Abstract
Despite recent advances, allografting remains the only potential cure for myeloma. From July 1999 to June 2005, 100 newly diagnosed patients younger than 65 years were enrolled in a prospective multicenter study. First-line treatment included vincristin, adriamycin, and dexamethasone (VAD)–based induction chemotherapy, a cytoreductive autograft (melphalan 200 mg/m2) followed by a single dose of nonmyeloablative total body irradiation and allografting from an human leukocyte antigen (HLA)–identical sibling. Primary end points were the overall survival (OS) and event-free survival (EFS) from diagnosis. After a median follow-up of 5 years, OS was not reached, and EFS was 37 months. Incidences of acute and chronic graft-versus-host disease (GVHD) were 38% and 50%, respectively. Complete remission (CR) was achieved in 53% of patients. Profound cytoreduction (CR or very good partial remission) before allografting was associated with achievement of posttransplantation CR (hazard ratio [HR] 2.20, P = .03) and longer EFS (HR 0.33, P < .01). Conversely, development of chronic GVHD was not correlated with CR or response duration. This tandem transplantation approach allows prolonged survival and long-term disease control in patients with reduced tumor burden at the time of allografting. We are currently investigating the role of “new drugs” in intensifying pretransplantation cytoreduction and posttransplantation graft-versus-myeloma effects to further improve clinical outcomes. (http://ClinicalTrials.gov; NCT-00702247.)
Introduction
Despite remarkable recent advances in its treatment, multiple myeloma remains incurable.1 Allografting is still regarded as the only potential cure on account of its well-documented graft-versus-myeloma effect observed in a subset of patients.2-5 However, its use remains controversial especially in newly diagnosed patients.
In the late 1990s, the introduction of reduced intensity/nonmyeloablative conditionings greatly renewed the interest in allografting, in particular for diseases such as myeloma where the transplantation-related mortality (TRM) with conventional transplantation regimens had been unacceptably high.5-7 Combining the cytoreductive effect of a high-dose melphalan-based autograft with the graft-versus-myeloma effects of a nonmyeloablative allograft reduced TRM even in elderly, medically unfit myeloma patients.8,9
Our recent comparison between autografting and nonmyeloablative allografting showed that the latter resulted in longer overall survival (OS) and event-free survival (EFS) in newly diagnosed patients younger than 65 years.10 Preliminary reports from other groups have confirmed our findings.11,12 Here, we report on an extended experience consisting of 100 newly diagnosed myeloma patients enrolled in a prospective clinical trial (http://ClinicalTrial.gov; NCT-00702247) and treated with nonmyeloablative allografts as part of their first-line treatment at 15 Italian Bone Marrow Transplantation Units of the Gruppo Italiano Trapianti di Midollo Osseo (GITMO).
Methods
Patients and donors
From July 1999 to June 2005, 100 newly diagnosed myeloma patients younger than 65 years were enrolled in a prospective multicenter trial. Informed consent was obtained upon enrollment in accordance with the Declaration of Helsinki. The protocol was approved by the Institutional Review Boards of the participating centers.
Inclusion criteria included diagnosis of untreated Durie & Salmon stage IIA to IIIB multiple myeloma or stage I progressed to require therapy; age less than 65 years; Karnofsky performance status greater than 60%; and presence of an human leukocyte antigen (HLA)–identical sibling donor eligible for peripheral blood stem cell (PBSC) donation. Exclusion criteria included prior treatment for myeloma, abnormal cardiac function and chronic respiratory disease defined as systolic ejection fraction less than 35% and carbon monoxide diffusing capacity less than 40% of predicted or need of continuous supplemental oxygen, respectively; serum bilirubins greater than twice normal and alanine amino transferase (ALAT) and/or aspartate amino transferase (ASAT) greater than 4 times normal; poorly controlled hypertension; pregnancy; and seropositivity for HIV. Patients with active nonhematologic malignancies except nonmelanoma skin cancers or who were less than 5 years from the achievement of complete remission with a greater than 20% risk of disease recurrence were also excluded.
Sibling donors less than 75 years of age were serologically matched for HLA-A, -B, and -C antigens, and by high-resolution typing for HLA-DRB1 and -DQB1 alleles. Donors gave consent to granulocyte colony-stimulating factor (G-CSF) administration and to leukapheresis for PBSC collections. Pregnant women, identical twins, HIV-positive people, and people with known allergy to G-CSF were excluded from donation.
Induction therapy, PBSC mobilization, and autografting
Initial treatment plan included induction chemotherapy, mainly consisting of 2 to 3 courses of vincristine-adriamycin-dexamethasone (VAD)–based regimens, followed by PBSC mobilization and harvest (target of at least 2 × 106 CD34 cells/kg) after 1 or 2 cycles of 3 to 4 g/m2 cyclophosphamide, with or without 250 mg/m2 paclitaxel, and 10 μg/kg G-CSF given intravenously or subcutaneously. After at least 1 month from PBSC collection, autografting consisted of 200 mg/m2 melphalan, on day −2 and cryopreserved PBSC infusion on day 0. Patients received 5 μg/kg G-CSF, from days 1 or 3 until neutrophil counts greater than 1000/μL were achieved.
Donor mobilization
HLA-identical sibling donors, mean age 54 (range 32-69) years, were mobilized with 16 μg/kg per day G-CSF (day −4 to 0), with aphereses on days −1 and 0. PBSC harvested on day −1 were stored overnight at 4°C and freshly infused with the day 0 collection. The entire collections were infused, and a minimum target of 5 × 106 CD34+ cells/kg of recipient body weight was recommended. No upper limit was defined.
Nonmyeloablative allografting
Upon recovery from autografting, defined as resolved mucositis, no evidence of cytomegalovirus (CMV) reactivation or disease, and no need for intravenous medications, planned range of 2 to 4 months, patients were conditioned for allografting with a single dose of nonmyeloablative 200 cGy total body irradiation (TBI) on day 0. Postgrafting immunosuppression consisted of mycophenolate mofetil, 15 mg/kg orally twice a day from the evening of day 0 until day 27, and cyclosporine, 6.25 mg/kg orally twice a day from day −3 or 1.5 mg/kg intravenously twice a day from day −1 through day 80, and then tapered.9,10 No maintenance/consolidation therapy was allowed by protocol after nonmyeloablative allografting.
Analyses of chimerism
Chimerism analyses of peripheral blood T cells, granulocytes, and unfractionated marrow were carried out at days 28, 56, 180, 360 after allografting and every 6 months thereafter or as clinically indicated with fluorescence in situ hybridization (FISH) in sex-mismatched pairs or polymerase chain reaction (PCR)–based analyses of polymorphic microsatellite regions in sex-matched pairs as previously described.9,10
Chromosomal abnormalities
Chromosome 13q deletion [del(13)] was analyzed by interphase FISH techniques on freshly purified bone marrow plasma cells as previously described.13
Supportive care and GVHD grading
After allografting, all patients received standard prophylaxis against bacterial and fungal infections; herpes simplex and varicella-zoster virus reactivation; and Pneumocystis carinii. CMV reactivation was monitored through levels of CMV antigenemia and/or serum CMV DNA levels and treated with ganciclovir or foscarnet as clinically indicated. Standard criteria were used for diagnosis and grading of acute and chronic graft-versus-host disease (GVHD).14,15
Salvage therapy
Standard chemotherapy and/or thalidomide- or bortezomib-containing regimens, as per institutional guidelines of the participating centers, with/without donor lymphocyte infusions (DLIs) were allowed to treat progression or relapse posttransplantation. DLIs were administered in the absence of GVHD clinical manifestation and after a rapid taper and discontinuation of the immunosuppression.
Disease response
Response was evaluated before each treatment, monthly for the first 6 months after allografting and at least every 3 months thereafter or as clinically indicated. Response criteria were defined according to the International Uniform Response Criteria for multiple myeloma.16 Complete remission (CR) required absence of serum monoclonal immunoglobulins and/or Bence-Jones proteinuria by electrophoresis and immunofixation, less than 5% plasma cell infiltration in bone marrow aspirates, absence of soft tissue lesions, and no increase in size or number of osteolytic lesions. Very good partial remission (VGPR) was defined as detection of serum monoclonal immunoglobulins and/or Bence-Jones proteinuria by immunofixation but not by electrophoresis or at least 90% reduction in Bence-Jones proteinuria with excretion lower than 100 mg per 24 hours and no increase in size or number of osteolytic lesions. Partial remission (PR) was defined as greater than 75% reduction in the levels of serum monoclonal immunoglobulin, at least 90% reduction in 24-hour urinary light chain excretion, and no increase in size or number of lytic bone lesions. Patients with less than a PR after induction chemotherapy or autografting were considered refractory, whereas the disease was considered stable if no response, meeting the criteria of CR, VGPR, or PR, was observed after allografting. Response criteria had to be met on at least 2 consecutive occasions at least 6 weeks apart. Progressive disease (PD) was considered an increase in serum monoclonal proteins or urine light chains of a least 25% in patients with refractory or stable disease, whereas relapse was considered as the reappearance of bone marrow infiltration, serum monoclonal immunoglobulins, or urine light chains or new bone lesions in patients in previous CR, or a 25% increase in any disease marker for patients in prior PR.
Statistical analysis
Primary end points of the study were OS and EFS from diagnosis according to the intention-to-treat principle and in patients who completed the program. Secondary end points included transplantation-related toxicity and TRM and incidence of acute and chronic GVHD. OS and EFS were calculated according to the Kaplan-Meier technique from the date of diagnosis until death from any cause and from the date of diagnosis until the date of first relapse or progression of death from any cause, respectively.17 Deaths not related to myeloma or to nonhematologic malignancies were classified as deaths from transplantation-related toxicity. Moreover, an estimation of the probability that a patient was alive in the original remission or in a subsequent remission after salvage treatment was carried out by the “Current Progression Free Survival” (CPFS) method as described by Klein et al18 Estimates of the incidence of acute and chronic GVHD, TRM, and disease-related mortality were calculated with the cumulative incidence method described by Gooley et al, in which risks of death in CR and of relapse were considered as competing risks.19 The individual effect of patient characteristics on time from allografting to 5 different events (relapse/progression, death, CR after transplantation, chronic extensive GVHD, and acute grade 2-IV GVHD) were evaluated using Cox proportional-hazards regression models with the Wei-Lin-Weissfeld estimators.20-23 Proportional hazard assumptions were checked with the Grambsh and Therneau test.24 Predictors were chosen for each outcome in the light of potential clinical impact and sample size as follows: prognostic role of age, isotype of myeloma protein, International Staging System (ISS) score, disease in remission, defined as VGPR or CR, at the time of allografting, and comorbidity index greater than or equal to 3 for both OS and EFS; chronic GVHD, as time-dependent covariate, age, isotype of myeloma protein, ISS, disease in remission at the time of allografting for both the achievement of CR and the risk of relapse/progression after allografting; effects of CD34+ cells and CD3+ T cells infused, age, and donor gender for the risk of developing acute and chronic GVHD.25,26 Results are presented as hazard ratios (HR) with corresponding 95% confidence intervals (CIs) and P values. SAS 8.2 statistical software (SAS Institute, Cary, NC) and R.2.1.0 software, package cmprsk were used.
Results
Patients
Patient characteristics are shown in Table 1. Overall, 100 patients with at least one HLA-identical sibling were enrolled at 15 Italian Bone Marrow Transplantation Units. Ninety-six of 100 (96%) completed the protocol, whereas 4 did not because of consent withdrawal (n = 2); infectious complications after the autograft (n = 1); and ineligible donor at pretransplantation work-up (n = 1). Fifty-two, also described in an earlier report, have been included in this series after updating their follow-up.10
Patient characteristics
| Characteristic . | Patients enrolled, no. (%)* . | Patients who completed program, no. (%)† . |
|---|---|---|
| Male | 52 (52) | 52 (54) |
| Mean age, y (range) | 54 (30-65) | 54 (30-65) |
| Durie & Salmon stage II | 29 (29) | 26 (27) |
| Durie & Salmon stage III | 67 (67) | 66 (69) |
| International Staging System 2 | 22/92 (24) | 22/88 (25) |
| International Staging System 3 | 14/92 (15) | 12/88 (14) |
| IgG myeloma | 57 (57) | 56 (58) |
| IgA myeloma | 18 (18) | 18 (19) |
| IgD myeloma | 1 (1) | 0 |
| Bence-Jones myeloma | 18 (18) | 17 (18) |
| Nonsecretory myeloma | 6 (6) | 5 (5) |
| β-2-microglobulin > 3.5 mg/dL | 33/95 (35) | 31/91 (34) |
| Albumin < 3.5 g/dL | 21/95 (22) | 21/92 (23) |
| Creatinine > 2 mg/dL | 11 (11) | 11 (11) |
| LDH above normal level | 17/91 (19) | 16/88 (18) |
| Presence of chromosome 13 deletion | 14/43 (33) | 13/39 (33) |
| HCT-specific comorbidity index ≥ 3 | 11 (11) | 10 (10) |
| Characteristic . | Patients enrolled, no. (%)* . | Patients who completed program, no. (%)† . |
|---|---|---|
| Male | 52 (52) | 52 (54) |
| Mean age, y (range) | 54 (30-65) | 54 (30-65) |
| Durie & Salmon stage II | 29 (29) | 26 (27) |
| Durie & Salmon stage III | 67 (67) | 66 (69) |
| International Staging System 2 | 22/92 (24) | 22/88 (25) |
| International Staging System 3 | 14/92 (15) | 12/88 (14) |
| IgG myeloma | 57 (57) | 56 (58) |
| IgA myeloma | 18 (18) | 18 (19) |
| IgD myeloma | 1 (1) | 0 |
| Bence-Jones myeloma | 18 (18) | 17 (18) |
| Nonsecretory myeloma | 6 (6) | 5 (5) |
| β-2-microglobulin > 3.5 mg/dL | 33/95 (35) | 31/91 (34) |
| Albumin < 3.5 g/dL | 21/95 (22) | 21/92 (23) |
| Creatinine > 2 mg/dL | 11 (11) | 11 (11) |
| LDH above normal level | 17/91 (19) | 16/88 (18) |
| Presence of chromosome 13 deletion | 14/43 (33) | 13/39 (33) |
| HCT-specific comorbidity index ≥ 3 | 11 (11) | 10 (10) |
Ig indicates immunoglobulin; LDH, lactate dehydrogenase; and HCT, hematopoietic cell transplantation.
n = 100.
n = 96.
Engraftment and response
Ninety-six allografts were carried out at a median of 90 (range 44-396) days after the autograft. One patient underwent the allograft 13 months after the autograft because of viral encephalitis. The median numbers of CD34+ cells and CD3+ T cells infused were 7.5 × 106/kg (range, 2.6-26.4 × 106) and 3.2 × 107/kg (range, 0.7-18.9 × 107) recipient body weight, respectively. All patients readily achieved engraftment with median percentages of donor cells at 1 month after transplantation of 97%, 97%, and 97% among blood T cells, granulocytes, and unfractionated bone marrow, respectively.
Forty-eight of 100 patients had chemosensitive disease (2 CR, 6 VGPR, and 40 PR) at the time of autografting, while, among the 96 patients who completed the program, 6 (6%) were in CR, 29 (30%) were in VGPR, and 38 (40%) were in PR at the time of allografting. Overall, after the allograft, 51 patients (53%) achieved CR at a median of 4 months (range 1-45), 15 patients (16%) achieved VGPR, and 21 patients (22%) achieved PR, giving an overall response of 91%. With a median follow-up of 5 (range, 2.3-8.4+) years from diagnosis and 4.3 (1.8-7.4+) from allografting, 14 of 51 patients (27%), 10 of 15 patients (67%), and 12 of 21 patients (57%) had relapsed from CR, VGPR, and PR, respectively, giving an overall relapse rate of 41% (36/87). Overall response and relapse rates for the entire cohort of patients were 88% (88/100) and 44% (39/88), respectively. Disease-related mortality was 5.2% at 2 years (95% CI, 0.8%-9.6%) and 20.5% at 5 years (95% CI, 11.7-29.3; Figure 1C).
Cumulative incidence estimates of GVHD and mortality. (A) Acute GVHD: grade 2 to 4 GVHD 38% (solid line); grade 4.3% (dotted line). (B) Transplantation-related mortality: 11%. (C) Disease-related mortality: 5.2% at 2 years; 20.5% at 5 years.
Cumulative incidence estimates of GVHD and mortality. (A) Acute GVHD: grade 2 to 4 GVHD 38% (solid line); grade 4.3% (dotted line). (B) Transplantation-related mortality: 11%. (C) Disease-related mortality: 5.2% at 2 years; 20.5% at 5 years.
Salvage therapy
Thirty-six patients were treated for disease relapse and 6 for progression of stable disease. First-line salvage therapy consisted of bortezomib- or thalidomide-containing regimens in 12 and 16 patients, respectively, and standard chemotherapy and/or radiotherapy in 8 patients. Moreover, 9 of these patients received DLI as consolidation therapy. Five patients received DLI alone. In 1 patient, the immunosuppression was tapered and eventually discontinued without further therapy. Overall, 6 of 42 patients (14%) obtained CR, and 13 of 42 (31%) obtained PR. Five patients experienced a second relapse.
Only 4 patients received DLI for posttransplantation stable disease. Overall, among the 18 patients treated with DLI, only 1 (7%) reached CR and 3 (17%) others reached PR.
Transplantation-related toxicity
Thirty-six patients developed grade 2 to 4 acute GVHD at a median of 41 (range, 20-115) days. This was grade 4 in 3 patients. Cumulative incidence of grade 2 to 4 and grade 4 GVHD was 38% and 3%, respectively (Figure 1A). Forty-seven of 94 patients (50%) with a follow-up of at least 120 days developed extensive chronic GVHD. Overall, 53 of 85 patients, 27 of 73, and 10 of 36 remained on immunosuppression at 1, 2, and 4 years after allografting, respectively. Six of 53 patients (11%) who developed acute GVHD had a flare after initial therapy that required an immunosuppression taper longer than 1 year. Most patients had a Karnofsky performance status of 90% to 100% despite immunosuppression. One patient in CR with severe bronchiolitis obliterans successfully underwent lung transplantation. Twenty-nine patients (30%) have died: 15 (16%) from disease progression, 11 (11%) from transplantation-related toxicity, and 3 from another malignancy. TRM was due to progressive encephalopathy (n = 1), complications associated with acute or chronic GVHD (n = 8), and hemolytic-uremic syndrome thrombotic thrombocytopenic purpura (HUS-TTP) syndrome (n = 2). Overall TRM was 11.4% (95% CI, 4.8-17.2; Figure 1B). By multivariable analysis, an increasing number of CD34+ cells infused, but not CD3+ T cells, was associated with a significant risk of developing acute GVHD, but not chronic GVHD (HR 1.12; CI 95%, 1.04-1.21; P < .01, and HR 1.03; CI 95%, 0.94-1.13; P = .55; Table 2). Furthermore, the development of chronic GVHD was not significantly associated with either the subsequent achievement of CR or disease relapse/progression (HR 0.80; CI 95%, 0.43-1.47; P = .47, and HR 0.85; CI 95%, 0.51-1.42; P = .54; Table 2).
Cox models for achievement of complete remission, for progression/relapse, and for development of acute and chronic GVHD
| Variable . | Multivariable analyses . | |||
|---|---|---|---|---|
| Achievement of CR . | Progression/relapse . | |||
| HR (95% CI) . | P . | HR (95% CI) . | P . | |
| Development of chronic GVHD* | 0.80 (0.43-1.47) | .47 | 0.85 (0.51-1.42) | .54 |
| Age† | 0.96 (0.92-1.00) | .05 | 1.08 (1.03-1.13) | < .01 |
| IgG myeloma | 0.77 (0.45-1.30) | .33 | 0.61 (0.35-1.06) | .08 |
| International Staging System 3 | 0.66 (0.22-1.98) | .46 | 1.39 (0.69-2.79) | .35 |
| Disease in remission‡ at allografting | 2.20 (1.18-4.08) | .03 | 0.30 (0.15-0.62) | < .01 |
| Variable . | Multivariable analyses . | |||
|---|---|---|---|---|
| Achievement of CR . | Progression/relapse . | |||
| HR (95% CI) . | P . | HR (95% CI) . | P . | |
| Development of chronic GVHD* | 0.80 (0.43-1.47) | .47 | 0.85 (0.51-1.42) | .54 |
| Age† | 0.96 (0.92-1.00) | .05 | 1.08 (1.03-1.13) | < .01 |
| IgG myeloma | 0.77 (0.45-1.30) | .33 | 0.61 (0.35-1.06) | .08 |
| International Staging System 3 | 0.66 (0.22-1.98) | .46 | 1.39 (0.69-2.79) | .35 |
| Disease in remission‡ at allografting | 2.20 (1.18-4.08) | .03 | 0.30 (0.15-0.62) | < .01 |
| . | Development of acute GVHD . | Development of chronic GVHD . | ||
|---|---|---|---|---|
| HR (95% CI) . | P . | HR (95% CI) . | P . | |
| CD3+ cells/kg recipient weight† | 0.98 (0.81-1.18) | .85 | 1.05 (0.97-1.13) | .23 |
| CD34+ cells/kg recipient weight† | 1.12 (1.04-1.21) | < .01 | 1.03 (0.94-1.13) | .55 |
| Age† | 1.03 (0.98-1.09) | .25 | 1.05 (1.01-1.10) | .02 |
| Female donor | 2.11 (1.00-4.45) | .05 | 1.06 (0.54-2.08) | .86 |
| . | Development of acute GVHD . | Development of chronic GVHD . | ||
|---|---|---|---|---|
| HR (95% CI) . | P . | HR (95% CI) . | P . | |
| CD3+ cells/kg recipient weight† | 0.98 (0.81-1.18) | .85 | 1.05 (0.97-1.13) | .23 |
| CD34+ cells/kg recipient weight† | 1.12 (1.04-1.21) | < .01 | 1.03 (0.94-1.13) | .55 |
| Age† | 1.03 (0.98-1.09) | .25 | 1.05 (1.01-1.10) | .02 |
| Female donor | 2.11 (1.00-4.45) | .05 | 1.06 (0.54-2.08) | .86 |
Ig indicates immunoglobulin.
Time-dependent variable.
Continuous variable.
Defined as VGPR and CR.
Outcome
By the intention-to-treat principle, after a median follow-up of 5 years (range, 0.7-8.4+) from diagnosis, median OS was not reached, and median EFS was 2.9 years (range, 2.4-4.3; Figure 2A,B). Among the 96 patients who completed the program, after a median follow-up of 5 years (range, 2.3-8.4+) from diagnosis, median OS was not reached, whereas median EFS was 3.1 years (range, 2.6-4.5; Figure 2C,D). No differences in both the updated OS and EFS between the previously reported cohort of 52 patients10 and the newly described 44 were observed (HR 0.58; CI 95%, 0.28-1.2; P = .14, and HR 0.93; CI 95%, 0.55-1.56; P = .78, respectively). Furthermore, the probability of a patient being alive in first remission or in a subsequent remission due to salvage therapy is illustrated in Figure 3.
Kaplan-Meier estimates of OS and EFS after a follow up of 5 years. OS (A) and EFS (B) by intention-to-treat principle; OS (C) and EFS (D) among patients who completed the program.
Kaplan-Meier estimates of OS and EFS after a follow up of 5 years. OS (A) and EFS (B) by intention-to-treat principle; OS (C) and EFS (D) among patients who completed the program.
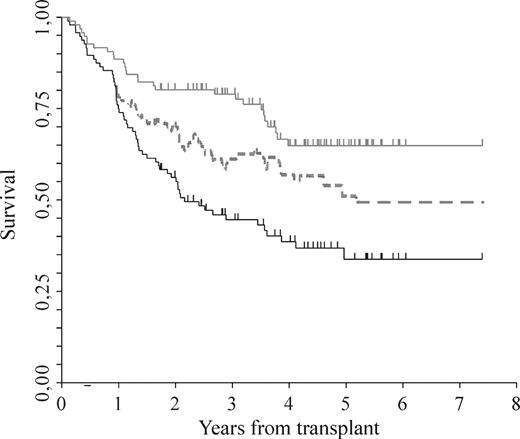
Clinical outcome. Standard OS (gray solid line) by Kaplan-Meier, “current progression-free survival” (dotted line) as described by Klein et al (see “Methods”), which includes responses to salvage therapies and standard EFS (black solid line) by Kaplan-Meier.
Clinical outcome. Standard OS (gray solid line) by Kaplan-Meier, “current progression-free survival” (dotted line) as described by Klein et al (see “Methods”), which includes responses to salvage therapies and standard EFS (black solid line) by Kaplan-Meier.
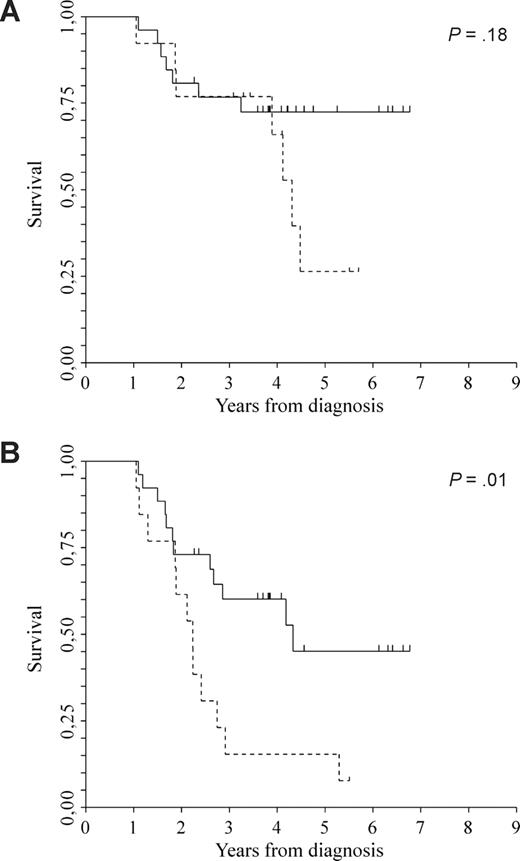
Multivariable analyses for OS and EFS are reported in Table 3. Irrespective of myeloma isotype, ISS, comorbidity index greater than or equal to 3, disease in remission was significantly associated with longer OS and EFS (HR 0.20; CI 95%, 0.06-0.67; P = .01, and HR 0.33; CI 95%, 0.17-0.65; P < .01). Age, as a continuous variable, was also significantly associated with both OS and EFS (HR 1.06; CI 95%, 1.00-1.14; P = .05, and HR 1.07; CI 95%, 1.02-1.12; P < .01, respectively). Chromosome 13 abnormalities, del(13), were studied in 39 of 96 patients (41%); 13 of 39 (33%) showed del(13), whereas 26 of 39 (67%) did not. There was no significant difference in median OS between the 2 cohorts of patients (not reached versus 4.3 years, P = .18), whereas EFS was better in patients without del(13) (4.3 versus 2.2 years, P = .01; Figure 4A,B).
Cox models for OS and EFS
| Variable . | Multivariable analyses . | |||
|---|---|---|---|---|
| OS . | EFS . | |||
| HR (95% CI) | P | HR (95% CI) | P | |
| Age* | 1.06 (1.00-1.14) | .05 | 1.07 (1.02-1.12) | < .01 |
| IgG myeloma | 0.60 (0.26-1.34) | .21 | 0.52 (0.28-0.96) | .03 |
| International Staging System 3 | 1.91 (0.74-4.90) | .18 | 1.53 (0.73-3.20) | .26 |
| Disease in remission† at allografting | 0.20 (0.06-0.67) | .01 | 0.33 (0.17-0.65) | < .01 |
| HCT-specific comorbidity index ≥ 3 | 0.96 (0.27-3.36) | .95 | 0.87 (0.33-2.28) | .78 |
| Variable . | Multivariable analyses . | |||
|---|---|---|---|---|
| OS . | EFS . | |||
| HR (95% CI) | P | HR (95% CI) | P | |
| Age* | 1.06 (1.00-1.14) | .05 | 1.07 (1.02-1.12) | < .01 |
| IgG myeloma | 0.60 (0.26-1.34) | .21 | 0.52 (0.28-0.96) | .03 |
| International Staging System 3 | 1.91 (0.74-4.90) | .18 | 1.53 (0.73-3.20) | .26 |
| Disease in remission† at allografting | 0.20 (0.06-0.67) | .01 | 0.33 (0.17-0.65) | < .01 |
| HCT-specific comorbidity index ≥ 3 | 0.96 (0.27-3.36) | .95 | 0.87 (0.33-2.28) | .78 |
Ig indicates immunoglobulin; and HCT, hematopoietic cell transplantation.
Continuous variable.
Defined as VGPR and CR.
Clinical outcome. (A) OS between patients without del(13)q (solid line; median not reached) and patients with del(13)q (dotted line; median 4.3 years). (B) EFS between patients without del(13)q (solid line; median 4.3 years) and patients with del(13)q (dotted line; median 2.2 years). P = .01.
Clinical outcome. (A) OS between patients without del(13)q (solid line; median not reached) and patients with del(13)q (dotted line; median 4.3 years). (B) EFS between patients without del(13)q (solid line; median 4.3 years) and patients with del(13)q (dotted line; median 2.2 years). P = .01.
Discussion
Progress in myeloma treatment has been impressive in the past 10 years with the introduction of high-dose melphalan followed by autologous transplantation and, recently, through the identification of new agents with molecular targets such as thalidomide, lenalidomide, and bortezomib.27-32 OS has been significantly prolonged especially in good risk patients, whereas eradication of the disease seems unlikely. Conversely, in the light of a well-documented graft-versus-myeloma effect, allografting may be curative in a subset of patients.5,33,34 However, its role has never been thoroughly investigated. First, the high transplantation-related toxicity associated with myeloablative conditionings has severely limited its application; second, the retrospective nature of several studies with strong patient selection bias and the lack of large prospective controlled trials have not allowed definitive conclusions.7 One of the strengths of our study is the rigid enrollment at diagnosis of untreated myeloma patients who underwent the same VAD-based induction treatment before the autologous cytoreductive transplantation. This strategy meant that they were all treated uniformly and any statistical bias was greatly reduced. By contrast, prospective studies, which include allografting as part of the up-front treatment regardless of the induction therapy, inevitably result in bias that may highly affect the clinical outcomes.
In the present study, median OS was not reached after a follow-up of 5 years. Before the era of “new drugs,” after a median follow-up of 75 months, Attal et al reported median OS of 48 and 58 months after 1 and 2 autologous transplantations, respectively.28 Barlogie et al35 reported that 17% of the patients enrolled in the Total Therapy 1 trial were alive at 15 years and 7% were event-free after a median follow-up of 12 years.29 More recently, with a follow-up of 7 years, the thalidomide arm of Total Therapy 2 has appeared to further improve clinical outcomes.35
Overall, 53% of patients (51/96) reached CR, 73% (37/51) of whom are in continuous CR, including molecular remissions (M. Ladetto, Division of Hematology, S. Giovanni Battista Hospital, University of Torino, Torino, Italy, oral communication, November 2008), prelude to a cure, with a follow-up extending to 8 years. Thus, the depth of response was crucial for prolonged response in our study (Table 3).36 Given the high rate of CR obtained without the use of so-called new drugs, it is imperative to thoroughly explore their role in the setting of allografting. Graft-versus-myeloma effects and the new drugs with molecular targets, in fact, are by no means mutually exclusive. Bortezomib and thalidomide have already been shown to reinduce remissions in patients who relapsed after allografting.37-39 Antimyeloma activity has also been shown at relapse in the series of patients reported in this study (Figure 3). We are currently investigating the role of lenalidomide in reducing the tumor burden before and enhancing graft-versus-myeloma effect after transplantation. Maintenance therapy may lead to a significant increase in response rates and prolonged response duration. Furthermore, this strategy may also overcome the higher risk of relapse in patients with poor prognostic factors.40
Overall, nonrelapse mortality was 11%. GVHD and its complications accounted for most TRM. Its incidence, however, may be further reduced as progress is made in the understanding of its pathogenesis.41 Chronic GVHD has often been associated with longer response duration and better OS.42 In our study, however, its development did not correlate with either subsequent achievement of CR or response duration (Table 2). In a subset of patients, therefore, a graft-versus-myeloma effect may be distinct from detrimental chronic GVHD or associated with subclinical graft-versus-host reactions. The potential biologic effects of the number of donor CD34+ cells infused have been debated in several studies.43,44 Interestingly, we noted that an increasing number of CD34+ cells infused, but not CD3+ T cells, was significantly correlated with a higher risk of developing acute GVHD (Table 2). An upper limit of CD34+ cells infused was not included in our trial. Evaluation of their number in a larger series of patients may help to set a range that allows consistent donor engraftment while reducing the risk of acute GVHD.
It is widely assumed that chromosomal abnormalities are important prognostic factors for both OS and EFS.45 Garban et al reported OS and EFS of 35 and 31.7 months, respectively, after a median follow-up of 2 years, in high risk newly diagnosed myeloma patients, with either elevated β-2–microglobulin levels or presence of del(13), who received reduced-intensity allografts.46 Perhaps because the conditioning with high-dose antithymocyte globulin may have attenuated graft-versus-myeloma effects, a survival benefit with allografting compared with melphalan-based autografting was not observed.47 In another comparison, the advantage of having an HLA-identical sibling, therefore the chance of undergoing an allograft, compared with not having an HLA-identical sibling was not offset by the presence of del(13).10 This finding, however, did not imply that in patients given allografts, del(13) might not have had a prognostic role. In the present series, del(13) appeared to significantly affect EFS (4.3 vs 2.2 years, P = .01), but not OS (not reached vs 4.3 years, P = .18). The data reported in all these studies should, however, be evaluated with larger and more comprehensive analyses that include a complete spectrum of the chromosomal abnormalities associated with myeloma rather than a single abnormality. Recent reports clearly showed that del(13) alone did not affect OS after transplantation unless it was associated with other abnormalities such as del(17) or t(4;14).47
Whether an allograft should be offered as part of first-line treatment plan or as salvage therapy for refractory or relapsed patients is a matter of debate.48 Although allografting with reduced intensity/nonmyeloablative conditionings has evolved into a less toxic procedure, new methods to further reduce toxicity while maintaining graft-versus-myeloma effects are being investigated. In our experience, the use of low-dose TBI conditioning regimens up-front proved significantly more effective in terms of graft-versus-myeloma effects than waiting with transplantation until relapse.49 Poor response to posttransplantation donor lymphocyte infusions at relapse, without prior cytoreduction, was also observed. These might be due to an antigen expression profile of potential targets for allogeneic cytotoxic T cells that progressively changed. For instance, Siegel et al identified HLA-A*0201–presented T-cell epitopes derived from the oncofetal antigen-immature laminin receptor protein in hematologic malignancies including myeloma.50 Expression of these antigens on myeloma cell was lost over time. Rosinol et al recently reported on the Program for the Study and Treatment of Hematological Malignances (PETHEMA) study.12 Patients who did not achieve at least near-CR after a first autograft were randomized to receive either a second autograft or an allograft after a reduced intensity-conditioning in the light of the presence/absence of an HLA-identical sibling donor. There was a significantly higher incidence of CR and a longer progression-free survival in patients treated with an allograft. However, there was also a higher TRM and no statistical difference in EFS and OS. The authors concluded that, although the progression-free survival plateau was encouraging, the procedure should be investigated in prospective clinical trials. Although different in design, the findings of the PETHEMA study are not conflicting with our trial. In particular, we clearly observed that disease response at the time of the allograft was significantly associated with posttransplantation EFS and OS (Tables 2 and 3).
In summary, our findings suggested that allografting was effective in the treatment of newly diagnosed myeloma patients. The combination of graft-versus-myeloma effects with new drugs should be clinically evaluated in well-designed phase 3 clinical trials, in which control groups should include patients treated with new agents with potent antimyeloma activity with/without autografting. Moreover, stratification of patients by prognostic factors, especially chromosomal abnormalities, is imperative to determine those who may most benefit from a “tandem transplant approach.”
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
Our thanks to the nurses and medical staff for caring for the patients, to the study coordinators who collected the trial and follow-up data, and to Antonio Capaldi, Giovannino Ciccone, Paolo Di Bartolomeo, Michele Falda, Andrea R. Filippi, Robin Foà, Giorgio Lambertenghi-Deliliers, Alessandro Levis, Massimo Massaia, Vittorio Montefusco, Nicola Mordini, Mario Petrini, Enrico Pogliani, Brenda M. Sandmaier, and Barry E. Storer for their contributions to the manuscript.
This research was supported in part by Progetti di Ricerca ex-60% (Torino, Italy), Ministero dell'Università e della Ricerca Scientifica (MIUR; Rome, Italy); Regione Piemonte (Torino, Italy): Ricerca Finalizzata 2005 (Progetto Clinico-scientifico e di Coordinamento Regionale), 2006, 2007; Compagnia di San Paolo (Torino, Italy); Fondazione Cassa di Risparmio di Torino (CRT; Torino, Italy) and Comitato Regionale Piemontese Gigi Ghirotti (Progetto Vita Vitae; Rome, Italy); Fondazione Neoplasie Sangue Onlus (Torino, Italy); grant CA78902 from the National Institutes of Health, Department of Health and Human Services (Bethesda, MD).
National Institutes of Health
Authorship
Contribution: B.B and M.B. designed and directed the study and edited the manuscript; M.R. and R. Sorasio contributed patients to the study, verified data, and assisted in drafting the manuscript; F.P., D. Mattei, B.A., F.C.-S., A.R., M.C., M.P., P.B., F.O., A.B., L.C., E.B., A.P.I., L.G., A.P., P.C., and R.F. contributed patients to the study and reviewed manuscript; D. Maloney and R. Storb contributed to study design and reviewed the manuscript; I.B. performed statistical analyses and reviewed the manuscript; and U.R. participated in designing research/protocol and reviewed the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Benedetto Bruno, Divisione Universitaria di Ematologia, Azienda Ospedaliera San Giovanni Battista, Via Genova 3, 10126, Torino, Italy; e-mail: benedetto.bruno@unito.it.