Abstract
Dendritic cell (DC)–based immunotherapy of malignant diseases relies on 2 critical parameters: antigen transport from the periphery to draining lymph nodes and efficient priming of primary and stimulation of secondary immune responses. Prostaglandin E2 (PGE2) signaling has been shown to be pivotal for DC migration toward lymph node–derived chemokines in vitro and in vivo. Here, we demonstrate that PGE2 induced the expression of the costimulatory molecules OX40L, CD70, and 4-1BBL on human DCs. Short triggering by PGE2 early during DC maturation was sufficient to induce the costimulatory molecules. The expression of the costimulatory molecules was independent of the maturation stimulus but strictly dependent on PGE2 on both monocyte-derived (Mo) DCs and peripheral blood myeloid (PB) DCs. PGE2-matured MoDCs showed enhanced costimulatory capacities resulting in augmented antigen-specific CD4+ and CD8+ T-cell proliferation in primary and recall T-cell responses. Blocking OX40/OX40L signaling impaired the enhanced T-cell proliferation induced by PGE2-matured MoDCs. Moreover, MoDCs matured in the presence of PGE2 induced the expression of OX40, OX40L, and CD70 on T cells facilitating T-cell/T-cell interaction that warrant long-lasting costimulation. This newly identified parameter will help to further optimize DC-based immunotherapy.
Introduction
Dendritic cells (DCs) are key players in the defense against pathogens because of their unique capacity to induce primary and secondary T-cell responses. By presenting specific antigens on major histocompatibility complex and by expressing costimulatory molecules, DCs provide essential signals for optimal T-cell activation, prevention of tolerance, and development of T-cell immunity. The most important costimulatory receptor for early proliferation of naive T cells is CD28, which interacts with CD80 or CD86 on DCs.1 However, CD28-CD80/86 costimulatory interactions cannot fully account for an efficient long-lasting T-cell response or the generation of memory T cells.2 T-cell proliferation and survival at later phases and the development of memory T cells depend on additional costimulatory molecules belonging to the tumor necrosis factor (TNF)/TNF receptor (TNFR) superfamily and include OX40L/OX40, 4-1BBL/4-1BB, and CD70/CD27.3
OX40 is induced on activated T cells and provides an essential signal for optimal CD4+ T-cell function4–7 as well as for the generation of memory T cells8–10 by prolonging the survival of effector T cells.9 Signaling through OX40 is controlled in vivo by the availability of its ligand OX40L. The expression of OX40L is tightly regulated and can be induced on antigen-presenting cells (APCs), such as DCs11,12 and B cells13 and microglia.14 The lack of OX40L on APCs results in a marked reduction of cytokine production and proliferation of T-helper cells.5,7 Murine DCs transfected with mRNA encoding OX40L induce enhanced antitumor activity in vivo, whereas transfection of OX40L mRNA in human monocyte-derived (Mo) DCs improves the induction of antigen-specific cytotoxic T lymphocytes (CTLs) in vitro.15
CD27 is expressed on naive CD4+ and CD8+ T cells16 and on primed B cells,17 and triggering of CD27 by CD70 supports T-cell survival. Consequently, constant activation of CD27 by persistent transgenic expression of CD70 on mouse B cells leads to excessive T-cell proliferation.18,19 CD70 expression is transiently induced on T cells, B cells, and DCs after antigen receptor engagement or Toll-like receptor (TLR) stimulation.20–22 CD70 expression on activated DCs is important for expansion and survival of primed CD8+ T cells in mice.23–25 In addition to its expression on DCs, OX40L and CD70 can be induced on T cells, providing a mechanism by which costimulatory survival signals are delivered by T-cell/T-cell contacts responsible for keeping T cells alive after APCs and antigens are no longer available to generate memory cells.26
Because of their natural features, DCs are currently used as cellular vaccines against tumors and infectious diseases.27 Because DCs in peripheral blood are very rare, the development of a protocol to differentiate blood monocytes into immature DCs in vitro,28 a process that also occurs in vivo,29,30 boosted the feasibility of DC-based immunotherapies. DC maturation can be induced by addition of sCD40L, poly I:C, lipopolysaccharide (LPS), or a cocktail of proinflammatory cytokines comprising TNF-α, interleukin-1β (IL-1β), IL-6, and prostaglandin E2 (PGE2).28,31,32 The presence of the arachidonic acid metabolite PGE2 during DC maturation is fundamental for the acquisition of a migratory DC phenotype both in vitro and in vivo,32–34 and hence essential for the transport of antigen from the periphery to draining lymph nodes and the induction of an antigen-specific immune response. Langerhans cells deficient in the PGE2 receptor EP4 display a significantly reduced T cell–stimulatory capacity.33 Moreover, stimulation of human MoDCs with PGE2 enhances their ability to induce allogeneic as well as antigen-specific T-cell proliferation.32,35,36 The mechanism by which PGE2 induces enhanced T cell–stimulatory capacities in mature DCs has not been identified yet.
In the present study, we demonstrate that the expression of the costimulatory molecules OX40L and CD70 on mature MoDCs depends on PGE2 stimulation resulting in a significantly enhanced capacity to induce antigen-specific CD4+ and CD8+ T-cell proliferation.
Methods
Generation of MoDCs
Human monocytes were positively selected from whole blood of healthy donors as previously described.32,34 Briefly, peripheral blood mononuclear cells (PBMCs) were enriched by density gradient centrifugation on Ficoll Paque Plus (GE Healthcare, Little Chalfont, United Kingdom), and CD14+ monocytes were isolated using anti-CD14–conjugated microbeads (Miltenyi Biotec, Bergisch Gladbach, Germany). Monocytes were cultured at 106 cells/mL in serum-free AIM-V medium (Invitrogen, Carlsbad, CA) supplemented with 50 ng/mL granulocyte-macrophage colony-stimulating factor (GM-CSF; Leukomax; Novartis, Basel, Switzerland) and IL-4 (recombinant protein from PromoCell, Heidelberg, Germany, or supernatant of an IL-4–producing J558 cell line; both revealed identical results). Where indicated, MoDCs were generated in AIM-V containing 5% human AB serum (Lonza, Basel, Switzerland). After 5 to 6 days, immature DCs were harvested and matured for 2 days by addition of 0.5 μg/mL soluble trimeric CD40L (sCD40L; PromoCell), 20 μg/mL poly I:C (Sigma-Aldrich, St Louis, MO), 10 μg/mL LPS (Salmonella abortus equi; Sigma-Aldrich), or a combination of 20 ng/mL TNF-α, 10 ng/mL IL-1β, and 20 ng/mL IL-6 (all PromoCell). Maturation occurred in the absence or presence of 1 μg/mL PGE2 (Minprostin E2; GE Healthcare), a concentration found in inflammation and used for clinical applications. For antigen loading, 10 μg/mL KLH (Sigma-Aldrich) or tetanus toxoid (TT; Berna Biotech AG, Berne, Switzerland) was added during DC maturation.
Peripheral blood DCs
Myeloid CD1c+ DCs were isolated from PBMCs using the CD1c (BDCA-1) DC Isolation Kit (Miltenyi Biotec) according to the manufacturer's instructions. Peripheral blood DCs (PBDCs) were analyzed by flow cytometry directly after isolation (ex vivo) or cultured at 106 cells/mL in AIM-V medium supplemented with 50 ng/mL GM-CSF and 50 ng/mL IL-4 (PromoCell). After 2 days of maturation with 0.5 μg/mL soluble trimeric CD40L in the absence or presence of 1 μg/mL PGE2, cells were analyzed by flow cytometry.
Cocultures and T-cell proliferation assay
Purified CD3+ T cells (Pan T-Cell Isolation Kit; Miltenyi Biotec) or PBMCs were labeled with CFSE (Invitrogen) according to the manufacturer's protocol. Subsequently, 105 autologous MoDCs were cultured together with 106 PBMCs or purified T cells in 1 mL AIM-V medium. Where indicated, graded amounts of a goat anti–human OX40L-neutralizing antibody (R&D Systems, Minneapolis MN) or control goat IgG (R&D Systems) were added. After 6 or 12 days of coculture, T-cell proliferation was assessed by carboxyfluorescein succinimidyl ester (CSFE) dilution assays using flow cytometry.
Flow cytometry
For cell-surface staining of costimulatory molecules, MoDCs were incubated for 45 minutes at 4°C in phosphate-buffered saline (PBS) containing 1% fetal calf serum (FCS) and 0.02% sodium azide with anti-CD83–phycoerythrin (PE), anti-CD80–PE, anti-CD86–PE, anti-OX40L–PE (clone Ik-1), anti-CD70–PE (clone Ki-24), or IgG-PE isotype control antibodies (all from BD Biosciences, San Jose, CA) and analyzed by flow cytometry (LSRII; BD Biosciences). T-cell proliferation was assessed by measuring CFSE dilution of cells stained with anti-CD3–allophycocyanin/Cy7, anti-CD4–PE/Cy7, anti-CD8–allophycocyanin (BD Biosciences). Dead cells were excluded by Sytox Blue (Invitrogen) staining. Surface expression of costimulatory molecules of nonlabeled T cells was measured using anti-CD3–allophycocyanin/Cy7, CD4-PE/Cy7, CD8–Pacific Blue in combination with either anti-OX40L–PE and anti-OX40–FITC or anti-CD70–PE (BD Biosciences).
Quantitative real-time RT-PCR
Total RNA from MoDCs matured with sCD40L in the absence or presence of PGE2 was isolated using the RNeasy mini kit (QIAGEN, Hilden, Germany) and transcribed into cDNA using random hexamer primers and the TaqMan reverse transcription kit (Applied Biosystems, Foster City, CA) according to the manufacturer's instructions. Amplification of TNFSF4, TNFSF7, and TNFSF9 mRNA was performed using the QuantiTect SYBR Green PCR Master Mix (QIAGEN) on a TaqMan 7700 (Applied Biosystems) with an initial denaturation step at 95°C for 15 minutes followed by 40 cycles of 15 seconds at 94°C, 30 seconds at 60°C, and 30 seconds at 72°C. Forward and reverse primers were used at a concentration of 200 nM with the following sequences: TNFSF4: 5′-CACCTACATCTGCCTGCACTTCT, 5′-GTTGTTCTGCACCTTCATGATTTC; TNFSF7: 5′-CCTCGTGGTGTGCATCCA, 5′-ATGCCATCACGATGGATACGTA; TNFSF9: 5′-GAGCTTTCGCCCGACGAT, 5′-GCAGCTCTAGTTGAAAGAAGACATAGTAGA. Expression of mRNA was normalized to β-2 microglobulin (β2M) and ubiquitin C using SYBR Green PCR Master Mix (Applied Biosystems) containing 200 nM forward and reverse primer (β2M: 5′-GCTATCCAGCGTACTCCAAAGATTC and 5′-CAACTTCAATGTCGGATGGATGA; ubiquitin C: 5′-ATTTGGGTCGCGGTTCTTG and 5′-TGCCTTGACATTCTCGATGGT). Relative mRNA expression was calculated by the ΔΔ cycle-threshold (Ct) method.
Statistical evaluation
Differences between groups were assessed by the Student paired t test.
Results
PGE2 potentiates the capacity of human MoDCs to induce T-cell proliferation
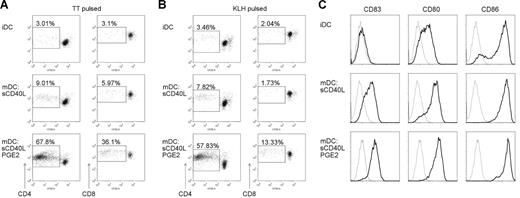
It is well established that PGE2 is the crucial factor during MoDC maturation to generate DCs with a migratory phenotype under serum-free conditions.32,34 Moreover, MoDCs matured in the presence of PGE2 show an enhanced capacity to stimulate allogenic T-cell proliferation.32,35 To determine whether this effect also applies to antigen-specific T-cell stimulation, we generated MoDCs matured with soluble trimeric CD40L (sCD40L) in the absence or presence of PGE2 under serum-free conditions and cocultured them with autologous CFSE-labeled PBMCs. MoDCs from TT-vaccinated donors were pulsed either with TT to induce secondary (memory) T-cell responses or keyhole limpet hemocyanin (KLH) to promote primary (naive) T-cell responses. Immature antigen-pulsed MoDCs elicited only weak T-cell proliferation after 6 days of coculture (Figure 1A,B), as expected because of the lack of CD83 expression and weaker expression of CD80 and CD86 compared with mature MoDCs (Figure 1C). MoDC maturation by CD40 ligation did not strongly improve T-cell proliferative responses (Figure 1A,B), despite high surface expression of CD83, CD80, and CD86 (Figure 1C). By contrast, MoDCs matured in the presence of PGE2 promoted strong antigen-specific primary and secondary T-cell proliferation (Figure 1A,B), although expression levels of CD83, CD80, and CD86 were not further increased by the addition of PGE2 (Figure 1C). Moreover, the augmented stimulatory capacity of PGE2-matured MoDCs affected both CD4+ and CD8+ T cells (Figure 1A,B). The latter must occur by cross-presentation as both antigens, if delivered exogenously to DCs, are known to be presented to CTLs by this pathway.36,37
PGE2 enhances T-cell-stimulatory capacities of human MoDCs. Human CSFE-labeled PBMCs were stimulated with tetanus toxoid (TT; A) or keyhole limpet hemocyanin (KLH; B) pulsed autologous immature (iDC) and MoDCs matured with trimeric soluble CD40L (mDC) in the absence or presence of PGE2. On day 6 of the coculture, cells were harvested, stained for CD3, CD4, CD8, and Sytox Blue to identify live T cells, and analyzed by flow cytometry. CSFE fluorescence of live CD3+CD4+ and CD3+CD8+ T cells from a representative experiment of at least 5 is shown. Percentages of proliferating, CSFE low, T cells are indicated. (C) Immature (iDC) or matured MoDCs (mDC) were analyzed for the expression of costimulatory molecules by flow cytometry. MoDCs were matured with sCD40L in the absence or presence of PGE2. Gray thin lines represent isotype controls; black bold lines, specific staining for CD83, CD80, or CD86 as indicated.
PGE2 enhances T-cell-stimulatory capacities of human MoDCs. Human CSFE-labeled PBMCs were stimulated with tetanus toxoid (TT; A) or keyhole limpet hemocyanin (KLH; B) pulsed autologous immature (iDC) and MoDCs matured with trimeric soluble CD40L (mDC) in the absence or presence of PGE2. On day 6 of the coculture, cells were harvested, stained for CD3, CD4, CD8, and Sytox Blue to identify live T cells, and analyzed by flow cytometry. CSFE fluorescence of live CD3+CD4+ and CD3+CD8+ T cells from a representative experiment of at least 5 is shown. Percentages of proliferating, CSFE low, T cells are indicated. (C) Immature (iDC) or matured MoDCs (mDC) were analyzed for the expression of costimulatory molecules by flow cytometry. MoDCs were matured with sCD40L in the absence or presence of PGE2. Gray thin lines represent isotype controls; black bold lines, specific staining for CD83, CD80, or CD86 as indicated.
PGE2 induces the expression of costimulatory molecules of the TNF superfamily on mature MoDCs and PBDCs
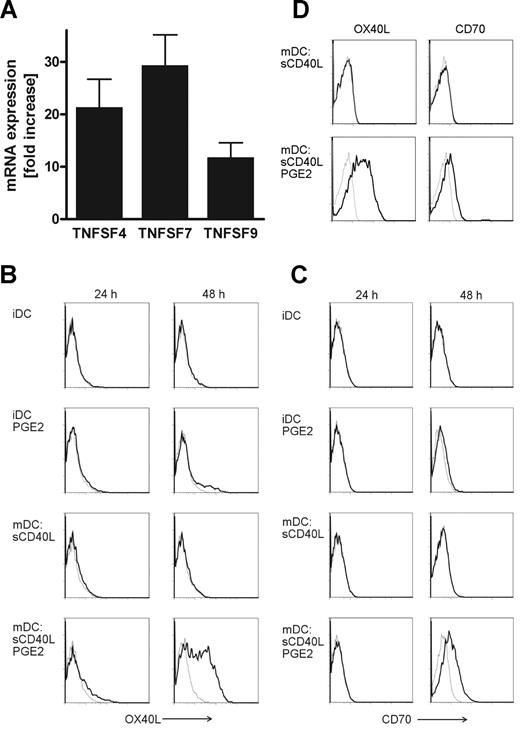
To investigate the mechanism responsible for the enhanced T cell–activating properties of PGE2-matured MoDCs, we analyzed the gene expression profiles of MoDCs matured with sCD40L in the absence or presence of PGE2 derived from 5 individual donors by Affymetrix GeneChip arrays (Santa Clara, CA). We found nonclassic costimulatory molecules of the TNF superfamily, namely, TNFSF4 (OX40L, CD134), TNFSF7 (CD70, CD27L), and TNFSF9 (4-1BBL, CD137L) to be up-regulated in MoDCs matured in the presence of PGE2, whereas the expression levels of CD80 and CD86 remained unchanged (data not shown). We corroborated these findings by quantitative real-time polymerase chain reaction (PCR) revealing a 10- to 30-fold higher mRNA expression of OX40L, CD70, and 4-1BBL in PGE2-matured MoDCs (Figure 2A). Next, we assessed cell-surface expression of the costimulatory molecules on MoDCs by flow cytometry. Immature MoDCs did not express OX40L and CD70, and stimulation with PGE2 alone had no effect (Figure 2B,C). Both costimulatory molecules were also absent in DCs after 24 hours or 48 hours of maturation with sCD40L. However, MoDCs matured by CD40 ligation in the presence of PGE2 markedly expressed OX40L and CD70 (Figure 2B,C) on the cell surface on day 2. Because we cultivated MoDCs under serum-free conditions, a prerequisite for immunotherapy, we investigated whether the observed necessity for PGE2 to induce OX40L and CD70 expression on mature MoDCs was the result of serum deprivation in our culture system. Therefore, we generated MoDCs in medium containing human AB serum and induced maturation using sCD40L with or without addition of PGE2. Even in the presence of serum, PGE2 was crucial for OX40L and CD70 expression on mature MoDCs as MoDCs matured in the absence of PGE2 did not express those costimulatory molecules (Figure 2D). We could not detect surface expression of 4-1BBL on MoDCs, which may be because of the poor quality of antibodies specific for human 4-1BBL.
