Abstract
As the risks of allogeneic blood transfusion (ABT)–transmitted viruses were reduced to exceedingly low levels in the US, transfusion-related acute lung injury (TRALI), hemolytic transfusion reactions (HTRs), and transfusion-associated sepsis (TAS) emerged as the leading causes of ABT-related deaths. Since 2004, preventive measures for TRALI and TAS have been implemented, but their implementation remains incomplete. Infectious causes of ABT-related deaths currently account for less than 15% of all transfusion-related mortality, but the possibility remains that a new transfusion-transmitted agent causing a fatal infectious disease may emerge in the future. Aside from these established complications of ABT, randomized controlled trials comparing recipients of non–white blood cell (WBC)–reduced versus WBC-reduced blood components in cardiac surgery have documented increased mortality in association with the use of non-WBC–reduced ABT. ABT-related mortality can thus be further reduced by universally applying the policies of avoiding prospective donors alloimmunized to WBC antigens from donating plasma products, adopting strategies to prevent HTRs, WBC-reducing components transfused to patients undergoing cardiac surgery, reducing exposure to allogeneic donors through conservative transfusion guidelines and avoidance of product pooling, and implementing pathogen-reduction technologies to address the residual risk of TAS as well as the potential risk of the next transfusion-transmitted agent to emerge in the foreseeable future.
Introduction
Today, the leading causes of allogeneic blood transfusion (ABT)–related mortality in the United States—in the order of reported number of deaths—are transfusion-related acute lung injury (TRALI), ABO and non-ABO hemolytic transfusion reactions (HTRs), and transfusion-associated sepsis (TAS).1 Other than TAS, the infectious causes of death have been declining as a proportion of all deaths caused by ABT over the past 3 decades.1-7 ABT, however, can still transmit lethal infections due to known pathogens,8 while novel transfusion-transmitted,9,10 or potentially transfusion-transmitted,11 pathogens continue to emerge.8,12 In addition to these deaths caused by ABT, randomized controlled trials (RCTs) comparing patients undergoing cardiac surgery and randomized to receive unmanipulated (“non–white blood cell [WBC]–reduced”) red blood cells (RBCs) versus RBCs from which the WBCs had been removed by WBC-reduction filters (“WBC-reduced” RBCs) have attributed increased mortality to the receipt of non-WBC–reduced (compared with WBC-reduced) ABT.13-15 This review will consider estimates of the mortality from noninfectious and infectious transfusion complications today and will recommend several currently available strategies that can further reduce ABT-related mortality.
Reporting of ABT-related fatalities
Hemovigilance systems have been set up in various countries—including France, the United Kingdom, other European countries, and the province of Quebec in Canada—to monitor the risk of ABT-related adverse events.2-5 Reporting to the recently established US Biovigilance Network will commence in 2009.16 Since 1976, the United States has required the reporting of all transfusion-associated deaths to the US Food and Drug Administration (FDA).1,6 Comparisons of the figures obtained from these surveillance systems are difficult, because: (1) the definitions of transfusion complications vary among systems; (2) the denominators necessary for the estimation of the risk of a transfusion-related death are often unavailable or inconsistent between systems; and (3) the criteria used to definitely, probably, or possibly attribute a death to ABT also vary significantly. Moreover, because surveillance systems usually rely on passive reporting, they generally underestimate the incidence of transfusion-related adverse events, including deaths.
Adverse-event reporting is mandatory in France, and in 1994 to 1999, 82 transfusion-related deaths were reported to the French hemovigilance network.2 There were 18 deaths due to TAS and 6 deaths due to ABO HTRs. TRALI was not identified as a specific entity by the French hemovigilance system at that time.2 More recent hemovigilance data presented from France have been limited to the risk of HTRs.17
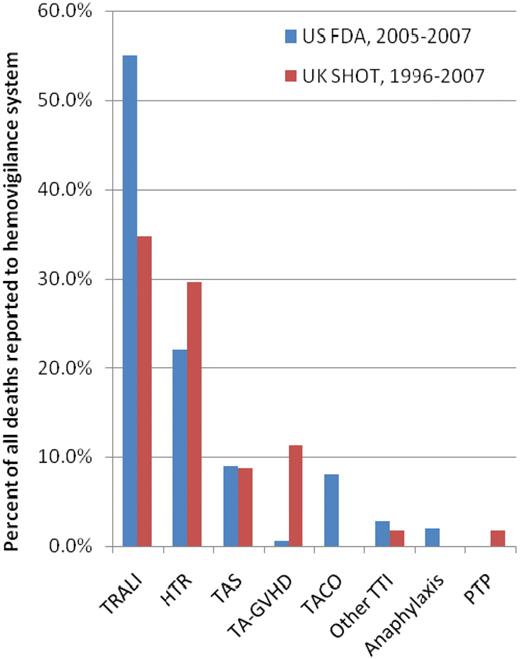
Over the first 11 years of reporting (1996-2007), transfusion was considered to have a causal or contributory role in 115 deaths reported to the United Kingdom Serious Hazards of Transfusion (SHOT) surveillance system.3,4 The lowest transfusion-related mortality rate, since SHOT began in 1996, was recorded in 2007 when there was no death definitely attributable to ABT; only 1 death was probably attributed to TRALI, and transfusion was deemed to have contributed to the deaths of 3 more patients.4 Figure 1 shows the causes of death that each accounted for greater than 1% of these 115 fatalities.
Causes of allogeneic blood transfusion–related deaths as a percentage of all deaths reported to SHOT (1996-2007)4 or the FDA (2005-2007).1 The figure shows the causes of death that accounted for at least 1% of all deaths in either of these 2 reports.1,4 Transfusion-associated circulatory overload (TACO) was not specifically captured, and anaphylaxis not specifically reported, by the United Kingdom SHOT surveillance system in 1996 to 2007.4 There were no deaths due to posttransfusion purpura (PTP) reported to the US Food and Drug Administration (FDA) from 2005 to 2007.1 TA-GVHD indicates transfusion-associated graft-versus-host disease.
Causes of allogeneic blood transfusion–related deaths as a percentage of all deaths reported to SHOT (1996-2007)4 or the FDA (2005-2007).1 The figure shows the causes of death that accounted for at least 1% of all deaths in either of these 2 reports.1,4 Transfusion-associated circulatory overload (TACO) was not specifically captured, and anaphylaxis not specifically reported, by the United Kingdom SHOT surveillance system in 1996 to 2007.4 There were no deaths due to posttransfusion purpura (PTP) reported to the US Food and Drug Administration (FDA) from 2005 to 2007.1 TA-GVHD indicates transfusion-associated graft-versus-host disease.
Although the definitions of causes of ABT-related deaths and the proportion of reported adverse events have evolved since 1996, the SHOT data show no death definitely attributed to TRALI after 2004.4 It may be noteworthy that in October 2003 the United Kingdom started using fresh frozen plasma (FFP) primarily or exclusively from male donors,4 discarding plasma from female donors as the latter is sometimes implicated in TRALI1,3 because it may contain WBC antibodies due to alloimmunization during previous pregnancies.4 None of the 12 probable TRALI cases reported to SHOT in 2007 was associated with the transfusion of FFP.4 SHOT concluded that the reduction in TRALI observed in 2006 and 2007—when the lowest TRALI mortality rate was recorded since SHOT began—was “likely” due to the United Kingdom decision to change to preferential use of male plasma in 2003/2004.4
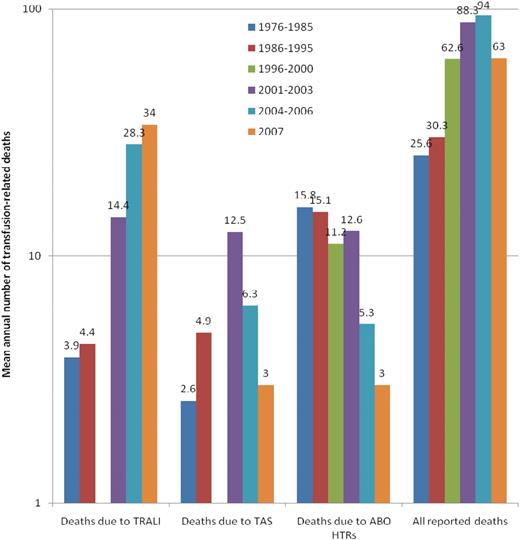
The number of transfusion-related deaths annually reported to the FDA (Figure 2) increased steadily from 16 in 1976 to 105 in 2005.1 Of the latter, however, only 62 were recipient deaths in which the transfusion was the likely or major cause of death. Of 63 deaths reported in 2007, 52 were determined to be due to the ABT.1 Because approximately 22.3 million units of RBCs, platelets, and plasma were transfused in the United States in 2006,18 the risk of a transfusion-related death can be estimated at approximately 2.3 per million transfused components. The decline in TAS deaths since 2004 (Figure 2) followed the implementation of bacterial detection of apheresis platelets in March 2004.
The 3 leading causes of known and reported allogeneic blood transfusion-related deaths, based on data reported passively to the US FDA over 32 years (1976-2007).1,6 For each of the 5 periods for which data have been made available, the figure shows the mean annual number of deaths deemed to be due to TRALI, TAS, or ABO hemolytic transfusion reactions (HTRs), along with the mean total number of deaths reported to the FDA plotted on a logarithmic scale. Deaths reported to the FDA include donor fatalities, recipient fatalities in which allogeneic blood transfusion (ABT) was not deemed to be the likely or major cause of death, and recipient fatalities due to TRALI, TAS, ABO HTRs, as well as other transfusion complications. Data on TRALI and TAS are not available for the period 1996 to 2000.
The 3 leading causes of known and reported allogeneic blood transfusion-related deaths, based on data reported passively to the US FDA over 32 years (1976-2007).1,6 For each of the 5 periods for which data have been made available, the figure shows the mean annual number of deaths deemed to be due to TRALI, TAS, or ABO hemolytic transfusion reactions (HTRs), along with the mean total number of deaths reported to the FDA plotted on a logarithmic scale. Deaths reported to the FDA include donor fatalities, recipient fatalities in which allogeneic blood transfusion (ABT) was not deemed to be the likely or major cause of death, and recipient fatalities due to TRALI, TAS, ABO HTRs, as well as other transfusion complications. Data on TRALI and TAS are not available for the period 1996 to 2000.
Table 1 summarizes the data reported by 3 major surveillance systems.1-3 Although ABT-related mortality would appear to be more than twice as high in France2 as in the United States,1 data from different periods (1994-1999 vs 2007) are presented and the reporting systems differ between the 2 countries; in France, reporting is mandatory and part of a centrally coordinated hemovigilance system. Absence of such a system greatly reduces reporting of adverse events, as illustrated by the Canadian experience in 2000, the year when the Quebec hemovigilance system was established. In 2000, all 4 ABT-related deaths reported to the Canadian equivalent of the FDA had also been captured by the Quebec hemovigilance system. Although possible, it is unlikely that in 2000 there were no transfusion-related deaths in the rest of Canada, which represents approximately 3 times the population of Quebec; and it appears that underreporting by hospitals outside Quebec produced this improbable finding.19
ABT-related mortality based on deaths reported to three major hemovigilance systems
| Country . | Type of surveillance system . | Reporting period . | ABT-related mortality* . |
|---|---|---|---|
| France | Hemovigilance/mandatory reporting2 | 1994-1999 | 5.6 |
| United Kingdom | Hemovigilance/voluntary reporting3 | 1996-2004 | 3.5 |
| United States | Passive reporting of deaths1 | 2007 | 2.3 |
| Country . | Type of surveillance system . | Reporting period . | ABT-related mortality* . |
|---|---|---|---|
| France | Hemovigilance/mandatory reporting2 | 1994-1999 | 5.6 |
| United Kingdom | Hemovigilance/voluntary reporting3 | 1996-2004 | 3.5 |
| United States | Passive reporting of deaths1 | 2007 | 2.3 |
Infectious causes accounted for 10.8% (18 of 167), 12.1% (15 of 124), or 22.0% (18 of 82) of the ABT-related deaths reported to SHOT,4 the FDA,1 or the French hemovigilance system2 in 1996 to 2007, 2005 to 2007, or 1994 to 1999, respectively. Except for 5 deaths from transfusion-transmitted babesiosis reported to the FDA,1 1 death from malaria, and 1 death from variant Creutzfeldt-Jakob disease (vCJD) reported to SHOT,4 all other deaths from infectious causes appearing in these 3 reports1,2,4 (44 of 51 deaths) were due to TAS. A more recent analysis of 3 FDA databases (Biological Product Deviation Reports and Adverse Event Reporting System in addition to the Fatality Reports)20 increased the number of deaths from transfusion-transmitted babesiosis in 2005 to 2007 from 5 to 8, with the infectious causes now accounting for 14.2% (18 of 127) of all ABT-related deaths. Thus, underreporting of fatalities aside, after the implementation of bacterial detection of platelets, deaths from infectious causes have been less than 15% of all reported deaths.1,4
Mortality from noninfectious complications of ABT
Table 2 describes the noninfectious causes of ABT-related deaths that accounted for more than 1% of all ABT-related deaths reported to the FDA1 and/or SHOT.4
Potentially fatal noninfectious complications of allogeneic blood transfusion
| Complication . | Definition . | Mechanism . |
|---|---|---|
| Transfusion-related acute lung injury (TRALI) | New acute lung injury (ALI) occurring within 6 hours after a transfusion, with a clear temporal relationship to the transfusion, in patients without risk factors for ALI other than transfusion21 * | Donor anti-WBC antibodies attacking the recipient's WBCs in the microcirculature of the lungs |
| “Two-hit” hypothesis implicating biologic response modifiers accumulating in supernatant plasma during storage† | ||
| Hemolytic transfusion reactions (HTRs) | Immune destruction of the transfused donor RBCs which are attacked by the recipient's: “naturally occurring” antibodies to the A or B antigens of the ABO blood group system, and/or alloantibodies to other RBC antigens produced following immunization through a previous transfusion or pregnancy | Acute HTR: Destruction of “incompatible” donor RBCs intravascularly or extravascularly (in the liver and/or spleen) by preexisting circulating antibody within 24 hours of a transfusion‡ |
| Delayed HTR: Destruction of “compatible” donor RBCs 7-10 days after a transfusion, following an anamnestic immune response to a donor RBC antigen to which the recipient has been alloimmunized by a previous transfusion or pregnancy | ||
| Transfusion-associated graft-versus-host disease (TA-GVHD) | Immune attack against the recipient's tissues and organs by donor lymphocytes which engraft, proliferate, and mount an immune assault against the recipient | Donor lymphocytes not cleared by:immunocompromised patients andpatients who receive components from donors (eg, relatives) with whom they partially share HLA haplotypes survive and engraft in the recipient |
| Transfusion-associated circulatory overload (TACO) | Acute pulmonary edema secondary to congestive heart failure precipitated by transfusion of a volume of blood greater than what the recipient's circulatory system can tolerate | Usually rapid infusion or massive transfusion of blood in patients with diminished cardiac reserve, chronic anemia, infants, and the elderly, although no patient is immune |
| Anaphylaxis | Anaphylactic response of a presensitized patient to various proteins contained in donor plasma | Often, donor IgA infused into an IgA-deficient recipient with preexisting circulating anti-IgA triggers anaphylaxis |
| Posttransfusion purpura (PTP) | Sudden, severe thrombocytopenia occurring 7-10 days after transfusion in a patient previously alloimmunized (by pregnancy or transfusion) to a platelet-specific antigen | Production of potent alloantibody to a platelet-specific antigen through an anamnestic immune response that follows reexposure to the antigen on the donor's platelets. Paradoxically, the antibody destroys the recipient's own (antigen-negative) platelets as well |
| Complication . | Definition . | Mechanism . |
|---|---|---|
| Transfusion-related acute lung injury (TRALI) | New acute lung injury (ALI) occurring within 6 hours after a transfusion, with a clear temporal relationship to the transfusion, in patients without risk factors for ALI other than transfusion21 * | Donor anti-WBC antibodies attacking the recipient's WBCs in the microcirculature of the lungs |
| “Two-hit” hypothesis implicating biologic response modifiers accumulating in supernatant plasma during storage† | ||
| Hemolytic transfusion reactions (HTRs) | Immune destruction of the transfused donor RBCs which are attacked by the recipient's: “naturally occurring” antibodies to the A or B antigens of the ABO blood group system, and/or alloantibodies to other RBC antigens produced following immunization through a previous transfusion or pregnancy | Acute HTR: Destruction of “incompatible” donor RBCs intravascularly or extravascularly (in the liver and/or spleen) by preexisting circulating antibody within 24 hours of a transfusion‡ |
| Delayed HTR: Destruction of “compatible” donor RBCs 7-10 days after a transfusion, following an anamnestic immune response to a donor RBC antigen to which the recipient has been alloimmunized by a previous transfusion or pregnancy | ||
| Transfusion-associated graft-versus-host disease (TA-GVHD) | Immune attack against the recipient's tissues and organs by donor lymphocytes which engraft, proliferate, and mount an immune assault against the recipient | Donor lymphocytes not cleared by:immunocompromised patients andpatients who receive components from donors (eg, relatives) with whom they partially share HLA haplotypes survive and engraft in the recipient |
| Transfusion-associated circulatory overload (TACO) | Acute pulmonary edema secondary to congestive heart failure precipitated by transfusion of a volume of blood greater than what the recipient's circulatory system can tolerate | Usually rapid infusion or massive transfusion of blood in patients with diminished cardiac reserve, chronic anemia, infants, and the elderly, although no patient is immune |
| Anaphylaxis | Anaphylactic response of a presensitized patient to various proteins contained in donor plasma | Often, donor IgA infused into an IgA-deficient recipient with preexisting circulating anti-IgA triggers anaphylaxis |
| Posttransfusion purpura (PTP) | Sudden, severe thrombocytopenia occurring 7-10 days after transfusion in a patient previously alloimmunized (by pregnancy or transfusion) to a platelet-specific antigen | Production of potent alloantibody to a platelet-specific antigen through an anamnestic immune response that follows reexposure to the antigen on the donor's platelets. Paradoxically, the antibody destroys the recipient's own (antigen-negative) platelets as well |
A category of possible TRALI encompasses cases in which patients have other risk factors for ALI temporally related to the transfusion.21
See text in the “TRALI” subsection.
Hemolysis often starts early during the transfusion in the case of an ABO-incompatible transfusion.
TRALI
Transfusion-related acute lung injury has a clinical presentation mirroring that of adult respiratory distress syndrome (ARDS) after ABT.21 In contrast to ARDS, however, patients typically recover with resolution of pulmonary infiltrates within 96 hours. The case-fatality ratio is only 5% to 10%.22 The incidence of TRALI is unknown, because a standard definition22 has not been available until recently. Early reports quoted an incidence of 1 per 5000 transfused blood components,23 with subsequent reports ranging from 1 per 432 pooled whole-blood-derived platelets to 1 per 557 000 RBCs.21 TRALI has been reported as occurring with all types of blood components, including components containing less than 50 mL plasma. Recent data on the relative frequency of TRALI from RBCs and whole blood–derived platelets underscore this fact.1,4 Historically, however, most implicated products have contained more than 50 mL plasma, and FFP has been the most frequently implicated component.24
In 65% to 90% of TRALI cases, WBC (including class I and II HLA or neutrophil-specific) antibodies have been identified in the plasma of the implicated donor.23,25 Most implicated donors have been multiparous women, because pregnancy can result in alloimmunization and 17% to 26% of women with more than 3 pregnancies are immunized to paternal HLA antigens.26 A crossover RCT in 105 intensive care unit patients showed impaired pulmonary function in patients receiving FFP from multiparous women versus controls.27 Thus, in November 2006, the United States28 followed the United Kingdom in recommending the exclusion of multiparous women as FFP and plateletpheresis donors, although women without a history of pregnancy or shown to be negative for WBC antibodies could continue to donate. This policy has already been applied almost uniformly for FFP, albeit not for plateletpheresis donors. Presently, US establishments are adopting various approaches to whether (or when) to exclude female donors with a history of pregnancy or test them for WBC antibodies.
Eder et al reviewed 550 reports of suspected TRALI, including 72 fatalities, submitted to ARC in 2003 to 2005.24 Thirty-eight fatalities were categorized as probable TRALI, and 24 (63%) of them had occurred after FFP transfusion. A female, WBC-antibody–positive donor was involved in 27 (71%) of all TRALI fatalities and in 18 of 24 (75%) deaths implicating FFP transfusion. As US blood establishments moved toward “male-only” FFP between November 2006 and November 2007,28 the number of TRALI fatalities reported to the FDA as associated with FFP decreased from 22 in 2006 to 12 in 2007.1 Eventually, all apheresis platelets could be collected from male donors or female donors without a history of pregnancy or shown to be negative for WBC antibodies. These donors alone, however, are unlikely to produce an adequate supply of RBCs, and the risk of TRALI secondary to RBC transfusion will remain. Five of the 12 probable United Kingdom and 12 of the 34 US 2007 TRALI cases were associated with transfusion of RBCs.1,4
When WBC antibodies are not present in the donor's serum, the “2-hit” model proposed by Silliman et al may explain the development of TRALI in recipients of stored blood components.29 According to this model, the initial insult to the vascular endothelium (due to infection, surgery, trauma, or massive transfusion) attracts and primes neutrophils that adhere to the endothelium. A second “hit” is then mediated by biologic response modifiers contained in transfused plasma (eg, lipid-priming molecules found in the plasma supernatant of stored [as opposed to fresh] RBCs and platelets; cytokines; CD40 ligand; and/or WBC antibodies). These molecules activate the sequestered neutrophils to release oxidases and proteases that damage the endothelium and produce capillary leak and acute lung injury. This model of TRALI pathogenesis would support the transfusion of fresher RBC and platelet components to prevent TRALI.
HTRs
Of the deaths reported to the FDA in 1976 to 1985,6 158 were due to acute HTRs (131 to ABO incompatibility) and 26 to delayed HTRs (mostly due to anti-c and anti-Jka alloantibodies). If all these deaths were attributed to the ABT, mortality from HTRs would approximate 1 per 250 000 RBC units transfused during the decade 1976 to 1985. It is very rare for a delayed HTR to result in death, yet such deaths have been reported.4,6 The outcome of an acute HTR depends on the potency of the (usually ABO) recipient antibody and the volume of blood transfused; infusion rate may also be a factor. Most fatalities have been associated with RBC transfusions of 200 mL or more, and mortality approaches 44% for transfusions exceeding 1000 mL.6,30
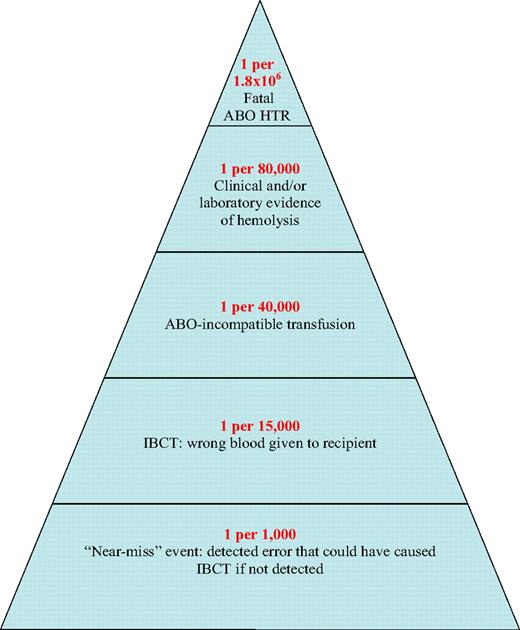
A 10-year (1990-1999) study in New York State documented mistransfusion (or incorrect blood-component transfusion [IBCT]) in 1 per 19 000 transfused RBC units, ABO-incompatible transfusion in 1 per 38 000, and acute HTR (or laboratory evidence of hemolysis) in 1 per 76 000. Five deaths were reported (case-fatality ratio of 2%), resulting in a mortality from acute HTR of 1 per 1.8 million transfused RBC units.31 This is similar to the figures calculated from 1994 to 1999 French hemovigilance2 (1 per 1.8 million transfused RBCs) and SHOT3 data (1 per 1.4 million components issued for transfusion). Figure 3 shows how clerical errors might result in IBCT, including ABO-incompatible transfusions that can result in ABO HTRs. A reduction in these avoidable deaths was observed recently (2004-20071 ), coinciding with the February 2004 FDA requirement that machine-readable information be included on blood-container labels by April 2006.32
Likelihood of a serious ABO HTR, shown as a pyramid whose base represents the probability of events predisposing to incorrect blood component transfusion, whose successive layers show the likelihood of increasingly more hazardous (as well as less likely) events sometimes leading to mortality from ABO HTR, and whose tip represents mortality. The likelihoods indicated are based on data reported by surveillance systems operating in several countries1-6 and are expressed per number of red blood cell (RBC) units transfused.
Likelihood of a serious ABO HTR, shown as a pyramid whose base represents the probability of events predisposing to incorrect blood component transfusion, whose successive layers show the likelihood of increasingly more hazardous (as well as less likely) events sometimes leading to mortality from ABO HTR, and whose tip represents mortality. The likelihoods indicated are based on data reported by surveillance systems operating in several countries1-6 and are expressed per number of red blood cell (RBC) units transfused.
A similar reduction in ABO-HTR fatalities was recently reported from Europe. SHOT reported no death due to IBCT in 2007.4 Between 1996 and 2007, there had been 213 ABO-incompatible RBC transfusions, with 24 deaths and 107 cases of major morbidity due to IBCT. Only 3 acute HTRs were reported in 2007, however, with only 1 of them causing major morbidity from intravascular hemolysis after an ABO-incompatible platelet transfusion.4 French hemovigilance data show a 3-fold reduction in ABO-incompatible RBC transfusions between 2000 and 2005 (from 35 to 13 events per year).17
Systems for checking the identity of units and patients lead to a very low error rate. Such systems, however, are often not used or are used incorrectly. Evolving technologies (such as barrier systems and bar codes) improve safety and efficiency,33,34 while the presence of national patient identification systems in Sweden and Finland has been associated with rates of miscollected samples too low to estimate.35
Reported fatalities due to HTRs secondary to non-ABO antibodies have, however, increased in the United States. In 2005 to 2007, non-ABO antibodies were implicated in 69.2% of all fatal HTRs.1 No single antibody-detection method can detect all clinically significant RBC antibodies, and these figures argue for the development of better antibody-detection methods, as well as for the evaluation of novel approaches to ensuring compatibility between donor and recipient (eg, phenotypic matching of donor and recipient for clinically significant RBC antigens—as currently practiced for sickle-cell anemia36 —to prevent formation of up to 83% of Rhesus, Jka, and Fya antibodies37 ; unequivocal determination of donor blood groups by sequence-specific PCR, oligohybridization, and sequencing approaches, as well as genotyping recipients for the provision of phenotypically matched RBCs38 ; and/or removal or camouflaging of the culprit RBC antigens on the surface of donor RBCs39 ).
Transfusion-associated graft-versus-host disease
Gamma-irradiation of cellular blood components prevents graft-versus-host disease (GVHD), yet sporadic cases of this usually fatal disease do occur. In 1996 to 1999, 12 fatal cases were captured by the SHOT system (4 per year),3,4 although there has been only 1 further case after universal WBC reduction was implemented.40 Of the 13 cases, 2 patients were not known to be immunocompromised at the time of their transfusion, 6 had B-cell malignancies (not listed as an indication for irradiation in the United Kingdom), and 5 were apparently immunocompetent (although there may have been partial haplotype sharing between donor and recipient).3
A higher risk of transfusion-associated (TA)–GVHD has been reported from Japan, where the population is racially quite homogeneous and thus likely to have shared HLA haplotypes between donors and recipients. Four of 847 patients receiving fresh (< 7 days old) blood for cardiac surgery developed TA-GVHD.41 Rososhansky et al estimated that 1 per 2000 patients transfused in the United States may share an HLA haplotype(s) with a donor.42 This figure is much higher than the reported number of TA-GVHD cases, perhaps because blood transfused in the United States is more than 7 days old and does not contain viable lymphocytes. Kleinman et al concluded that the risk of TA-GVHD in Canada is probably less than 1 per 1 000 000 units transfused.19
Approximately 10% of blood components transfused in the United States in 2006 were gamma-irradiated.18 Some hospitals (especially cancer and pediatric centers) gamma-irradiate all blood transfused to their patients, because: (1) patients at risk for TA-GVHD may not receive irradiated blood due to errors of omission, (2) there is no consensus on the list of conditions that render a patient at risk for TA-GVHD,43 and (3) some apparently immunocompetent patients do develop TA-GVHD. WBC-reduced components do not eliminate the risk of TA-GVHD, although it may be possible to develop WBC reduction technologies in the future capable of doing so.44
Mortality attributed to ABT through poorly understood pathophysiologic mechanisms
An RCT designed to investigate a WBC-mediated ABT effect predisposing to postoperative infection in cardiac surgery found, instead of an association between non-WBC–reduced (compared with WBC-reduced) ABT and postoperative infection, an association between non-WBC–reduced (compared with WBC-reduced) ABT and short-term (up to 3 months after transfusion) mortality from all causes.13 The association between ABT and mortality was reported as a data-derived hypothesis,13 and the authors postulated that non-WBC–reduced ABT may predispose to multiple-organ failure (MOF), which might in turn predispose to mortality. These investigators undertook another cardiac-surgery RCT that confirmed the association between ABT and mortality but did not find an association between non-WBC–reduced ABT and increased MOF.14
Hitherto, 11 RCTs comparing recipients of non-WBC–reduced versus WBC-reduced allogeneic RBCs have presented information on short-term (up to 3 months after transfusion) mortality from all causes.13-15,45-52 Across 5 RCTs conducted in cardiac surgery13-15,49,51 that had transfused RBCs filtered before storage to the WBC-reduced arm, non-WBC–reduced (vs WBC-reduced) ABT was associated with a 72% increase in postoperative mortality (Figure 4A). In contrast, across 6 RCTs conducted in other settings,45-48,50,52 non-WBC–reduced (vs WBC-reduced) ABT was not associated with any increase in postoperative mortality (Figure 4B).53
Postoperative mortality in cardiac surgery versus other surgical settings, as calculated from randomized controlled trials investigating the association of nonwhite blood cell–reduced ABT with short-term (up to 3 months after transfusion) mortality from all causes.13-15,45-52 The figure shows the odds ratio (OR) of mortality in recipients of nonwhite blood cell–reduced versus white blood cell (WBC)–reduced allogeneic RBCs, as calculated from each randomized controlled trial (RCT), across RCTs conducted in cardiac surgery (A)13-15,49,51 (summary OR = 1.72; 95% CI, 1.05-2.81), and across RCTs conducted in other settings (B)45-48,50,52 (summary OR = 0.99; 95% CI, 0.73-1.33).53 A WBC-mediated deleterious ABT effect (and thus a benefit from WBC reduction) is demonstrated by an OR more than 1, provided that the effect is statistically significant (P < .05; ie, provided that the associated 95% confidence interval [CI] does not include the null value of 1).
Postoperative mortality in cardiac surgery versus other surgical settings, as calculated from randomized controlled trials investigating the association of nonwhite blood cell–reduced ABT with short-term (up to 3 months after transfusion) mortality from all causes.13-15,45-52 The figure shows the odds ratio (OR) of mortality in recipients of nonwhite blood cell–reduced versus white blood cell (WBC)–reduced allogeneic RBCs, as calculated from each randomized controlled trial (RCT), across RCTs conducted in cardiac surgery (A)13-15,49,51 (summary OR = 1.72; 95% CI, 1.05-2.81), and across RCTs conducted in other settings (B)45-48,50,52 (summary OR = 0.99; 95% CI, 0.73-1.33).53 A WBC-mediated deleterious ABT effect (and thus a benefit from WBC reduction) is demonstrated by an OR more than 1, provided that the effect is statistically significant (P < .05; ie, provided that the associated 95% confidence interval [CI] does not include the null value of 1).
The adverse effect selectively seen across the open heart surgery studies13-15,49,51 may be associated with factors prevalent in patients undergoing cardiac surgery. During cardiac surgery, exposure to the extracorporeal circuit, hypothermia, and reperfusion injury may generate a systemic inflammatory response syndrome (SIRS) that is counteracted by a compensatory anti-inflammatory response syndrome (CARS).54 Any intervention by biologic response modifiers (such as the soluble mediators contained in stored non-WBC–reduced allogeneic RBCs55 ) during an already-existing inflammatory cascade could thus produce an imbalance in the SIRS-CARS equilibrium toward SIRS. An overwhelming SIRS causes a dormant state of cell metabolism referred to as multiple-organ-dysfunction syndrome (MODS), which can ultimately lead to MOF and death.54
The association between prolonged storage of transfused non-WBC–reduced allogeneic RBCs and increased mortality reported from some observational studies56 could also be explained if soluble mediators accumulating in a time-dependent manner during storage were associated with adverse outcomes.55 In this context, the longer the non-WBC–reduced allogeneic RBCs are stored, the higher the level of such mediators they will contain. Silliman et al proposed that ABT may exercise a neutrophil-priming effect mediated by bioactive lipids that accumulate during storage.57 They postulated that rapidly deteriorating WBCs in stored RBCs release cytotoxic enzymes that may act on fragmented RBC membranes to produce mediators responsible for neutrophil priming and endothelial-cell activation. They demonstrated that plasma obtained from stored RBCs primes neutrophils for superoxide production and enhanced cytotoxicity, and also activates pulmonary endothelial cells in a dose- and age-dependent fashion; however, no evidence of neutrophil priming was obtained when plasma stored for short periods was used.57,58 Hitherto, 7 of 21 observational studies and small, pilot RCTs have shown an association between prolonged RBC storage and adverse outcomes.59 Several large RCTs of the effect of length of storage are about to be undertaken.59
In the Transfusion Requirements in Critical Care (TRICC) study,60 normovolemic critically ill patients were randomized to a restrictive-strategy arm and a liberal-strategy arm. Patients transfused for a hemoglobin concentration less than 7.0 g/dL received approximately 3 fewer RBC units than patients transfused for a hemoglobin concentration less than 10.0 g/dL; 33% versus 0% of the patients, respectively, avoided ABT (P < .01). The primary outcome, 30-day mortality, was 18.7% in the restrictive-strategy arm, compared with 23.3% in the liberal-strategy arm (P = .11), but the multiple-organ dysfunction score and in-hospital mortality differed significantly between the arms. The latter were secondary outcomes of the TRICC study. The trend (P = .11) indicated by the primary outcome of the TRICC RCT60 has not been substantiated by any further RCTs.
Mortality from infectious complications of ABT
Since the mid-1980s, the incidence of hepatitis B virus (HBV), hepatitis C virus (HCV), and human immunodeficiency virus (HIV) infections has declined in blood donors, thanks to both better predonation screening criteria and a decrease in the incidence of these infections in the general population.7 At the same time, several measures were introduced in the United States to reduce the risk of transfusion-transmitted infections (TTIs; Table 3). Figure 5 shows the estimated reduction in the risk of transmission of TTIs from the mid-1980s to today.61 Because of the transfusion recipients' advanced age and underlying disease, only a minority survive long enough62 to develop fatal complications of HIV, HCV, or HBV infection. Pereira modeled that, on average, patients acquiring these viruses through transfusion lose 3.26,63 0.75,64 and 0.1865 years of life, respectively, because of the TTI.
Categories of transfusion-transmitted agents
| Agents causing transfusion-transmitted disease for which donors are routinely screened* |
| Hepatitis B virus (HBV; 1970 [surface antigen]; 1986-1987 [core antibody]) |
| Human immunodeficiency virus (HIV; 1985 [antibody]; 2000 [nucleic acid]) |
| Hepatitis C virus (HCV; 1986-1987 [alanine aminotransferase]; 1990 [antibody]; 1999 [nucleic acid]) |
| Human T-cell lymphotropic virus (HTLV; 1988 [antibody]) |
| West Nile virus (WNV; 2003 [nucleic acid]) |
| Bacteria (in platelets only; 2004†) |
| Trypanosoma cruzi (2007 [antibody]) |
| Cytomegalovirus (CMV)‡ |
| Agents that are transfusion transmissible, but have not caused any known disease when acquired through transfusion |
| Agents initially thought to cause hepatitis (GBV-C/HGV, SEN-V, TTV) |
| Human herpes virus 8 (HHV-8) |
| Agents causing transfusion-transmitted disease for which donors are not currently routinely screened |
| Hepatitis A virus (HAV) |
| Parvovirus B19 |
| Dengue fever virus (DFV) |
| Babesia sp§ |
| Plasmodium sp |
| Leishmania sp |
| Brucella sp |
| Variant Creutzfeldt-Jakob disease (vCJD) prions |
| Other¶ |
| Agents causing transfusion-transmitted disease for which donors are routinely screened* |
| Hepatitis B virus (HBV; 1970 [surface antigen]; 1986-1987 [core antibody]) |
| Human immunodeficiency virus (HIV; 1985 [antibody]; 2000 [nucleic acid]) |
| Hepatitis C virus (HCV; 1986-1987 [alanine aminotransferase]; 1990 [antibody]; 1999 [nucleic acid]) |
| Human T-cell lymphotropic virus (HTLV; 1988 [antibody]) |
| West Nile virus (WNV; 2003 [nucleic acid]) |
| Bacteria (in platelets only; 2004†) |
| Trypanosoma cruzi (2007 [antibody]) |
| Cytomegalovirus (CMV)‡ |
| Agents that are transfusion transmissible, but have not caused any known disease when acquired through transfusion |
| Agents initially thought to cause hepatitis (GBV-C/HGV, SEN-V, TTV) |
| Human herpes virus 8 (HHV-8) |
| Agents causing transfusion-transmitted disease for which donors are not currently routinely screened |
| Hepatitis A virus (HAV) |
| Parvovirus B19 |
| Dengue fever virus (DFV) |
| Babesia sp§ |
| Plasmodium sp |
| Leishmania sp |
| Brucella sp |
| Variant Creutzfeldt-Jakob disease (vCJD) prions |
| Other¶ |
The target of the screening assay (antibody, microbial antigen, or microbial nucleic acid) and the year of assay implementation are indicated in parentheses.
See text in the “TAS” subsection.
Prevention by detecting CMV antibody in donors or removing the mononuclear donor cells (in which CMV resides) by means of WBC reduction.
Risk of death from transfusion-transmitted babesiosis is increasing in the United States.20 Of 9 deaths reported to the FDA in 1997-2007, 8 occurred in 2005-2007.20 Thus, in 2005-2007, there were 8 deaths from transfusion-transmitted babesiosis (for which donors are not currently screened) compared to 10 deaths1 from TAS (for which screening of platelet components is imperfect; see text in the “TAS” subsection).
Sixty-eight potentially or known transfusion-transmitted agents have been identified by an AABB Task Force.8
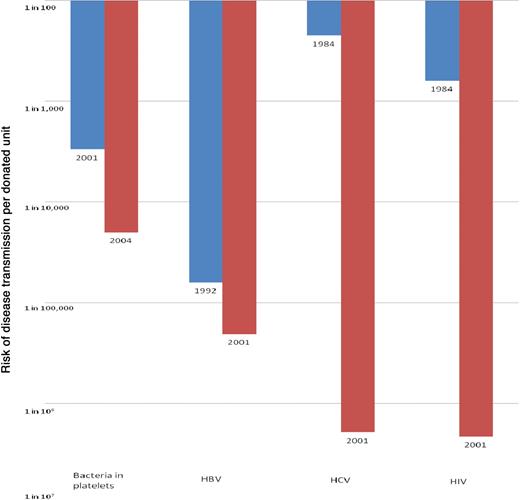
Reduction in the risk of transmission of the 4 most frequently transmitted, potentially fatal, transfusion-acquired infections in the United States since the mid-1980s. The figures plotted pertain to risk reduction documented between 2001 and 2004 (for bacteria in platelets), 1992 and 2001 (for HBV), and 1984 and 2001 (for HCV and HIV). The risk of bacteria in platelets today is considered to be the same as in 2004, and the risk of HBV, HCV, and HIV the same as in 2001, because no further measures to protect the blood supply from these pathogens have been introduced since the latest risk estimates61 were published. The depicted risk estimates reflect the approximate per-unit risk based on various sources (recipient follow-up studies, donor prevalence studies, or mathematical models of the risk of transmission).7 There are no published US data on the risk of bacterial contamination of platelets after several measures (bacterial detection, substitution of single-donor platelets for pools of 4 to 6 whole blood–derived platelets, and/or various process improvements to reduce risk) were implemented in or around 2004. Based on other available literature reviewed in the text in the “TAS” subsection,78-80 the depicted risk reduction represents the authors' estimate of the effect of these combined approaches.
Reduction in the risk of transmission of the 4 most frequently transmitted, potentially fatal, transfusion-acquired infections in the United States since the mid-1980s. The figures plotted pertain to risk reduction documented between 2001 and 2004 (for bacteria in platelets), 1992 and 2001 (for HBV), and 1984 and 2001 (for HCV and HIV). The risk of bacteria in platelets today is considered to be the same as in 2004, and the risk of HBV, HCV, and HIV the same as in 2001, because no further measures to protect the blood supply from these pathogens have been introduced since the latest risk estimates61 were published. The depicted risk estimates reflect the approximate per-unit risk based on various sources (recipient follow-up studies, donor prevalence studies, or mathematical models of the risk of transmission).7 There are no published US data on the risk of bacterial contamination of platelets after several measures (bacterial detection, substitution of single-donor platelets for pools of 4 to 6 whole blood–derived platelets, and/or various process improvements to reduce risk) were implemented in or around 2004. Based on other available literature reviewed in the text in the “TAS” subsection,78-80 the depicted risk reduction represents the authors' estimate of the effect of these combined approaches.
In 2002, the US mosquito-borne West Nile virus (WNV) epidemic resulted in 23 confirmed cases of transfusion-transmitted WNV infection with 7 WNV-related deaths.9 WNV nucleic-acid amplification technology testing was introduced in 2003, but WNV transmissions and deaths have occurred even after the introduction of such testing.66 Although lacking an intermediate avian host that could facilitate its spread to the United States, dengue fever virus (DFV) is transmitted by mosquitoes already present in the United States, has a median viremia of 5 days, and causes asymptomatic infection in most cases. At least 2 cases of transfusion transmission of DFV have been documented,67 and DFV could replicate the 2002 WNV experience in the future. Other arboviruses may pose a similar threat. Chickungunya virus caused several outbreaks on islands in the Indian Ocean; and, in 2007, 205 cases of infection (imported by a visitor from India) occurred in Italy where mosquitoes capable of transmitting the virus exist.12
The number of vCJD cases worldwide has barely exceeded 200, of which 167 have occurred in the United Kingdom.68 The incidence appears to be falling, and recent mathematical projections have suggested an upper limit of approximately 70 further clinical cases in the United Kingdom,69 or that 3800 people aged 10 to 30 years (1 per 11 000 in the United Kingdom population) may be incubating the disease.70 The transmissible spongiform encephalopathies (such as vCJD) may, however, have incubation periods of up to 50 years, with infectious proteinaceous particles (prions) potentially circulating in the peripheral blood for much of the presymptomatic phase of the infection.71 Thus, measures to protect the blood supply from vCJD are being actively evaluated in the United Kingdom, and implementation of a protective measure(s) is expected in the near future. Possible measures include the filtration of blood components through prion-retention filters and/or testing of donor blood for the abnormally conformed prion protein by such methods as PMCA (protein misfolding cyclic amplification).71 Four probable cases of transfusion transmission of vCJD have been reported from the United Kingdom in patients who had received blood products from asymptomatic donors who later developed vCJD.10
TAS
The number of reported TAS deaths today is half what it used to be before the introduction of bacterial detection of (single-donor) apheresis platelets in March 2004 (Figure 1).1,6 Bacterial contamination of blood components results from the introduction of low concentrations of skin bacteria at the time of phlebotomy; less commonly, from asymptomatic donor bacteremia; or rarely, during blood processing.
Before 2004, TAS occurred with a frequency of approximately 1 per 25 000 platelet transfusions (range 1 per 13 000 to 1 per 100 000).72-74 Actual contamination rates of platelet components were substantially higher. Data from 7 studies of apheresis and 12 studies of pooled whole blood–derived platelets showed bacterial contamination rates of 0.09% (31 of 35 122) and 0.43% (376 of 87 922), respectively.75 This (nearly 5-fold) difference could be due to the number of venipunctures (1 vs 4-6) involved in the collection of single-donor versus pooled whole blood–derived platelets.72 Thus, when an institution changed from 51.7% to 99.4% apheresis platelets, correspondingly eliminating pooled whole blood–derived platelets, a two-thirds reduction in TAS was observed.72
In 5 reviewed studies, the RBC contamination rate was 0.1% (60/61 136),75 probably because the 4°C storage temperature of RBCs inhibits bacterial growth during RBC storage. Platelets are responsible for 70% of the fatalities and RBCs for 30%.75 At the 22°C storage temperature of platelets, a wide range of bacteria are capable of proliferation to levels of 106 to 1011 cfu/mL.76,77 Moreover, platelets are most often given to patients who have an impaired immune system and are also often neutropenic. Such recipients are likely more susceptible than other patients to severe bacterial infections. Such recipients, however, also frequently succumb to infections from other sources, and thus the platelet transfusion may not be considered as a source of the bacterial infection. As a result, many episodes of TAS secondary to platelet transfusion are neither recognized nor reported as being transfusion-transmitted.
Before or around 2004, improved donor selection (intended to exclude prospective donors with bacteremia), improved donor-arm disinfection, diversion of the initial flow of donor blood (presumed to contain the skin contaminants) from the collection bag into a pouch, overnight hold of the collected whole blood, and/or WBC reduction had already considerably reduced the risk of bacterial contamination of blood components.75 In 2004, the AABB instituted the requirement to limit and detect bacterial contamination in all platelet components. Various methods were used to meet this requirement, the most effective among them (with sensitivities in the order of 1 cfu/mL) using culture media targeting CO2 production and systems detecting the oxygen consumption by bacteria.75
At that time in the United States, such automated bacterial-culture systems were available only for apheresis platelets, and hospitals releasing pooled whole blood–derived platelets for transfusion had to rely on ineffective methods for bacterial detection that detected only high bacterial levels. Such methods included multiple-reagent dipsticks to detect lowered glucose and pH secondary to bacterial metabolism, and visual inspection of components for poor platelet viability (due to low pH) reflected in absent or decreased platelet “swirling.”75 These methods had species-specific sensitivities in the order of 105 to 108 and more than 107 cfu/mL, respectively,75 thus meeting the letter—but not the spirit—of the 2004 AABB standard.
Since then, automated bacterial-culture systems have become available for pooled whole blood–derived platelets as well, but in the interim many US blood centers had converted to an all-apheresis platelet supply to protect recipients from TAS. As a result, 87.5% of the therapeutic platelet doses transfused in the United States today are single-donor platelets,18 offering patients the benefits of a lower risk of bacterial contamination from platelets as well as a reduced risk of other blood-borne pathogens thanks to reduced donor exposures—1 rather than 4 to 6 exposures. Along these lines, to reduce the number of potential exposures to vCJD, the United Kingdom is also striving to increase collections of single-donor (apheresis) platelets.4
Because even the most sensitive methods currently available for automated bacterial-culture testing have sensitivities of less than 50%,78,79 increased reliance on single-donor platelets may have had more of an effect in reducing fatalities from TAS72 (Figure 1) than did the implementation of bacterial-culture testing per se. In 2004 to 2006, the American Red Cross (ARC) performed bacterial-culture testing on 1 000 000 apheresis platelet donations.80 During this period, 20 episodes of TAS secondary to (screened) apheresis platelets were passively reported, including 3 fatalities (1 per 498 711 distributed components). Comparison of the risk of TAS and TAS-related death from apheresis platelets before and after implementation of bacterial-culture testing of single-donor platelets indicated a downward trend (P = .11) in reported events (1 per 75 000 vs 1 per 40 000) and fatalities (1 per 500 000 vs 1 per 240 000 components).
Strategies for preventing ABT-related deaths
Table 4 presents 5 currently available interventions that can further reduce ABT-related mortality today. Four strategies (conservative transfusion guidelines and avoidance of pooled products, avoidance of female FFP and plateletpheresis donors who have a history of pregnancy and have not tested negative for WBC antibodies, WBC reduction of cellular blood components administered in cardiac surgery, as well as some measures to prevent HTRs) have already been, at least partly, implemented in the United States. The last strategy (pathogen reduction of platelets and FFP) is not yet available in the United States, because no such technologies have been licensed by the FDA. Those strategies that have been introduced, however, have not been adopted universally or uniformly, and the benefit they can confer in preventing ABT-related deaths has not yet been fully realized. For example, 12.5% of therapeutic platelet doses continue to be provided as pooled, whole blood–derived platelets18 ; also, female plateletpheresis donors with a history of pregnancy who have not been tested for WBC antibodies continue to be used, and approaches presently envisioned or implemented by blood establishments for handling these donors vary from testing all women with a history of pregnancy to testing only women with 4 or more pregnancies.
Five currently available interventions for further reducing allogeneic blood transfusion-related mortality
| Strategy . | Causes of ABT-related deaths mitigated by the strategy . |
|---|---|
| Reduction in the number of exposures to allogeneic blood donors through: conservative transfusion guidelines avoidance of pooled blood products | Transmission of any emerging, potentially fatal TTI |
| Residual risk from known TTIs (especially TAS and babesiosis; Table 3) | |
| Other potentially fatal transfusion complications (Table 2) | |
| Use of FFP and single-donor platelets collected exclusively from male donors or female donors without a history of pregnancy or shown not to have WBC antibodies | TRALI |
| Information systems for checking the identity of units and intended recipients*/other measures to prevent HTRs† | Acute HTRs due to ABO-incompatible transfusions |
| Acute and delayed HTRs due to non-ABO alloantibodies to RBC antigens | |
| WBC reduction of RBCs and platelets administered in cardiac surgery | Adverse effects of ABT attributable to WBCs and their biologically active products elaborated during storage |
| Pathogen reduction (PR) of platelets and FFP‡ | Transmission of most emerging, potentially fatal TTIs |
| Residual risk of known TTIs (especially TAS and babesiosis; Table 3) | |
| Transfusion-associated GVHD |
| Strategy . | Causes of ABT-related deaths mitigated by the strategy . |
|---|---|
| Reduction in the number of exposures to allogeneic blood donors through: conservative transfusion guidelines avoidance of pooled blood products | Transmission of any emerging, potentially fatal TTI |
| Residual risk from known TTIs (especially TAS and babesiosis; Table 3) | |
| Other potentially fatal transfusion complications (Table 2) | |
| Use of FFP and single-donor platelets collected exclusively from male donors or female donors without a history of pregnancy or shown not to have WBC antibodies | TRALI |
| Information systems for checking the identity of units and intended recipients*/other measures to prevent HTRs† | Acute HTRs due to ABO-incompatible transfusions |
| Acute and delayed HTRs due to non-ABO alloantibodies to RBC antigens | |
| WBC reduction of RBCs and platelets administered in cardiac surgery | Adverse effects of ABT attributable to WBCs and their biologically active products elaborated during storage |
| Pathogen reduction (PR) of platelets and FFP‡ | Transmission of most emerging, potentially fatal TTIs |
| Residual risk of known TTIs (especially TAS and babesiosis; Table 3) | |
| Transfusion-associated GVHD |
Technologies such as barrier systems, bar codes, and/or national patient identification systems.
Including donor genotyping for RBC antigens, phenotypic matching of donor and recipient for clinically significant RBC antigens, and/or RBC antibody detection methods with improved sensitivity.
No FDA-licensed technology is available in the United States. Moreover, the benefit from this intervention will be suboptimal until PR is also available for RBCs.
Why non-WBC–reduced (compared with WBC-reduced) ABT in cardiac surgery is associated with increased mortality13-15 remains unknown. Non-WBC–reduced ABT has not been associated with a specific cause(s) of death in these RCTs.55 Nevertheless, the magnitude of the absolute risk reduction in mortality attributed to WBC reduction in the RCT of van de Watering et al (4.3%) was impressive: 7.8% of 305 patients receiving non-WBC–reduced RBCs, compared with 3.5% of 604 subjects receiving WBC-reduced RBCs, died within 60 days of having surgery.13 Although overall mortality was lower in the United States15,51 than the Dutch13,14 RCTs, such potentially WBC-mediated adverse ABT effects13-15 could account for a larger number of ABT-related deaths than all the currently established complications of ABT combined. The need for further research to elucidate the mechanism of the apparent increase in mortality in recipients of non-WBC–reduced (compared with WBC-reduced) ABT notwithstanding, we believe that, where RCTs have attributed excess mortality to such a WBC-mediated effect, WBC reduction of cellular blood components should be implemented to prevent such excess deaths. This situation has hitherto arisen only in cardiac surgery (Figure 4), a setting in which idiosyncratic effects of ABT could be expected and would not necessarily be generalizable to other settings.53-55
Approximately 80% of platelet and 55% of RBC units transfused in the US are WBC-reduced.18 The number of WBC-reduced components declined by 12% between 2004 and 2006 (from 12 to 10.6 million components).18 US hospitals use WBC-reduced components either universally or selectively, depending primarily on their location and the practices of the local blood provider (which may manufacture only WBC-reduced or both WBC-reduced and non-WBC–reduced cellular blood components). When selective WBC reduction is used, administration of WBC-reduced components to cardiac-surgery patients is usually not included among the established indications for WBC reduction.81 Thus, in some US hospitals, WBC-reduced RBCs are administered routinely to patients whose mortality has not been shown to be affected by WBC reduction (Figure 4B), whereas elsewhere cardiac-surgery patients whose mortality has been shown to be reduced by WBC reduction (Figure 4A) may not always receive WBC-reduced components.
The past 2 decades have witnessed an impressive reduction in the probability of transmission of HIV and HCV through ABT by approximately 4 log (Figure 5). Yet the risk of bacterial contamination of platelets was addressed only in 2004, and this risk remains significant also today. Also, although the number of transfusion-related deaths has been greatly reduced, the risk of a new, or poorly understood, infectious disease with a long incubation period that can be transmitted by ABT, while it is accumulating in the blood-donor base before its clinical consequences become apparent, remains a “fixed and inevitable property of transfusion medicine.”82 Although only 3 years had elapsed between the recognition of the threat that HIV posed to blood safety (1982) and the implementation of donor testing (1985), approximately 12 000 cases of transfusion-acquired HIV infection were contracted in the US.83 When the interval between recognition and introduction of testing was longer, the number of transfusion-acquired infections was correspondingly higher. Thus, in 1970 to 1990, there were 4.8 million HCV transmissions through ABT.8 Even if only 3% of these resulted in fatal cirrhosis or hepatocellular carcinoma in long-term survivors,84 and only 30% of recipients survived long enough to develop such complications,62 43 200 ABT-related deaths could have ensued.
Our current “agent-by-agent” reactive approach to transfusion safety necessitates that blood establishments implement further safety measures in response to each new transfusion-transmitted, or potentially transfusion-transmitted, agent that emerges. Rather than this reactive “agent-by-agent” approach, there can be a more all-encompassing approach to blood safety that would address most transfusion-transmitted pathogens: pathogen reduction (PR) by nucleic-acid intercalating agents such as psoralens or riboflavin that, in the presence of ultraviolet light, bind to the nucleic acids of pathogens and inactivate them, while permitting the nucleic-acid-free constituents of donor blood (plasma proteins, platelets, and RBCs) to continue to function.85 These technologies can eliminate most of the residual risk of bacteria, as well as the risk associated with a long list of transfusion-transmitted pathogens for which the blood supply is not screened (Table 3). Even more importantly, these technologies offer probable, preemptive protection against the next potentially lethal transfusion-transmitted agent that could emerge in the future, possibly replicating the experience with HIV, HCV, or WNV. Pathogen reduction will also prevent rare deaths from TA-GVHD in patients receiving unirradiated components either by mistake or because they do not have a recognized indication for gamma-irradiation.86
These technologies are currently available for platelets and FFP, have an acceptable safety profile, and have already been licensed in western Europe and elsewhere. Although the safety improvement they confer is widely acknowledged, there is debate as to whether PR for all platelets and FFP should be introduced before suitable technologies also become available for RBCs. Pathogen reduction's contribution to safety can be only suboptimal until such time as such technology can also be applied to RBCs. Also, until there is a comprehensive system of PR that also encompasses RBCs, thereby permitting various cost savings from the discontinuance of other safety measures,8 the cost of PR is bound to be incremental and significant. The Canadian Consensus Conference concluded that economic evaluations of PI procedures should be conducted, but implementation of PI should be based on other considerations in addition to the results of economic analyses.87 In discussing the cost-effectiveness of PI, Custer and Hoch wondered whether, “as a society, we want to adopt this expensive technology that has broad-spectrum capabilities and—because of the investment already made in PI—face the budgetary reality that other safety interventions could be beyond our means but also not needed due to PI's efficacy.”88(p10)
In our opinion, because it cannot be predicted when the next potentially lethal, transfusion-transmitted agent will emerge, PR technologies for platelets and FFP should be implemented when they are licensed, rather than waiting for PR technologies for RBCs to also be developed and licensed, hopefully over the next 5 to 10 years.87 PR cannot protect recipients from all future transfusion-transmitted agents. It is ineffective against pathologic prions, intracellular pathogens, spore-forming bacteria, nonenveloped viruses, and viruses present in exceedingly high concentrations in blood. Its downside is that it causes cellular losses and thus reduces the therapeutic efficacy of blood components, necessitating the transfusion of greater volumes of blood and exposing patients to more donors, thereby increasing the risk of transmission of agents not inactivated by PR.
Reducing the number of donors to whom a patient is exposed is the only strategy that can reduce the risk of transmission of any transfusion-transmitted agent that could emerge in the future. This can be accomplished through conservative transfusion guidelines and avoidance of pooled products such as pooled whole blood–derived platelets. Muller et al outlined 5 key elements that contributed to effectiveness in reducing RBC use in orthopedic surgery.89 These included: (1) simplicity of the algorithm provided, (2) wide distribution in the hospital of the information contained in the algorithm, (3) no requirement for major change in practice, (4) endorsement by local opinion leaders, and (5) development of a sense of ownership by the local blood conservation program. When an intervention as simple as a 1-page flow chart graphically summarizing the perioperative decision pathways for anemic patients was implemented under these conditions, the proportion of patients receiving allogeneic RBC transfusion decreased from 35.5% to 19.8%, indicating that a change in culture can indeed be achieved by modest means, reducing recipient exposure to ABT.
Authorship
Contribution: E.C.V. and M.A.B. contributed equally to the development of the concepts relating to the contents of this article, the data analysis, and the writing of the manuscript.
Conflict-of-interest disclosure: E.C.V. declares no competing financial interests. M.A.B. is a member of the Scientific Advisory Board of Cerus Corporation (Concord, CA), a manufacturer of pathogen inactivation technology for blood products; holds a research contract with MacoPharma International GmbH (Langen, Germany) to do developmental and experimental animal experiments related to the pathogen inactivation of human platelets; was on the Medical Advisory Board of Pall Medical, a company involved in manufacturing filters used for the leukoreduction of blood components; is currently the Medical Director of the Hamilton and London Centres of Canadian Blood Services, 1 of the 2 major suppliers to hospitals of blood components in Canada; and is currently Chair of the Steering Committee (SC) of the National Heart, Lung and Blood Institute Transfusion Medicine/Hemostasis Clinical Trials Network (TMH CTN), which is not currently involved in any clinical studies relevant to this scientific review.
Correspondence: Morris A. Blajchman, Department of Pathology, McMaster University, 1200 Main St West, HSC 4N67, Hamilton, ON, Canada L8N 3Z5; e-mail: blajchma@mcmaster.ca.

![Figure 4. Postoperative mortality in cardiac surgery versus other surgical settings, as calculated from randomized controlled trials investigating the association of nonwhite blood cell–reduced ABT with short-term (up to 3 months after transfusion) mortality from all causes.13–15,45–52 The figure shows the odds ratio (OR) of mortality in recipients of nonwhite blood cell–reduced versus white blood cell (WBC)–reduced allogeneic RBCs, as calculated from each randomized controlled trial (RCT), across RCTs conducted in cardiac surgery (A)13–15,49,51 (summary OR = 1.72; 95% CI, 1.05-2.81), and across RCTs conducted in other settings (B)45–48,50,52 (summary OR = 0.99; 95% CI, 0.73-1.33).53 A WBC-mediated deleterious ABT effect (and thus a benefit from WBC reduction) is demonstrated by an OR more than 1, provided that the effect is statistically significant (P < .05; ie, provided that the associated 95% confidence interval [CI] does not include the null value of 1).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/113/15/10.1182_blood-2008-10-167643/7/m_zh80170934000004.jpeg?Expires=1769083961&Signature=3n4Alxm8fO1fUxzVRfZqyFgBCMrtJ-GPmt8qFJOiBb-bmdf2elCzxqXUvpjlWWyzqVSZ01yH~BLc5bhEpzWja5B3D~LmAQUWB1JBHsPnp7FPolRhWsVBPVxQPIrTF8kr-FUBHC87vGLg9JvAjNDZ38LpAywifYChth5boGokZIx1one39umJLcakyKq0uI6EfnpYmeKW4lbo4zfkDqseeVh0YWF9SkGcYJ3fm4SYORm3BTxLdB3abAaF8HVeRINqv0hfDAoGIOHW3Fs7eZlX~mMPp3R65dvtXBXo-bynqpVTG1Y1lqeCEneiprPpoprMxNQ4QSWWulYZHPsnFJawGA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)