Abstract
Although the authors of several studies report elevated numbers of immunosuppressive regulatory T cells (Tregs) in hematologic and solid malignancies, the underlying mechanism is not fully clarified. Cancer is associated with oxidative stress mediated through reactive oxygen species produced by malignant cells, granulocytes, tumor-associated macrophages, and myeloid-derived suppressor cells. Oxidative stress is known to have detrimental effects on natural killer (NK) and T cells during chronic inflammatory conditions and cancer. Paradoxically, greater numbers of Tregs can be detected at tumor sites, indicating that Tregs can persist in this environment of increased oxidative stress. We demonstrate that Tregs, especially naive CD45RA+, exhibit reduced sensitivity to oxidative stress–induced cell death and maintain their suppressive function, a phenomenon that may be attributed to their observed high antioxidative capacity. This newly described characteristic could explain their enrichment in malignancies associated with increased levels of oxidative stress.
Introduction
CD4+CD25brightFOXP3+CD127dim/low regulatory T cells (Tregs) represent 4% to 5% of the CD4+ T cells and have a potent immunosuppressive capacity. They are critical for the prevention of autoimmunity and play an emerging role in cancer immunology.1,2 The authors of several studies have demonstrated the presence of elevated levels of Tregs in cancer patients, a phenomenon that may promote tumor progression and influence the course of disease.3-6 Increasing evidence indicates that tumor cells can induce Tregs directly or indirectly through their microenvironment by, for example, nitric oxide (NO) production, COX-2/PGE2 expression, or interleukin (IL)-10.7,8 Malignant cells and, more importantly, cells recruited or induced by tumor cells within the microenvironment, such as tumor-associated macrophages, activated granulocytes, and myeloid-derived suppressor cells, produce significant amounts of reactive oxygen species (ROS).9,10 The detrimental effect of ROS such as hydrogen peroxide (H2O2) and reactive nitrogen species such as NO on natural killer (NK) and T cells is well established and described in malignant and chronic inflammatory diseases.11-14 Paradoxically, Treg levels can be increased in this hostile (for lymphocytes) milieu
We studied the sensitivity of freshly isolated Tregs from peripheral blood to oxidative stress–induced cell death and resulting functional alterations. Furthermore, we evaluated the antioxidative capacity and components of the antioxidative machinery to gain a better understanding of our observations.
We demonstrate for the first time that Tregs show reduced sensitivity to oxidative stress–induced cell death and maintained their suppressive activity even at H2O2 levels that are lethal for half of the conventional effector CD4+CD25−/low T-cell population. Greater expression levels of surface thiols and a stronger intracellular antioxidative capacity observed in Tregs may contribute to their reduced sensitivity to oxidative stress.
Methods
Cell isolation
Peripheral blood mononuclear cells from healthy donors were isolated by Ficoll-Paque separation (Amersham Biosciences, Sunnyvale, CA). Tregs and conventional CD4+ T cells were purified with the use of a CD4+CD25bright Treg isolation kit (purity >90%) and frequencies of memory (63.20% ± 13.81%) and naive (36.40% ± 13.42%) Tregs were in the expected range (Figure S1, available on the Blood website; see the Supplemental Materials link at the top of the online article). CD45RA+ and CD45RA− subpopulations (purity > 95%) were purified by the use of anti-CD45RA/RO microbeads (Miltenyi Biotec, Auburn, CA). Granulocytes were isolated from the pellet obtained after Ficoll-Paque gradient centrifugation, followed by erythrocyte lysis.
This study received institutional review board approval from the Karolinska Institutet, and informed consent was obtained from donors in accordance with the Declaration of Helsinki.
Antibodies and flow cytometry
Cells were stained with the use of mouse monoclonal antibody (mAb) against human CD3-FITC (UCHT1), CD4-FITC/-PE/-PerCP (RPA-T4), CD25-PE/-APC (M-A251), CD45RA-FITC/-APC (HI100), CD127-PE/-APC (HIL-7R-M21), Bcl-2-FITC (3F11), and FOXP3-FITC/PE (PCH101, eBioscience, San Diego, CA). Antibodies were purchased from BD Biosciences (San Jose, CA) if not stated otherwise. Surface thiols were detected with Alexa-Fluor-488–coupled maleimides (Invitrogen, Carlsbad, CA). Cells were acquired and analyzed on a FACSCalibur with the use of Cellquest Pro (BD Biosciences) and FlowJo software (TreeStar, Ashland, OR).
Suppression assay
Tregs were cultured (18 hours) in AIM-V serum-free medium (Invitrogen, Carlsbad, CA) in the absence or presence of 10 μmol/L H2O2, then washed thoroughly before coculture with carboxyfluorescein succinimidyl ester (CFSE)–labeled CD25−/low T cells. A total of 5 × 104 CD4+CD25−/low T cells was plated alone or together with 2.5 × 104 Tregs and stimulated with the use of anti-CD2, anti-CD3, and anti-CD28 beads (Miltenyi Biotec, Auburn, CA) for 3 or 5 days. Proliferation was assessed by flow cytometry by calculating the mean fluorescence index (MFI) of the CFSE staining (FL-1) of each cell-cycle division.
Intracellular oxidation
Intracellular oxidation levels were determined by 5-(and-6)-chloromethyl-2′,7′-dichlorodihydrofluorescein diacetate acetyl ester (Invitrogen) staining that is metabolized to fluorescent (FL-1) H2-2′,7′-dichlorofluorescein (DCF) upon oxidation.
Lymphocyte cell death
Short-term culturing conditions were compared (AIM-V vs X-VIVO with/without human serum) to minimize spontaneous cell death, and serum-free AIM-V medium was chosen. Oxidative stress was induced by either addition of H2O2 or coculture with autologous granulocytes. Granulocyte-generated ROS effects were antagonized by the addition of human catalase (100 U/mL; Sigma-Aldrich, St Louis, MO). Cell death was assessed by 7-amino-actinomycin D (7-AAD) staining and forward-to-side-scatter profile (see Figure S2).
Preparation of RNA and qRT-PCR
RNA was extracted (RNeasy mini kit; QIAGEN, Valencia, CA) and cDNA prepared (iScript cDNA synthesis kit; Bio-Rad, Hercules, CA). Messenger RNA levels were quantified by RT quantitative PCR (qRT-PCR; SYBR Green Supermix, iCycler; Bio-Rad). Relative gene expression was determined by normalizing the expression of each target gene to β-actin. For primer sequences (β-actin, catalase, Mn-/Zn-SOD, and FOXP3), see Table S1.
Results and discussion
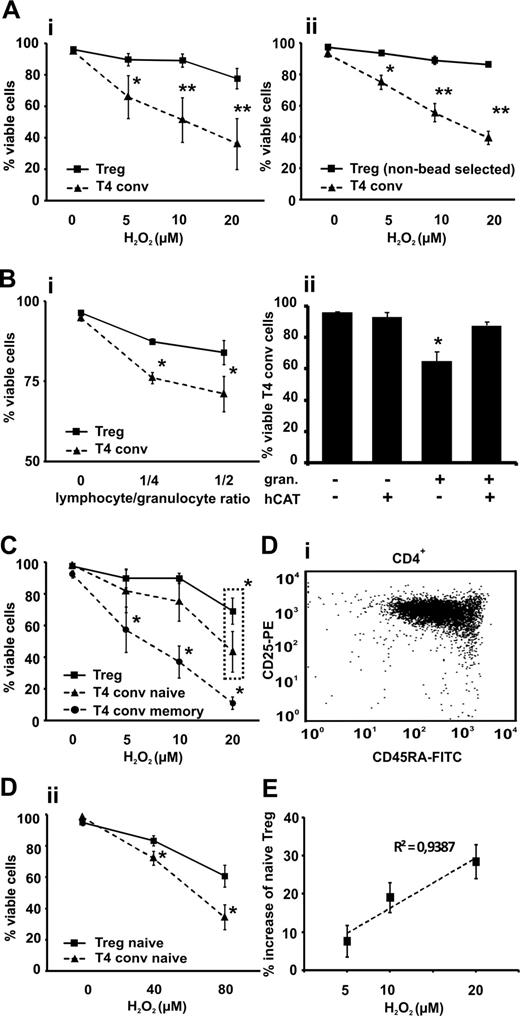
Previous reports have shown that T-cell subsets can differ in their susceptibility to oxidative stress–induced cell death.13,15 In comparison with their conventional CD4+CD25−/low counterparts, Tregs were significantly more resistant to cell death induced by exogenous H2O2 and granulocytes at all tested concentrations and cell-to-cell ratios (Figure 1A,B). In addition to the pure populations (majority of isolated Tregs were FOXP3+CD127dim/low; see Figure S3), we performed identical experiments with negatively selected mixed CD4+ T-cell populations to rule out any triggering effects through the CD25 receptor during cell selection. The majority of the gated “untouched” CD4+CD25bright Tregs were CD127dim/low (87% ± 5%; see Figure S4), ruling out any false-positive gating on activated conventional CD4 T cells and displayed the same pattern of resistance to H2O2 as the bead-selected Tregs (Figure 1Aii).16 The addition of human catalase almost completely protected conventional T cells from granulocyte-mediated cell death, indicating that this effect was mediated by ROS (Figure 1Bii). Naive CD4+CD25−/lowCD45RA+ T cells showed, as previously described, a significantly greater resistance to H2O2 than memory cells but were significantly less resistant to oxidative stress–induced cell death compared with Tregs when exposed to 20 μmol/L H2O2 (Figure 1C).15 This difference is even more prominent when comparing naive conventional cells with naive Tregs (Figure 1D), a recently described subpopulation that is less sensitive to death receptor (CD95) ligation-induced cell death.17 The proportion of naive Tregs among the viable Treg fraction increased upon induction of oxidative stress (Figure 1E) and correlated positively (R2 = 0.94; P = .03) with the extent of H2O2 exposure, indicating a potential oxidative stress–driven modification of the Treg pool.18
Sensitivity of CD4+CD25bright Tregs to ROS-induced cell death compared with CD4+CD25−/low T cells (T4 conv). Tregs and T4 conv were (A) treated with increasing concentrations of H2O2 (5-20 μmol/L) or (Bi) cocultured with autologous granulocytes (granulocyte to T-cell ratio 1:2 to 1:4) in (Bii) the presence or absence of 100 U/mL human catalase (hCAT) and cell viability was assessed by flow cytometry and 7-AAD labeling. Viable cells were identified using dual criteria: no shift in the forward and/or side scatter and no staining of 7-AAD. Survival data are shown for Tregs, both purified by magnetic beads (Ai and Bi; solid line) and T4 conv (dashed line) and “untouched” negatively selected gated Tregs (Aii; solid line) and T4 conv (dashed line). (C) CD4+CD25bright Tregs, CD4+CD25−/lowCD45RA+ naive and CD4+CD25−/lowCD45RA− memory T cells were treated with increasing concentrations of H2O2 (5-20 μmol/L) for 18 hours, and cell viability was assessed as described previously. (D) Bead-isolated naive CD45RA+ CD4+CD25bright Tregs (representative FACS data, Di) and CD4+CD25−/low T4 conv were treated with H2O2 (40 and 80 μmol/L, respectively) for 18 hours and cell viability was assessed as described previously (Dii). (E) CD4+CD25bright Tregs were treated with increasing concentrations of H2O2 (5-20 μmol/L) for 18 hours, stained with anti-CD45RA antibody, and a fraction of naive Tregs among viable population and its percent increase was measured. Cell viability was assessed as described previously. The initial proportion of CD45RA+ among the untreated Tregs served as the baseline value. Bars indicate standard error mean based on data from 6 (Ai), 4 (Aii), 4 (Bi), 5 (Bii), 6 (C), 3 (Dii), and 5 (E) different healthy donors in each and every panel. P values were calculated using a 2-tailed paired t test. Regression analyses were evaluated with analysis of variance testing. *P < .05, **P < .01.
Sensitivity of CD4+CD25bright Tregs to ROS-induced cell death compared with CD4+CD25−/low T cells (T4 conv). Tregs and T4 conv were (A) treated with increasing concentrations of H2O2 (5-20 μmol/L) or (Bi) cocultured with autologous granulocytes (granulocyte to T-cell ratio 1:2 to 1:4) in (Bii) the presence or absence of 100 U/mL human catalase (hCAT) and cell viability was assessed by flow cytometry and 7-AAD labeling. Viable cells were identified using dual criteria: no shift in the forward and/or side scatter and no staining of 7-AAD. Survival data are shown for Tregs, both purified by magnetic beads (Ai and Bi; solid line) and T4 conv (dashed line) and “untouched” negatively selected gated Tregs (Aii; solid line) and T4 conv (dashed line). (C) CD4+CD25bright Tregs, CD4+CD25−/lowCD45RA+ naive and CD4+CD25−/lowCD45RA− memory T cells were treated with increasing concentrations of H2O2 (5-20 μmol/L) for 18 hours, and cell viability was assessed as described previously. (D) Bead-isolated naive CD45RA+ CD4+CD25bright Tregs (representative FACS data, Di) and CD4+CD25−/low T4 conv were treated with H2O2 (40 and 80 μmol/L, respectively) for 18 hours and cell viability was assessed as described previously (Dii). (E) CD4+CD25bright Tregs were treated with increasing concentrations of H2O2 (5-20 μmol/L) for 18 hours, stained with anti-CD45RA antibody, and a fraction of naive Tregs among viable population and its percent increase was measured. Cell viability was assessed as described previously. The initial proportion of CD45RA+ among the untreated Tregs served as the baseline value. Bars indicate standard error mean based on data from 6 (Ai), 4 (Aii), 4 (Bi), 5 (Bii), 6 (C), 3 (Dii), and 5 (E) different healthy donors in each and every panel. P values were calculated using a 2-tailed paired t test. Regression analyses were evaluated with analysis of variance testing. *P < .05, **P < .01.
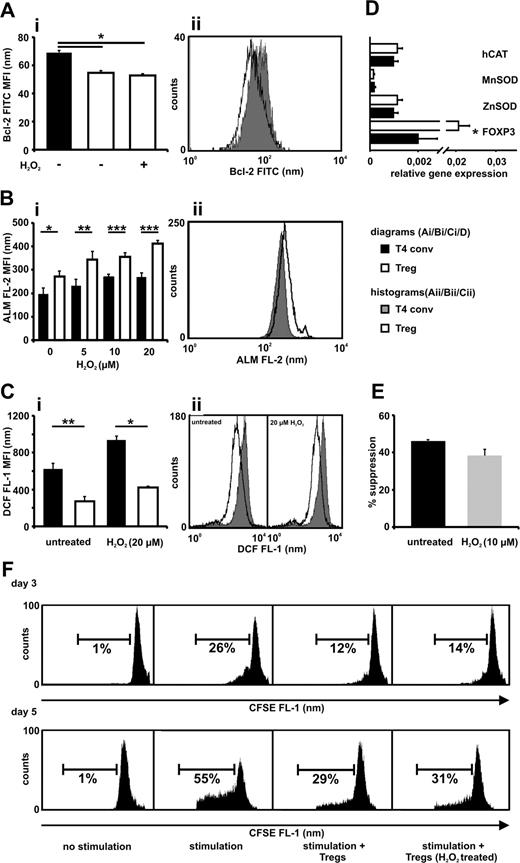
The sensitivity to oxidative stress can be modified by expression of pro- and antiapoptotic molecules such as Bcl-2.19 Bcl-2 is differently expressed in various lymphoid subsets.20 Tregs exhibited significantly lower Bcl-2 levels compared with conventional T cells, confirming previous results, and these levels remained stable upon exposure to oxidative stress (Figure 2A).21 Despite their lower Bcl-2 levels, Tregs show reduced activation-induced cell death and the underlying mechanism behind this action may be related to our findings.21,22 Several mechanisms contribute to cellular protection against oxidative stress, one being proteins containing free thiol groups expressed at various levels on lymphoid subsets.23,24 The amount of thiols per cell was significantly greater in the Treg population, which is similar to the observation made in oxidative stress-resistant CD56bright NK cells (Figure 2B).25 Tregs showed significantly lower basal intracellular oxidation levels when freshly isolated and upon H2O2 treatment, indicating a greater antioxidative capacity (Figure 2C). Well-described antioxidative enzymes protecting lymphocytes from ROS-induced cell death via up-regulation of Bcl-2 levels and direct metabolizing effects did not have a greater expression in the Tregs (Figure 2D).19
CD4+CD25bright Tregs display a lower Bcl-2 expression, a greater antioxidative capacity compared with CD4+CD25−/low T cells, and maintain suppressive activity after overnight incubation with H2O2. (A) Bcl-2 expression was measured in freshly isolated CD4+CD25bright Tregs (□ −), CD4+CD25−/low T cells (T4 conv; ■) and in Tregs (□ +) after treatment with H2O2 (10 μmol/L) for 18 hours. (B) Tregs (□) and T4 conv (■) were exposed to increasing concentrations of H2O2 (5-20 μmol/L) for 18 hours, and thiol expression was determined by the use of Alexa Fluor 488-coupled maleimide (ALM-488). Dead cells were excluded in the analysis to avoid artefacts caused by the release of intracellular thiols resulting from membrane instability. (C) The intracellular antioxidative capacity for Tregs (□) and T4 conv (■) was measured by staining the cells with CM-H2-DCFDA and exposing them to H2O2 (20 μmol/L) for 45 minutes. The results are presented as the MFI of Bcl-2-FITC (Ai), ALM-488 staining (ALM FL-2; Bi), and DCF fluorescence (DCF FL-1; Ci) and as representative histograms (Aii,Bii,Cii). (D) Purified Tregs and T4 conv were analyzed for their relative gene expression of human catalase (hCAT), manganese superoxide dismutase (MnSOD), zinc superoxide dismutase (ZnSOD), and FOXP3 as an internal control by real-time PCR by the use of gene-specific primers for each gene. (E,F) Tregs were incubated in the absence or presence of 10 μmol/L H2O2 for 18 hours. Subsequently, cells were washed thoroughly and cocultured (ratio 1:2) with CFSE-labeled T4 conv in the presence of stimulatory beads coated with anti-CD2, anti-CD3, and anti-CD28 Abs. After 3 or 5 days the proliferation of CD4+CD25−/low T cells was assessed with the use of flow cytometry. Dead cells were excluded by 7-AAD labeling. (E) Percentage of inhibition of proliferation on day 3 by non- and H2O2-treated Tregs. (F) Two representative and independent experiments with cells from 2 different healthy donors, each evaluated at day 3 or day 5. The unlabeled CFSE negative autofluorescent Tregs were gated out during fluorescence-activated cell sorting (FACS) analysis. Bars indicate standard error of the mean based on data from 4 (Ai), 5 (Bi), 4 (Ci), 8 (D), and 4 (E) different healthy donors in each and every panel. P values were calculated using a 2-tailed paired t test. *P ≤ .05, **P ≤ .01, ***P ≤ .001.
CD4+CD25bright Tregs display a lower Bcl-2 expression, a greater antioxidative capacity compared with CD4+CD25−/low T cells, and maintain suppressive activity after overnight incubation with H2O2. (A) Bcl-2 expression was measured in freshly isolated CD4+CD25bright Tregs (□ −), CD4+CD25−/low T cells (T4 conv; ■) and in Tregs (□ +) after treatment with H2O2 (10 μmol/L) for 18 hours. (B) Tregs (□) and T4 conv (■) were exposed to increasing concentrations of H2O2 (5-20 μmol/L) for 18 hours, and thiol expression was determined by the use of Alexa Fluor 488-coupled maleimide (ALM-488). Dead cells were excluded in the analysis to avoid artefacts caused by the release of intracellular thiols resulting from membrane instability. (C) The intracellular antioxidative capacity for Tregs (□) and T4 conv (■) was measured by staining the cells with CM-H2-DCFDA and exposing them to H2O2 (20 μmol/L) for 45 minutes. The results are presented as the MFI of Bcl-2-FITC (Ai), ALM-488 staining (ALM FL-2; Bi), and DCF fluorescence (DCF FL-1; Ci) and as representative histograms (Aii,Bii,Cii). (D) Purified Tregs and T4 conv were analyzed for their relative gene expression of human catalase (hCAT), manganese superoxide dismutase (MnSOD), zinc superoxide dismutase (ZnSOD), and FOXP3 as an internal control by real-time PCR by the use of gene-specific primers for each gene. (E,F) Tregs were incubated in the absence or presence of 10 μmol/L H2O2 for 18 hours. Subsequently, cells were washed thoroughly and cocultured (ratio 1:2) with CFSE-labeled T4 conv in the presence of stimulatory beads coated with anti-CD2, anti-CD3, and anti-CD28 Abs. After 3 or 5 days the proliferation of CD4+CD25−/low T cells was assessed with the use of flow cytometry. Dead cells were excluded by 7-AAD labeling. (E) Percentage of inhibition of proliferation on day 3 by non- and H2O2-treated Tregs. (F) Two representative and independent experiments with cells from 2 different healthy donors, each evaluated at day 3 or day 5. The unlabeled CFSE negative autofluorescent Tregs were gated out during fluorescence-activated cell sorting (FACS) analysis. Bars indicate standard error of the mean based on data from 4 (Ai), 5 (Bi), 4 (Ci), 8 (D), and 4 (E) different healthy donors in each and every panel. P values were calculated using a 2-tailed paired t test. *P ≤ .05, **P ≤ .01, ***P ≤ .001.
Lymphocytes exhibit aberrant function upon exposure to H2O2, a mechanism considered part of the immunosuppressive tumor microenvironment.13 Functional alteration of Tregs was evaluated by overnight exposure to 10 μmol/L H2O2, a concentration that is lethal for 50% of the conventional CD4+ T-cell population (LD50; Figure 1A). These Tregs were then subjected to a suppression assay at a 1:2 Treg to T4 conv responder ratio. A significant loss of suppressive function could not be observed (Figure 2E,F).
Taken together, we demonstrate that ROS, besides impairing the immune system by functional alterations and apoptosis induction of NK- and T cells, may further exacerbate the immune dysfunctions by selective enrichment of Tregs. Several mechanisms leading to induction of peripheral Tregs in cancer have been reported but here, for the first time, a potential mechanism contributing to the selective enrichment of naturally occurring Tregs is described.7,8
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Dr Raja Choudhury for the critical review of the manuscript.
This work was supported by grants from the Swedish Cancer Society, the Cancer Society of Stockholm, the Karolinska Institutet, and an ALF-Project grant from the Stockholm City Council.
Authorship
Contribution: D.M. and C.C.J. designed and performed research, analyzed data, and wrote the manuscript; and R.K. designed research and supervised data analysis and writing of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Rolf Kiessling, Professor, Department of Oncology and Pathology, Karolinska University Hospital, Solna, Cancer Center Karolinska R8:01, 171 76 Stockholm, Sweden; e-mail: Rolf.Kiessling@ki.se.
References
Author notes
*D.M. and C.C.J. contributed equally to this work.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal