The lymphatic vessel is a major conduit for immune cell transport; however, little is known about how lymphatic vessels regulate immune cell trafficking and how lymphatic vessels themselves respond to inflammation. Toll-like receptor 4 (TLR4) plays a central role in lipopolysaccharide (LPS)–induced inflammation, but the role of TLR4 in lymphatic endothelial cells (LECs) is poorly understood. Here, we found that LECs express high amounts of TLR4 in the intracellular region, and that the TLR4 of LECs is the main mediator of nuclear factor–κB (NF-κB) activation by LPS. LPS-TLR4 signaling in LECs resulted in the production of various chemokines for chemotaxis of macrophage. In addition, TLR4 in LECs actively contributed to the recruitment of macrophages to the draining lymphatic vessel. Furthermore, the macrophages that infiltrated into the lymphatic vessel induced lymphangiogenesis by secreting lymphangiogenic growth factors. These phenomena were largely attenuated not only in the mice defective in TLR4 signaling but also in the chimeric mice defective in TLR4 signaling that were recipients for bone marrow transplantation from normal TLR4-signaling mice. In conclusion, TLR4 in LECs plays an essential role in LPS-induced inflammatory lymphangiogenesis by chemotactic recruitment of macrophages.
Introduction
Toll-like receptor 4 (TLR4) is a pattern recognition receptor (PRR) that initiates the innate immune response against Gram-negative bacteria infection by recognizing lipopolysaccharide (LPS), the prominent component of the bacteria cell wall.1 The role of TLR4 and its signaling pathway has been extensively studied in macrophages, as these cells abundantly express TLR4 and are crucial players in LPS-induced immune responses.2,3 In response to infection by Gram-negative bacteria, resident macrophages recognize LPS via surface TLR4,1 process the pathogen, migrate to the regional draining lymph node (DLN), and present the antigen to T lymphocytes, resulting in induction of the adaptive immune response.4,–6 During this process, TLR4 activates nuclear factor–κB (NF-κB), a canonical transcription factor that mediates various inflammatory responses, which results in the activation of a variety of proinflammatory genes in macrophages.4,7,–9
TLR4 is also expressed in nonimmune cells, including those that constitute the vascular system.10,11 The lymphatic vessel has a specialized structure distinct from blood vessels in several aspects. Lymphatic endothelial cells (LECs), a major cell type in lymphatic vessels, express specific markers, respond to different growth factors, and constitute blind-ended loose initial lymphatic capillary.12,,–15 Moreover, the function of the lymphatic vessel is unique in that it absorbs macromolecules, transports interstitial fluid, and orchestrates proper trafficking and recirculation of immune cells to the regional lymph node, where the adaptive immune response occurs.16,17 However, despite the demonstrated function of the lymphatic vessel as a major player in several immune responses, the details on the expression level, signaling pathway, and precise role of TLR4 in LECs remain unknown.
De novo lymphangiogenesis occurs mainly by proliferation/migration of LECs from pre-existing lymphatic vessels.13 Recent reports18,,–21 indicate that lymphangiogenesis occurs largely by the paracrine effect of abundantly infiltrated macrophages, suggesting a crucial role of macrophages for lymphangiogenesis. However, little is known about the mechanism by which macrophages are recruited to the remodeling lymphatic vessel, particularly in the Gram-negative bacteria-induced inflammatory condition. Although recent papers have reported the expression of TLR4 in LECs,22,23 the in vivo role of TLR4 in LECs is poorly understood, not only in the aspect of immune cell transport but also in the aspect of inflammatory lymphangiogenesis.
In this study, we investigated the role of TLR4 in LECs using the LPS-induced peritoneal inflammation model that mimics peritoneal Gram-negative bacteria infection. We compared LPS-induced lymphangiogenesis between C3H/HeN (HeN, normal TLR4 signaling) and C3H/HeJ (HeJ, defective TLR4 signaling) mice.24 To define the role of LECs specific TLR4 during LPS-induced lymphangiogenesis, we generated chimeric “N/J” (bone marrow cells [BMCs] of HeJ was replaced with BMCs from HeN) mice10 and, as a control, “N/N” (BMCs of HeN was replaced with BMCs from HeN) mice. We also investigated the role of TLR4 in LECs on macrophage chemotaxis using the in vitro transwell migration assay and the adoptive cell transfer experiment in vivo.25 Our results indicate that TLR4 in LECs play a key role in LPS-induced lymphangiogenesis, mainly by chemotactic recruitment of macrophages to the lymphatic vessels.
Methods
Mice and LPS treatment
Pathogen-free C3H/HeN (HeN, normal TLR4 signaling mice) and C3H/HeJ (HeJ, mice with a TLR4 gene missense mutation, thus defective in TLR4 signaling) mice24 were purchased from SLC (Shizuoka, Japan). TLR4 knockout (KO) mice in C57BL/6 background3 were donated by Dr Seung-Hyun Han (Seoul National University; originally provided by S. Akira, Osaka University, Japan). C57BL/6 mice were purchased from the Jackson Laboratory (Bar Harbor, ME). κB-lacZ mice26 were transferred from the Institute Pasteur (Paris, France) to the KAIST Animal Facility; these mice harbor a fragment derived from the p105 promoter with 3 functional NF-κB responsive sites upstream of the gene encoding Escherichia coli β-galactosidase with a nuclear localization sequence. Animal care and experimental procedures were performed under approval of the Animal Care Committees of KAIST. All mice were maintained in a specific pathogen–free facility and fed a standard normal diet (PMI LabDiet; Purina Mills, St Louis, MO) ad libitum with free access to water. Male mice aged 8 to 10 weeks (21-25 g) were used for experiments. For LPS-induced peritoneal inflammation, the indicated dose of LPS (from E coli 0111:B4; Sigma-Aldrich, St Louis, MO) in 200 μL of phosphate-buffered saline (PBS) was injected daily into the peritoneal cavity for the indicated time. As a control, 200 μL of PBS was injected in the same manner. As a positive control, human VEGF-A (10 μg/day; R&D Systems, Minneapolis, MN) in 200 μL PBS was injected in the same manner.
Cell culture and LPS treatment
HEK-293A and THP-1 cells were purchased from ATCC (Manassas, VA) and maintained in Dulbecco modified Eagle medium (DMEM) and RPMI 1640 medium, respectively, with 10% FBS. BECs (primary blood microvascular endothelial cells derived from human adult dermis) and LECs (primary lymphatic microvascular endothelial cell derived from human adult dermis) were purchased from Cambrex (East Rutherford, NJ) and maintained in endothelial cell basal medium-2 with growth supplements (EBM-2 MV). Cells cultured through passage 4 to 6 were used for the in vitro experiments. LPS was diluted with the appropriate basal culture media containing 2% fetal bovine serum (FBS) without other supplements and added to the cells, which were preincubated in serum-deprived media for 8 hours. Mice LECs were isolated from the peritoneal lymphangioma model according to the previous report,27 and were maintained and cultured in EBM-2 MV up to passage 3. The purity of mice LEC was more than 90%, which was verified by double immunofluorescence staining of Prox-1 and CD31.
TLR4 knock-down by siRNA transfection
LECs (approximately 50% confluence in a 6-well plate) were transfected with 180 pmol of TLR4-specific siRNA (Santa Cruz Biotechnology, Santa Cruz, CA), 180 pmol of nonspecific scramble (sc) RNA (Santa Cruz Biotechnology), or control dilution buffer using X-tremeGene siRNA transfection reagent (Roche Applied Science, Indianapolis, IN). After 48 hours of transfection, cells were used for experiments as indicated.
MACS and RT-PCR
For enrichment of CD11b+ cells infiltrated into diaphragms, the diaphragms were incubated with collagenase type II (Sigma-Aldrich) for 1 hour at 37°C, and the collected single cells were incubated in the Blood Cell lysing buffer (Sigma-Aldrich) for 10 minutes. After several washes in cold PBS, CD11b+ cells were enriched using anti-mouse CD11b antibody-coupled MicroBeads (Miltenyi Biotec, Auburn, CA) and a magnetic-activated cell sorter (MACS; Miltenyi Biotec) according to the manufacturer's instructions. For reverse transcriptase–polymerase chain reaction (RT-PCR), total RNA from each sample was extracted using the Total RNA Isolation System (Promega, Madison, WI) according to the manufacturer's instructions. Each cDNA was generated with the Reverse Transcription System (Promega), and semi-quantitative and quantitative PCR were performed using Taq DNA polymerase (Bioneer, Daejon, Korea) and iQTM SYBR Green Supermix (Bio-Rad, Hercules, CA) with the appropriate primers (Table S1, available on the Blood website; see the Supplemental Materials link at the top of the online article). Gene expression was normalized against levels of human or mouse GAPDH.
Immunostaining and morphometric analysis
Immunostaining, morphometric analysis, and flow cytometric analysis were performed as described in Document S1.
Flow cytometric analysis
PE-conjugated anti–human TLR4 (eBioscience, San Diego, CA), PerCP Cy5.5–conjugated anti–mouse CD11b, FITC-conjugated anti–mouse CD11b, PerCP Cy5.5 conjugated anti–mouse F4/80, PE-conjugated anti–mouse CD11c (all from BD Pharmingen, San Diego, CA) antibodies and FITC-LPS (Sigma-Aldrich) were used for flow cytometric analysis. For surface staining, cells were incubated with antibody without permeabilization. For intracellular staining, cells were treated with BD FACS Permeabilizing Solution 2 (BD Pharmingen) before incubation with the antibody. The BD FACSCalibur machine (BD Pharmingen) and FlowJo software (TreeStar, Ashland, OR) were used for signal uptake and data analysis.
Immunoblotting and ELISA
Mouse anti-human phospho–IκB-α antibody, rabbit anti–human IκB-α antibody (Cell Signaling Technology, Danvers, MA), anti–human β-actin antibody (Sigma-Aldrich) were used for immunoblotting. Quantikine enzyme-linked immunosorbent assay (ELISA) kits (R&D Systems, Minneapolis, MN) were used to measure human CCL2 (MCP-1) and human CCL5 (RANTES) according to the manufacturer's protocol.
Chemotactic migration assay
THP-1 monocyte cells (1 × 106) resuspended in 0.4 mL of serum-free endothelial cell basal media (EBM) were seeded in the Hanging Cell Culture Inserts (pore size 5 μm; Millipore, Billerica, MA). A total of 1 mL of culture supernatant from LECs stimulated with the indicated dose of LPS for 48 hours or from LECs stimulated with 500 ng/mL of LPS for 48 hours in the presence of TLR4 siRNA (180 pM), scRNA (180 pM), or control dilution buffer was added into the space between the hanging insert and each well of a 12-well plate. After 6 hours, the number of cells that transmigrated outside of the hanging inserts toward the culture supernatant from LECs were counted with the BD FACSCalibur machine. Migration was determined as a percentage based on the number of transmigrated cells per the initially seeded cells.
Adoptive cell transfer and macrophage depletion
To harvest peritoneal macrophages, 1 mL of 3% thioglycollate was injected intraperitoneally into HeN mice. After 3 days of injections, the peritoneal macrophages were collected by peritoneal washing with PBS. After red blood cell (RBC) lysis, the cells were tagged with CFSE green dye (Molecular Probes [now Invitrogen; Carlsbad, CA]) and counted. CFSE tagged cells (∼2 × 107) were injected into the peritoneal cavity of HeN and HeJ mice pretreated with intraperitoneal injection of LPS (0.5 mg/kg). For depletion of endogenous macrophages, mice were either treated with intraperitoneal injections of clodronate liposome (CDL; 50 mg/kg; a kind gift from Dr Reto A. Schwendener, University of Zurich, Switzerland) as previously described28 3 days prior to the adoptive transfer experiment or treated with CDL (25 mg/kg) every 3 days from the beginning of 7 days of LPS (0.5 mg/kg per day) injection experiment. Empty control liposome (CL) was injected as a control.
Generation of N/N, N/J, J/N and J/J chimeric mice by bone marrow transplantation
Bone marrow cells were harvested from the femurs and tibias of HeN or HeJ mice by flushing with ice-cold Dulbecco PBS (Sigma-Aldrich). The recipient mice (8-week-old HeN and HeJ) were lethally irradiated twice (5 Gy) with a 3-hour interval using a gamma irradiator (Gammacell 3000; MDS Nordion, Ottawa, ON). After irradiation, approximately 8 × 106 bone marrow cells derived from the HeN or HeJ mice were injected intravenously into HeN and HeJ mice to generate N/N, N/J, J/N and J/J chimeric mice. The mice were bred in microisolator cages for 2 months prior to the experiments.
Statistics
Values are presented as means plus or minus standard deviation (SD). Significant differences between the means were determined by analysis of variance followed by the Student-Newman-Keuls test. Statistical significance was set at P values less than .05.
Results
TLR4 signaling is essential for LPS-induced lymphangiogenesis and CD11b+ macrophage infiltration in the peritoneal side of diaphragm
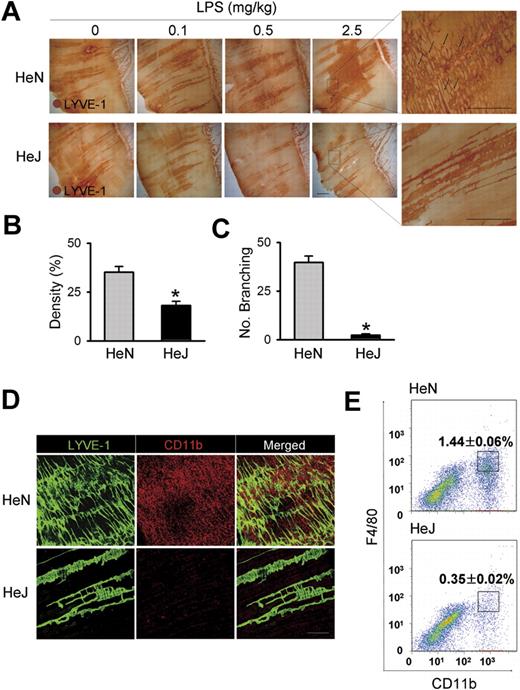
To assess the function of TLR4 signaling on lymphatic vessels, we compared LPS-induced inflammatory lymphangiogenesis in mice with normal (C3H/HeN) and defective (C3H/HeJ) TLR4 signaling.24 Daily intraperitoneal injections of LPS for 7 days induced robust and dose-dependent increases of lymphatic vessel density in the peritoneal side of diaphragmatic muscle of HeN mice (Figure 1A,B). To investigate whether the increased lymphatic density resulted from proliferation of LECs, we examined the number of lymphatic vessel endothelial hyaluronan receptor 1 (LYVE-1) and PH3 (a nuclear protein of dividing cells) double-positive LECs. A greater number of LYVE-1+/PH3+ LECs were observed in the enlarged lymphatic vessels of mice treated with LPS for 7 days (Figure S1). Moreover, this LPS-induced lymphangiogenesis was characterized by an increased number of lymphatic capillary branching (Figure 1C). These findings indicate that the LPS-induced increased lymphatic densities mainly resulted from LEC proliferation and active branching process, which are major cellular processes of lymphangiogenesis.13,16,19,29 In contrast, no notable changes in the density and structure of the lymphatic vessels in HeJ mice in response to LPS-induced inflammation were observed (Figure 1A). In comparison, daily intraperitoneal injections of VEGF-A (10 μg/day) for 7 days increased lymphatic densities and the amount of lymphatic capillary branching in both HeN and HeJ mice without any significant differences (Figure S2), indicating induction of lymphangiogenesis in response to lymphangiogenic growth factor is indistinguishable between the HeN and HeJ mice. Because CD11b+ macrophages are closely involved in inflammatory lymphangiogenesis,20,30,31 we examined the extent of CD11b+ macrophage infiltration in the same region. A marked infiltration of CD11b+ macrophages was detected adjacent to the diaphragmatic lymphatic vessels in HeN mice, whereas only a few CD11b+ macrophages were detected in HeJ mice (Figure 1D). Flow cytometric analysis revealed that CD11b+/F4/80+ macrophages that infiltrated into the diaphragm constituted 1.44% of the total number of cells in the diaphragm of HeN mice compared with 0.35% in HeJ mice (Figure 1E). TLR4 KO mice (C57BL/6 background)3 showed similar largely attenuated responses in LPS-induced lymphangiogenesis and CD11b+ macrophage infiltration in the peritoneal side of the diaphragm (data not shown). These data indicate that TLR4 signaling is essential for LPS-induced lymphangiogenesis and adjacent CD11b+ macrophage infiltration to the lymphatic vessels in the peritoneal side of the diaphragm.
Comparison of LPS-induced lymphangiogenesis in HeN and HeJ mice and CD11b+ macrophage infiltration in the peritoneal side of diaphragm. (A) HeN and HeJ mice were treated intraperitoneally with the indicated doses of LPS for 7 days, and diaphragms were immunostained for LYVE-1 (brown) and visualized with DAB. Representative images of LYVE-1+ lymphatic vessels and lymphatic branching (black arrows) in the peritoneal side of diaphragm muscle. Right panels are higher magnifications of the black dotted rectangle and show lymphatic branching (n = 4). Scale bars indicate 300 μm. (B-E) HeN and HeJ mice were treated with LPS (0.5 mg/kg/day) intraperitoneally for 7 days. (B,C) Densities of LYVE-1+ diaphragmatic lymphatic vessels were measured in each given area (3.64 mm2); values were presented as a percentage per each area (n = 4). Numbers of LYVE-1+ lymphatic branching exceeding 50 μm in a given area (1 mm2) were counted and presented as the actual number per field (n = 4). Bars represent means plus or minus SD. *P < .05 versus HeN. (D) Diaphragms were double immunostained for LYVE-1 (green) and CD11b (red) and visualized with fluorescent dyes. Note the robust infiltration of CD11b+ macrophages in the diaphragmatic lymphatic vessels of HeN but not in HeJ mice (n = 3). Scale bars indicate 200 μm. (E) Whole diaphragms were harvested from HeN and HeJ mice, digested into single cells, and analyzed by flow cytometry. The number of CD11b+/F4/80+ macrophages that infiltrated into the diaphragm was presented as a percentage of the total cell number in the whole diaphragm. Data are presented as means plus or minus SD. Information on immunostaining and morphometric analysis is available in Document S1.
Comparison of LPS-induced lymphangiogenesis in HeN and HeJ mice and CD11b+ macrophage infiltration in the peritoneal side of diaphragm. (A) HeN and HeJ mice were treated intraperitoneally with the indicated doses of LPS for 7 days, and diaphragms were immunostained for LYVE-1 (brown) and visualized with DAB. Representative images of LYVE-1+ lymphatic vessels and lymphatic branching (black arrows) in the peritoneal side of diaphragm muscle. Right panels are higher magnifications of the black dotted rectangle and show lymphatic branching (n = 4). Scale bars indicate 300 μm. (B-E) HeN and HeJ mice were treated with LPS (0.5 mg/kg/day) intraperitoneally for 7 days. (B,C) Densities of LYVE-1+ diaphragmatic lymphatic vessels were measured in each given area (3.64 mm2); values were presented as a percentage per each area (n = 4). Numbers of LYVE-1+ lymphatic branching exceeding 50 μm in a given area (1 mm2) were counted and presented as the actual number per field (n = 4). Bars represent means plus or minus SD. *P < .05 versus HeN. (D) Diaphragms were double immunostained for LYVE-1 (green) and CD11b (red) and visualized with fluorescent dyes. Note the robust infiltration of CD11b+ macrophages in the diaphragmatic lymphatic vessels of HeN but not in HeJ mice (n = 3). Scale bars indicate 200 μm. (E) Whole diaphragms were harvested from HeN and HeJ mice, digested into single cells, and analyzed by flow cytometry. The number of CD11b+/F4/80+ macrophages that infiltrated into the diaphragm was presented as a percentage of the total cell number in the whole diaphragm. Data are presented as means plus or minus SD. Information on immunostaining and morphometric analysis is available in Document S1.
Profound expression but intracellular localization of TLR4 in the primary cultured LECs
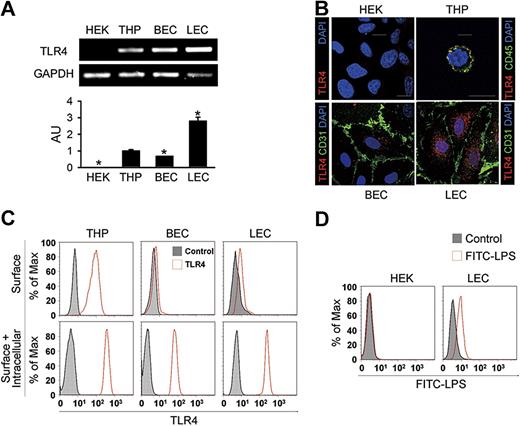
These findings led us to assess the expression and localization of TLR4 in primary cultured human LECs from the adult dermis. Similar to primary cultured BECs from the adult dermis, LEC exhibited a typical cobblestone appearance (Figure S3A). LECs expressed not only endothelial cell marker CD31, but also LEC-specific and distinct markers such as LYVE-1, podoplanin, and Prox-1 (Figure S3B). Semiquantitative and quantitative RT-PCR analysis revealed that the expression level of TLR4 mRNA in LECs was 2.8- and 4.2-fold higher than in THP-1 cells (THP) from a human monocyte cell line and BECs, respectively; HEK-293A cells (HEK) from a human fibroblast cell line showed no detectable range of TLR4 expression (Figure 2A). Immunofluorescent analysis revealed that TLR4 was abundant and mainly localized as granules in the intracellular area in LECs (Figure 2B). In BECs, a lesser amount of TLR4 was mainly localized in the intracellular-perinuclear area as granules, whereas TLR4 localized as granules largely at the cell surface in THP (Figure 2B), consistent with a previous report.11 No detectable TLR4 signal was found in HEK. To further define the localization of TLR4 in LECs, flow cytometric analysis was next performed. THP showed both surface and intracellular TLR4 expression, whereas most TLR4 was detected in the intracellular compartment in LECs and BECs (Figure 2C). In addition, LECs incubated with FITC-LPS generated a positive signal for binding, while no binding was observed with HEK (Figure 2D), suggesting that LECs have a functional binding receptor for LPS, such as TLR4. Together, these suggest that LECs express a high amount of TLR4, mainly in the intracellular area, and that this TLR4 may function as an internal receptor for LPS.