PGE2-dependent expression of costimulatory molecules of the TNF superfamily on MoDCs. (A) Quantitative real-time RT-PCR analysis of TNFSF4 (OX40L), TNFSF7 (CD70), and TNFSF9 (4-1BBL) mRNA expression in MoDCs matured in the absence or presence of PGE2. Up-regulation of specific mRNA expression levels is depicted as fold increase by PGE2. Mean values and SEM derived from 7 individual donors are shown. Cell-surface expression of OX40L (B, black bold lines) and CD70 (C, black bold lines) on immature MoDCs (iDC) or sCD40L-matured DCs in the absence or presence of PGE2 after 24 hours or 48 hours of culture was analyzed by flow cytometry. MoDCs were generated either in serum-free AIM-V medium (A-C) or AIM-V medium containing 5% human AB serum (D). Gray thin lines represent isotype control stainings. A representative of 3 (D) or at least 5 (B,C) independent experiments with different donors is shown.
PGE2-dependent expression of costimulatory molecules of the TNF superfamily on MoDCs. (A) Quantitative real-time RT-PCR analysis of TNFSF4 (OX40L), TNFSF7 (CD70), and TNFSF9 (4-1BBL) mRNA expression in MoDCs matured in the absence or presence of PGE2. Up-regulation of specific mRNA expression levels is depicted as fold increase by PGE2. Mean values and SEM derived from 7 individual donors are shown. Cell-surface expression of OX40L (B, black bold lines) and CD70 (C, black bold lines) on immature MoDCs (iDC) or sCD40L-matured DCs in the absence or presence of PGE2 after 24 hours or 48 hours of culture was analyzed by flow cytometry. MoDCs were generated either in serum-free AIM-V medium (A-C) or AIM-V medium containing 5% human AB serum (D). Gray thin lines represent isotype control stainings. A representative of 3 (D) or at least 5 (B,C) independent experiments with different donors is shown.
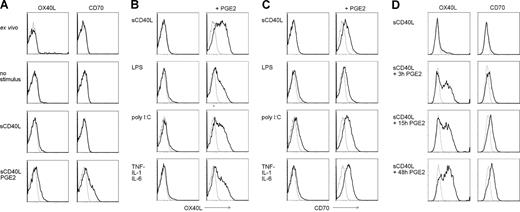
Next, we investigated the expression of costimulatory molecules on human peripheral blood myeloid (PB) DCs. Ex vivo PBDCs are CD83−, CD80−, CD86low (data not shown)34 and do not express OX40L and CD70 (Figure 3A). Stimulation of ex vivo PBDCs with sCD40L for 2 days induced surface expression of CD83, CD80, and CD86, but not OX40L and CD70. As for MoDCs, PBDCs expressed OX40L and CD70 exclusively if PGE2 was added to the maturation stimulus (Figure 3A).
Expression of OX40L and CD70 on ex vivo PBDCs and MoDCs strictly depends on PGE2 stimulation. (A) Myeloid DCs were isolated from peripheral blood of healthy donors, and cell-surface expression of OX40L and CD70 (black bold lines) was measured by flow cytometry on ex vivo DCs, or PBDCs cultured for 48 hours in medium alone (no stimulus), or matured with sCD40L in presence or absence of PGE2. (B,C) MoDCs were matured for 48 hours with sCD40L, LPS, poly I:C, or a cytokine cocktail consisting of TNF-α, IL-1β, and IL-6, in the absence or presence of PGE2 and analyzed for surface expression of OX40L (B, black bold lines) and CD70 (C, black bold lines) by flow cytometry. (D) MoDCs were matured with sCD40L, whereas PGE2 was present for the first 3 hours or 15 hours, or for the full period of maturation. Cell-surface expression of OX40L and CD70 (black bold lines) was assessed by flow cytometry after 48 hours of maturation. Isotype control stainings are presented as gray thin lines. A representative of 4 independent experiments with different donors is shown.
Expression of OX40L and CD70 on ex vivo PBDCs and MoDCs strictly depends on PGE2 stimulation. (A) Myeloid DCs were isolated from peripheral blood of healthy donors, and cell-surface expression of OX40L and CD70 (black bold lines) was measured by flow cytometry on ex vivo DCs, or PBDCs cultured for 48 hours in medium alone (no stimulus), or matured with sCD40L in presence or absence of PGE2. (B,C) MoDCs were matured for 48 hours with sCD40L, LPS, poly I:C, or a cytokine cocktail consisting of TNF-α, IL-1β, and IL-6, in the absence or presence of PGE2 and analyzed for surface expression of OX40L (B, black bold lines) and CD70 (C, black bold lines) by flow cytometry. (D) MoDCs were matured with sCD40L, whereas PGE2 was present for the first 3 hours or 15 hours, or for the full period of maturation. Cell-surface expression of OX40L and CD70 (black bold lines) was assessed by flow cytometry after 48 hours of maturation. Isotype control stainings are presented as gray thin lines. A representative of 4 independent experiments with different donors is shown.
Expression of OX40L and CD70 on mature MoDCs is PGE2-dependent
To test whether up-regulation of OX40L and CD70 on DCs generally depends on PGE2, we matured MoDCs by TLR4 or TLR3 stimulation or by adding a cytokine cocktail consisting of TNF-α, IL-1β, and IL-6. Interestingly, MoDC maturation by sCD40L, LPS, poly I:C, or a cocktail of cytokines in the absence of PGE2 was never accompanied by the expression of OX40L (Figure 3B), whereas MoDCs matured in the presence of PGE2 always expressed OX40L, independently of the maturation stimulus used (Figure 3B). Similarly, CD70 expression on MoDCs was only detectable if PGE2 was added during maturation (Figure 3C). In some donors, maturation via TLR3 signaling alone led to low expression of CD70, which was strongly enhanced in the presence of PGE2 (Figure 3C). In vivo, peripheral DCs are exposed to PGE2 that is produced very early during inflammation by monocytes, macrophages, fibroblast, and keratinocytes,38,39 only for a short period of time as they rapidly leave the inflammatory milieu. Therefore, we determined whether a short stimulation with PGE2 during the first hours of maturation was sufficient to up-regulate OX40L and CD70 expression on mature MoDCs. As depicted in Figure 3D, both OX40L and CD70 were expressed on MoDCs after a maturation time of 48 hours if PGE2 was added exclusively for the initial 3 hours of maturation. A prolonged presence of PGE2 further increased surface expression of both costimulatory molecules in some donors but was dispensable in others.
PGE2-induced augmentation of T cell–stimulatory capacities of MoDCs is inhibited by OX40L blockage
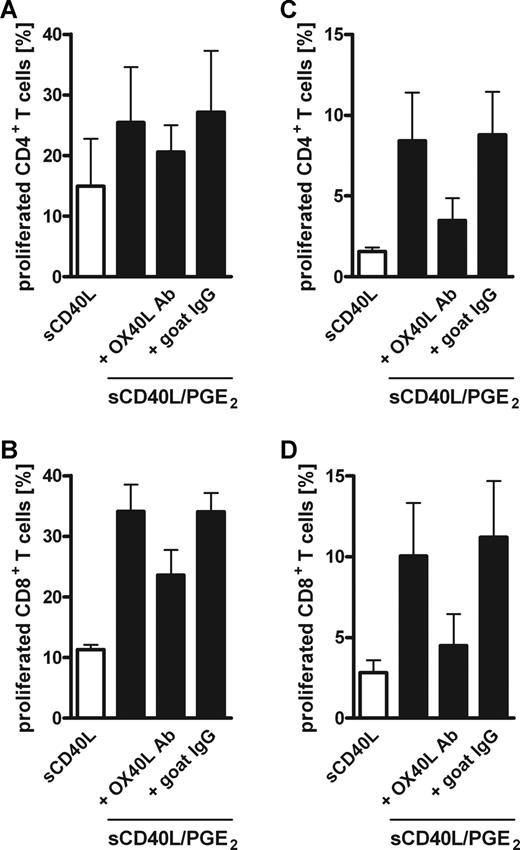
To determine the impact of PGE2-induced OX40L expression on human MoDCs, we cocultured antigen-pulsed MoDCs matured in the absence or presence of PGE2 with autologous PBMCs while blocking the OX40L/OX40 interaction using a neutralizing goat anti–human OX40L antibody. First, we used TT-pulsed MoDCs from TT-vaccinated donors to investigate the role of OX40/OX40L signaling on a recall, secondary immune response. After 6 days of coculture, neutralizing OX40L antibody inhibited the PGE2-dependent enhanced proliferation of both CD4+ and CD8+ T-cell populations in a concentration-dependent manner, whereas an irrelevant control goat IgG had no effect (Figure 4A,B). The effect of OX40L blockage was even more pronounced on day 12 of coculture (Figure 4C,D), which can be explained by the antiapoptotic signal provided by OX40 leading to survival of proliferating T cells.9 Noteworthy, the inhibition by OX40L neutralization was not complete, suggesting that CD70 and maybe 4-1BBL contribute to the enhanced T-cell proliferation induced by PGE2-matured MoDCs. Because there are currently no neutralizing antibodies for human CD70 and 4-1BBL commercially available, we were not able to address their contribution. Next, we tested whether the enhanced primary T-cell response induced by KLH-pulsed, PGE2-matured MoDCs was also mediated by OX40/OX40L. Indeed, neutralizing OX40L antibody significantly impaired the enhanced expansion of CD4+ and CD8+ T cells induced by PGE2-matured MoDCs at days 6 and 12 (Figure 5).
Blocking OX40L partially reversed the PGE2-induced enhanced capacity of MoDCs to stimulate memory T-cell proliferation. MoDCs were matured with sCD40L in the absence (□) or presence (■) of PGE2, pulsed with tetanus toxoid (TT), and cocultured with autologous CFSE-labeled PBMCs for 6 (A,B) or 12 days (C,D). Graded concentrations of a goat anti–human OX40L-neutralizing antibody (■) or control goat IgG (●) were added to cocultures with PGE2-matured MoDCs, and T-cell proliferation was analyzed by CFSE dilution of live (Sytox Blue negative) cells. Percentages of proliferating, CSFElowCD3+CD4+ (A,C) or CSFElowCD3+CD8+ (B,D) T cells are presented. Mean values and SEM of at least 4 independent experiments with different donors are shown. *P < .05; **P < .01; ***P < .001.
Blocking OX40L partially reversed the PGE2-induced enhanced capacity of MoDCs to stimulate memory T-cell proliferation. MoDCs were matured with sCD40L in the absence (□) or presence (■) of PGE2, pulsed with tetanus toxoid (TT), and cocultured with autologous CFSE-labeled PBMCs for 6 (A,B) or 12 days (C,D). Graded concentrations of a goat anti–human OX40L-neutralizing antibody (■) or control goat IgG (●) were added to cocultures with PGE2-matured MoDCs, and T-cell proliferation was analyzed by CFSE dilution of live (Sytox Blue negative) cells. Percentages of proliferating, CSFElowCD3+CD4+ (A,C) or CSFElowCD3+CD8+ (B,D) T cells are presented. Mean values and SEM of at least 4 independent experiments with different donors are shown. *P < .05; **P < .01; ***P < .001.
The enhanced primary T-cell response induced by PGE2-matured MoDCs can be inhibited by OX40L blockage. MoDCs were pulsed with KLH and matured with sCD40L in the absence (□) or presence of PGE2 (■) for 48 hours. Freshly isolated autologous PBMCs were labeled with CFSE and cocultured with MoDCs at a PBMC/MoDC ratio of 10:1. Where indicated, 5 μg/mL of a neutralizing goat anti–human OX40L antibody or a control goat IgG was added. CD4+ (A,C) and CD8+ (B,D) T-cell proliferation on day 6 (A,B) or 12 (C,D) of the coculture was analyzed by flow cytometry by CFSE dilution of live (Sytox Blue negative) cells. Mean values and SEM of 7 (A,B) or 4 (C,D) independent experiments with different donors are shown. *P < .05; **P < .01.
The enhanced primary T-cell response induced by PGE2-matured MoDCs can be inhibited by OX40L blockage. MoDCs were pulsed with KLH and matured with sCD40L in the absence (□) or presence of PGE2 (■) for 48 hours. Freshly isolated autologous PBMCs were labeled with CFSE and cocultured with MoDCs at a PBMC/MoDC ratio of 10:1. Where indicated, 5 μg/mL of a neutralizing goat anti–human OX40L antibody or a control goat IgG was added. CD4+ (A,C) and CD8+ (B,D) T-cell proliferation on day 6 (A,B) or 12 (C,D) of the coculture was analyzed by flow cytometry by CFSE dilution of live (Sytox Blue negative) cells. Mean values and SEM of 7 (A,B) or 4 (C,D) independent experiments with different donors are shown. *P < .05; **P < .01.
To rule out a possible role of non-T cells in our coculture system, we purified CD3+ T cells and cocultured them separately with autologous TT- and KLH-pulsed MoDCs matured in the presence or absence of PGE2. Similar to T cells in total PBMCs, cultivation of purified CD3+ T cells with MoDCs resulted in enhanced proliferation of CD4+ and CD8+ T cells when antigen-bearing MoDCs were matured in the presence of PGE2 (Figure 6). Moreover, the PGE2-induced augmentation of T cell–stimulatory capacities of MoDCs was reduced by blocking the OX40L/OX40 interaction (Figure 6).
Enhanced T-cell response induced by PGE2-matured MoDCs in cocultures with purified CD3+ T cells is inhibited by OX40L blockage. MoDCs were matured with sCD40L in the presence (■) or absence (□) of PGE2 and pulsed with either TT (A,B) or KLH (C,D) for 48 hours. Freshly isolated purified CD3+ T cells were labeled with CFSE and cocultured with autologous MoDCs at a T-cell/MoDC ratio of 10:1. Where indicated, 5 μg/mL of a neutralizing goat anti–human OX40L antibody or a control goat IgG was added. CD4+ (A,C) and CD8+ (B,D) T-cell proliferation was determined by flow cytometry on day 6 of the coculture by CFSE dilution of live (Sytox Blue negative) cells. Mean values and SEM of 3 independent experiments with different donors are shown.
Enhanced T-cell response induced by PGE2-matured MoDCs in cocultures with purified CD3+ T cells is inhibited by OX40L blockage. MoDCs were matured with sCD40L in the presence (■) or absence (□) of PGE2 and pulsed with either TT (A,B) or KLH (C,D) for 48 hours. Freshly isolated purified CD3+ T cells were labeled with CFSE and cocultured with autologous MoDCs at a T-cell/MoDC ratio of 10:1. Where indicated, 5 μg/mL of a neutralizing goat anti–human OX40L antibody or a control goat IgG was added. CD4+ (A,C) and CD8+ (B,D) T-cell proliferation was determined by flow cytometry on day 6 of the coculture by CFSE dilution of live (Sytox Blue negative) cells. Mean values and SEM of 3 independent experiments with different donors are shown.
PGE2-matured MoDCs induce expression of OX40L, OX40, and CD70 in T cells
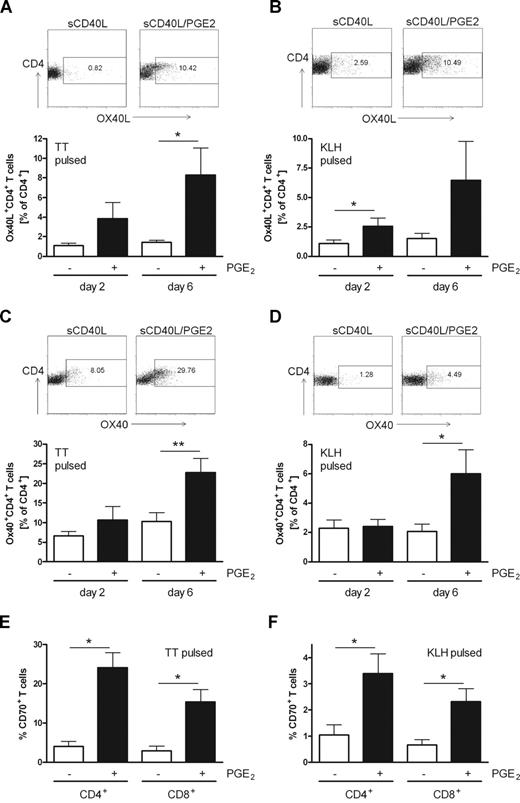
Costimulatory molecules are also involved in contacts between T cells. Expression of OX40L on activated CD4+ T cells has been reported to contribute to an enhanced T-cell survival by triggering OX40 on adjacent T cells.26 To test whether T cells interacting with (PGE2)–matured MoDCs up-regulate costimulatory molecules and thus deliver an amplification signal, we measured the expression of OX40L and OX40 on CD4+ T cells after 2 and 6 days of coculture of PBMCs with autologous antigen-pulsed MoDCs matured with sCD40L in the absence or presence of PGE2. The percentage of OX40L-expressing T helper cells increased in cocultures with PGE2-matured MoDCs (Figure 7A,B). This holds true for both primary KLH-mediated and secondary TT-mediated immune responses and was observed at day 2 and even more profoundly at day 6 of coculture. Moreover, OX40 is induced in CD4+ T cells after activation rendering them susceptible to OX40L-mediated survival signals,2 and CD4+ T cells from cocultures with TT-pulsed MoDCs matured in the presence of PGE2 expressed significantly more OX40 than those cocultured with MoDCs matured in the absence of PGE2 (Figure 7C). In addition, significantly more T cells expressed OX40 when responding to the primary antigen KLH presented by PGE2-matured MoDCs (Figure 7D). Because the expression of CD70 can be induced on T cells after T-cell receptor engagement,20,21 we determined the surface expression level of CD70 on CD4+ and CD8+ T cells after 6 days of stimulation by TT-pulsed (Figure 7E) or KLH-pulsed (Figure 7F) MoDCs matured in the absence or presence of PGE2. Significantly more CD4+ and CD8+ T cells expressed CD70 on the surface in cocultures with PGE2-matured MoDCs compared with MoDCs matured in the absence of the arachidonic acid metabolite. The expression of OX40L and CD70 on CD4+ T cells was not restricted to proliferating T cells but was also observed on a population of nonproliferating T cells; and a population of proliferating cells lacked expression of the 2 costimulatory molecules (data not shown).
Enhanced expression of OX40L, OX40, and CD70 on T cells stimulated with PGE2-matured MoDCs. MoDCs were matured with sCD40L in the absence or presence of PGE2, pulsed with TT (A,C,E) or KLH (B,D,F), and cocultured with autologous PBMCs. On days 2 and 6 of the coculture, surface expression of OX40L (A,B), OX40 (C,D), and CD70 (E,F) was analyzed on CD3+CD4+ or CD3+CD8+ T cells by flow cytometry. (A-D) One representative experiment on day 6 is shown as dot plots; diagrams represent mean values and SEM for at least 5 independent experiments with different donors. (E,F) Mean values and SEM of at least 4 independent experiments at day 6 are presented. *P < .05; **P < .01.
Enhanced expression of OX40L, OX40, and CD70 on T cells stimulated with PGE2-matured MoDCs. MoDCs were matured with sCD40L in the absence or presence of PGE2, pulsed with TT (A,C,E) or KLH (B,D,F), and cocultured with autologous PBMCs. On days 2 and 6 of the coculture, surface expression of OX40L (A,B), OX40 (C,D), and CD70 (E,F) was analyzed on CD3+CD4+ or CD3+CD8+ T cells by flow cytometry. (A-D) One representative experiment on day 6 is shown as dot plots; diagrams represent mean values and SEM for at least 5 independent experiments with different donors. (E,F) Mean values and SEM of at least 4 independent experiments at day 6 are presented. *P < .05; **P < .01.
In summary, we provide clear evidence that the inflammatory mediator PGE2 is critical during DC maturation, permitting efficient proliferation of antigen-specific CD4+ and CD8+ T cells. We demonstrate, for the first time, that PGE2 induces the up-regulation of the costimulatory molecules OX40L, CD70, and 4-1BBL on human DCs, correlating with an enhanced capacity to elicit primary and secondary T-cell responses. Blocking OX40/OX40L signaling impaired the enhanced T-cell proliferation induced by PGE2-matured MoDCs. Moreover, MoDCs matured in the presence of PGE2 induce the expression of OX40, OX40L, and CD70 on T cells, which provide prolonged survival signals. Together with the fact that PGE2 facilitates DC migration permitting the transport of antigens to draining lymph nodes, PGE2 also enables DCs to deliver an efficient costimulatory signal for naive and memory CD4+ and CD8+ T-cell responses.
Discussion
The exceptional ability of DCs to induce antigen-specific immune responses has brought them into the focus of cell-based immunotherapy of malignant diseases. As the quantity of sufficient numbers of primary human DCs is a limiting factor, MoDCs are used as source of DCs in most clinical studies. Large numbers of monocytes can be isolated from peripheral blood of patients and differentiated in vitro under serum-free conditions into immature MoDCs, which are fully competent to take up and process antigens. The pulsing with tumor antigens and induction of maturation result in antigen-presenting DCs, which can potently activate tumor antigen-specific immune responses. We and others have shown previously that supplementation of the maturation stimulus with PGE2 at concentrations found at inflammatory sites is critical to generate MoDCs with a migratory phenotype.32,34,40 Particularly, the presence of PGE2 in the initial phase of maturation is essential to promote DC migration toward lymph node–derived chemokines.34 Interestingly, PGE2 also affects T cell–stimulatory properties of MoDCs. Allogenic T-cell proliferation was enhanced in mixed lymphocyte reactions if MoDCs were matured in the presence of PGE2.32,35 In the present study, we provide evidence that PGE2-matured MoDCs have an enhanced potential to induce antigen-specific T-cell proliferation by up-regulating the expression of the costimulatory molecules OX40L, CD70, and 4-1BBL. The expression of OX40L on mature DCs was shown to prolong survival of OX40-bearing CD4+ T cells at the late phase of a primary immune response contributing to the development of a larger pool of memory T cells.4,9 Consequently, mice lacking OX40L expression on mature DCs are unable to mount a specific cellular antitumor response.41 Conversely, transfection of murine DCs with mRNA encoding OX40L augmented the induction of an antitumor response.15 On human MoDCs, a rather weak induction of OX40L expression has been described after TNF-α and CD40L stimulation.11 In the present study, we identify PGE2 as a key factor for the expression of OX40L and CD70 on the surface of mature MoDCs (Figure 2) and PBDCs (Figure 3A). For 4 different maturation stimuli, we observed OX40L and CD70 expression exclusively if DCs were matured in the presence of PGE2 (Figure 3B,C). Notably, addition of the TLR7/8 ligand resiquimod (R-848) to poly I:C and PGE2 for MoDC maturation resulted in migratory DCs that produced high amounts of IL-12p7042 (data not shown). However, addition of resiquimod does not induce full maturation of MoDCs43 (M.B., personal observation, August 2008). Consequently, we did not detect expression of OX40L and CD70 on MoDCs matured by poly I:C and resiquimod, even in the presence of PGE2 (data not shown). For all other maturation stimuli tested, PGE2 induced OX40L and CD70 on DCs.
The enhanced proliferation of antigen-specific T cells induced by MoDCs matured in the presence of PGE2 was partially reversed by antibody-mediated blockage of OX40/OX40L interactions (Figures 4,Figure 5–6). Blocking OX40L/OX40 signaling not only inhibited CD4+ T-cell proliferation but also strongly hampered the expansion of CD8+ T cells (Figures 4,Figure 5–6), even though OX40 is described to primarily affect CD4+ T-cell proliferation. However, we detected OX40 expression on 2% to 15% of CD8+ T cells after 6 days of coculture with PGE2-matured MoDCs, whereas OX40 expression was mostly absent if MoDCs were matured in the absence of PGE2 (data not shown). The inhibitory effect of OX40L-blockage on CD8+ T-cell proliferation may be indirect because of impaired CD4+ T-cell help occurring through multiple mechanisms, including cytokine production and supply of costimulatory signals. A role of OX40 on costimulation of CD8+ T cells is described, although the signal is weaker than that delivered by 4-1BB.44 Indeed, we also found a profound up-regulation of 4-1BBL mRNA on MoDCs matured in the presence of PGE2 (Figure 2A), which may contribute to the enhanced CTL response. Unfortunately, we could not investigate the role of human 4-1BBL/4-1BB signaling in our coculture system because of the lack of specific antibodies. Expression of CD70 on activated DCs contributes to the expansion of CD8+ T cells leading to the generation of memory CD8+ T cells, even in the absence of T cell help.45 Indeed, binding of CD70 to CD27 promoted survival of activated T cells, rather than augmenting cell-cycle progression,46 and blocking CD70 completely abrogated mouse CD8+ T-cell proliferation.23 Recently, it has been reported that CD70 must be expressed by DCs for efficient priming of CD8+ T cells to eliminate pathogens in vivo.24 The observation that antigen-induced expansion of CD8+ T cells is dependent on CD70 expression by DCs highlights the importance of our finding that CD70 expression in human MoDCs fully depended on the presence of PGE2 during maturation (Figure 3C). In addition, we found that PGE2-matured MoDCs induced CD70 expression on proliferating and nonproliferating CD4+ and CD8+ T cells (Figure 7E,F). T-cell/T-cell interaction mediated by CD70 and CD27 is a relatively early event after priming47 and plays a critical role not only in T-cell activation but also in T cell–dependent modulation of B-cell functions.16 Similar to CD70, OX40L expression was induced on CD4+ T cells on stimulation by MoDCs matured in the presence of PGE2 (Figure 7A,B). OX40L expressed on T cells provides a potent costimulatory signal, leading to augmented CD4+ T-cell proliferation and sustained CD4+ T-cell longevity.26,48 OX40/OX40L interaction between T cells was suggested to occur in T-cell zones of lymph nodes or spleen and to sustain T-cell survival after OX40L expression on APCs had diminished.48 Therefore, the induction of OX40L and CD70 on T cells by PGE2-matured MoDCs ensures the maintenance of survival signals through T-cell/T-cell contacts resulting in sustained and enhanced antigen-specific immune responses.
Triggering costimulatory molecules on APCs and T cells also modulates the profile of cytokine production16 and hence strongly influences the outcome of a T cell–mediated immune response. CD4+ T cells producing high amounts of IFN-γ are referred to as Th1 cells and play a major role in the defense against intracellular pathogens and tumors, whereas CD4+ T cells producing IL-4, IL-5, and IL-13 are termed Th2 cells and mediate immunity against helminth infection.49 Interestingly, a recent report points to CD70 as a decision marker for an IL-12–independent IFN-γ production/Th1 differentiation by a DEC205+ DC subset in vivo.50 The role of OX40 in T-cell differentiation is more complex. On the one hand, OX40 engagement was shown to enhance IL-4 production of T cells resulting in Th2 effector cell differentiation.48,51,52 On the other hand, in experimental allergic encephalomyelitis and an inflammatory bowel disease model, OX40/OX40L interactions promoted the generation of Th1 responses.14,53 Moreover, OX40L triggering on DCs enhanced its production of IL-12,11 favoring Th1 differentiation, and CD4+ T cells cocultured with MoDCs matured in the presence of PGE2 produced the Th1 cytokines IFN-γ and TNF-α, but only little IL-4.36 Because of these controversial observations, it may be that OX40L/OX40 interactions rather support the survival of activated T cells, which then differentiate dependent on the balance of existing cytokines, than serving as decision maker for Th1/Th2 differentiation.
An antitumor immune response is usually weaker than a response to pathogens because of the lack of danger signals. Moreover, the tumor environment can be immunosuppressive, resulting in antigen-specific tolerization of T cells. Stimulation of OX40 can restore antigen-specific proliferation and cytokine production by anergic CD4+ T cells, suggesting that OX40 signals can reverse established tolerance. Along this line, OX40 signaling has been shown to enhance antitumor immune responses, resulting in tumor rejection in a variety of tumor models.54–56 Breaking tumor-specific tolerance is of pivotal importance for the success of immunotherapy. The induction of OX40L on MoDCs by PGE2 could hence be a crucial parameter for DC-based immunotherapy. However, overcoming tolerance is not the only challenge immunotherapy is facing. The presence and induction of regulatory T cells (Treg) in tumors pose another problem. Interestingly, OX40 stimulation on T cells not only increases the frequencies of antigen-specific effector and memory T cells, but renders CD25−CD4+ T cells insusceptible to Treg-mediated suppression.57 Moreover, costimulation by OX40 inhibited TGF-β–driven Foxp3 expression in CD25−CD4+ T cells and prevented the induction of new Tregs from effector T cells and suppressed the functions of natural Foxp3+ Tregs.58,59 Thus, up-regulation of OX40L on MoDCs by PGE2 may fulfill major functions relevant for DC-based immunotherapies: enhancing survival of effector T cells, promoting the generation of memory T cells, breaking established tolerance, and suppressing natural Tregs.
Besides their role in the initiation of an adaptive immune response, DCs also play a role in the innate immune system. A recent study demonstrated that the antitumor activity of NKT cells depended on the expression of OX40L on DCs,41 thus further emphasizing the importance of PGE2-mediated OX40L up-regulation on MoDCs.
In conclusion, we demonstrate that human MoDCs greatly enhance their capacity to activate naive and memory, as well as CD4+ and CD8+ T cells if matured in the presence of PGE2 by up-regulating costimulatory molecules of the TNF superfamily. Surface expression of OX40L and CD70 on PBDCs and MoDCs strictly depended on PGE2 stimulation. Expression of OX40L and CD70 on migratory DCs has the potential to improve the efficacy of immunotherapy by promoting protective effector and memory T-cell responses, by hampering antigen-specific tolerance, and by attenuating the suppressor activity of Tregs. Besides its established role in facilitating DC migration, PGE2-dependent expression of OX40L and CD70 on mature human MoDCs as described herein represents a new parameter that will help to further optimize DC-based immunotherapy.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Dr Eva-Maria Boneberg for helpful discussions and Stefanie Buerger for excellent technical assistance.
This study was supported in part by research funding from the Vontobel Stiftung, the Swiss National Science Foundation (SNF 310000-112421/1), the Thurgauische Stiftung für Wissenschaft und Forschung, the Swiss State Secretariat for Education and Research, and the Thurgauische Krebsliga (D.F.L.). D.F.L. is a recipient of a Career Development Award from the Prof Dr Max Cloëtta Foundation.
Authorship
Contribution: P.K., M.B., M.G., and D.F.L. designed the research; P.K., M.B., C.U., and D.F.L. performed experiments and analyzed data; E.S. was responsible for blood donations; P.K., M.G., and D.F.L. wrote the paper; and D.F.L. organized and supervised research.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Daniel F. Legler, Biotechnology Institute Thurgau, Unterseestrasse 47, CH-8280 Kreuzlingen, Switzerland; e-mail: daniel.legler@bitg.ch.
References
Author notes
*P.K. and M.B. contributed equally to this work.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal