Abstract
Montreal platelet syndrome (MPS), hitherto described in only one kindred, is a hereditary thrombocytopenia associated with mucocutaneous bleeding, giant platelets, and spontaneous platelet aggregation in vitro. These are features shared with some forms of type 2B von Willebrand disease (VWD); however, the MPS kindred had not been investigated for VWD. We found that all affected MPS family members had borderline to normal von Willebrand factor antigen (VWF:Ag; 0.43-0.75 U/mL), discrepantly low ristocetin cofactor activity (VWF:RCo; 0.16-0.29 U/mL), and normal factor VIII coagulant activity (FVIII:C; 0.57-1.04 U/mL). Unaffected family members all had normal VWF:Ag, VWF:RCo, and FVIII:C levels. In addition, persons with MPS, but not unaffected family members, had loss of plasma (but not platelet) high molecular weight VWF multimers, and were heterozygous for the previously reported V1316M type 2B VWD mutation. Thus, in reevaluating this kindred, we determined that patients with MPS have type 2B VWD with the V1316M VWF mutation.
Introduction
In 1963, a mother (L.T.) and all 3 of her children (L.T.B., I.T., and X.X. [Note: Subject X.X. has been lost to follow-up; therefore, permission could not be obtained to print identifying information.]), of French Canadian descent, who had lifelong easy bruising and bleeding tendencies were reported to have hereditary giant platelets, thrombocytopenia, and spontaneous platelet clumping in vitro.1 Although there were features of Bernard-Soulier syndrome, the inheritance pattern appeared autosomal dominant. Phase-contrast microscopy showed that many platelets from affected members of this family were normal-sized and apparently assumed a larger volume only after activation and shape change in vitro,2,3 explaining the giant platelets seen on peripheral blood film. The disorder was named Montreal platelet syndrome (MPS).2
Several studies have now shown that some patients with type 2B von Willebrand disease (VWD) present with macrothrombocytopenia4-8 and spontaneous platelet aggregation in vitro,4-7,9 features found in MPS.10 A diagnosis of type 2 VWD is suggested by a discrepantly low ristocetin cofactor activity (VWF:RCo) compared with the VWF antigen (VWF:Ag; [VWF:RCo/VWF:Ag ratio < 0.7]). Type 2B VWD is typically characterized by (1) increased ristocetin-induced platelet agglutination (RIPA) at low ristocetin concentration,11-13 (2) selective loss of plasma high molecular weight (HMW) multimers, with normal platelet VWF multimer distribution,13 and (3) mutations involving exon 28 of the VWF gene that codes for the A1 domain of VWF, the known contact site for platelet glycoprotein Ib (GPIb).14,15
Patients with MPS have previously been shown to have normal RIPA at high ristocetin concentration, but low-dose RIPA and VWD studies were not performed.16 We now present investigations to show that persons previously diagnosed with MPS have type 2B VWD associated with heterozygosity for the previously reported V1316M VWF mutation.
Methods
We investigated 5 members of this family (L.T.B., I.T., X.X., I.B., and M.B.), including one unaffected member (M.B.), who have been previously described (Figure 1A).2 All affected members show lifelong bruising, and in some cases severe postoperative bleeding, postpartum hemorrhage, and gastrointestinal bleeding. All unaffected members, including 2 previously unreported (B.B. and C.B.), have no bruising or bleeding tendencies. Approval was obtained from the Faculty of Medicine, University of Calgary Institutional Review Board for these studies. Informed consent was obtained in accordance with the Declaration of Helsinki.
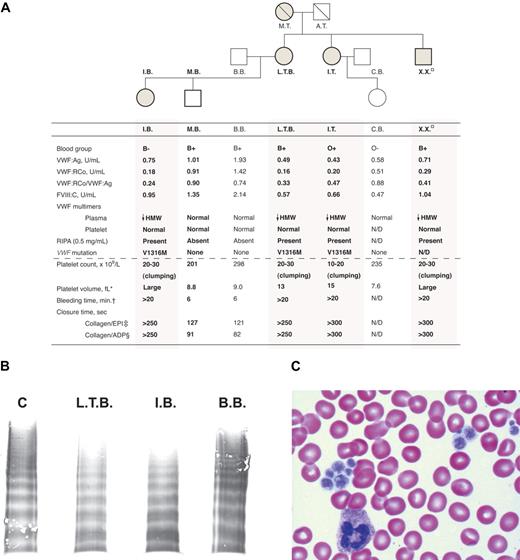
Characteristics of the Montreal platelet syndrome kindred. (A) Genogram and laboratory data with affected family members indicated by shading and previously studied subjects by bold text and bold genogram symbol outline. In the hemostatic variables, HMW indicates high molecular weight; N/D, not determined; □, subject lost to follow-up; therefore, permission was not obtained to print identifying information. *Normal range, 7 to 11 fL; †Normal, < 9.5 minutes; ‡normal range, 84 to 176 seconds; §normal range, 63 to 111 seconds. (B) Sodium dodecyl sulfate–agarose gel electrophoresis of plasma VWF multimers showing loss of HMW VWF multimers in representative subjects affected with MPS (L.T.B., I.B.) and normal plasma HMW VWF multimers in a representative unaffected family member (B.B.) and a control (C). (C) Peripheral blood film showing large platelets and platelet clumping.
Characteristics of the Montreal platelet syndrome kindred. (A) Genogram and laboratory data with affected family members indicated by shading and previously studied subjects by bold text and bold genogram symbol outline. In the hemostatic variables, HMW indicates high molecular weight; N/D, not determined; □, subject lost to follow-up; therefore, permission was not obtained to print identifying information. *Normal range, 7 to 11 fL; †Normal, < 9.5 minutes; ‡normal range, 84 to 176 seconds; §normal range, 63 to 111 seconds. (B) Sodium dodecyl sulfate–agarose gel electrophoresis of plasma VWF multimers showing loss of HMW VWF multimers in representative subjects affected with MPS (L.T.B., I.B.) and normal plasma HMW VWF multimers in a representative unaffected family member (B.B.) and a control (C). (C) Peripheral blood film showing large platelets and platelet clumping.
Platelet counts were performed with phase-contrast microscopy when significant clumping was present. Factor VIII coagulant activity (FVIII:C) was assayed with the use of a one-stage partial thromboplastin time (PTT) method. VWF:Ag was quantified by a latex particle immunoturbidity assay (HemosIL-ACL Advance Analyzer; Beckman-Coulter, Fullerton, CA). VWF:RCo was determined by aggregometry (PAP-4; BioData, Horsham, PA) with the use of 1.2 g/L ristocetin and lyophilized washed normal platelets (BioData). Platelet-rich plasma (PRP) for RIPA was prepared by centrifuging whole blood anticoagulated with acid-citrate-dextrose at 1000g for 3 successive 1-minute intervals, with careful removal and pooling of the PRP after each centrifugation. RIPA was assessed with the use of 0.5- and 1.5-mg/mL concentrations of ristocetin. Bleeding times were performed by the template method (Surgicutt; ITC, Edison, NJ), and PFA-100 (Dade Behring, Deerfield, IL) closure times were assessed with the collagen/epinephrine (collagen/EPI) and collagen/ADP cartridges. Multimer analysis was performed by sodium dodecyl sulfate–agarose gel electrophoresis (adapted for a vertical minigel apparatus) followed by electroblotting,17 and visualization with sequential incubation with biotinylated rabbit anti-VWF antibody, streptavidin-alkaline phosphatase and alkaline phosphatase substrate BCIP/NBT (5-bromo-4-chloro-3-indolyl-phosphate/nitro blue tetrazolium; Zymed Laboratories, South San Francisco, CA). For mutational analysis, genomic DNA was extracted from leukocytes by standard methods, primers specific to intron 27 and 28 of the VWF gene were used to amplify exon 28 and approximately 50 base pairs of flanking intron sequence. Amplicons were purified using the Qiaquick polymerase chain reaction (PCR) purification kit (QIAGEN, Valencia, CA) and sequenced directly at the Center for Applied Genomics (Toronto, ON).
Results and discussion
All subjects with a diagnosis of MPS (L.T.B., I.T., X.X., and I.B.) had normal to borderline low VWF:Ag with discrepantly lower VWF:RCo with the VWF:RCo/VWF:Ag ratio ranging from 0.24 to 0.47 (Figure 1A), suggesting the presence of type 2 VWD. Type 2B VWD classification was confirmed by showing (1) RIPA at low ristocetin concentration (0.5 mg/mL; Figure 1A), (2) loss of plasma HMW VWF multimers (Figure 1B) with normal platelet VWF multimer distribution (data not shown), and (3) heterozygosity for a VWF 3946 G>A (V1316M) mutation at exon 28 by DNA sequence analysis (Figure 1A). These subjects also displayed macrothrombocytopenia and platelet clumping on blood film (Figure 1C). In keeping with the very long bleeding times previously reported,1 bleeding times and PFA-100 closure times with both cartridges were significantly prolonged (Figure 1A).
Subjects without a diagnosis of MPS (M.B., B.B., and C.B.) showed normal platelet number and size, absence of platelet clumping on blood film, normal VWF phenotype, normal bleeding and PFA-100 closure times and, importantly, absence of the exon 28 mutation (Figure 1). Thus, there was complete segregation of type 2B VWD with the MPS phenotype, suggesting that MPS may actually be type 2B VWD with macrothrombocytopenia (Figure 1A), although extremely close genetic linkage of 2 distinct defects cannot be ruled out.
The gain-of-function V1316M substitution mutation resulting from the nucleotide 3946 G>A transition has been reported in several patients.14,18-20 As anticipated, the mutant VWF displays a high affinity to platelets,19 and many patients have persistent or transient thrombocytopenia,21-23 large platelets, and spontaneous platelet aggregation.4,6 One illustrative example is a 5-year-old boy with the heterozygous V1316M VWF mutation who presented with macrothrombocytopenia (platelet count ∼ 20 × 109/L), platelet clumping on blood film, and loss of plasma high and intermediate molecular weight VWF multimers.4 This boy had severe epistaxis that was successfully treated with VWF/FVIII concentrates, although concomitant platelet transfusions were occasionally required. Patient L.T.B., like her mother (L.T.), began to experience regular lower gastrointestinal bleeding associated with angiodysplasia at approximately 60 years of age. Bleeding has become less frequent and severe after she was started on prophylactic infusions of VWF/FVIII concentrate once weekly. Severe bleeding responded better clinically to VWF/FVIII concentrate than to human leukocyte antigen–matched platelets alone. However, concurrent treatment with both has provided the best results.
Thrombocytopenia, with or without platelet clumping, has also been observed in other exon 28 mutations such as 1304InsM, P1337L, and R1308P.8,24 Thrombocytopenia is probably related to in vivo clearance of mutant VWF-bound platelets/platelet clumps.11 In 2 related patients with a heterozygous R1308P mutation, defective proplatelet/platelet production during megakaryocytopoiesis was also shown to be contributory.24
Attempts to identify the determinants of spontaneous platelet aggregation (SPA) in this MPS cohort have previously been performed.16,25 MPS platelets were reported to aggregate spontaneously in whole blood or PRP with microaggregates typically containing 2 to 6 disc-shaped platelets.16 However, no SPA occurred when washed and concentrated normal platelets or normal PRP were added to patient platelet-free plasma, leading Milton et al16 to postulate that MPS platelets had an intrinsic defect. However, the findings did not exclude the possibility that much of the platelet-reactive HMW VWF multimers in MPS plasma were depleted because of binding to MPS platelets in vivo. This would explain increased in vivo platelet clearance contributing to the thrombocytopenia and account for (1) the lack of SPA of normal platelets in MPS plasma and (2) the continuing MPS platelet aggregation in normal plasma.
MPS has been, for the past 45 years, an ill-defined inherited platelet disorder described in only one known kindred. It was beyond the scope of the present study to verify the abnormal in vitro shape change, reduced thrombin-induced aggregation at very low platelet counts, and decreased platelet calpain previously reported in MPS platelets.16,25 However, our phenotypic and genotypic studies indicate that the MPS kindred has defective VWF and macrothrombocytopenia associated with type 2B VWD and the V1316M mutation. Further studies are needed to determine whether the described platelet characteristics for MPS are always associated with type 2B VWD with or without the V1316M mutation.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Note added in proof.
Since submission of our manuscript, Federici et al26 reported a study of thrombocytopenia and bleeding in a cohort of 38 unrelated families with type 2B VWD. In comparison to several other type 2B mutations, the 4 families with V1316M showed (1) the most severe thrombocytopenia, (2) higher bleeding scores, (3) the presence of small platelet aggregates, and (4) a high mean platelet volume of 10 fL. They also found a direct correlation between the level of VWF in the GPIbα-binding conformation (as a measure of VWF gain of function) and degree of thrombocytopenia, which was most pronounced with the V1316M VWF mutation. These findings are consistent with our observation that the clinical and laboratory manifestations of MPS are primarily those of type 2B VWD with the V1316M VWF mutation.
Acknowledgments
We thank Dr David Lillicrap, Ms Colleen Notley, and Ms Jayne Leggo (all from Queen's University, Kingston, ON) for performing the VWF genotyping; Ms Louise Vu (CHA-Hôpital de l'Enfant-Jesus, Quebec, QC) and Ms Joyce Morrison (Calgary Laboratory Services, Calgary, AB) for performing the hemostasis testing; Dr Georges Rivard (University of Montreal, Hôpital St-Justine, Montreal, QC) for his helpful discussions. We thank the patients and their families for their participation, as well as Dr Xiu Jiang (Calgary Laboratory Services, Calgary, AB) and Mr Robert Teteruk (Hospital for Sick Children, Toronto, ON) for assistance in the preparation of Figure 1.
This work was supported by a Canadian Hemophilia Society grant (M.-C.P., G.D.S.). S.C.J. is the recipient of a Bayer Clinical Scholarship Award in Hemophilia.
Authorship
Contribution: S.C.J. wrote the paper and analyzed data; G.D.S., S.C., and Z.D. performed research; M.L.R. analyzed data and revised the paper; and M.-C.P. designed the study, performed research, analyzed data, and revised the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Man-Chiu Poon, University of Calgary, Foothills Hospital, 1403 29th St NW, Calgary, AB, Canada T2N 2T9; e-mail: mcpoon@ucalgary.ca.