Elevated expression but intracellular localization of TLR4 in the primary cultured LECs. (A) Semiquantitative (top panel) and quantitative (bottom panel) RT-PCR analysis of TLR4 mRNA in cultured HEK-293A (HEK), THP-1 (THP), BECs, and LECs. Quantitative RT-PCR analysis is presented in arbitrary units (AU) after normalization to GAPDH, with THP set as 1. Bars represent means plus or minus SD (n = 3). *P < .05 versus THP. (B) Immunostainings of TLR4, CD45, or CD31, and DAPI nuclear staining in HEK, THP, BECs, and LECs. Scale bars indicate 10 μm. (C) Flow cytometric analysis of the distribution of TLR4 before permeabilization (top panel) and after permeabilization (bottom panel). (D) Flow cytometric analysis of the distribution of FITC-LPS in HEK and LECs. Independent flow cytometric analyses (n = 3-4) show similar findings.
Elevated expression but intracellular localization of TLR4 in the primary cultured LECs. (A) Semiquantitative (top panel) and quantitative (bottom panel) RT-PCR analysis of TLR4 mRNA in cultured HEK-293A (HEK), THP-1 (THP), BECs, and LECs. Quantitative RT-PCR analysis is presented in arbitrary units (AU) after normalization to GAPDH, with THP set as 1. Bars represent means plus or minus SD (n = 3). *P < .05 versus THP. (B) Immunostainings of TLR4, CD45, or CD31, and DAPI nuclear staining in HEK, THP, BECs, and LECs. Scale bars indicate 10 μm. (C) Flow cytometric analysis of the distribution of TLR4 before permeabilization (top panel) and after permeabilization (bottom panel). (D) Flow cytometric analysis of the distribution of FITC-LPS in HEK and LECs. Independent flow cytometric analyses (n = 3-4) show similar findings.
TLR4 in LECs is a functional receptor mediating LPS-induced NF-κB signaling
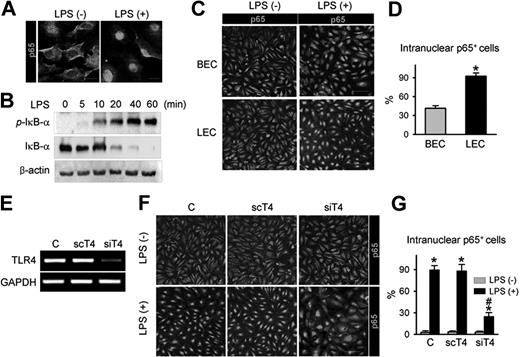
As NF-κB signaling is one of the major pathways that convey LPS-TLR4–mediated inflammation in macrophages,32 the interactions among LPS, TLR4, and NF-κB signaling were examined in primary cultured LECs. LPS treatment produced a marked intranuclear translocation of p65, a subunit of NF-κB (Figure 3A), and a substantial increase in phosphorylation of the NF-κB inhibitor, IκB-α, in a time-dependent manner (Figure 3B). Intriguingly, upon LPS stimulation, approximately 93% of LECs exhibited nuclear localization of p65, whereas just approximately 41% of BECs did (Figure 3C,D), indicating that activation of NF-κB signaling in response to LPS is more sensitive in LECs than BECs. To determine whether LPS-induced NF-κB signaling activation is mediated through TLR4 in LECs, we used TLR4-specific siRNA (siTLR4) to knock down TLR4 in LECs (Figure 3E). Upon LPS stimulation, approximately 25% of siTLR4-transfected LECs exhibited nuclear localization of p65, whereas approximately 90% of control-transfected and approximately 86% of scTLR4 (nonspecific scrambled siRNA)–transfected LECs exhibited nuclear localization of p65 (Figure 3F,G), indicating that TLR4 is essential for LPS-induced NF-κB signaling activation in LECs. We also analyzed the activity of NF-κB in vivo using a reporter mouse expressing lacZ under control of the p105 promoter, which contains 3 NF-κB responsive elements.26 Upon NF-κB activation in these mice, one of the NF-κB subunits, p65, translocates to the nucleus and binds the p105 promoter, which induces lacZ expression. Consistent with a previous report,33 NF-κB was predominantly expressed in LYVE-1+ lymphatic capillaries, but not in the blood vessel of the diaphragm (Figure S4 top panel). Moreover, the LPS-induced, newly developed branching lymphatic vessels also expressed NF-κB (Figure S4 bottom panel). These data suggest that TLR4-NF-κB signaling in LECs could be a crucial pathway that conveys LPS-induced inflammation and lymphangiogenesis both in vitro and in vivo.
TLR4 in LECs is a functional receptor mediating LPS-induced NF-κB signaling. (A) p65 immunostaining (a subunit of NF-κB; green) in LECs in the absence (LPS−) and presence (LPS+) of LPS stimulation (50 ng/mL for 30 minutes). Scale bars indicate 10 μm. (B) Immunoblotting analysis for phosphorylated IκB-α, IκB-α, and β-actin proteins in LECs after LPS (50 ng/mL) stimulation for indicated times. (C,D) p65 immunostaining in BECs and LECs after LPS stimulation (500 ng/mL for 60 minutes). Scale bars indicate 100 μm. (D) Cells positive for p65 intranuclear staining were counted; the values are presented as a percentage of the total cell number. Bars represent mean plus or minus SD (n = 4). *P < .05 versus BECs. (E) TLR4 knock-down by siTLR4 (siT4) but not scTLR4 (scT4) was confirmed by semiquantitative RT-PCR in LECs. (F,G) LECs were transfected with C (control transfection), scT4, and siT4, stimulated with LPS (500 ng/mL for 60 minutes), and immunostained for p65. Scale bars indicate 100 μm. Cells positive for p65 intranuclear staining were counted and presented as a percentage of total cell number. Bars represent means plus or minus SD (n = 4). *P < .05 versus LPS−; #P < .05 versus scT4 plus LPS+.
TLR4 in LECs is a functional receptor mediating LPS-induced NF-κB signaling. (A) p65 immunostaining (a subunit of NF-κB; green) in LECs in the absence (LPS−) and presence (LPS+) of LPS stimulation (50 ng/mL for 30 minutes). Scale bars indicate 10 μm. (B) Immunoblotting analysis for phosphorylated IκB-α, IκB-α, and β-actin proteins in LECs after LPS (50 ng/mL) stimulation for indicated times. (C,D) p65 immunostaining in BECs and LECs after LPS stimulation (500 ng/mL for 60 minutes). Scale bars indicate 100 μm. (D) Cells positive for p65 intranuclear staining were counted; the values are presented as a percentage of the total cell number. Bars represent mean plus or minus SD (n = 4). *P < .05 versus BECs. (E) TLR4 knock-down by siTLR4 (siT4) but not scTLR4 (scT4) was confirmed by semiquantitative RT-PCR in LECs. (F,G) LECs were transfected with C (control transfection), scT4, and siT4, stimulated with LPS (500 ng/mL for 60 minutes), and immunostained for p65. Scale bars indicate 100 μm. Cells positive for p65 intranuclear staining were counted and presented as a percentage of total cell number. Bars represent means plus or minus SD (n = 4). *P < .05 versus LPS−; #P < .05 versus scT4 plus LPS+.
LPS-TLR4 signaling in LECs does not directly promote cell proliferation/migration, tube formation, and expression of lymphangiogenic molecules
To examine whether LPS had any direct effect on lymphangiogenesis, primary cultured human LECs were stimulated with several concentrations of LPS. However, none of the concentrations examined (50, 500, and 5000 ng/mL) significantly promoted LEC proliferation/migration (Figure S5A,B) or tube formation (Figure S5C,D), whereas the media containing 10% serum with growth supplements markedly induced LEC proliferation/migration and tube formation. To assess whether LPS induces the expression of lymphangiogenic growth factors, such as VEGF-C and VEGF-D, and their receptors, VEGFR2 and VEGFR3, quantitative RT-PCR was performed. No significant change in expression of VEGF-C, VEGF-D, VEGFR3, and VEGFR2 in response to LPS (5-50 000 ng/mL) was detected (Figure S5E). Thus, LPS could not directly induce lymphangiogenesis in a cell-autonomous manner.
TLR4 in LECs mediates LPS-induced chemokine up-regulation and macrophage chemotaxis
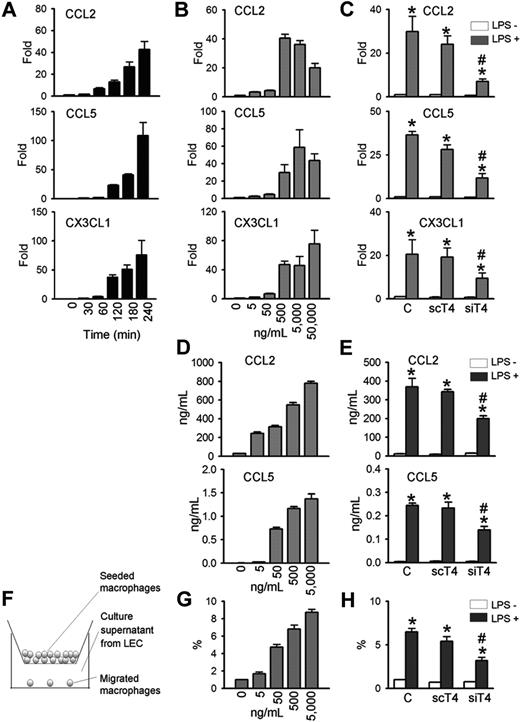
Because LPS-induced lymphangiogenesis is accompanied with CD11b+ macrophage infiltration in a TLR4-dependent manner, we next determined whether primary cultured human LECs produce chemokines for macrophages in a TLR4-dependent manner. LPS markedly increased the mRNA expressions of major chemokines for macrophage, such as CCL2, CCL5, and CX3CL1,34,,,,,–40 in time- and dose-dependent manners (Figure 4A,B). Treatment with LPS (500-50 000 ng/mL for 4 hours) induced CCL2 by approximately 20- to 40-fold, CCL5 by approximately 30- to 110-fold, and CX3CL1 by approximately 47- to 76-fold (Figure 4A,B). The LPS-induced increase of CCL2, CCL5, and CX3CL1 in human LECs were largely dependent on TLR4, as shown by the significant reduction in gene levels in human LECs treated with siTLR4 compared with scTLR4 (Figure 4C). The basal expression level of these chemokines in human LECs were not markedly altered by either siTLR4 or scTLR4 treatment. ELISA assays revealed that LPS increased CCL2 and CCL5 protein levels in the culture media of human LECs in dose-dependent and TLR4-dependent manners (Figure 4D,E). Notably, LPS treatment (5000 ng/mL) increased the level of CCL2 protein from approximately 29 ng/mL up to approximately 780 ng/mL for 48 hours. Consistent with these findings, LPS treatment (500 ng/mL for 4 hours) increased the level of CCL2 mRNA (HeN vs HeJ, 118.9-fold vs 2.6-fold; P < .05), CCL5 (HeN vs HeJ, 1023.9-fold vs 3.3-fold; P < .05), and CX3CL1 (HeN vs HeJ, 2.5-fold vs 1.1-fold; P < .05) mRNA in the primary cultured mice LECs derived from HeN and HeJ (Figure S6B). Moreover, expression of CCL2 was markedly up-regulated in the diaphragmatic lymphatic vessels by LPS treatment in HeN mice, whereas it was not changed in the diaphragmatic lymphatic vessels by LPS treatment in HeJ mice (Figure S6C). We further determined the chemotactic activity of the secreted chemokines from LPS-stimulated LECs using a transwell migration assay (Figure 4F). The media supernatant from LECs stimulated with different concentrations of LPS (0-5000 ng/mL) for 48 hours increased the chemotactic migration of THP-1 monocytes in a dose-dependent manner (Figure 4G). Notably, the chemotactic migration of monocytes was reduced when monocytes were incubated with media supernatant from siTLR4-transfected LECs compared with control- or scTLR4-transfected LECs with LPS stimulation (Figure 4H). These data indicate that TLR4 signaling in LECs is critical for LPS-induced up-regulation of chemokines for trafficking and chemotactic migration of macrophages.
TLR4 mediates LPS-induced chemokine up-regulation and monocytes chemotaxis in LECs. LECs were stimulated with LPS (500 ng/mL) for indicated times (A), 4 hours (C), or 48 hours (E,H), or stimulated with indicated amounts of LPS for 4 hours (B) or 48 hours (D,G). Prior to LPS stimulation, LECs were transfected with control (C), scTLR4 (scT4), or siTLR4 (siT4) (C,E,H). (A-C) The CCL2, CCL5, and CX3CL1 mRNA levels were analyzed by quantitative RT-PCR. The mRNA level of each gene were normalized to GAPDH and presented as a fold increase compared with the control. (D,E) CCL2 and CCL5 protein levels in the LEC culture supernatant were measured by ELISA. (F) Schematic diagram of the monocyte chemotactic migration assay. (G,H) The number of THP-1 monocytes that migrated from the hanging transwell to the culture supernatants of the LPS-stimulated LECs in the bottom were counted and presented as a percentage of the number of initially seeded cells. Bars represent mean plus or minus SD (n = 4). *P < .05 versus LPS−; #P < .05 versus scT4+ LPS+.
TLR4 mediates LPS-induced chemokine up-regulation and monocytes chemotaxis in LECs. LECs were stimulated with LPS (500 ng/mL) for indicated times (A), 4 hours (C), or 48 hours (E,H), or stimulated with indicated amounts of LPS for 4 hours (B) or 48 hours (D,G). Prior to LPS stimulation, LECs were transfected with control (C), scTLR4 (scT4), or siTLR4 (siT4) (C,E,H). (A-C) The CCL2, CCL5, and CX3CL1 mRNA levels were analyzed by quantitative RT-PCR. The mRNA level of each gene were normalized to GAPDH and presented as a fold increase compared with the control. (D,E) CCL2 and CCL5 protein levels in the LEC culture supernatant were measured by ELISA. (F) Schematic diagram of the monocyte chemotactic migration assay. (G,H) The number of THP-1 monocytes that migrated from the hanging transwell to the culture supernatants of the LPS-stimulated LECs in the bottom were counted and presented as a percentage of the number of initially seeded cells. Bars represent mean plus or minus SD (n = 4). *P < .05 versus LPS−; #P < .05 versus scT4+ LPS+.
TLR4 signaling in LECs contributes to macrophage recruitment and infiltration to draining lymphatic vessels in vivo
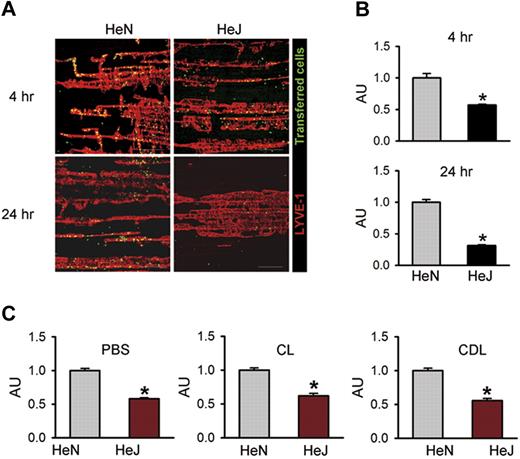
To investigate whether TLR4 signaling in LECs contributes to macrophage chemotaxis in vivo, we performed an adoptive cell transfer experiment25 by intraperitoneal transfer of CFSE green dye–tagged peritoneal macrophages from HeN mice into the peritoneal cavity of HeN and HeJ mice (Figure S7A). Flow cytometric analysis revealed that approximately 80% to 85% of the inflammatory cells collected from HeN mice with thioglycollate-induced peritonitis were CD11b+/F4/80+ macrophages (Figure S7B). Confocal images of the diaphragms revealed higher numbers of the transferred cells along the diaphragmatic lymphatic vessels in HeN mice compared with HeJ mice at 4 hours (∼1.75-fold) and 24 hours (∼3.18-fold) following the intraperitoneal injection of LPS (Figure 5A,B). The transferred cells were mainly located in the diaphragmatic lymphatic vessel, but not in the lymphatic vessels of peritoneal cavity sites, such as mesentery or abdominal wall (data not shown). To exclude the effect of endogenous macrophages on recruitment and infiltration of the transferred macrophages to the draining lymphatic vessel, we first depleted endogenous macrophages by intraperitoneal injection of clodronate liposome (CLD)28 and then performed the adoptive cell transfer experiment. Even after CLD treatment, the recruitment of macrophages to draining lymphatic vessel in HeN mice was higher (approximately 1.8-fold) compared with HeJ mice. Similar differences in the extent of recruitment and infiltration of macrophages between HeN and HeJ mice were observed in the treatment of PBS and CL (Figure 5C). Thus, LPS-TLR4 signaling in LECs substantially contributes to chemotaxis for the recruitment and infiltration of macrophages to the draining lymphatic vessel, which could be mediated mainly through the secretion of various chemokines from the LECs. Z-stack analysis of confocal images in the lymphatic vessels was performed to further define the location of these transferred macrophages. Horizontal and vertical sections of Z-stack images showed that all transferred cells were inside the lumen of the lymphatic capillary in HeN mice (Figure S8). These data indicate that the transferred cells might be drained into the initial lymphatic capillary and were in the process of transmigration to the DLN.
TLR4 signaling contributes to macrophage trafficking to the draining lymphatic vessels. The diaphragms were analyzed at 4 and 24 hours after intraperitoneal injection of LPS (0.5 mg/kg) and transfer of approximately 2 × 107 of CFSE-tagged peritoneal macrophages (green) into the peritoneal cavity. (A) Whole diaphragms were harvested and then immunostained with LYVE-1; LYVE-1+ diaphragmatic lymphatic vessels (red) and injected cells (green) were visualized. Scale bars indicate 200 μm. (B) The number of transferred cells (green) along the lymphatic vessel (red) was divided by the total area of LYVE-1+ lymphatic vessel in a given area (1 mm2). (C) At 3 days after intraperitoneal injection of PBS, CL (50 mg/kg), or CDL (50 mg/kg) into HeN and HeJ mice, LPS (0.5 mg/kg) and approximately 2 × 107 of CFSE-tagged peritoneal macrophages were injected intraperitoneally. After 4 hours, whole diaphragms were harvested. The number of transferred cells along the lymphatic vessel was divided by the total area of the LYVE-1+ lymphatic vessel in a given area (1 mm2). The values are presented as arbitrary units (AU) compared with the value from HeN mice as 1. Bars represent means plus or minus SD (n = 4). *P < .05 versus HeN.
TLR4 signaling contributes to macrophage trafficking to the draining lymphatic vessels. The diaphragms were analyzed at 4 and 24 hours after intraperitoneal injection of LPS (0.5 mg/kg) and transfer of approximately 2 × 107 of CFSE-tagged peritoneal macrophages (green) into the peritoneal cavity. (A) Whole diaphragms were harvested and then immunostained with LYVE-1; LYVE-1+ diaphragmatic lymphatic vessels (red) and injected cells (green) were visualized. Scale bars indicate 200 μm. (B) The number of transferred cells (green) along the lymphatic vessel (red) was divided by the total area of LYVE-1+ lymphatic vessel in a given area (1 mm2). (C) At 3 days after intraperitoneal injection of PBS, CL (50 mg/kg), or CDL (50 mg/kg) into HeN and HeJ mice, LPS (0.5 mg/kg) and approximately 2 × 107 of CFSE-tagged peritoneal macrophages were injected intraperitoneally. After 4 hours, whole diaphragms were harvested. The number of transferred cells along the lymphatic vessel was divided by the total area of the LYVE-1+ lymphatic vessel in a given area (1 mm2). The values are presented as arbitrary units (AU) compared with the value from HeN mice as 1. Bars represent means plus or minus SD (n = 4). *P < .05 versus HeN.
TLR4 in LECs is a prime initiator in the LPS-induced lymphangiogenesis by macrophage recruitment
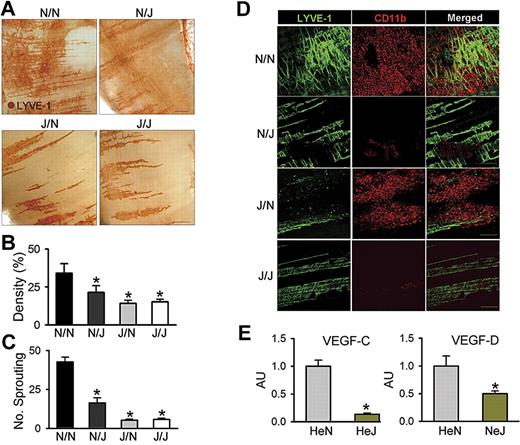
To define the role of LEC-specific TLR4 in LPS-induced diaphragmatic lymphangiogenesis in the context of interaction with macrophage, equal numbers of BMCs from HeN mice were transplanted to lethally irradiated HeN and HeJ mice (referred to as N/N and N/J mice, respectively), and equal numbers of BMCs from HeJ mice were transplanted to lethally irradiated HeN and HeJ mice (referred to as J/N and J/J mice, respectively). After 2 months, the N/N, N/J, J/N, and J/J mice were intraperitoneally treated with LPS for 7 days. Similar to the effect of LPS in HeN mice, LPS profoundly increased lymphatic densities and branching in the peritoneal side of the diaphragm in N/N mice (Figure 6A-D). In contrast, these phenomena were largely attenuated or absent in N/J, J/N, and J/J mice, indicating that TLR4 in both LECs and macrophages have an interactive role in LPS-induced lymphangiogenesis (Figure 6A-D). To clarify this interactive role in the LPS-induced lymphangiogenesis, we first evaluated the contribution of LEC-specific LPS-TLR4 signaling on the extent of CD11b+ macrophage infiltration into the lymphatic vessels. Similar to the effect of LPS in HeN mice, LPS markedly increased CD11b+ macrophage infiltration adjacent to the lymphatic vessels in N/N and J/N mice, but had little effect in N/J and J/J mice, indicating that LEC-specific TLR4 contributes to macrophage infiltration by secretion of chemokines regardless of the presence or absence of TLR4 in macrophages (Figure 6D). Second, to evaluate the role of macrophages in LPS-induced lymphangiogenesis, we depleted macrophages by CDL treatment during LPS stimulation in the HeN mice. Treatment of CDL but not CL largely abolished the LPS-induced increase of lymphatic densities and branching in the peritoneal side of diaphragm (Figure S9), indicating that the infiltrated macrophages play an important role in LPS-induced lymphangiogenesis. Finally, to elucidate the exact mechanism of lymphangiogenesis by the infiltrating CD11b+ macrophages, we analyzed VEGF-C and VEGF-D mRNA levels in the CD11b+ macrophages that infiltrated into the diaphragm of HeN and HeJ mice after LPS stimulation. The CD11b+ cells from HeN mice expressed higher levels of VEGF-C (approximately 7.5-fold) and VEGF-D (approximately 2.0-fold) than those from HeJ mice (Figure 6E). This suggests that the infiltrating CD11b+ macrophages by LPS-TLR4 signaling in LECs could be the major source for lymphangiogenic growth factors. Furthermore, the lack of lymphangiogenesis in the J/N mice again suggests that activated TLR4 in LECs is a prime initiator for the macrophage recruitment, whereas activated TLR4 in macrophages is a secondary critical component for the LPS-induced lymphangiogenesis through enhanced production of lymphangiogenic growth factors. Taken together, our data indicate that TLR4 in LECs is a critical initiator of LPS-induced lymphangiogenesis by chemotactic recruitment of activated macrophages, which are the major source for lymphangiogenic growth factors.
LEC-specific LPS-TLR4 signaling is critical in LPS-induced lymphangiogenesis. N/N, N/J, J/N, and J/J mice were treated with intraperitoneal injection of LPS (0.5 mg/kg per day) for 7 days; the diaphragms were then harvested, whole-mounted, and immunostained for LYVE-1 (brown; scale bars indicate 300 μm) (A) or coimmunostained for LYVE-1 (green) and CD11b (red) (D). Scale bars indicate 200 μm. (A,D) Note that the LPS-induced increase of lymphatic densities and branchings and infiltration of CD11b+ cells adjacent to lymphatic vessels of the diaphragm in N/N mice are largely abrogated in N/J mice. (B,C) Densities of the LYVE-1+ diaphragmatic lymphatic vessels were measured in each given area (3.64 mm2), and the values are presented as the percentage of total area of the field. The number of LYVE-1+ lymphatic branching exceeding 50 μm in length in each given area (1 mm2) was counted, and the absolute numbers are presented. Bars represent means plus or minus SD (n = 4). *P < .05 versus N/N. (E) The CD11b+ cells in the diaphragm of HeN and HeJ mice after LPS stimulation (0.5 mg/kg per day) for 5 days were collected and enriched by MACS. The VEGF-C and VEGF-D mRNA levels in CD11b+ cells were analyzed by quantitative RT-PCR. mRNA levels for each gene were normalized to GAPDH, and the values are presented in arbitrary units (AU) compared with the value from HeN mice set as 1. Bars represent means plus or minus SD (n = 4). *P < .05 versus HeN.
LEC-specific LPS-TLR4 signaling is critical in LPS-induced lymphangiogenesis. N/N, N/J, J/N, and J/J mice were treated with intraperitoneal injection of LPS (0.5 mg/kg per day) for 7 days; the diaphragms were then harvested, whole-mounted, and immunostained for LYVE-1 (brown; scale bars indicate 300 μm) (A) or coimmunostained for LYVE-1 (green) and CD11b (red) (D). Scale bars indicate 200 μm. (A,D) Note that the LPS-induced increase of lymphatic densities and branchings and infiltration of CD11b+ cells adjacent to lymphatic vessels of the diaphragm in N/N mice are largely abrogated in N/J mice. (B,C) Densities of the LYVE-1+ diaphragmatic lymphatic vessels were measured in each given area (3.64 mm2), and the values are presented as the percentage of total area of the field. The number of LYVE-1+ lymphatic branching exceeding 50 μm in length in each given area (1 mm2) was counted, and the absolute numbers are presented. Bars represent means plus or minus SD (n = 4). *P < .05 versus N/N. (E) The CD11b+ cells in the diaphragm of HeN and HeJ mice after LPS stimulation (0.5 mg/kg per day) for 5 days were collected and enriched by MACS. The VEGF-C and VEGF-D mRNA levels in CD11b+ cells were analyzed by quantitative RT-PCR. mRNA levels for each gene were normalized to GAPDH, and the values are presented in arbitrary units (AU) compared with the value from HeN mice set as 1. Bars represent means plus or minus SD (n = 4). *P < .05 versus HeN.
Discussion
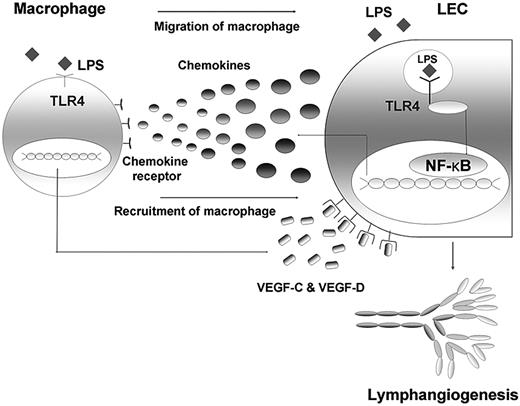
Here, we found that TLR4 in LECs plays a key role in LPS-induced inflammatory lymphangiogenesis by enhancing the recruitment and infiltration of macrophages around lymphatic vessels. LECs actively participate in these processes by producing major chemokines through the LPS-TLR4-NF-κB signaling pathway, and the macrophages recruited to the lymphatic vessels by chemotactic stimuli from LECs act as the major source of lymphangiogenic growth factors, including VEGF-C and VEGF-D (Figure 7). Taken together, our findings not only clarify the mechanism of the role of TLR4 in LEC involvement in LPS-induced inflammatory lymphangiogenesis, but also highlight close functional interactions between LECs and macrophages in these processes.
Schematic diagram on the role of TLR4 in LECs in the context of LPS-induced inflammation, resulting in lymphangiogenesis by chemotactic migration of macrophages that produce VEGF-C and VEGF-D. The LEC actively responds to LPS with intracellular TLR4 by secreting various chemokines, such as CCL2, CCL5, and CX3CL1. Here, NF-κB may contribute to the signaling pathway. The migrated and recruited macrophages, in turn, produce lymphangiogenic factors, such as VEGF-C and -D, resulting in robust lymphangiogenesis. Therefore, an active crosstalk connects LECs and macrophages in the context of LPS-induced inflammation, the process of which is under the control of TLR4 in LECs.
Schematic diagram on the role of TLR4 in LECs in the context of LPS-induced inflammation, resulting in lymphangiogenesis by chemotactic migration of macrophages that produce VEGF-C and VEGF-D. The LEC actively responds to LPS with intracellular TLR4 by secreting various chemokines, such as CCL2, CCL5, and CX3CL1. Here, NF-κB may contribute to the signaling pathway. The migrated and recruited macrophages, in turn, produce lymphangiogenic factors, such as VEGF-C and -D, resulting in robust lymphangiogenesis. Therefore, an active crosstalk connects LECs and macrophages in the context of LPS-induced inflammation, the process of which is under the control of TLR4 in LECs.
We observed profound changes in the lymphatic vessels in response to LPS stimulation in HeN mice with normal TLR4 signaling, whereas almost no changes were observed in in HeJ mice with defective TLR4 signaling. This striking difference led us to explore the role of TLR4 in LECs in LPS-induced inflammatory lymphangiogenesis at in vitro and in vivo levels. Intriguingly, a significant amount of TLR4 was expressed in the intracellular area of LECs and LPS was able to bind to the intracellular TLR4, even though the functional implication of this subcellular localization warrants future investigation. Furthermore, LPS-TLR4-NF-κB signaling was approximately 2-fold more sensitive in LECs than in BECs, and the NF-κB signal was constitutively active in the diaphragmatic lymphatic vessels but not blood vessels in κB-lacZ mice. This constitutive and active NF-κB signaling in the lymphatic vessels might be the result of high expression and activation of TLR4 and other PRRs by various agonists, including various Gram-negative bacteria, which are the main normal flora of the peritoneal cavity. These results clearly indicate that the LEC is a crucial cell type responding sensitively to LPS. Immune cells, including monocytes/macrophages, are major cell types that respond to pathogen-induced inflammation and also use TLR4-NF-κB signaling upon LPS stimulation for maturation and mobilization to DLN during the adaptive immune response.4,41 Recently, growing evidence has shown that nonimmune cells, such as platelets,42 fibroblasts,43,44 pulmonary epithelial cells,45 and BECs,11 also have functional TLR4, which mediates the production of various inflammatory mediators by LPS. Based on the high levels of TLR4 and the sensitive activation of NF-κB signaling in LECs compared with BECs upon LPS stimulation, the lymphatic vessel may not just be a passive conduit for immune cell transport, but may also be one of the active players in immune modulation.
Because we also observed marked infiltration of CD11b+ macrophages around the lymphatic vessels by LPS stimulation in HeN mice, compared with nearly no infiltration in HeJ mice, we hypothesized that TLR4 in LECs may function as a recruiter of macrophages by secreting chemokines upon LPS stimulation. Therefore, we analyzed the expression and production levels of major chemokines for macrophages, such as CCL2, CCL5, and CX3CL1,34,,,,,–40 in cultured LECs derived from human, and HeN and HeJ mice. Interestingly, our data showed that LPS induced a robust increase in production of the chemokine mRNAs in a TLR4-dependent manner. In addition, our in vitro transwell migration assay and in vivo adoptive cell transfer experiment revealed that the chemotactic migration of macrophages to LECs is largely dependent on TLR4 signaling in LECs. Macrophages are also known as a major supplier for the production of chemokines during LPS-induced inflammation.46,47 Therefore, LECs and macrophages may closely interact for the production of chemokines on the recruitment and infiltration of immune cells, including macrophages to draining lymphatic vessels during LPS-induced inflammation (Figure 7).
Our in vitro data in Figure S3 argue against the idea that the LPS-TLR4 pathway in LECs may directly induce lymphangiogenesis in a cell-autonomous manner. Rather, the LPS-TLR4 pathway in LECs indirectly induces lymphangiogenesis by the production of major chemotactic factors for macrophages that were able to secrete lymphangiogenic factors. Our data clearly revealed that TLR4 in LECs is essential in this scenario, as shown by marked reductions in LPS-induced chemokine expression and production in the primary cultured human LECs knocked down for TLR4, LECs from the HeJ mouse, and lymphatic vessels in the HeJ mouse. Interestingly, the contribution of lymphatic vessels on macrophage trafficking also affected the lymphatic vessels, resulting in robust lymphangiogenesis by lymphangiogenic growth factors secreted from the infiltrated macrophages. In support of this, our data showed that LPS-stimulated peritoneal macrophages expressed significant amounts of VEGF-C and VEGF-D. Moreover, depletion of macrophages by CLD largely abolished LPS-induced inflammatory lymphangiogenesis, and J/N mice, which have defective TLR4 signaling in macrophages, did not develop LPS-induced lymphangiogenesis even though abundant macrophages infiltrated into the diaphragm, strongly indicating that lymphangiogenic growth factors secreted from the infiltrated macrophages are the main sources for LPS-induced inflammatory lymphangiogenesis. This LPS-induced extensive lymphangiogenesis may play an important role in draining inflammatory macromolecules, immune cells, and debris from inflamed tissues.
The incidence of fatal Gram-negative bacterial sepsis is increasing in proportion to the growing numbers of immunocompromised patients.48 Several new drugs are currently being developed to treat this, including TLR4-blocking agents.49 In the past, TLR4 in “effector cells” such as macrophages were considered the main target of these drugs.50 However, as “conduit cells” for immune cell transport, the active contribution of LECs in LPS-induced inflammation in our study implies that the control and modulation of TLR4 function in LECs can be a potential and novel alternative target to overcome Gram-negative bacteria–induced mortality.
In conclusion, TLR4 in LECs is an essential molecule in the production of major chemokines for macrophages and in the subsequent recruitment of macrophages to the lymphatic vessel during LPS-induced lymphangiogenesis. In addition, the macrophages induced to infiltrate by the LPS-TLR4 pathway in LECs contribute to robust lymphangiogenesis (Figure 7). These TLR4-mediated interactions between LECs and macrophages are central mechanisms in the innate/adaptive immune response and pathogen clearance.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Dr R. A. Schwendener for CDL and CD, Dr S.-H. Han and Dr S. Akira for TLR4 KO mice, and Seong-Ik Hwang and Jinah Han for technical support.
This work was supported by the Korea Science and Engineering Foundation (KOSEF) through the National Research Laboratory Program (2004-02376 to G.Y.K.) funded by the Ministry of Science and Technology.
Authorship
Contribution: S.K., S-P.L., and G.Y.K. designed and organized the experiments, performed the animal studies, analyzed the data, generated the figures, and wrote the manuscript; K.E.K. and H.-Z.K. performed the histologic and molecular analysis; and S.M. provided κB-lacZ mice.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Gou Young Koh, Department of Biological Sciences, KAIST, 373-1, Guseong-dong, Daejeon, 305-701, Republic of Korea; e-mail: gykoh@kaist.ac.kr.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal