.S.H. and N.A.W. obtained funding, designed research, and cowrote the paper; and V.D. and S.L.J. obtained funding, designed research, and proofread the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Sarah Glennie, Malawi-Liverpool-Wellcome Trust Clinical Research Programme, Blantyre PO Box 30096, Malawi; e-mail: sarah.glennie@liverpool.ac.uk.
References
Author notes
*B.M.-A. and S.J.G. contributed equally to this work.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal