Abstract
Although the mechanisms of cross-talk that regulate the hematopoietic and epithelial compartments of the thymus are well established, the interactions of these compartments with the thymic endothelium have been largely ignored. Current understanding of the thymic vasculature is based on studies of adult thymus. We show that the neonatal period represents a unique phase of thymic growth and differentiation, marked by endothelium that is organized as primitive, dense networks of capillaries dependent on vascular endothelial growth factor (VEGF). VEGF dependence in neonates is mediated by significantly higher levels of both VEGF production and endothelial VEGF receptor 2 (VEGF-R2) expression than in the adult thymus. VEGF is expressed locally in the neonatal thymus by immature, CD4−CD8− “double negative” (DN) thymocytes and thymic epithelium. Relative to adult thymus, the neonatal thymus has greater thymocyte proliferation, and a predominance of immature thymocytes and cortical thymic epithelial cells (cTECs). Inhibition of VEGF signaling during the neonatal period results in rapid loss of the dense capillaries in the thymus and a marked reduction in the number of thymocytes. These data demonstrate that, during the early postnatal period, VEGF mediates cross-talk between the thymocyte and endothelial compartments of the thymus.
Introduction
The anatomy of the adult thymus and the mechanisms of cross-talk between its hematopoietic and epithelial compartments have been extensively studied through a variety of murine genetic and transplantation models.1 The thymic epithelial cells (TECs) of the medulla and cortex have specialized roles in directing thymocyte proliferation, differentiation, and survival through both membrane-bound and soluble molecules. In turn, TEC development and survival is influenced by the presence of thymocytes at specific stages of development, through as yet undefined molecular mechanisms.2,3 The role of the third compartment of the thymus, the vascular endothelium, has been generally considered to be limited to its function as a conduit for the delivery of circulating, marrow-derived thymic precursor cells; little attention has been paid to possible mechanisms of cross-talk that may regulate the thymic vascular compartment and the role of the endothelium in thymic development.
The anatomy of the blood vessels in the adult thymus that deliver circulating stem and progenitor cells is closely linked to the organization of the TEC compartment.4 The architecture of the adult thymic vasculature is hierarchic, consisting of arterioles that enter the thymus at the corticomedullary junction (CMJ) and form capillaries that ascend into the cortex in a series of anastomoses. At the periphery of the cortex, the capillaries form a fine network of branching arcades that then curve back down to the medulla, where they merge into larger postcapillary venules.4,5
Vascular endothelial growth factor (VEGF) is a potent mitogen that plays a role in angiogenesis, promoting the migration, growth, and survival of endothelial cells.6 There are several members of the VEGF family; of these, VEGF-A (referred to here as “VEGF”) is critical for vascular development during embryonic organogenesis7,8 and for the angiogenesis related to tumor growth.9,10 In contrast, the vasculature of normal adult organs is largely VEGF independent, resulting in relatively low toxicity seen with VEGF inhibitors used in antitumor therapy.11,12 During neonatal development, although most vasculature exhibits a mature phenotype,11,13 specific organs, such as the liver, have been found to contain immature, VEGF-dependent endothelium,12,13 suggesting that a critical interplay may exist between vascular development and the growth and/or function of certain organs during early postnatal life. Despite the characterization of the VEGF dependency of several organs, a role for VEGF in the postnatal thymus has not been described.
During the neonatal period, the volume of the thymus increases rapidly relative to body size, peaking around the first year of human life4,14 and the first 1 to 2 weeks of murine life.15 Thus, signals clearly exist during early postnatal life, which provide profound stimulation of thymic growth or sustain growth initiated during embryogenesis. In this report, we show that both the hematopoietic and nonhematopoietic compartments of the murine neonatal thymus are significantly different from those of the adult thymus. During the first week of postnatal life, increased thymocyte proliferation is associated with a phenotypic skewing toward more immature thymocytes and a predominance of cortical TECs in contrast to the medullary TEC predominance of adult thymus. During the same time frame, the neonatal thymus contains a dense, immature, VEGF-dependent vasculature consisting of fine, branching capillaries, higher levels of CD31+ endothelium, and very few pericytes. Inhibition of VEGF signaling during neonatal life results in a rapid loss of the dense capillaries in the thymus and profoundly reduces thymopoiesis. VEGF is expressed predominantly by the neonatal thymic epithelium and stroma with low-level expression in specific thymocyte subsets. VEGF-R2 is expressed predominantly on the neonatal thymic endothelium, with little VEGF-R2 expression on epithelium and no expression on thymocytes, suggesting that VEGF acts directly on the endothelial compartment. Taken together, our data demonstrate the existence of a novel form of cross-talk mediated by VEGF between the vascular, hematopoietic, and epithelial compartments of the thymus that occurs during the neonatal phase of rapid thymic growth and differentiation.
Methods
Animals
C57Bl/6J mice (The Jackson Laboratory, Bar Harbor, ME) were kept in specific pathogen-free facilities at CHLA. CHLA's Institutional Animal Care and Use Committee approved all protocols. Unless otherwise indicated, “neonate” refers to mice 1 day old and “adult” refers to mice 6 to 8 weeks old.
In vivo labeling of the thymic vasculature
Anaesthetized mice were perfused with a mixture of biotinylated tomato lectin (TL; 10 mg/kg; Vector Laboratories, Burlingame, CA) and streptavidin-Cy3 (SA-Cy3; 3.1 mg/kg; Jackson Immunoresearch, West Grove, PA)11 via facial vein (neonates) or cardiac injection (adults). Two minutes later, mice were perfused with PBS followed by 4% paraformaldehyde (PFA). Isolated thymi were further fixed in 4% PFA. Sections (200 μm each) of 3% agarose-embedded thymi were examined for lectin labeling using a Leica TCS SP1 confocal microscope with a Plan Apo 10×/0.4NA objective and images were acquired using Leica LCS 2.5 software (Heidelberg, Germany). For each 200-μm section, 20 Z-sections of 6.5 μm each were captured. MetaMorph software (Molecular Devices, Downingtown, PA) was used to analyze fluorescence intensity. Control (no lectin) sections were used to establish the lowest threshold of pixel intensity.
Morphologic and IF studies
For morphologic analyses, 5-μm sections of paraffin-embedded thymi were stained with hematoxylin and eosin (H&E). Sections were imaged using a Zeiss Axioplan microscope (Carl Zeiss, Heidelberg, Germany) equipped with Spot Insight QE camera. For immunofluorescent (IF) studies, 5-μm sections of thymi were stained with goat polyclonal antibodies against CD31 at 1:100 (Santa Cruz Biotechnology, Santa Cruz, CA), or rabbit polyclonal VEGF-R2 (Upstate, Lake Placid, NY) at 1:50 dilution, followed by incubation with biotinylated anti–goat or anti–rabbit secondary antibodies and streptavidin-conjugated DTAF or Cy3, respectively (Jackson Immunoresearch). Pericytes were detected using FITC-conjugated anti–mouse smooth muscle actin monoclonal antibody (SMA; Sigma-Aldrich, St Louis, MO) at 1:250 dilution.16 Slides were examined on a Leica DM RXA upright microscope equipped with a SkyVision-2/VDS camera (Chroma Technology, Rockingham, VT) using a Plan-Apo 20×/0.9 NA objective lens. Images were acquired using easyFISH software (Applied Spectral Imaging, Vista, CA). Quantitation of fluorescence intensity was obtained using MetaMorph software as described in “In vivo labeling of the thymic vasculature.”
In vivo administration of VEGF-Trap
Mice were 1, 4, 7, and 30 days old (D1, D4, D7, D30) at the time of first intraperitoneal administration of VEGF-Trap and Fc control (25 mg/kg). Based on previous studies using VEGF inhibitors during the neonatal period,12 D1 and D4 mice received a single dose of drug or control and were killed 24 and 48 hours after treatment, respectively. The D7 and D30 mice received 2 doses, 3 days apart, and were killed 3 days after the second dose. Before killing, TL-SA-Cy3 was administered to label the vasculature. In experiments to analyze the effect of VEGF-Trap on other thymic compartments, D1 mice were given a single dose of VEGF-Trap or Fc control and killed 2 to 7 days later.
VEGF protein assay
Isolated thymi were washed with PBS to remove serum on the capsule, and disaggregated in PBS using 26-gauge needles to release the majority of thymocytes. The remaining stroma provided the “thymocyte-depleted fraction.” Protein extracts from the thymocyte and thymocyte-depleted fractions were prepared and VEGF levels were assayed by enzyme-linked immunosorbent assay (ELISA) according to the manufacturer's protocols (R&D Laboratories, Minneapolis, MN).
Thymocyte analysis, isolation, BrdU labeling, and flow cytometry
To analyze or sort thymocytes, thymi from individual adults or pooled litters were dissociated through a 70-mm sieve (BD Biosciences, Bedford, MA) to obtain thymocytes. After blocking with Mouse BD Fc Block (BD Biosciences, San Jose, CA), thymocytes were stained with the following rat anti–mouse antibodies: CD45-FITC, CD4 (APC or FITC), CD8 (PE or PerCP), CD3-PerCP, CD25-PE, and CD44-APC (all from BD Biosciences). Fluorescence-activated cell sorting (FACS) data were acquired on a FACSCalibur running CellQuest Pro (BD Biosciences) and analyzed using FlowJo software (TreeStar, Eugene, OR) or sorted on a FACSAria. Cycling cells were analyzed by injecting animals intraperitoneally with bromodeoxyuridine (BrdU; 10 mg/kg; BD Biosciences). Two hours later, mice were killed and thymocytes stained with anti–BrdU-FITC antibody according to the manufacturer's protocol and analyzed on a FACSCalibur.
Epithelial cell isolation
TECs were obtained according to the method of Gray et al.17 After enzymatic digestion of thymic stroma, cell fractions were further depleted of CD45+ cells using CD45+ microbeads and Miltenyi LS columns (Miltenyi Biotec, Auburn, CA). The resulting enriched CD45− cells were then labeled with MHCII-APC (Miltenyi Biotec), UEA-1 (Vector Laboratories), Ly51 (BD Biosciences), and CD45-APC-Cy7 (BD Biosciences). Fifty thousand to 100 000 non-TEC cells (CD45−MHCII−), TECs (CD45−MHCII+), or hematopoietic cells (CD45+MHCII−) were collected. The TEC population was further analyzed to determine medullary TECs (mTECs; CD45−MHCII+UEA-1+Ly51−) or cortical TECs (cTECs; CD45-MHCII+UEA-1−Ly51+).
FACS analysis of VEGF-R2–expressing cells
After enzymatic digestion, cells from neonate or adult thymi were stained with the following antibodies: CD45-FITC, VEGF-R2-PE, and either MHCII-APC or CD31-APC and analyzed on a FACSCalibur.
RNA extraction and RT-PCR
RNA was isolated using an mRNA isolation kit (QIAGEN, Valencia, CA) and first-strand cDNA synthesis was performed using the OmniscriptIII reverse-transcription–polymerase chain reaction (RT-PCR) kit (QIAGEN). Total cDNA from 7500 to 10 000 cells were interrogated with primers recognizing mouse VEGF (5′-CTGCTCTCTTGGGTGCACTG-3′ [forward] and 5′-CACCGCCTTGGCTTGTCACAT-3′ [reverse]). β-Actin primers (5′-TGTTACCAACTGGGACGACA-3′ [forward] and 5′-GGGGTGTTGAAGGTCTCAAA-3′ [reverse]) were used as loading controls. PCRs used HotStar Taq polymerase (QIAGEN) and consisted of 35 to 40 cycles of 30 seconds at 95°C, 30 seconds at 56°C, and 1 minute at 72°C, with a final elongation cycle of 7 minutes at 72°C. cDNA synthesized from mouse reference RNA (Stratagene, La Jolla, CA) was used as a positive control.
Results
Differences in thymocyte and epithelial cell subsets between the neonatal and adult thymus
Although the thymus undergoes a rapid increase in volume during the neonatal period, little is known about the hematopoietic and other microenvironment compartments of the thymus during this period of growth. Analysis of BrdU incorporation 2 hours after in vivo administration revealed that thymocyte proliferation was significantly higher in neonatal (1-2 day old) than adult (6-8 weeks old) thymocytes (10.8% vs 6.8%, respectively; P = .01; Figure 1A). FACS analysis showed significant differences in immunophenotype between neonatal and adult thymocytes with a higher proportion of immature thymocytes in the neonatal thymus (Figure 1B). The frequency of the most immature thymocytes, CD45+CD4−CD8− DN cells, was 3-fold higher in the neonates (10.5% ± 1.1%, n = 5) compared with the adults (3.0% ± 0.5%, n = 3; Figure 1C; P < .001). In contrast, later stages of thymopoiesis were significantly more common in adult thymus (CD4+CD8+ cells in neonate 83.1 ± 1.3% vs adult 85.4% ± 1.3% [P = .05]; CD3+ cells in neonate 5.2% ± 1.0% vs adult 10.8% ± 1.6% [P < .001]; CD4+CD8− cells in neonate 4.53% ± 0.8% vs adult 8.3% ± 0.8% [P < .001]; CD4−CD8+ cells in neonate 1.8% ± 0.8% vs adult 3.23% ± 0.2% [P = .03]; Figure 1C). When thymocyte distribution was correlated with BrdU uptake, it was observed that BrdU incorporation was higher at all stages of thymocyte differentiation in neonate compared with adult thymus, suggesting that no single thymocyte subset is responsible for the increased BrdU incorporation observed in the neonate (data not shown).
Thymocyte and TEC profiles in neonatal and adult thymus. (A) Average frequency of BrdU incorporation in cells from total thymi from 3 independent pooled litters of neonates and 3 individual adult animals (P = .01). (B) Representative FACS profiles of neonatal (left panel) and adult (right panel) thymocytes after gating on CD45+ cells. (C) Thymocyte distribution (mean and SD, shown as % of total CD45+ cells) from neonatal (n = 5) and adult (n = 3) mice. *P < .001; **P = .03. (D) H&E stain of sections of adult and neonatal thymus acquired according to “Morphologic and IF studies.” Lighter colored areas are medulla (M) and darker areas are cortex (C). (E) Frequency of TEC subsets, based on FACS profiles gated from CD45−MHCII+ cells. ■ indicates UEA-1+ mTECs;  , Ly51+ cTECs. *P = .006; **P = .003. n = 3 pooled neonatal, and n = 3 individual adult samples. Error bars represent standard deviation.
, Ly51+ cTECs. *P = .006; **P = .003. n = 3 pooled neonatal, and n = 3 individual adult samples. Error bars represent standard deviation.
Thymocyte and TEC profiles in neonatal and adult thymus. (A) Average frequency of BrdU incorporation in cells from total thymi from 3 independent pooled litters of neonates and 3 individual adult animals (P = .01). (B) Representative FACS profiles of neonatal (left panel) and adult (right panel) thymocytes after gating on CD45+ cells. (C) Thymocyte distribution (mean and SD, shown as % of total CD45+ cells) from neonatal (n = 5) and adult (n = 3) mice. *P < .001; **P = .03. (D) H&E stain of sections of adult and neonatal thymus acquired according to “Morphologic and IF studies.” Lighter colored areas are medulla (M) and darker areas are cortex (C). (E) Frequency of TEC subsets, based on FACS profiles gated from CD45−MHCII+ cells. ■ indicates UEA-1+ mTECs;  , Ly51+ cTECs. *P = .006; **P = .003. n = 3 pooled neonatal, and n = 3 individual adult samples. Error bars represent standard deviation.
, Ly51+ cTECs. *P = .006; **P = .003. n = 3 pooled neonatal, and n = 3 individual adult samples. Error bars represent standard deviation.
The thymus is arranged into 2 morphologically and functionally distinct compartments: the cell-dense cortex containing immature DN and DP T cells that are subjected to positive selection through cortical epithelium, and the less dense medulla containing more mature T cells that undergo negative selection by dendritic cells and Aire-expressing medullary epithelium.18,19 Consistent with the predominance of immature thymocytes seen by flow cytometry, histologic analysis revealed that the neonatal thymus is dominated by cortex with a poorly defined corticomedullary junction (CMJ) and very small medullary areas compared with the adult thymus (Figure 1D). Quantitation of cytokeratin K5 (expressed by medullary TECs [mTECs]) and K8 (cortical TECs [cTECs]) expression by fluorescent immunohistochemistry revealed a cTEC/mTEC ratio of 1.78 for neonatal thymus, demonstrating that in neonates, cTECs are more common than mTECs. In contrast, in the adult thymus mTECs predominate (cTEC/mTEC ratio of 0.56). Rapid reversal of the cortical-medullary ratio with a transition to adult morphology occurred during the first week of postnatal life (data not shown).
The histologic and immunohistochemical findings were confirmed by analysis of the frequency of cTECs and mTECs by flow cytometry. In the adult, the majority of TECs (ie, CD45−MHCII+ cells) consisted of UEA-1–positive mTECs (60.3% ± 9.5% mTECs vs 24.8% ± 3.9% cTECs, n = 3), in line with previously published results17,20 (Figure 1F). In the neonate, the frequencies were reversed, with Ly51-positive cTECs making up the majority of TECs (29.1% ± 4.2% mTECs vs 58.2% ± 8.1% cTECS, n = 3; P = .006 for mTECs, P = .003 for cTECs).
The neonatal thymus contains a unique vascular architecture, qualitatively and quantitatively different from adult thymus
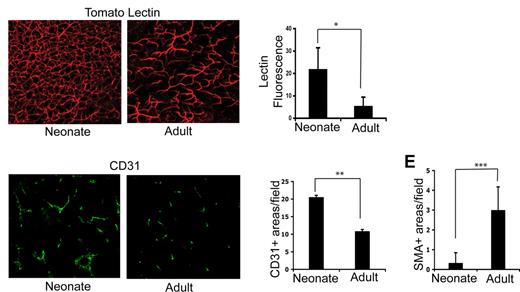
Having observed significant differences in both thymocyte and TEC distribution in neonates and adults, we further investigated age-related changes in the thymic microenvironment by examining the thymic vascular compartment of neonates and adults. Fluorescent labeling of the thymic vasculature of neonates and adults was undertaken using in vivo tomato lectin staining, which labels the luminal surface of endothelial cells of all blood vessel types.21 Examination of 200-μm-thick sections of thymus scanned by confocal microscopy revealed marked differences between neonates and adults. The mature and hierarchical architecture of the adult vasculature consisted of a range of blood vessel types with larger arterioles and venules predominating and relatively little vessel branching (Figure 2A right panel), as previously described.22 In contrast, a dense, highly branched network of capillaries and small vessels was seen throughout the neonatal thymus (Figure 2A left panel; also see Videos S1,S2, available on the Blood website; see the Supplemental Materials link at the top of the online article). Low-power examination of neonatal and adult thymus confirmed that the dense vascularity observed in the neonate (Figure 2A) persists throughout the entire thymus and is not biased toward medullary or cortical areas (Figure S1). Quantitation of lectin binding revealed a significantly greater vascular density in neonatal compared with adult thymus (Figure 2B; P < .001; n = 6 areas from neonatal and adult thymi). The difference in vascular density was confirmed using in vitro immunofluorescence analysis of expression of the endothelial cell marker CD31 (Figure 2C,D; P = .05). Differences in vascular appearance between Figure 2A and 2C reflect use of Z-series to provide a 3-D picture of the vasculature (Figure 2A) versus examination of a thin section (Figure 2C). When tomato lectin–labeled thymi are scanned to a shallower depth (Figure S1), the vasculature resembles that of Figure 2C. Analysis of smooth muscle actin (SMA) staining revealed that pericytes were almost absent in neonatal thymi, consistent with the presence of immature vasculature (Figure 2E; P < .001).
The neonatal thymus contains an immature, dense vasculature. (A) In vivo tomato lectin staining acquired by confocal microscopy as described in “In vivo labeling of the thymic vasculature” showing the vasculature of a neonatal (left panel) and adult (right panel) mouse thymus. (B) Graph shows the average percentage increase (± SD) in fluorescent intensity above the control (no lectin staining) threshold. *P < .001. (C) Immunofluorescent staining of sections of neonatal (left panel) and adult (right panel) thymus using anti-CD31 antibody. (D) Quantitation of average numbers of CD31+ vessels per section in adult and neonate. **P = .05; n = 7 sections for neonate and adult. (E) Average number of SMA-positive areas per field examined in adults (n = 12 fields) and neonate (n = 6 fields). ***P < .001. Error bars represent standard deviation.
The neonatal thymus contains an immature, dense vasculature. (A) In vivo tomato lectin staining acquired by confocal microscopy as described in “In vivo labeling of the thymic vasculature” showing the vasculature of a neonatal (left panel) and adult (right panel) mouse thymus. (B) Graph shows the average percentage increase (± SD) in fluorescent intensity above the control (no lectin staining) threshold. *P < .001. (C) Immunofluorescent staining of sections of neonatal (left panel) and adult (right panel) thymus using anti-CD31 antibody. (D) Quantitation of average numbers of CD31+ vessels per section in adult and neonate. **P = .05; n = 7 sections for neonate and adult. (E) Average number of SMA-positive areas per field examined in adults (n = 12 fields) and neonate (n = 6 fields). ***P < .001. Error bars represent standard deviation.
