Abstract
Although complement lysis is frequently used for the purification of lymphocyte subpopulations in vitro, how lymphocytes escape complement attack in vivo has not been clearly delineated. Here, we show that conditional gene targeting of a murine membrane complement regulator Crry on thymocytes led to complement-dependent peripheral T-cell lymphopenia. Notably, despite evidence of hypersensitivity to complement attack, Crry-deficient T cells escaped complement injury and developed normally in the thymus, because of low intrathymic complement activity. Crry-deficient T cells were eliminated in the periphery by a C3- and macrophage-mediated but C5-independent mechanism. Thus, Crry is essential for mature T-cell survival in the periphery but not for lymphogenesis in the thymus. The observation that the thymus is a complement-privileged site may have implications for complement-based antitumor therapies.
Introduction
The complement system is a form of humoral innate immunity that plays an important role in host defense. Recognition of nonself by the complement system is achieved by natural or elicited antibodies that trigger the classical pathway of complement activation, by mannose-binding lectins that activate the lectin pathway on interaction with microbial sugar molecules, and by spontaneous activation of the alternative pathway that occurs as a default process in the absence of adequate regulation.1 Activated complement defends the host through generation of the proinflammatory anaphylatoxins C5a and C3a, opsonization of pathogens with C4 and C3 cleavage fragments, and formation of the lytic membrane attack complex.1-3 Host cells avoid collateral complement damage, particularly by the constitutively active alternative pathway, through the expression of complement regulatory proteins.4-6 These proteins are present in the host as membrane-bound or soluble plasma proteins.4-6 Plasma complement regulators confer their protection by preferentially interacting with host cells through eukaryote-specific signature molecules such as glycosaminoglycans and sialic acid.1,7,8
Complement receptor 1–related gene/protein y (Crry) is a broadly expressed murine membrane complement regulatory protein.9,10 Crry inhibits both classical and alternative pathway C3 convertases by decay acceleration and by serving as a cofactor for the C4b/C3b-cleaving enzyme factor I.11 These activities of Crry resemble those of human decay-accelerating factor (DAF) and membrane cofactor protein (MCP).10,11 Because MCP expression in the mouse is restricted to the testis, Crry is considered a functional homolog of human MCP.5,12-14 Both human MCP and murine Crry are expressed on numerous cell types, including endothelial cells, platelets, bone marrow stem cells, thymocytes, and mature lymphocytes. The significance of their expression on these cells, with relation to other membrane and fluid-phase regulators, has not been well delineated. Human MCP mutations are rare but have recently been linked to atypical hemolytic uremic syndrome,15 suggesting a role of MCP in preventing complement-mediated endothelial and/or platelet injury and activation. Interestingly, deletion of Crry in the mouse by conventional gene targeting resulted in embryonic lethality as a result of maternal complement attack of the fetus.16 This outcome prevented the in vivo analysis of Crry as a complement regulator in normal physiology.
In the present study, we have used the Cre-Lox system and generated a thymocyte-specific Crry knockout mouse to address the role of Crry on T lymphocytes. We describe here that protection from complement attack by Crry is essential for mature T-lymphocyte survival in the periphery but not for T-cell development and survival in the thymus. We investigated the mechanism of the observed differential survival of thymic and peripheral T cells and discuss the potential implications of our findings for complement-based antitumor therapies.
Methods
Generation of a floxed Crry mouse by gene targeting
To construct the Crry conditional targeting vector, we amplified both the short-arm and long-arm homologous sequences by polymerase chain reaction (PCR) using the Expand Long Template PCR System (Roche Diagnostics, Indianapolis, IN) with 129S6/SvEvTac mouse genomic DNA as a template. For the short-arm homologous sequence, we amplified a 5.2-kb KpnI-EcoRI fragment containing the sixth exon of the Crry gene with the use of 5′-GGTACCGGTTCTGGATGAGGGAATACAGT-3′ (forward) and 5′-GAATTCCAAGGGTCATGGCCTACAGGTT-3′ (reverse) as primers (KpnI and EcoRI sites underlined). This fragment was cloned into the pND1 vector17 at KpnI and EcoRI sites. For the long-arm homologous sequence, we first amplified a 6.3-kb fragment containing the fourth exon of the Crry gene with the use of 5′-GCATTCCCTTGTCTAGGTATTC-3′ (forward) and 5′-GGATCCGACTTTCCTATTTATGCAG-TTGACC-3′ (reverse; BamHI site underlined) as primers, and cloned it into the pCR 2.1 vector (Invitrogen, Carlsbad, CA). This fragment was subcloned into the pBluescript vector at NotI-BamHI sites. We then amplified a 0.6-kb BamHI-XhoI fragment containing the fifth exon of the Crry gene with the use of 5′-GGATCCATAACTTCGTATAATGTATGCTATACGAAGTTATGAGCACATGTTATGTATGCATC-3′ (forward) and 5′-CTCGAGAACCCTGTCCCTAGTCCTCCAATA-3′ (reverse) as primers (BamHI and XhoI sites underlined and loxP sequence italicized). This fragment was subscloned into the above pBluescript vector at BamHI and XhoI site to link with the 6.3 kb fragment in a tail to head orientation. The combined 6.9-kb fragment was then excised from the pBluescript plasmid and subcloned into the pND1 vector at NotI-XhoI sites (Figure 1A).
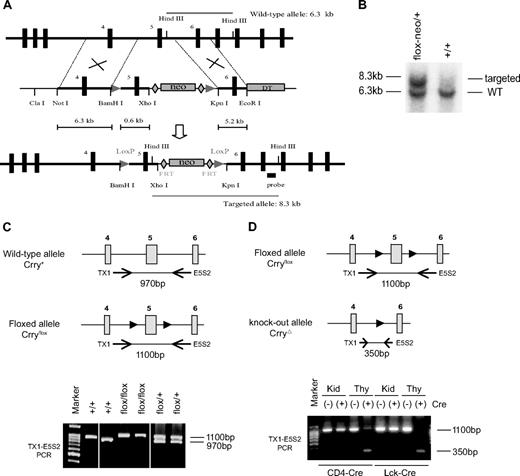
Generation of a T cell–specific Crry mutant mouse. (A) Schematic diagram of the wild-type Crry gene locus, targeting vector, and recombinant Crry gene locus (from top to bottom). The targeting vector contained 2 loxP sites (arrowheads) flanking exon 5. The neomycin (neo) gene cassette was flanked by 2 FRT sites (diamonds). HindIII cut produces a 6.3-kb fragment in the wild-type allele (top) and a 8.3-kb fragment in the recombinant allele (bottom). DT indicates diphtheria toxin. (B) Southern blot data showing that the positive ES cell clone (flox-neo/+) produced 2 HindIII restriction fragments, whereas a representative nontargeted ES cell clone (+/+) produced only the 6.3-kb band. (C) PCR genotyping of WT and floxed Crry gene alleles (after neo excision) with the use of a pair of primers flanking exon 5. WT alleles produced a 970-bp product, whereas the floxed allele produced a 1100-bp product. Horizontal arrows denote the approximate locations of primers (Tx1, E5S2) used in PCR, and solid arrowheads indicate the loxP sites. (D) PCR analysis showing thymus-specific deletion of exon 5 of the Crry gene in CD4-Cre+ and Lck-Cre+ Crryflox/flox mice. A 350-bp fragment indicative of mutated Crry gene allele was detected in Cre-positive mouse thymus (Thy) only. It was not present in the kidney (Kid) or in Cre-negative mouse thymus.
Generation of a T cell–specific Crry mutant mouse. (A) Schematic diagram of the wild-type Crry gene locus, targeting vector, and recombinant Crry gene locus (from top to bottom). The targeting vector contained 2 loxP sites (arrowheads) flanking exon 5. The neomycin (neo) gene cassette was flanked by 2 FRT sites (diamonds). HindIII cut produces a 6.3-kb fragment in the wild-type allele (top) and a 8.3-kb fragment in the recombinant allele (bottom). DT indicates diphtheria toxin. (B) Southern blot data showing that the positive ES cell clone (flox-neo/+) produced 2 HindIII restriction fragments, whereas a representative nontargeted ES cell clone (+/+) produced only the 6.3-kb band. (C) PCR genotyping of WT and floxed Crry gene alleles (after neo excision) with the use of a pair of primers flanking exon 5. WT alleles produced a 970-bp product, whereas the floxed allele produced a 1100-bp product. Horizontal arrows denote the approximate locations of primers (Tx1, E5S2) used in PCR, and solid arrowheads indicate the loxP sites. (D) PCR analysis showing thymus-specific deletion of exon 5 of the Crry gene in CD4-Cre+ and Lck-Cre+ Crryflox/flox mice. A 350-bp fragment indicative of mutated Crry gene allele was detected in Cre-positive mouse thymus (Thy) only. It was not present in the kidney (Kid) or in Cre-negative mouse thymus.
The targeting vector was linearized with NotI and introduced by electroporation into TL-1 embryonic stem (ES) cells (kindly provided by P. Labosky, Vanderbilt University, Nashville, TN). ES cell culture and DNA transfection were carried out as previously described,18 and transfected cells were subjected to G418 selection (200 μg/mL) starting from 48 hours after electroporation. ES cells with homologous recombination were screened by Southern blot analysis of genomic DNA after HindIII digestion with a 397-bp 3′ probe amplified with 5′-AGGGTGTCATGAGATTGGCATC-3′ and 5′-TGTAGGTGGCATCATTCTCAG-3′ as primers. Wild-type allele produced a 6.3-kb HindIII fragment, whereas the targeted allele produced a 8.3-kb fragment (Figure 1A,B). Correctly targeted ES cells (Crryflox-neo/+) were injected into 3.5-day postcoital C57BL/6J blastocysts, and the resulting male chimeras were crossed with female C57BL/6J mice to determine germ line transmission of the targeted allele. As shown in Figure 1A, the neomycin-resistance cassette (neo) in the targeting vector is flanked by 2 flippase (FLP) recombinase target (FRT) sites to allow its subsequent removal by FLP recombinase. Heterozygous Crry-targeted mice (Crryflox-neo/+) were crossed with FLPe transgenic mice (expressing the enhanced version of FLP)19 to remove the neo from the Crry allele and to generate a Crry-floxed mouse (Crryflox/+). Crryflox/+ mice were intercrossed to generate Crryflox/flox mice. All Crryflox/flox mice used in the study were on a B6 × 129 mixed background.
Generation of T cell–specific Crry knockout mice and source of other experimental mice
To generate T cell–specific Crry-knockout mice, Lck-Cre+ and CD4-Cre+ transgenic mice (The Jackson Laboratory, Bar Harbor, ME, and Taconic Farms, Germantown, NY, respectively) were crossed with Crryflox/+ mice. The resulting Lck-Cre+-Crryflox/+ or CD4-Cre+-Crryflox/+ mice were then crossed with Crryflox/flox mice to generate Lck-Cre+-Crryflox/flox or CD4-Cre+-Crryflox/flox mice, as well as their Cre-negative littermate controls. Mouse genotyping was performed by PCR of tail DNAs. For the wild-type, Crryflox allele and CrryΔ allele, a fragment of 970 bp, 1100 bp and 350 bp, respectively, was expected with the use of the following pair of primers, TX-1 (5′-CAGAGTAATCTACAGTTTCACC-3′) and E5S2 (5′-GTTCACTGTATTCCCTCATCCAGA-3′). CD4-Cre and LCK-Cre transgenic mice were identified using a pair of Cre-specific primers, 5′-ATTCTCCCACCGTCAGTACG-3′ and 5′-CGTTTTCTGAGCATACCTGGA-3′. The generation of C3−/− (C57BL/6 background), CRIg−/−, Crry−/−/C3−/−, and Crry−/−/DAF−/−C3−/− mice was described previously.20-22 Congenic C57BL/6 wild-type mice (CD45.1, CD45.2) and C5-deficient mice were obtained from The Jackson Laboratory. CRIg−/−CD4-Cre+-Crryflox/flox, CRIg−/−CD4-Cre−-Crryflox/flox, and CRIg+/+CD4-Cre+-Crryflox/flox mice were generated as littermates by crossing CD4-Cre+-Crryflox/flox and CRIg−/− mice. Mice were maintained under specific pathogen-free conditions at the University of Pennsylvania. All animal experiments were approved by the Institutional Animal Care and Use Committees of the University of Pennsylvania.
Generation of BM chimera mice
We produced BM chimera mice in 2 experiments. In the first experiment, C3−/− mice were used as recipients, and WT, Crry−/−/C3−/−, Lck-Cre+-Crryflox/flox, CD4-Cre+-Crryflox/flox, and CD4-Cre−-Crryflox/flox mice were used as BM donors. Recipient mice were lethally irradiated (5.25 Gy [525 rads] × 2 times, 3-hour interval), and each was infused through the tail vein with 1 × 107 donor BM cells. In the second experiment, WT and C3−/− mice (both CD45.2) were used as recipients. They were similarly irradiated and received mixed (at 1:1 ratio) BM cells from C57BL/6 WT (CD45.1) and CD4-Cre+-Crryflox/flox (CD45.2) mice through tail vein injection (total 2 × 107 cells per recipient). Chimera mice were studied 2 months after BM transplantation.
Complement activation assays on erythrocytes and thymocytes
Erythrocytes were opsonized with a monoclonal IgG2a antierythrocyte autoantibody 34-3C (50 μg/mL) and subjected to classical pathway complement activation assays as described.18 Thymocytes (4 × 106) were incubated with 100 μL of diluted mouse serum as specified in GVBS++ at 37°C for 45 minutes, washed 3 times, and analyzed for C3 deposition by fluorescence-activated cell sorting (FACS) after staining with anti-C3.
In vivo macrophage depletion study
Clodronate liposomes were prepared as previously described.23 Mice were treated intravenously with 2 mg/20 g body weight of clodronate liposomes 24 hours before lymphocyte transfer experiment. This treatment resulted in greater than 90% reduction of splenic and bone marrow F4/80-positive macrophages.23,24 In another experiment, mice were treated intraperitoneally with 2 mg/20 g body weight clodronate liposomes on day 1, and thereafter every 4 days with 1 mg/20 g body weight for a period of 4 weeks. Liposome-encapsulated PBS was used as a control treatment. Blood was obtained by retroorbital bleeding at 0, 2, and 4 weeks after liposome treatment for flow cytometric analysis of CD4+ and CD8+ T cells.
Assessment of thymocyte clearance in vivo
Thymocytes (5 × 107) were labeled ex vivo with carboxyfluorescein diacetate succinimidyl ester (CFSE; Molecular Probes, Eugene, OR) in 1 mL PBS containing 50 nM or 5 nM of CFSE. Cells from Lck-Cre+-Crryflox/flox and Lck-Cre−-Crryflox/flox mice were mixed at a 1:1 ratio and injected into recipient mice through the tail vein (5 × 107 cells/recipient). Alternatively, thymocytes were first incubated in 5% mouse serum at 37°C for 45 minutes, washed, labeled with CFSE, and injected into recipient mice as described earlier. Splenocytes were harvested 1 hour after injection and analyzed by FACS for the ratio of Crry-deficient and -sufficient cells among CFSE-labeled cells after staining with anti-SCR3/4.
Statistical analysis
Data were analyzed by Student t test. P values less than .05 were considered significant.
Results
Generation of a Crryflox/flox mouse by gene targeting
To circumvent embryonic lethality caused by global Crry knockout,16 we used the Cre/loxP approach to generate a conditional Crry knockout mouse. Our strategy for targeting the Crry gene is shown in Figure 1A. We inserted 2 LoxP sites to flank exon 5 of the mouse Crry gene. Exon 5 encodes short-consensus repeat (SCR) 3 and 4 in the Crry protein, and these domains are critical for its function.16,25,26 Neomycin (neo), used as a positive selection marker during ES cell screening, was inserted between exon 5 and 6 (Figure 1A). To allow its subsequent excision from the recombinant Crry allele by FLP recombinase, the neo gene was flanked by 2 FRT sequences from the yeast Saccharomyces cerevisiae27 (Figure 1A).
We screened more than 600 drug-resistant ES cell clones by Southern blot analysis after HindIII digestion of genomic DNA and identified a single positive clone (Figure 1B). This ES cell clone was then expanded and used to produce 6 chimeras, all of which subsequently transmitted the recombinant Crry allele through germ line. Heterozygous Crry mice containing LoxP and neo insertions (Crryflox−neo/+) were crossed with transgenic mice expressing the enhanced version of FRT site-specific FLP recombinase (FLPe)19 to excise the neo gene and produce Crryflox/+, and eventually Crryflox/flox mice (Figure 1C).
Thymus-specific mutation of the Crry gene
To inactivate Crry on T lymphocytes, we crossed the Crryflox/flox mouse with CD4-Cre and Lck-Cre transgenic mice.28,29 As expected, PCR analysis of thymus DNA showed that exon 5 of the Crry gene was deleted in CD4-Cre+- and Lck-Cre+-Crryflox/flox but not in Cre−-Crryflox/flox mice (Figure 1D). Importantly, deletion of exon 5 was not observed in the liver or the kidney of the same mice, suggesting that mutation of the Crry gene was thymus specific (Figure 1D; data not shown).
We next analyzed the Crry gene mutation at the mRNA and protein levels. We performed reverse transcriptase PCR (RT-PCR) and, as expected, detected a shortened Crry mRNA species in the thymus of CD4-Cre+- and Lck-Cre+-Crryflox/flox mice (Figure 2A). Again, production of this mutant Crry mRNA was detected only in Cre+ mice and was thymus specific (Figure 2A). cDNA cloning and sequence analysis confirmed the absence of exon 5 sequence in the shortened Crry mRNA and showed an open-reading frame encoding a mutant Crry protein lacking SCR 3 and 4 (CrryΔ3-4; Figure 2B). The latter finding was unexpected, because deletion of exon 5 should have caused a frame shift and introduced an early stop codon to terminate protein translation beyond SCR 2. It appeared that a cryptic donor splice site was used in transcribing the mutant Crry mRNA, enabling the coding sequence downstream of SCR 3 to 4 to remain in frame.
Confirmation of Crry gene mutation at mRNA and protein levels. (A) RT-PCR analysis confirming thymus-specific production of a shortened Crry mRNA in CD4-Cre+ and Lck-Cre+-Crryflox/flox mice. LS indicates leader sequence; SCR, short consensus repeat; UTR, untranslated region; TM, transmembrane domain; Kid, kidney; Thy, thymus. (B) Domain structures of the WT (full-length, containing SCR 1-5) and mutant Crry protein (CrryΔ3-4, containing SCR 1, 2, and 5) predicted from the shortened Crry mRNA. (C) FACS analysis of CHO cells stably transfected with the full-length Crry cDNA or the shortened Crry cDNA (CrryΔ3-4). Both proteins were expressed and detected on the cell surface by a polyclonal rabbit anti–mouse Crry antibody (α-Crry). Cells transfected with the empty pCDNA3 vector were used as controls (CHO). (D) Western blot analysis of CHO cells stably transfected with the full-length Crry (lane 1), CrryΔ3-4 (lane 2), or pCDNA vector (lane 3). As expected, CrryΔ3-4 produced a protein of smaller size. A polyclonal rabbit anti–mouse Crry antibody (α-Crry) was used. (E) Left: Western blot using α-Crry analysis indicating thymus-specific production of the mutant Crry protein (smaller size between 36.9 kDa and 50.2 kDa). Right: Absorbed SCR3/4-specific anti-Crry antibody detected WT Crry protein but not the mutant protein in the thymus. (F) Confirmation of the specificity of α-SCR3/4 by FACS analysis of transfected CHO cells. It detected full-length Crry but not CrryΔ3-4 expressed on CHO cells.
Confirmation of Crry gene mutation at mRNA and protein levels. (A) RT-PCR analysis confirming thymus-specific production of a shortened Crry mRNA in CD4-Cre+ and Lck-Cre+-Crryflox/flox mice. LS indicates leader sequence; SCR, short consensus repeat; UTR, untranslated region; TM, transmembrane domain; Kid, kidney; Thy, thymus. (B) Domain structures of the WT (full-length, containing SCR 1-5) and mutant Crry protein (CrryΔ3-4, containing SCR 1, 2, and 5) predicted from the shortened Crry mRNA. (C) FACS analysis of CHO cells stably transfected with the full-length Crry cDNA or the shortened Crry cDNA (CrryΔ3-4). Both proteins were expressed and detected on the cell surface by a polyclonal rabbit anti–mouse Crry antibody (α-Crry). Cells transfected with the empty pCDNA3 vector were used as controls (CHO). (D) Western blot analysis of CHO cells stably transfected with the full-length Crry (lane 1), CrryΔ3-4 (lane 2), or pCDNA vector (lane 3). As expected, CrryΔ3-4 produced a protein of smaller size. A polyclonal rabbit anti–mouse Crry antibody (α-Crry) was used. (E) Left: Western blot using α-Crry analysis indicating thymus-specific production of the mutant Crry protein (smaller size between 36.9 kDa and 50.2 kDa). Right: Absorbed SCR3/4-specific anti-Crry antibody detected WT Crry protein but not the mutant protein in the thymus. (F) Confirmation of the specificity of α-SCR3/4 by FACS analysis of transfected CHO cells. It detected full-length Crry but not CrryΔ3-4 expressed on CHO cells.
To determine whether a protein could be translated from the mutant Crry mRNA, we first transfected CrryΔ3-4 and full-length Crry cDNAs into Chinese hamster ovary (CHO) cells with the use of pCDNA3 as an expression vector. As shown in Figure 2C and D, flow cytometry and Western blot analyses showed that both full-length Crry and CrryΔ3-4 were expressed in the transfected CHO cells. As expected, we also detected by Western blot analysis thymus-specific production of CrryΔ3-4 in CD4-Cre+-Crryflox/flox and Lck-Cre+-Crryflox/flox mice but not in Cre−-Crryflox/flox mice (Figure 2E left). To facilitate subsequent analysis of the mutant mice, we prepared an antiserum that recognized full-length Crry but not CrryΔ3-4 by absorbing a rabbit anti–mouse Crry serum against CrryΔ3-4–expressing CHO cells. In principle, the absorbed antiserum should contain SCR 3/4–specific antibodies only. As shown in Figure 2E (right) and F, we confirmed that the absorbed antibody detected full-length Crry but not CrryΔ3-4 in the mouse thymus and in transfected CHO cells. This antiserum will be referred to as anti-SCR3/4 hereafter. Although the production of a truncated Crry protein was unexpected, functional analysis showed that CrryΔ3-4 was inactive as a complement regulator (Figures S1,S2, available on the Blood website; see the Supplemental Materials link at the top of the online article).
CD4-Cre+-Crryflox/flox and Lck-Cre+-Crryflox/flox mice had severe T-cell lymphopenia but normal thymus cellularity
To evaluate the consequence(s) of thymus-specific Crry mutation, we analyzed by flow cytometry the cellular composition of the thymus, spleen, lymph nodes, and peripheral blood mononuclear cells (PBMCs) in CD4-Cre+-Crryflox/flox, Lck-Cre+-Crryflox/flox mice and their Cre−-Crryflox/flox littermates. We detected no significant difference between Cre−-Crryflox/flox and CD4-Cre+-Crryflox/flox or Lck-Cre+-Crryflox/flox mice in the number of total thymocytes or the number of CD4−CD8− (double negative [DN]), CD4+CD8+ (double positive [DP]), CD4+, and CD8+(single positive [SP]) thymocytes (Figure 3; data not shown). There was also no significant change in the percentages of various thymocyte populations in the mutant mice, except that the percentage of CD8+ cells was moderately decreased (5.67% vs 3.66%; P = .029) and that of CD4−CD8− cells slightly increased (2.43% vs 3.96%; P = .004) in Lck-Cre+-Crryflox/flox mice. In contrast, we found that both CD4+ and CD8+ T-cell numbers were strikingly reduced in the spleens and lymph nodes of CD4-Cre+-Crryflox/flox and Lck-Cre+-Crryflox/flox mice (Figure 3 right). With the exception of lymph node CD4+ T cells, the percentages of CD4+ and CD8+ T cells in the spleen, lymph nodes, and PBMCs of the mutant mice were also markedly reduced (Figure 3 left). Notably, total numbers of B lymphocytes (B220+) remained normal in the spleens and lymph nodes of CD4-Cre+-Crryflox/flox and Lck-Cre+-Crryflox/flox mice, but the percentages of B cells in PBMCs were significantly elevated (Figure 3 right), reflecting a relative abundance of B cells because of the severe reduction in T lymphocytes.
CD4-Cre+-Crryflox/flox and Lck-Cre+-Crryflox/flox mice developed peripheral T-cell lymphopenia. (A) Left: Representative flow cytometric analysis of thymocyte composition and CD4+, CD8+ T-cell populations in the spleens, lymph nodes, and blood of CD4-Cre−-Crryflox/flox and CD4-Cre+-Crryflox/flox littermates. Right: Numbers of total cells, B220+, CD4 SP (CD4+), and CD8 SP (CD8+) cells in the thymi, spleens, and lymph nodes of CD4-Cre−-Crryflox/flox and CD4-Cre+-Crryflox/flox littermates (n = 4 per group). Because blood collection varied in volume, the percentage, instead of total number, of B220+, CD4+, and CD8+ cells in the blood of the 2 groups of mice was shown. Values shown are mean plus or minus SEM. NS indicates not significant (P > .05). *P < .05, Student t test. (B) Data from Lck-Cre−-Crryflox/flox and Lck-Cre+-Crryflox/flox littermates (n = 4 per group). Analysis and data presentation are the same as in panel A.
CD4-Cre+-Crryflox/flox and Lck-Cre+-Crryflox/flox mice developed peripheral T-cell lymphopenia. (A) Left: Representative flow cytometric analysis of thymocyte composition and CD4+, CD8+ T-cell populations in the spleens, lymph nodes, and blood of CD4-Cre−-Crryflox/flox and CD4-Cre+-Crryflox/flox littermates. Right: Numbers of total cells, B220+, CD4 SP (CD4+), and CD8 SP (CD8+) cells in the thymi, spleens, and lymph nodes of CD4-Cre−-Crryflox/flox and CD4-Cre+-Crryflox/flox littermates (n = 4 per group). Because blood collection varied in volume, the percentage, instead of total number, of B220+, CD4+, and CD8+ cells in the blood of the 2 groups of mice was shown. Values shown are mean plus or minus SEM. NS indicates not significant (P > .05). *P < .05, Student t test. (B) Data from Lck-Cre−-Crryflox/flox and Lck-Cre+-Crryflox/flox littermates (n = 4 per group). Analysis and data presentation are the same as in panel A.
Crry-deficient thymocytes but not peripheral T lymphocytes had C3 opsonization
We next analyzed by flow cytometry Crry expression on various populations of T cells in the thymus, spleen, and PBMCs in WT and mutant mice. With the use of anti-SCR3/4, we detected Crry expression on thymocytes of all developmental stages as well as on peripheral T and B lymphocytes in Cre−-Crryflox/flox mice (Figure 4). As expected, we observed stage-specific deletion of Crry on thymocytes of both CD4-Cre+-Crryflox/flox and Lck-Cre+-Crryflox/flox mice (Figure 4A,B). Thus, in both mutants, most DN thymocytes were Crry-positive, whereas most CD4 and CD8 SP cells were Crry-negative (Figure 4A,B). A higher percentage of DP thymocytes in Lck-Cre+-Crryflox/flox mice than in CD4-Cre+-Crryflox/flox mice were Crry deficient, consistent with the known earlier expression of the Lck-Cre transgene.29 Importantly, we observed that a substantial proportion of, although not all, Crry-deficient DP and SP thymocytes in the mutant mice were positive for C3 deposition (Figure 4A,B), suggesting that Crry-deficient thymocytes were susceptible to complement activation. Notably, a relatively higher proportion of CD8 SP thymocytes than CD4 SP thymocytes were C3-opsonized, suggesting that Crry deficiency had a greater effect on complement sensitivity of CD8 T cells, which could help to explain why peripheral CD8 T cells were reduced to a greater degree in the mutant mice.
Flow cytometric analysis of Crry expression and C3 deposition on WT and mutant mouse thymocytes and peripheral T and B cells. (A) Analysis of thymocytes in CD4-Cre−-Crryflox/flox and CD4-Cre+-Crryflox/flox mice. (B) Analysis of thymocytes in Lck-Cre−-Crryflox/flox and Lck-Cre+-Crryflox/flox mice. (C,E) Analysis of CD4+ and CD8+ T lymphocytes (C) and B220+ B lymphocytes (E) in CD4-Cre−-Crryflox/flox and CD4-Cre+-Crryflox/flox mice. (D,F) Analysis of CD4+ and CD8+ T lymphocytes (D) and B220+ B lymphocytes (F) in Lck-Cre−-Crryflox/flox and Lck-Cre+-Crryflox/flox mice. In panels E and F, open histogram represents Cre(-) and filled histogram represents Cre(+) genotype (the 2 histograms completely overlap), respectively. The absorbed antibody, α-Crry3/4, was used for all Crry staining.
Flow cytometric analysis of Crry expression and C3 deposition on WT and mutant mouse thymocytes and peripheral T and B cells. (A) Analysis of thymocytes in CD4-Cre−-Crryflox/flox and CD4-Cre+-Crryflox/flox mice. (B) Analysis of thymocytes in Lck-Cre−-Crryflox/flox and Lck-Cre+-Crryflox/flox mice. (C,E) Analysis of CD4+ and CD8+ T lymphocytes (C) and B220+ B lymphocytes (E) in CD4-Cre−-Crryflox/flox and CD4-Cre+-Crryflox/flox mice. (D,F) Analysis of CD4+ and CD8+ T lymphocytes (D) and B220+ B lymphocytes (F) in Lck-Cre−-Crryflox/flox and Lck-Cre+-Crryflox/flox mice. In panels E and F, open histogram represents Cre(-) and filled histogram represents Cre(+) genotype (the 2 histograms completely overlap), respectively. The absorbed antibody, α-Crry3/4, was used for all Crry staining.
Analysis of splenic and PBMC CD4+ and CD8+ T cells showed that the small number of peripheral T cells in the mutant mice were composed of a mixture of Crry-positive and -negative cells (Figure 4C,D). Interestingly, we detected little C3 opsonization on Crry-deficient splenic and PBMC T cells in CD4-Cre+-Crryflox/flox and Lck-Cre+-Crryflox/flox mice (Figure 4C,D). These results suggested that C3-opsonized T cells could not survive outside the thymus and that the remaining peripheral T cells were those that escaped Crry deletion (Crry-positive) and those that did have Crry deletion but nevertheless survived, presumably because of other compensatory mechanism(s) to resist complement attack (Crry-negative, C3-negative). As expected, splenic and circulating B lymphocytes in the mutant mice had normal Crry expression and lacked C3 deposition (Figure 4E,F; data not shown), confirming the tissue-specific nature of CD4-Cre– and Lack-Cre–mediated Crry mutation.
T-cell lymphopenia in CD4-Cre+-Crryflox/flox mice depended on systemic C3
To prove that complement activation on Crry-deficient thymocytes and T cells was directly responsible for peripheral T-cell lymphopenia, we adoptively transferred BM stem cells from CD4-Cre+- or CD4-Cre−-Crryflox/flox mice to irradiated C3−/− mice. After 2 months, we analyzed T-cell populations in the thymus, spleen, lymph nodes, and PBMCs of the BM chimeras. We detected no difference between the 2 types of chimeras in thymocyte or peripheral T-cell number or percentage (Figure 5A). Furthermore, although, as expected, thymocytes in CD4-Cre+-Crryflox/flox/C3−/− BM chimeras were Crry negative, they incurred no C3 deposition (Figure 5B). These data established that systemic complement activity caused the observed peripheral T-cell lymphopenia in the mutant mice.
T-cell lymphopenia in conditional Crry gene knockout mice depends on systemic complement. (A) Bone marrow cells from CD4-Cre−-Crryflox/flox and CD4-Cre+-Crryflox/flox mice were transplanted into lethally irradiated C3−/− recipients. After 2 months, total thymus cellularity and CD4+, CD8+ T-cell numbers and percentages in the thymi, spleens, lymph nodes, and blood of the 2 types of chimeras (n = 4 per group) were analyzed as in Figure 4. No difference was detected between the 2 types of chimeras. Values are mean plus or minus SEM. (B) Flow cytometry analysis of Crry expression and C3 deposition on thymocytes of chimera mice. CD4-Cre−-Crryflox/flox → C3−/− chimeras had normal Crry expression on thymocytes, whereas CD4-Cre+-Crryflox/flox → C3−/− chimeras had no thymocyte Crry expression nor C3 deposition. (C) FACS analysis of Crry and DAF expression on thymocytes (left) and splenic CD4+ T cells (right) in CD4-Cre−-Crryflox/flox (denoted as −) and CD4-Cre+-Crryflox/flox (denoted as +) mice. DAF is not expressed on thymocytes, and average DAF level on surviving splenic CD4+ T cells in CD4-Cre+-Crryflox/flox mice (denoted as +) was higher than that on splenic CD4+ T cells of CD4-Cre−-Crryflox/flox mice (denoted as −). (D) FACS analysis of Crry and DAF expression on thymocytes (left) and splenic CD4+ T cells (right) in CD4-Cre−-Crryflox/flox → C3−/− (denoted as −) and CD4-Cre+-Crryflox/flox → C3−/− (denoted as +) chimeras. No difference in DAF expression was detected on splenic CD4+ T cells of the 2 groups of mice in this case.
T-cell lymphopenia in conditional Crry gene knockout mice depends on systemic complement. (A) Bone marrow cells from CD4-Cre−-Crryflox/flox and CD4-Cre+-Crryflox/flox mice were transplanted into lethally irradiated C3−/− recipients. After 2 months, total thymus cellularity and CD4+, CD8+ T-cell numbers and percentages in the thymi, spleens, lymph nodes, and blood of the 2 types of chimeras (n = 4 per group) were analyzed as in Figure 4. No difference was detected between the 2 types of chimeras. Values are mean plus or minus SEM. (B) Flow cytometry analysis of Crry expression and C3 deposition on thymocytes of chimera mice. CD4-Cre−-Crryflox/flox → C3−/− chimeras had normal Crry expression on thymocytes, whereas CD4-Cre+-Crryflox/flox → C3−/− chimeras had no thymocyte Crry expression nor C3 deposition. (C) FACS analysis of Crry and DAF expression on thymocytes (left) and splenic CD4+ T cells (right) in CD4-Cre−-Crryflox/flox (denoted as −) and CD4-Cre+-Crryflox/flox (denoted as +) mice. DAF is not expressed on thymocytes, and average DAF level on surviving splenic CD4+ T cells in CD4-Cre+-Crryflox/flox mice (denoted as +) was higher than that on splenic CD4+ T cells of CD4-Cre−-Crryflox/flox mice (denoted as −). (D) FACS analysis of Crry and DAF expression on thymocytes (left) and splenic CD4+ T cells (right) in CD4-Cre−-Crryflox/flox → C3−/− (denoted as −) and CD4-Cre+-Crryflox/flox → C3−/− (denoted as +) chimeras. No difference in DAF expression was detected on splenic CD4+ T cells of the 2 groups of mice in this case.
To understand why certain Crry-deficient T cells in CD4-Cre+-Crryflox/flox mice managed to avoid C3 opsonization and survived in the periphery (Figure 4C), we analyzed by flow cytometry the expression level of DAF, another membrane complement regulator, on thymocytes and peripheral T cells. Figure 5C shows that DAF is normally expressed at a much lower level on thymocytes than on splenic T cells in WT mice. Significantly, we found that splenic T cells from CD4-Cre+-Crryflox/flox mice had elevated DAF expression than those from CD4-Cre−-Crryflox/flox mice (Figure 5C right; mean fluorescence intensity, 149 and 86, respectively). However, this difference in DAF expression was not observed on splenic T cells between CD4-Cre+-Crryflox/flox/C3−/− and CD4-Cre−-Crryflox/flox/C3−/− BM chimeras (Figure 5D right; mean fluorescence intensity, 130 and 130, respectively). Thus, up-regulation of DAF may have compensated for the lack of Crry and contributed to complement resistance and viability of the residual Crry-deficient T cells in CD4-Cre+-Crryflox/flox mice.
Crry-deficient T cells survived normally in the thymus despite complement attack
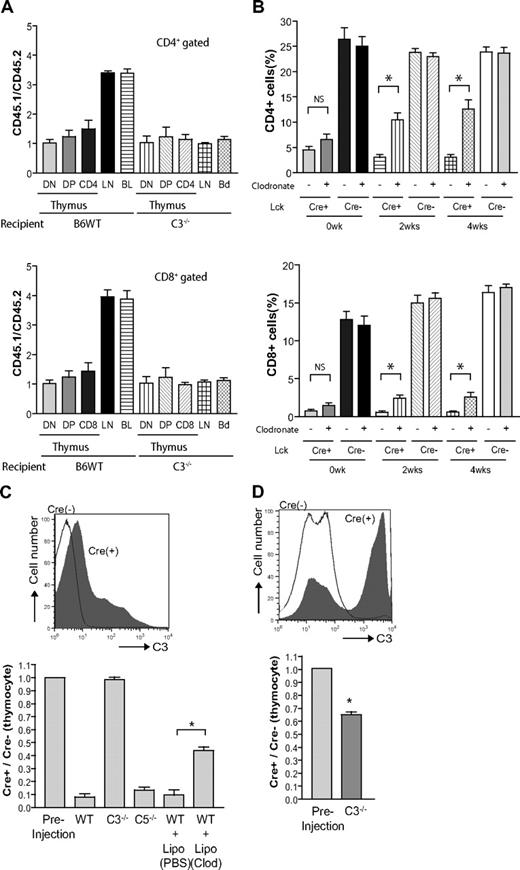
As described in Figures 3 and 4, despite evidence of complement activation on thymocytes and severe peripheral T-cell lymphopenia, we observed normal thymic cellularity and composition in the mutant mice. However, this observation did not conclusively establish that Crry-deficient thymocytes were resistant to intrathymic destruction because normal cellularity and composition could have been maintained by increased lymphogenesis. To test the latter hypothesis, we transplanted mixed (1:1 ratio) bone marrow cells from WT (CD45.1) and CD4-Cre+-Crryflox/flox (CD45.2) mice into irradiated WT and C3−/− mice. As shown in Figure 6A, we observed marked enrichment of WT (CD45.1) T cells in the lymph nodes and PBMCs but not the thymus of the reconstituted WT recipients. As expected, no enrichment of WT T cells was observed in reconstituted C3−/− recipients, either in the periphery or in the thymus (Figure 6A). These results clearly established that peripheral but not thymic Crry-deficient T cells were susceptible to complement-dependent elimination. Consistent with these data, although we detected by immunohistochemistry C3-positive cells in the thymi of CD4-Cre+-Crryflox/flox and Lck-Cre+-Crryflox/flox mice, we observed no gross abnormality in the size or structure of the mutant mouse thymi (Figure S3).
Differential survival of thymic and peripheral T cells in the mutant mice and the underlying mechanism. (A) Bone marrow cells from Lck-Cre+-Crryflox/flox mice (CD45.2) and WT mice (CD45.1) were mixed at 1:1 ratio and transplanted into lethally irradiated WT or C3−/− recipients (n = 4 per group). After 2 months, the ratios of CD45.1/CD45.2 thymocytes (DN, DP, CD4 single positive, CD8 single positive), and peripheral (lymph node and blood) CD4+ or CD8+ T cells were determined by flow cytometry. CD45.1 T cells were enriched in the lymph nodes (LNs) and blood (BL) but not in the thymus of WT recipients. As expected, no enrichment was observed at any sites in C3−/− recipients. (B) Lck-Cre+-Crryflox/flox and Lck-Cre−-Crryflox/flox mice (n = 4 per group) were treated twice weekly with clodronate liposome (+) or control liposome (−) for 4 weeks, and blood CD4+ and CD8+ T-cell counts were monitored by flow cytometry. Clodronate liposome but not control liposome treatment time dependently increased blood CD4+ and CD8+ T-cell counts (NS, not statistically significant; *P < .05, Student t test). Clodronate-liposome treatment had no significant effect on blood T-cell counts in Lck-Cre−-Crryflox/flox mice. (C) Lck-Cre+-Crryflox/flox and Lck-Cre−-Crryflox/flox mouse thymocytes were labeled with CFSE, mixed at 1:1 ratio, and injected into different recipient mice (n = 2 per group). The ratios of Cre+/Cre− thymocytes (determined with the use of Crry as a marker among CFSE-positive cells) in the spleens of the recipient mice were determined 1 hour after cell injection. Some WT mice received clodronate-liposome or clodronate-PBS (control) treatment 24 hours before thymocyte transfer. (D) Same experimental procedure as in panel C except that the thymocytes were first incubated in 5% WT mouse serum at 37°C for 45 minutes before adoptive transfer. The top FACS histograms in panels C and D show C3 deposition levels on the thymocytes with (D) or without (C) ex vivo incubation in WT mouse serum. All values shown are mean plus or minus SEM.
Differential survival of thymic and peripheral T cells in the mutant mice and the underlying mechanism. (A) Bone marrow cells from Lck-Cre+-Crryflox/flox mice (CD45.2) and WT mice (CD45.1) were mixed at 1:1 ratio and transplanted into lethally irradiated WT or C3−/− recipients (n = 4 per group). After 2 months, the ratios of CD45.1/CD45.2 thymocytes (DN, DP, CD4 single positive, CD8 single positive), and peripheral (lymph node and blood) CD4+ or CD8+ T cells were determined by flow cytometry. CD45.1 T cells were enriched in the lymph nodes (LNs) and blood (BL) but not in the thymus of WT recipients. As expected, no enrichment was observed at any sites in C3−/− recipients. (B) Lck-Cre+-Crryflox/flox and Lck-Cre−-Crryflox/flox mice (n = 4 per group) were treated twice weekly with clodronate liposome (+) or control liposome (−) for 4 weeks, and blood CD4+ and CD8+ T-cell counts were monitored by flow cytometry. Clodronate liposome but not control liposome treatment time dependently increased blood CD4+ and CD8+ T-cell counts (NS, not statistically significant; *P < .05, Student t test). Clodronate-liposome treatment had no significant effect on blood T-cell counts in Lck-Cre−-Crryflox/flox mice. (C) Lck-Cre+-Crryflox/flox and Lck-Cre−-Crryflox/flox mouse thymocytes were labeled with CFSE, mixed at 1:1 ratio, and injected into different recipient mice (n = 2 per group). The ratios of Cre+/Cre− thymocytes (determined with the use of Crry as a marker among CFSE-positive cells) in the spleens of the recipient mice were determined 1 hour after cell injection. Some WT mice received clodronate-liposome or clodronate-PBS (control) treatment 24 hours before thymocyte transfer. (D) Same experimental procedure as in panel C except that the thymocytes were first incubated in 5% WT mouse serum at 37°C for 45 minutes before adoptive transfer. The top FACS histograms in panels C and D show C3 deposition levels on the thymocytes with (D) or without (C) ex vivo incubation in WT mouse serum. All values shown are mean plus or minus SEM.
Macrophage depletion ameliorated T-cell lymphopenia in Lck-Cre+-Crryflox/flox mice
To determine whether C3-opsonzied T cells were eliminated in the periphery by complement receptor–mediated phagocytosis, we depleted macrophages in Lck-Cre+-Crryflox/flox mice by clodronate liposome treatment. Mice were treated with clodronate liposome or control liposome every 4 days for 4 weeks, and their PBMC T-cell numbers were analyzed before and after treatment. As shown in Figure 6B, we observed a time-dependent increase in PBMC T lymphocytes in clodronate liposome–treated but not control liposome–treated Lck-Cre+-Crryflox/flox mice. This result provided evidence that lymphopenia in Lck-Cre+-Crryflox/flox mice, and in all likelihood in CD4-Cre+-Crryflox/flox mice too, was caused at least in part by macrophage- and complement receptor–mediated phagocytosis. To begin to define the complement receptors (CRs) involved in this process, we crossed CD4-Cre+-Crryflox/flox mice with mice deficient in CRIg, a recently discovered complement receptor primarily expressed on Kupfer cells.22 We detected no difference between CRIg-sufficient and CRIg-deficient CD4-Cre+-Crryflox/flox mice in their T-cell populations (Figure S4), suggesting that either CRIg was not involved or that there is redundancy between CRIg and other complement receptors.
Mechanism for differential survival of thymic and peripheral T cells in the mutant mice
The differential survival of thymic and peripheral T cells in the mutant mice suggested that thymocytes might be less accessible to complement factors. Furthermore, the failure to completely correct T-cell lymphopenia in clodronate-treated mice indicated the potential involvement of the lytic complement pathway. To test these possibilities, we labeled thymocytes from Lck-Cre+-Crryflox/flox and Lck-Cre−-Crryflox/flox mice, mixed them at 1:1 ratio, and introduced them through intravenous injection into WT, C3−/−, and C5−/− mice. As shown in Figure 6C, we confirmed C3 deposition on Lck-Cre+-Crryflox/flox but not Lck-Cre−-Crryflox/flox thymocytes before injection and found the former to be preferentially eliminated in WT and C5−/− mice but not in C3−/− mice. Consistent with results shown in Figure 6B, treatment of WT recipient mice with clodronate partially prevented Lck-Cre+-Crryflox/flox thymocytes from accelerated elimination (Figure 6C). These results suggested that (1) Lck-Cre+-Crryflox/flox mouse T cells were eliminated in the periphery by a C3- and macrophage-mediated but C5- and lytic pathway-independent mechanism, and (2) the level of intrathymic C3 opsonization on Lck-Cre+-Crryflox/flox thymocytes was not sufficient to enhance phagocytosis in the absence of additional complement opsonization in the periphery. Supporting the latter conclusion, we observed that when Lck-Cre+-Crryflox/flox thymocytes were incubated with WT mouse serum ex vivo, they incurred substantially higher C3 opsonization and were subjected to accelerated elimination when introduced into C3−/− mice (Figure 6D). Thus, the lytic pathway was not responsible for the T-cell lymphopenia phenotype, and the underlying mechanism for normal survival of mutant T cells in the thymus was insufficient complement activity.
Discussion
By conditional gene targeting, we have circumvented the embryonic lethality phenotype of global Crry gene deficiency16 and have shown an essential role of Crry in T-cell survival. Several lines of evidence supported the conclusion that T-cell lymphopenia in the mutant mice was caused by complement attack of thymocytes and peripheral T cells, followed by complement receptor–mediated phagocytosis. First, a large proportion of Crry-deficient thymocytes incurred C3 deposition. Second, bone marrow transfer from the conditional knockout mice to irradiated C3−/− mice rescued the T-cell lymphopenia phenotype. Third, macrophage depletion in the knockout mice time-dependently increased peripheral T-cell counts.
It is interesting that despite susceptibility to complement attack as evidenced by C3 opsonization, developing Crry-deficient T cells were resistant to intrathymic elimination. We observed normal thymic cellularity in the mutant mice, and our mixed bone marrow transplantation experiment further confirmed normal survival of Crry-deficient T cells in the thymus. We considered and examined several potential mechanisms for the differential survival of thymic and peripheral T cells. First, Crry-deficient T cells might be removed by terminal complement-mediated lysis in the periphery, whereas such lytic activity could be limiting or absent in the thymus. However, the thymocyte transfer experiment with C5−/− mice showed that C5 and the terminal complement pathway were not involved. Instead, multiple lines of evidence pointed to a C3- and macrophage-dependent mechanism. The fact that clodronate treatment did not completely rescue the lymphopenia phenotype suggested the involvement of phagocytic cells that were resistant to clodronate depletion. Second, thymic macrophages, although known to be active at phagocytosing apoptotic thymocytes,30,31 might be devoid of the relevant CR(s) and were therefore incapable of ingesting C3-opsonized Crry-deficient thymocytes. The CR(s) responsible for peripheral lymphopenia remains to be identified, but it appeared that the recently described CRIg, a Kupffer cell-restricted CR,22 was not critical for this process. Although we have not excluded differential CR expression and/or phagocytic activity among thymic and peripheral macrophages, our data strongly suggested a difference in intrathymic and peripheral complement activity as the most likely explanation for the observed phenotype. Indeed, we observed that not all Crry-deficient thymocytes in CD4-Cre+-Crryflox/flox and Lck-Cre+-Crryflox/flox mice were positive for C3 opsonization, suggesting either limited intrathymic complement activity or unequal access to the complement system by Crry-deficient thymocytes. The finding that T-cell lymphopenia was rescued in CD4-Cre+-Crryflox/flox → C3−/− bone marrow chimeras also implied that systemic rather than bone marrow–derived complement proteins were responsible for the phenotype.
The surviving T cells in the periphery appeared to consist of 2 populations, those that escaped Cre-mediated Crry mutation (remained Crry-positive) and those that did have Crry mutation but expressed a higher level of DAF. Presumably, the level of DAF expressed on the latter group of cells was sufficient to compensate for the lack of Crry, although the possibility cannot be excluded that they had additional mechanisms of complement resistance (eg, higher membrane sialic acid content to favor interaction with the plasma complement inhibitor factor H). Both Crry and DAF are membrane-anchored proteins that inhibit complement activation through regulation of the C3 convertases.5,6 DAF promotes the decay of C3 convertases, whereas Crry has both decay-accelerating activity and cofactor activity for factor I.11 Earlier studies led to the speculation that Crry may be more critical than DAF at inhibiting alternative pathway complement activation.9,18,21 The present data support the notion that the relative importance of Crry and DAF on a given cell type largely depends on their levels of expression. The fact that most Crry-deficient T cells could not survive in the periphery suggested that DAF expression was low and not sufficient for lymphocyte protection. Conversely, the finding that DAF knockout mice did not develop T-cell lymphopenia32,33 implied that Crry is highly expressed on T cells and acted as the main membrane regulator.
It is notable that, despite evidence of intrathymic complement activation, we observed no inflammatory injury in the thymus. Complement activation generates potent anaphylatoxins C3a and C5a, as well as the membrane attack complex, and is typically associated with inflammation and tissue destruction.1-3 We detected no damage to the thymic architecture by light microscopy and observed no increase in neutrophil infiltration by immunohistochemistry in the thymi of CD4-Cre+-Crryflox/flox and Lck-Cre+-Crryflox/flox mice (data not shown). However, the finding of peripheral T-cell lymphopenia but normal thymic cellularity in the mutant mice is consistent with earlier observations that neutralizing mAbs effectively depleted peripheral CD4+ T cells but had minimal effect on thymic T-cell populations.34 Collectively, these results suggested that the thymus is a complement-privileged site, a conclusion that may have significant implications for complement-based antitumor therapies. For example, the anti-CD20 mAb, rituximab, has been widely used in the treatment of human non-Hodgkin lymphomas, and there is experimental evidence to suggest that complement-mediated tumoricidal activity contributes to its therapeutic efficacy.35-37 Our findings here imply that, to minimize the risk of relapses, adjuvant treatment may be required in antibody therapy of metastatic lymphomas to eradicate tumor cells infiltrated into complement-privileged sites such as the thymus.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Dr J. Lambris for the anti-Crry antibody, Dr M. van Lookeren Campagne for the CRIg−/− mice, and Dr R. Schwendener for clodronate liposomes.
This work was supported by the National Institutes of Health (grants AI-44970, AI-49344, and AI-63288) and the National Multiple Sclerosis Society (grant RG3671-A-1).
National Institutes of Health
Authorship
Contribution: T.M. designed and performed the research, analyzed data, and contributed to the writing of the paper; L.Z., Y.K., and D.K. performed research and analyzed data; A.B. contributed to the research design and data analysis; and W.-C.S. designed the research, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Wen-Chao Song, Institute for Translational Medicine and Therapeutics and Department of Pharmacology, University of Pennsylvania School of Medicine, Rm 1254 BRBII/III, 421 Curie Blvd, Philadelphia, PA 19104; e-mail: Songwe@upenn.edu.





This feature is available to Subscribers Only
Sign In or Create an Account Close Modal