The vasculature of the neonatal thymus is VEGF dependent
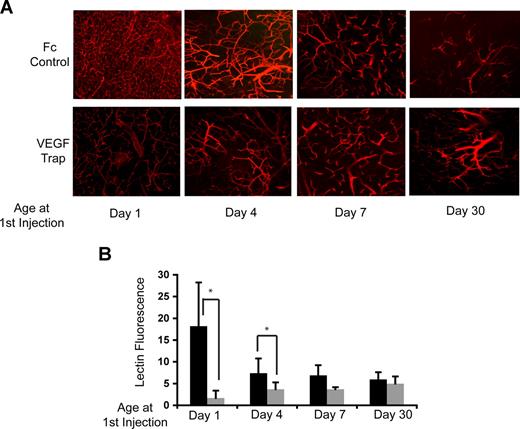
The highly branched and dense capillary network with minimal pericyte coverage observed in the neonatal thymus was highly suggestive of the presence of immature, VEGF-responsive vasculature.12 To test whether the differences in neonatal and adult thymic vasculature were mediated through VEGF, VEGF-Trap, a fusion protein that encompasses the VEGF ligand-binding domains of both VEGF-R1 and VEGF-R2,23 was used to inhibit VEGF signaling in vivo. One-day-old (day-1), and day-4, day-7, and day-30 mice were administered either VEGF-Trap or control Fc protein, and then perfused in vivo with labeled tomato lectin before killing. Visually, the vascular architecture of the thymus from untreated, control animals progressively matured over the first week of life, with vascular density decreasing to an adult level by day 7 (Figure 3A top panels). VEGF inhibition dramatically altered the vascular architecture during the early neonatal period. Mice treated with VEGF-Trap at either day 1 or day 4 lost the dense branching and capillary predominance observed in age-matched control mice (Figure 3A bottom panels), and were converted to an adult vascular phenotype. Quantitative analyses of lectin binding to the thymic vasculature of control- and VEGF-Trap–treated mice confirmed that VEGF-Trap significantly reduced the vascular density in the thymus when administered at day 1 and day 4 of life (Figure 3B; P < .001). No significant change in the thymic vasculature architecture or density was observed between control mice and mice treated with VEGF-Trap at day 7 or day 30. These findings show that the thymic vasculature is VEGF dependent during the first week of life, but becomes VEGF independent after this period.
The vasculature of the neonatal thymus is VEGF dependent. (A) Tomato lectin staining acquired by confocal microscopy as described in “Methods” showing the vasculature of mice injected with VEGF-Trap (bottom panels) or hFc control (top panels) at 1, 4, 7, and 30 days of age. (B) Quantitation of tomato lectin fluorescence above the control (no lectin staining) threshold. ■ indicates controls;  , VEGF-Trap. *P = .001. Error bars represent standard deviation.
, VEGF-Trap. *P = .001. Error bars represent standard deviation.
The vasculature of the neonatal thymus is VEGF dependent. (A) Tomato lectin staining acquired by confocal microscopy as described in “Methods” showing the vasculature of mice injected with VEGF-Trap (bottom panels) or hFc control (top panels) at 1, 4, 7, and 30 days of age. (B) Quantitation of tomato lectin fluorescence above the control (no lectin staining) threshold. ■ indicates controls;  , VEGF-Trap. *P = .001. Error bars represent standard deviation.
, VEGF-Trap. *P = .001. Error bars represent standard deviation.
VEGF inhibition in neonates reduces thymic cellularity and influences thymopoiesis
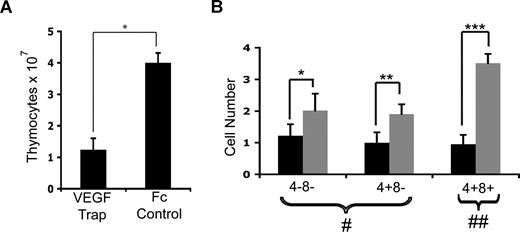
Given that inhibition of VEGF signaling had a dramatic effect on the thymic vasculature, we next explored the effect of VEGF inhibition on thymocyte proliferation and differentiation. Day-1 mice were injected once with VEGF-Trap and examined 24 hours, 72 hours, or 7 days later. No change in BrdU uptake or apoptosis of thymocytes was detected after VEGF inhibition in any of the cohorts compared with mice given Fc control (data not shown). In addition, there was no difference in body weight, thymocyte numbers, or thymocyte distribution in mice harvested 24 and 72 hours after treatment with either VEGF-Trap or Fc control (data not shown). However, by 7 days after treatment, total thymocyte numbers were significantly reduced in VEGF-Trap–treated mice compared with controls (Figure 4A; P < .001). FACS analysis of thymocyte distribution showed that thymi of mice treated with VEGF-Trap contained significantly lower numbers of DN, DP, and CD4+CD8− SP cells compared with controls, with the largest reduction in the DP subset (Figure 4B).
Effect of VEGF-Trap on neonatal mouse thymocytes. (A) Graph showing total thymocyte numbers isolated from mice given VEGF-Trap or Fc control on day 1, and analyzed 7 days later. *P < .001. (B) Effect of VEGF inhibition on T-cell subset numbers. The CD4−CD8− and CD4+CD8− cells are shown as cell number × 106 (#), whereas the CD4+CD8+ cell subset is cell number × 107 (##). *P = .04; **P = .004; ***P < .001. n = 4 pooled VEGF-Trap litters (■) and n = 5 individual Fc control mice ( ). Error bars represent standard deviation.
). Error bars represent standard deviation.
Effect of VEGF-Trap on neonatal mouse thymocytes. (A) Graph showing total thymocyte numbers isolated from mice given VEGF-Trap or Fc control on day 1, and analyzed 7 days later. *P < .001. (B) Effect of VEGF inhibition on T-cell subset numbers. The CD4−CD8− and CD4+CD8− cells are shown as cell number × 106 (#), whereas the CD4+CD8+ cell subset is cell number × 107 (##). *P = .04; **P = .004; ***P < .001. n = 4 pooled VEGF-Trap litters (■) and n = 5 individual Fc control mice ( ). Error bars represent standard deviation.
). Error bars represent standard deviation.
VEGF is produced by thymocytes and epithelial cells in the neonatal but not the adult thymus
Previous studies have shown that locally produced VEGF, rather than systemic VEGF, acts on the endothelium within specific organs.24-26 The level of intracellular VEGF in neonatal and adult thymocytes was below the level of detection by ELISA (data not shown). However, the thymic microenvironment (a combination of extracellular and intracellular fractions of the thymocyte-depleted fraction) showed significantly higher VEGF protein levels in the neonatal compared with the adult thymus (neonate: 88.53 pg/mg total protein, n = 7; adult: 29.9 pg/mg total protein, n = 3; P = .02; Figure 5A).
VEGF expression patterns in neonatal and adult thymus. (A) VEGF protein levels from “thymocyte-depleted” lysates from neonate and adult thymi. *P = .02. (B) VEGF and β-actin expression from RNA isolated from bulk thymic cells from neonates and adults. “+ control” lane contains mouse reference RNA. (C) VEGF and β-actin mRNA expression in stromal (CD45−MHCII−), hematopoietic (CD45+MHCII−), and epithelial (CD45−MHCII+) cells from neonatal thymus. (D) VEGF and actin mRNA expression from FACS-isolated DN (CD4−CD8−) thymocyte subsets in neonatal thymus. The vertical lines in panels B through D indicate repositioned gel lanes. Error bars represent standard deviation.
VEGF expression patterns in neonatal and adult thymus. (A) VEGF protein levels from “thymocyte-depleted” lysates from neonate and adult thymi. *P = .02. (B) VEGF and β-actin expression from RNA isolated from bulk thymic cells from neonates and adults. “+ control” lane contains mouse reference RNA. (C) VEGF and β-actin mRNA expression in stromal (CD45−MHCII−), hematopoietic (CD45+MHCII−), and epithelial (CD45−MHCII+) cells from neonatal thymus. (D) VEGF and actin mRNA expression from FACS-isolated DN (CD4−CD8−) thymocyte subsets in neonatal thymus. The vertical lines in panels B through D indicate repositioned gel lanes. Error bars represent standard deviation.
Given that VEGF is a secreted molecule, to confirm that VEGF protein measured in the thymic microenvironment fraction was produced locally in the thymus and was not due to the presence of contaminating systemic VEGF, RNA was isolated from identical numbers of cells obtained from whole neonatal or adult thymus, the majority of which were thymocytes. RT-PCR detected VEGF mRNA in cells from neonatal but not adult mice (Figure 5B). Analysis of VEGF expression in more defined fractions of hematopoietic (thymocyte) and stroma (TEC and non-TEC) cell types was undertaken, based on MHCII and CD45 expression. TECs were isolated as CD45−MHCII+; non-TEC stromal cells, as CD45−MHCII−; and hematopoietic cells, as CD45+MHCII−.20 RT-PCR revealed that VEGF mRNA was expressed in all 3 subsets of the neonatal thymus, with the highest levels found in the TEC subset (Figure 5C). RT-PCR analysis of similar fractions from adult thymus revealed no VEGF mRNA expression (data not shown). Both neonatal and adult hematopoietic cells were further fractionated into DP, SP, and DN cells. Across 5 independent experiments, VEGF was consistently detected in the rare DN but not in the more numerous DP subset of neonatal thymocytes (data not shown). No VEGF mRNA was detected in the same cell fractions from adult thymus (data not shown).
When the immature DN thymocyte population from both neonates and adults was further fractionated into the 4 sequential developmental stages (DN1, DN2, DN3, and DN4) based on CD44 and CD25 expression,27,28 VEGF expression was consistently and strongly expressed only in the DN2 and DN3 subpopulations of neonatal thymocytes (Figure 5D). No VEGF mRNA was detected in B lymphocytes or in macrophages isolated from the neonatal thymus, nor was it detected in any DN subpopulations, B lymphocytes, or macrophages in adults (data not shown). Thus during the first week of life, thymic vascular development is dependent on VEGF produced locally in the thymus by TECs and also by specific, rare immature thymocyte subsets. By early adulthood, however, VEGF is no longer expressed in the thymus.
VEGF-R2 is expressed on the thymic endothelium
The dramatic reduction of thymopoiesis without an associated change in either cell proliferation or apoptosis argues against a direct effect from VEGF on neonatal thymocytes. Further, the delay in reduction of thymocyte numbers after inhibition also suggests that thymopoiesis was affected by VEGF indirectly, most likely through its effects on the neonatal vasculature. We thus examined expression of VEGF-R2 on the endothelial, thymocyte, and epithelial compartments. Immunohistochemistry revealed that VEGF-R2 colocalizes with CD31 in the neonate thymus (Figure 6A), with significantly lower VEGF-R2 expression in the adult thymus (Figure 6B; P < .001). Using the same cell populations as in Figure 5C, RT-PCR confirmed that VEGF-R2 is predominantly found in CD45− cells (data not shown). FACS analysis showed that 33.5% of all CD45−CD31+ endothelial cells in the neonatal thymus (which make up 0.55% of all CD45− cells) expressed VEGF-R2. In contrast, only 11.6% of all CD45−MHCII+ epithelial cells (accounting for 4.9% of all CD45− cells) expressed VEGF-R2. (Figure 6C). In the adult, only 5.13% of CD31+ and 1.02% of MHCII+ cells (which comprised 0.27% and 3.08% of the total CD45− cell population, respectively) coexpressed VEGF-R2. No convincing expression of VEGF-R2 was detected on neonatal or adult thymocytes (Figure 6C). Furthermore, ELISA of thymocytes showed no detectable VEGF-R2 expression (data not shown). Our data suggest that VEGF acts predominantly on the neonatal thymic endothelium and the effect of VEGF inhibition on thymocytes is likely to be indirect, through its profound effects on the neonatal thymic vasculature.
VEGF-R2 expression in neonatal and thymus. (A) Immunofluorescent staining of sections of neonatal (top row) and adult (bottom row) thymus with VEGF-R2 (leftmost panel) and CD31 (2nd from left) as described in “Methods.” The merged images (2nd from right) show overlap between VEGF-R2 and CD31 represented by yellow areas. The rightmost panels represent the merged images with DAPI included. (B) Quantitation of VEGF-R2 expression by immunofluorescence in neonatal and adult thymus. *P < .001. (C) Percentage of VEGF-R2+ cells in each subset (endothelial cells: CD45-CD31+; TECs: CD45−MHCII+; hematopoietic cells: CD45+) of neonatal and adult thymus measured by FACS analysis. Error bars represent standard deviation.
VEGF-R2 expression in neonatal and thymus. (A) Immunofluorescent staining of sections of neonatal (top row) and adult (bottom row) thymus with VEGF-R2 (leftmost panel) and CD31 (2nd from left) as described in “Methods.” The merged images (2nd from right) show overlap between VEGF-R2 and CD31 represented by yellow areas. The rightmost panels represent the merged images with DAPI included. (B) Quantitation of VEGF-R2 expression by immunofluorescence in neonatal and adult thymus. *P < .001. (C) Percentage of VEGF-R2+ cells in each subset (endothelial cells: CD45-CD31+; TECs: CD45−MHCII+; hematopoietic cells: CD45+) of neonatal and adult thymus measured by FACS analysis. Error bars represent standard deviation.
Discussion
The bidirectional interactions between thymocytes and TECs are well established.2,3 However, the role of the thymic endothelium in thymopoiesis and the regulation of its growth and differentiation by the other thymic compartments have been largely ignored. In this report, we demonstrate that growth and development of the neonatal murine thymic endothelium are regulated by local production of VEGF from the epithelial and possibly also the hematopoietic compartments of the thymus. These novel interactions were revealed by studies of the early postnatal period during which thymopoiesis is marked by proliferative and relatively immature thymocytes and by a dominance of cortical over medullary TECs. We show for the first time that the vascular architecture of the neonatal murine thymus is quantitatively and qualitatively different from that of the adult thymus, consisting of a dense network of highly branched capillaries, with high VEGF-R2 expression and few pericytes, all features typical of immature, VEGF-dependent vasculature.29 Inhibition of VEGF signaling converted the neonatal thymic vasculature to a more mature, hierarchic architecture, confirming that the endothelium of the neonatal thymus is uniquely VEGF dependent.
Although the highest levels of VEGF message were detected in neonatal TECs, VEGF mRNA was also detected in discrete subsets of thymocytes. The relative contribution to local VEGF secretion from hematopoietic versus nonhematopoietic cells (TECs and stroma) is difficult to ascertain as VEGF is a secreted molecule, and thus intracellular VEGF protein in thymocytes was difficult to detect. However, the restriction of VEGF message to specific thymocyte subsets suggests a possible involvement of upstream regulatory signals affecting VEGF expression during thymocyte differentiation. Interestingly, both the DN2 and DN3 subsets, which express VEGF, also express interleukin-2 receptor alpha (IL-2Rα, CD25),30 and IL-2 has been reported to stimulate VEGF expression in adult rat T-cell lines.31
During the same 7-day period of postnatal life in which the thymic vasculature matures into an adult form, the neonatal pattern of TEC dominance also undergoes a rapid transition to an adult pattern, changing from predominantly cTECs to dominance of mTECs. These findings in murine thymus are in line with age-related morphologic changes reported in the human thymus.14 The question thus arises as to whether local VEGF production from TECs might induce the spatial organization of the thymic vasculature, or alternatively whether vascular maturation influences differentiation of the thymic epithelium. The former possibility is supported by studies of brain development, showing that local VEGF production by neural progenitors induces the anatomic positioning and growth of capillaries in specific areas of the developing brain, and that VEGF expression is critical for normal brain morphogenesis.32 The latter possibility was raised by Anderson et al in their 3-dimensional imaging and electron microscope analysis of thymi from adult mice.33 They found medullary epithelium is closely associated with larger, more mature vessels such as venules and arterioles rather than smaller capillaries. This association persisted even in Rag2−/− mice, which lack thymocytes and thus would not influence TEC growth. Although the authors did not examine the correlation between cortical epithelium and vasculature, they postulated, “Neovascularization may be centrally involved in the initial organization of the medullary compartment.”33 p 1109
In addition to changes to the thymic vasculature, VEGF inhibition resulted in a dramatic reduction in thymocyte numbers. This provides evidence of possible cross-talk, between endothelium and thymocytes, in which thymocyte production of VEGF supports and/or maintains thymic endothelium, which in turn influences thymopoiesis. In addition to the well-established fact that the epithelial compartment plays a role in proper thymocyte development, our data suggest that the thymic epithelial cell compartment, though the production of high levels of VEGF, can also indirectly influence thymopoiesis through the ability to influence the endothelium. In a previous report that used a blastocyst complementation strategy to ablate TEC-specific VEGF expression in fetal mice, thymic vascular architecture was dramatically altered with a reduction of the dense capillary network within the thymic parenchyma.34 However, in contrast to our studies in neonatal animals, no reduction in thymic size or thymocyte number was seen in the studies of inhibition of VEGF during fetal life. It should be noted however that in the fetal studies, unlike our studies with VEGF-TRAP, VEGF expression was manipulated only in TECs; thus expression of local, nonepithelial (eg, from mesenchymal cells or thymocytes), and systemic VEGF was maintained, minimizing the full effect of VEGF inhibition on the developing thymus.
Based on the data presented here, it appears unlikely that VEGF acts directly on thymocytes. Inhibition of VEGF caused rapid changes in thymic vasculature within 24 hours, but the reduction in thymocyte number was delayed until between 4 and 7 days after inhibition. VEGF inhibition had no effect on BrdU incorporation or apoptosis of thymocytes. Furthermore, exhaustive analysis of VEGF-R2 expression on various cell populations suggests that there is little, if any, VEGF-R2 expressed on thymocytes. Although we cannot exclude the possibility that rare subsets of thymocytes may express VEGF-R2, it seems unlikely that such expression would be sufficient to account for the magnitude of the reduction in thymocyte number produced by VEGF inhibition. Thus, it seems most likely that the effect of VEGF inhibition on thymopoiesis is indirect, secondary to changes induced in the thymic vasculature.
Studies have reported that high systemic VEGF levels in patients with malignancies are associated with immune dysregulation including a decline in lymphocyte cell numbers and function.35 A murine model devised to study this observation found that prolonged systemic administration of VEGF in adult mice caused a decrease in T cells in the thymus leading to thymic atrophy; the effects were mediated specifically through VEGF-R2 rather than VEGF-R1.36,37 However, no direct effect of VEGF on thymocytes either through changes in thymocyte cycling or apoptosis was identified. Of note, no effect on thymopoiesis was observed until 21 to 28 days of continuous administration of VEGF. In addition, the effect of VEGF on the vascular and epithelial compartments of the thymus was not assessed. Thus prolonged, systemic administration of supraphysiologic levels of VEGF resulted in gradual thymocyte loss, whereas in our own study, blocking the high local endogenous VEGF present during the neonatal period led to a reduction in thymocyte numbers. The explanation for this apparent contradiction is unclear but several critical differences between the experimental models could be involved, including the different stages of thymic development studied, the local versus systemic delivery of VEGF, and the different duration of VEGF exposure between the models.
In the studies presented here, the neonatal thymus contained a significantly higher frequency of immature thymocytes than the adult thymus, suggesting that influx of thymocyte precursors is higher during neonatal life. Typically, VEGF-responsive endothelium is highly fenestrated, has few pericytes and is more permeable than that of mature vasculature. In studies of tumor-associated angiogenesis, inhibition of VEGF leads to a reduction in the fenestrations of the tumor vasculature and can block the extravasation of macromolecules into the tumor parenchyma.16,38 It is therefore possible that similar changes to the neonatal thymic vasculature could influence ingress of thymocyte precursors, and that permeable, VEGF-responsive vasculature may permit highly efficient migration into the neonatal thymus. Interestingly, the perinatal, but not the adult, thymus is the source of gut CD8αα T cells,39,40 which have recently been shown to be generated after the export, or “leaking from,”41 the thymus of immature (DN2 and DN3) thymocytes.42 Thus, the VEGF-responsive endothelium in the thymus may be responsible for the unique patterns of both thymic import and export that exist in the neonatal thymus.
In summary, the neonatal period represents a stage of rapid change in thymocyte, TEC, and vascular growth and differentiation. Analysis of the unique biology of this period has revealed that the thymic vasculature, which provides the gateway for the initiation and completion of thymopoiesis, is in turn regulated by the hematopoietic and epithelial compartments of the thymus. This novel form of thymic cross-talk is mediated through the local production of VEGF.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Laura Gordon, and The Saban Research Institute FACS Core and Animal Core Facilities at CHLA. We are indebted to Xingchao Wang for assistance with morphologic analysis. Drs Erica Sloan, Gautam Dravid, Denis Evseenko, and Robertson Parkman provided insightful discussion and critical comments.
This work was supported by National Institutes of Health (NIH, Bethesda, MD) grants RO1 HL77912, PO1 HL073104, and R21 HL095165 (G.M.C.). A.R.C. was supported by a fellowship from the California Institute for Regenerative Medicine (San Francisco, CA).
National Institutes of Health
Authorship
Contribution: A.R.C., G.T., and G.M.C. designed research; A.R.C., S.G., J.Z., and J.J. performed research; A.C., G.T., and R.B. provided vital new reagents or analytic tools; A.R.C., A.C., and G.M.C analyzed data; and A.R.C. and G.M.C. wrote the paper.
Conflict-of-interest disclosure: G.T. is an employee of Regeneron Pharmaceuticals. The remaining authors declare no competing financial interests.
Correspondence: Gay M. Crooks, University of California, Los Angeles, A7-149 CHS, 10833 Le Conte Ave, Los Angeles, CA 90095-1732; e-mail: gcrooks@mednet.ucla.edu.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal