Natural killer (NK) cells express inhibitory receptors for major histocompatibility complex (MHC) class I. If self-MHC is down-regulated or absent, lack of inhibition triggers “missing self” killing. NK cells developing in the absence of MHC class I are hypo-responsive, demonstrating that MHC class I molecules are required for NK-cell education. Here, we show that the number and the type of MHC class I alleles that are present during NK-cell education quantitatively determine the frequency of responding NK cells, the number of effector functions in individual NK cells, and the amount of interferon-γ production in NK cells of specific Ly49 subsets. A relationship between the extent of inhibitory signals during education and functional responsiveness was corroborated by an enhanced probability of NK cells expressing more than one inhibitory receptor for a single host self–MHC class I allele to degranulate after activation. Our data suggest that the capacity of an individual NK cell to respond to stimulation is quantitatively controlled by the extent of inhibitory signals that are received from MHC class I molecules during NK-cell education.
Introduction
Natural killer (NK) cells take part in the defense against virus-infected, neoplastic, and allogeneic cells.1,2 NK-cell function is balanced by signals from inhibitory and activating receptors. If the balance is tipped toward activation, cytolysis and cytokine production are initiated.3 Murine inhibitory receptors belong to the Ly49 or the NKG2/CD94 family.4 Their ligands are MHC class I molecules, and MHC class I deficiency, or the presence of MHC class I alleles that fail to bind the inhibitory receptors, therefore results in “missing self” recognition.5
NK cells from mice and humans without MHC class I expression are incapable of rejecting MHC class I–deficient cells, showing that they have developed hyporesponsiveness in the MHC-deficient environment.6,–8 Conversely, NK cells lacking inhibitory receptors for self-MHC class I are hyporesponsive despite normal MHC class I expression.9,–11 Studies with MHC class I transgenic mice provided evidence that individual MHC class I alleles deliver educational signals to NK cells, and this conveys novel reactivity to the NK-cell system.12,13 Altogether, these data imply an education mechanism, in which host MHC class I molecules both secure self-tolerance and convey functional capacity to NK cells.6,7,9,10
The question of how MHC class I alleles influence NK-cell functionality is important for the understanding of hematopoietic stem cell transplantation across killer cell Ig-like receptor (KIR)/HLA donor-recipient mismatched barriers, in which NK cells elicit therapeutically beneficial missing self-rejections of recipient leukemic cells.14 Epidemiologic correlations between certain KIR/HLA genotypes and disease susceptibility and outcome constitute another area in which NK-cell education is of potential importance.15,–17 Several models have been proposed to explain the educational effects of MHC class I molecules on NK-cell function. Kim and colleagues suggested that NK cells are initially hypo-responsive and become “licensed” when their Ly49 receptors engage self–MHC class I during maturation.10,18 In another model, NK cells are responsive by default, and become hyporesponsive, or “disarmed,” in the absence of inhibitory input.9,19
A commonly held view in NK-cell education is that an NK cell is either educated or not, depending on whether its inhibitory receptors are engaged during NK-cell education. This view is based on the notions that Ly49 receptors discriminate sharply between MHC class I alleles20,21 and that the functional capacity of NK-cell subsets to develop missing self-activity depend on the MHC setup.22 Our recent studies showed, however, that individual MHC class I alleles are not equally efficient in educating NK cells for the capacity to reject MHC-deficient cells in vivo; some display a good “educating impact” on the NK-cell system, while some MHC class I alleles are less efficient.23 Here, we provide data that explain the difference in educating impact between MHC class I alleles. We show that the number and type of host MHC class I alleles quantitatively tunes the responsiveness of individual Ly49 subsets. This impact controls the number of responsive NK cells as well as the strength by which each responsive NK cell exerts its effector functions.
Methods
Mice and cell lines
All mice were bred and maintained at the Department of Microbiology, Tumor and Cell Biology, Karolinska Institutet, Stockholm, Sweden. C57BL/6 (B6) mice were originally obtained from Bomholt Gaard breeding and research center (Ry, Denmark). All animal experiments have been approved by the animal ethics committee in Stockholm. D8 mice (transgenic for H-2Dd on B6 background) have been described.24 B6.Kb−/−Db−/− (depicted MHC−/− throughout) mice and mice expressing only H-2Db or only H-2Dd (B6.Kb−/−Db−/−Dd+) on the B6 background were generated as described elsewhere.23,25 MHC class I molecules are denoted by their allelic (eg, Db, Dd) names throughout this article. YAC-1 is a Moloney virus-induced lymphoma of the A/Sn strain. A.H-2− is a β2-microglobulin–deficient variant of YAC-1. As YAC-1 down-regulates its MHC class I expression upon prolonged in vitro culture, its MHC class I expression is virtually negative and identical to that of A.H-2−. The 2 target cell lines generated identical results and were used interchangeably.
A 5(6)-carboxyfluorescein diacetate N-succinimidyl ester–assay for in vivo rejection of target cells
This method for quantitative measurements of in vivo killing has been described previously.23,26 In brief, 2 different populations of spleen cells, 1 syngeneic and 1 expressing a complete missing self-phenotype were labeled with 0.4 to 0.5 μM and 4 to 5 μM, respectively, of the dye 5(6)-carboxyfluorescein diacetate N-succinimidyl ester (CFSE; Invitrogen, Carlsbad, CA) and mixed 1:1. The ratio between the 2 populations before coinjection (determined by fluorescence-activated cell sorting [FACS] analysis) was compared with the ratios in the blood or spleen at various time points after inoculation, allowing a quantitative measurement of the relative rejection of MHC−/− cells in each mouse strain. For NK-cell depletions, 200 μg of the TMβ-1 antibody (anti-interleukin [IL]–2Rβ; rat IgG2b) was given intraperitoneally 2 days before the experiment. TMβ-1 was purified from hybridoma supernatants by MabTech (Stockholm, Sweden).
Antibodies and FACS analysis
Before staining of surface markers, Fc receptors were blocked by incubation with anti-FcγRIII (2.4G2). Antibodies used for FACS staining were purchased from BD PharMingen (Stockholm, Sweden) and consisted of the following: anti-NK1.1 (PK136)–fluorescein isothiocyanate (FITC), anti-NK1.1 (PK136)–FITC-phycoerythrin (PE), PE-Cy7 and Cy7–allophycocyanin (APC), anti-CD3 (145-2C11)–peridinin chlorophyll protein (PerCP)–Cy5.5, anti-Ly49G2 (4D11)–APC, anti-Ly49G2 (4D11)–FITC, anti-Ly49F (HBF-719)–PE, anti-CD107a (1D4B)–FITC, anti–Mac-1 (M1/70)–FITC, anti-CD127 (IL-7Rα)–FITC, anti-Ly49D (4E5)–FITC, anti-Ly49D (4E5)–purified, anti-LFA-2/CD2 (RM2-5)–FITC, anti-CD16 (2.4G2)–PE, anti-CD11c (HL3)–PE, anti-CD244.2 (2B4)–PE, anti-CD44 (IM7)–PE, anti-LAG-3 (C9B7W)–PE, anti-NKG2D (A10)–PE, anti-NKG2D (A10)–purified, anti-NKG2A (16a11)–PE, anti-CD27 (LG.3A10)–PE, anti-CD69 (H1.2F3)–PerCP Cy5.5, anti-DX5 (HMα2)–APC, anti–IFN-γ (XMG1.2)–PE and streptavidin-PE, streptavidin-FITC, streptavidin-APC, PerCP Cy5.5. Anti-Ly49C (4LO3311) biotin and hybridoma was a kind gift from Suzanne Lemieux (INRS–Institut Armand-Frappier, Laval, Quebec) and was used with anti–mouse IgG3-APC-Cy7 (Southern Biotech, Birmingham, AL). Anti-Ly49I (YLI-90)–purified (eBiosciences, San Diego, CA) was used with Zenon Kit Alexa 700 (Invitrogen). Anti-Ly49A (YE1/48)–biotin (StemCell Technologies, Vancouver, BC) was used with streptavidin QD565 (Invitrogen). NKG2A/C/E (20d5)–purified (BD Biosciences, San Jose, CA) was conjugated to Pacific Blue using an antibody labeling kit (Invitrogen). NKp46 (NCR1)–biotin was purchased from R&D Systems (Abingdon, United Kingdom). For functional experiments, dead cells were excluded using the Vivid near IR reagent (Invitrogen). Results were acquired with a FACSAria (BD Biosciences) and analyzed using the FlowJo software (TreeStar, Ashland, OR).
In vitro stimulation assay
We added 1.5 × 106 splenocytes from naive mice (Figure 5D-F) and from mice pretreated intraperitoneally with 70 μg polyriboinosinic:polyribocytidylic acid (poly IC) on day 1 or spleen cells stimulated for 4 days with IL-15/18 (50 ng/mL) to plates precoated (for 2 hours, at 37°C) with 50 μg/mL anti-NK1.1 (PK136), anti-NKG2D (A10) or 10 μg/mL anti-Ly49D (4E5; BD Biosciences) or NKp46/Mar-1 (R&D Systems) antibodies. GolgiStop (BD PharMingen) and 500 U/mL IL-2 was added to the wells. For degranulation experiments, anti-CD107a (LAMP-1) was also included during the stimulation (5 μg/mL). For negative controls, cells were incubated in wells coated with phosphate-buffered saline (PBS) and for positive controls cells were stimulated with 20 ng/mL phorbol-12-myristate-13-acetate (PMA) plus 1 μg/mL of ionomycin (Sigma-Aldrich, St Louis, MO). Plates were incubated at 37°C for 4 to 6 hours followed by surface staining, as described in “Antibodies and FACS analysis.” Strategies to identify NK cells differed depending on the activating receptor targeted for stimulation, but usually DX5 or NK1.1 was used with CD3. For IFN-γ detection, cells were fixed and permeabilized using the Cytofix/cytoperm kit (BD PharMingen) followed by intracellular cytokine staining. For coculture experiments, 1.5 × 106 LAK cells activated by IL-15/18 (50 ng/mL, 4 days) were cocultured with 0.3 × 106 YAC-1 cells for 4 hours before surface staining, fixation, and intracellular staining as described in “Antibodies and FACS analysis.” In some of the experiments, a β2-microglobulin–deficient variant of YAC-1 (A.H-2−) was used. Both these cell lines have very low levels of surface MHC class I at the cell surface, and, as the results with the 2 variants were identical, we compiled all data into 1 figure.
Statistical calculations
Subset responses (percentages of CD107a+, IFN-γ+ or geometric mean fluorescence intensity [GeoMFI] IFN-γ+) were compared between groups using a nonparametric, paired, 2-tailed Wilcoxon signed rank test. For significant Ly49 receptor down-regulation (Figure 3A), the same test was used comparing the GeoMFI for the Ly49 receptor of interest in a given mouse with that of MHC−/− NK cells from the same experiment. Synergistic functional responses in Ly49 receptor subsets (percentages of CD107a+ NK cells) were related to the same subset in MHC−/− mice after subtracting the background activity seen in the PBS-negative control.
Results
More efficient missing self-rejection in vivo in mice with multiple MHC class I alleles
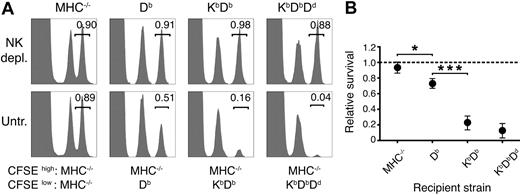
To quantify the strength of missing self-responses in vivo after successive addition of host MHC class I alleles, normal cells displaying a full missing self-phenotype were grafted to mice with no (MHC−/−), 1 (Db), 2 (KbDb), or 3 (KbDbDd) MHC class I alleles. Rejection was measured using a quantitative CFSE-based method.23,26 In accordance with our previous results,23 Db alone provided a clear educating impact on missing self-rejection (Figure 1). Adding Kb further enhanced rejection, while introduction of Dd to KbDb mice had only a small, although consistent, additional effect (Figure 1). These results demonstrate a combined action of several MHC class I alleles on NK-cell education, leading to stronger missing self-reactivity in vivo.
More efficient missing self-reactivity in mice expressing multiple MHC class I alleles. (A) Remaining CFSE+ donor spleen cells in the blood 2 days after injection. Numbers indicate the relative survival in the test population (CFSEhigh) compared with the syngeneic control population (CFSElow). Graphs show data from 1 representative mouse. (B) Summary of all 3 experiments performed. The dashed line represents complete survival, and error bars denote SD. Each data point represents n = 2-6. MHC−/− versus Db: *P < .05; Db versus KbDb: ***P < .001; KbDb versus KbDbDd: nonsignificant. depl. indicates depleted; untr., untreated.
More efficient missing self-reactivity in mice expressing multiple MHC class I alleles. (A) Remaining CFSE+ donor spleen cells in the blood 2 days after injection. Numbers indicate the relative survival in the test population (CFSEhigh) compared with the syngeneic control population (CFSElow). Graphs show data from 1 representative mouse. (B) Summary of all 3 experiments performed. The dashed line represents complete survival, and error bars denote SD. Each data point represents n = 2-6. MHC−/− versus Db: *P < .05; Db versus KbDb: ***P < .001; KbDb versus KbDbDd: nonsignificant. depl. indicates depleted; untr., untreated.
Increasing the number of MHC class I alleles enhances the number of functionally responsive NK cells
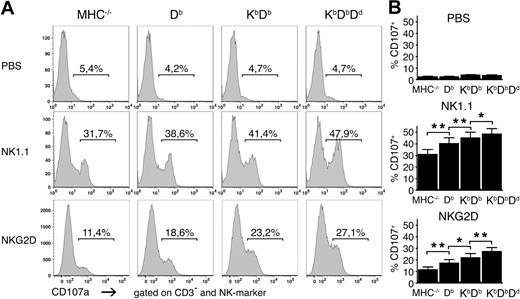
To test if differences in the strength of missing self-rejection was reflected in the number of responsive NK cells, we investigated the capacity of NK cells from the 4 mouse lines to degranulate after stimulation using antibodies against the activating receptors NK1.1 and NKG2D. The numbers of degranulating NK cells became larger as the number of MHC class I alleles increased in the host (Figure 2). Similar results were observed using antibodies against NKp46 and Ly49D (data not shown). This pattern was not due to differences in the frequencies or expression levels of the activating receptors targeted for stimulation, which were expressed similarly in the different mice (Figure S1, available on the Blood website; see the Supplemental Materials link at the top of the online article). We also studied 14 other cell-surface molecules: activating and inhibitory receptors, activation markers, and adhesion molecules. None of these showed any clear differences in expression between the different strains (Figure S2). Thus, we concluded that the number of functionally responsive NK cells is controlled by the number of MHC class I alleles and varies in the same direction as the strength of the missing self-response.
Higher frequencies of responding NK cells in the presence of multiple MHC class I alleles. (A) Frequencies of CD107a+ NK cells after stimulation with plate-bound antibodies against NK1.1 and NKG2D or in the absence of antibody (PBS) for 5 hours. One representative experiment of 11. (B) Summaries of all 11 experiments performed, expressed as mean percentage of CD107a+CD3−DX5+ NK cells with SEM. *P < .05 and **P < .01.
Higher frequencies of responding NK cells in the presence of multiple MHC class I alleles. (A) Frequencies of CD107a+ NK cells after stimulation with plate-bound antibodies against NK1.1 and NKG2D or in the absence of antibody (PBS) for 5 hours. One representative experiment of 11. (B) Summaries of all 11 experiments performed, expressed as mean percentage of CD107a+CD3−DX5+ NK cells with SEM. *P < .05 and **P < .01.
Addition of novel MHC class I alleles results in a cumulative educational effect on single Ly49/NKG2 subsets
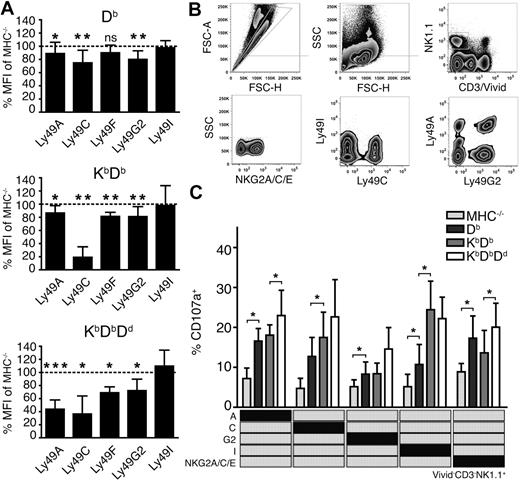
To investigate how individual Ly49 receptors were affected by the sequential addition of MHC class I alleles, we measured the expression levels of the inhibitory receptors Ly49A, C, F, G2 and I on NK cells from the 4 mouse lines. Notably, most Ly49 receptors were down-regulated in all mice, but to a different extent (Figure 3A). In Db single mice, 3 of 5 Ly49 subsets were weakly affected23 (Figure 3A), reflecting a history of receptor-ligand interactions in this particular host. After addition of Kb, Ly49A and Ly49G2 were unaltered while Ly49C and Ly49F were further down-regulated (Figure 3A). The addition of Dd to KbDb led to additional down-regulation of Ly49A, F and G2, but did not further affect Ly49C. Altogether, these data indicate a quantitative effect on a given Ly49 subset, based on the combined educating impact of MHC class I, reflected in the degree of receptor down-regulation.
More extensive Ly49 receptor down-regulation and improved functional responses of NK cells expressing single inhibitory receptors in the presence of multiple MHC class I alleles. (A) Surface expression of inhibitory Ly49 receptors (GeoMFI) relative to MHC−/− NK cells (dotted line). Summary of 6 to 15 experiments with n = 11 Db, n = 9 KbDb and n = 8 KbDbDd mice. *P < .05; **P < .01; ***P < .001. (B) Identification of viable NK cells expressing only single inhibitory receptors. Gates are indicated with gray lines. FSC-A indicates forward light scatter area and SSC, side light scatter. (C) Degranulation of naive NK cells from MHC−/−, Db, KbDb, and KbDbDd mice expressing only 1 inhibitory receptor (indicated in the matrix). Presented as mean percentage of CD107a+ NK cells with SEM, and PBS control background subtracted, within the specified single Ly49 subset after anti-NKG2D stimulation.
More extensive Ly49 receptor down-regulation and improved functional responses of NK cells expressing single inhibitory receptors in the presence of multiple MHC class I alleles. (A) Surface expression of inhibitory Ly49 receptors (GeoMFI) relative to MHC−/− NK cells (dotted line). Summary of 6 to 15 experiments with n = 11 Db, n = 9 KbDb and n = 8 KbDbDd mice. *P < .05; **P < .01; ***P < .001. (B) Identification of viable NK cells expressing only single inhibitory receptors. Gates are indicated with gray lines. FSC-A indicates forward light scatter area and SSC, side light scatter. (C) Degranulation of naive NK cells from MHC−/−, Db, KbDb, and KbDbDd mice expressing only 1 inhibitory receptor (indicated in the matrix). Presented as mean percentage of CD107a+ NK cells with SEM, and PBS control background subtracted, within the specified single Ly49 subset after anti-NKG2D stimulation.
To test the relationship between the effects on receptor down-regulation and effector competence, we next tested functional responses of NK cells expressing either one of the inhibitory receptors Ly49A, Ly49C, Ly49G2, Ly49I, or NKG2A, but none of the others. To allow such analysis, a multiparameter flow cytometry protocol was developed (Figure 3B) to measure functional responses in all these single receptor subsets after the triggering of activating receptors. All subsets were more functional in all MHC+ mice than in MHC−/− mice (Figure 3C), but differed in the development of functionality as MHC class I alleles were added. For example, NK cells expressing only Ly49A responded more frequently in mice expressing Db (a weak self-ligand for this receptor) than in mice lacking MHC class I completely (Figure 3C). Addition of Kb had little or no effect on functionality of the Ly49A single receptor subset, while a further increase in responsiveness was seen in mice also expressing Dd, a known strong ligand for Ly49A (Figure 3C). The results were similar for NK cells expressing only Ly49G2. Cells expressing only Ly49C or Ly49I were educated by Db and Kb, with no additional contribution from Dd (Figure 3C). We also studied NK cells expressing the NKG2A receptor, which recognizes Qa-1b in complex with peptides from classical MHC class I molecules. NKG2A+ NK cells (expressing NKG2A but none of the other receptors) displayed functional capacity in all mice, consistent with the presence of MHC class I alleles that can supply the correct peptide for Qa-1b in all situations. A significantly stronger response was seen upon addition of Dd to KbDb, suggesting the NKG2A-dependent education is also dependent on the number of MHC class I alleles that contribute leader peptides for Qa-1b presentation.
Single MHC class I alleles with strong and weak educating impact recruit different numbers of single Ly49A+ NK cells to the responsive pool
To directly test the role of individual MHC class I alleles in quantitative education of NK-cell subsets, we compared the responses of single Ly49A-expressing NK cells in mice expressing only Db, which is a weak ligand for this receptor21,27 or only Dd, which is a strong ligand.20,21,27,28 Missing self-rejection of MHC−/− splenocytes in vivo was stronger in Dd than in Db mice (Figure 4A), confirming the higher educating impact of the first allele.23 After NKG2D-stimulation, a higher frequency of single Ly49A+ NK cells degranulated in Db single mice than in MHC− mice, and this fraction was further increased in Dd single mice as compared with the same subset from Db single mice (Figure 4B,C). Similar results were obtained using antibodies against NK1.1, NKp46 and Ly49D (data not shown). These data conclusively show that a given Ly49 subset can be educated more or less well depending on the educating impact of the MHC class I allele that it interacts with in vivo.
Higher frequencies of responsive single Ly49A–expressing NK cells in the presence of a strong versus weak MHC class I ligand. (A) Survival of MHC−/− splenocytes in Db and Dd mice relative to MHC− mice. Kinetics over 1, 2, and 4 days. Dd versus Db: **P < .01 at all time points. depl. indicates depleted. (B) Percentage of CD107a+ NK cells expressing the inhibitory receptor Ly49A, but not Ly49C, I, G2, or NKG2A/C/E, from naive Db and Dd single mice after NKG2D stimulation. One representative experiment of 8. SSC indicates side light scatter. (C) Summary of all experiments performed. **P < .01; ***P < .001.
Higher frequencies of responsive single Ly49A–expressing NK cells in the presence of a strong versus weak MHC class I ligand. (A) Survival of MHC−/− splenocytes in Db and Dd mice relative to MHC− mice. Kinetics over 1, 2, and 4 days. Dd versus Db: **P < .01 at all time points. depl. indicates depleted. (B) Percentage of CD107a+ NK cells expressing the inhibitory receptor Ly49A, but not Ly49C, I, G2, or NKG2A/C/E, from naive Db and Dd single mice after NKG2D stimulation. One representative experiment of 8. SSC indicates side light scatter. (C) Summary of all experiments performed. **P < .01; ***P < .001.
A strong educating impact is associated with more efficient NK cells in the responsive pool
So far, our results indicated that the inhibitory input during the education process, controlled both by the number and the type of MHC class I alleles, is a critical element in the determination of the frequency of NK cells within the responsive pool. To test whether the amount of inhibitory input would also determine the efficiency by which each educated NK cell would respond, we analyzed degranulation and IFN-γ production together, after receptor stimulation in a single cell assay, allowing for the estimation of 2 effector responses simultaneously. The number of IFN-γ+ NK cells in the population after NK1.1 stimulation increased, just as the percentage of CD107a+ had increased with the number of MHC class I alleles (Figure 5A). IFN-γ–producing cells were entirely confined to the CD107a+ population, thus identifying NK cells capable of responding with 2 different effector responses to a given stimulus. Interestingly, the fraction of such NK cells increased in the presence of more MHC class I alleles (Figure 5A,B). Similar results were obtained using stimulating antibodies against Ly49D and NKp46 (Figure 5B).
Strong educating impact leads to more efficient NK cells within the responsive pool. (A) Degranulation and IFN-γ production in NK cells from naive MHC−/−, Db, KbDb, and KbDbDd mice in response to anti-NK1.1 stimulation (stim.). The large gate defines frequencies of total CD107a+ and the small gate CD107a+ and IFN-γ+ double-positive NK cells. One representative experiment of 7. (B) Summary of all 7 experiments performed, as in panel A, with the percentage of CD107a+ IFN-γ+ NK cells expressed as the mean with SEM. (C) Mean GeoMFI for IFN-γ+ with SEM for all 7 experiments performed. (D) GeoMFI of IFN-γ in Ly49A+C−I−Ly49G2− IL-15/18–activated NK cells from MHC−/−, Db, and Dd mice after stimulation with antibodies against Ly49D, NK1.1, and NKG2D. Numbers indicate the percentage of IFN-γ+ cells and GeoMFI for IFN-γ. One representative experiment of 3. (E) Similar experiment as in panel D. Anti-Ly49D stimulation of poly IC–activated NK cells, numbers indicate the percentage of IFN-γ+ cells and GeoMFI for IFN-γ+. (F) The percentage of IFN-γ+ (top graph) and GeoMFI (bottom graph) in Ly49A+C−I−Ly49G2− IL-15/18–activated NK cells from Db and Dd mice after stimulation with either YAC-1 or its β2m-deficient variant AH-2−. YAC-1 and A.H-2− both display very low levels of MHC class I at the cell surface and, as they gave identical results, all experiments were combined in the same figures. In total, 7 experiments were performed. Data are expressed as mean relative values compared with MHC−/− NK cells from the same experiment. *P < .05; **P < .01; ***P < .001.
Strong educating impact leads to more efficient NK cells within the responsive pool. (A) Degranulation and IFN-γ production in NK cells from naive MHC−/−, Db, KbDb, and KbDbDd mice in response to anti-NK1.1 stimulation (stim.). The large gate defines frequencies of total CD107a+ and the small gate CD107a+ and IFN-γ+ double-positive NK cells. One representative experiment of 7. (B) Summary of all 7 experiments performed, as in panel A, with the percentage of CD107a+ IFN-γ+ NK cells expressed as the mean with SEM. (C) Mean GeoMFI for IFN-γ+ with SEM for all 7 experiments performed. (D) GeoMFI of IFN-γ in Ly49A+C−I−Ly49G2− IL-15/18–activated NK cells from MHC−/−, Db, and Dd mice after stimulation with antibodies against Ly49D, NK1.1, and NKG2D. Numbers indicate the percentage of IFN-γ+ cells and GeoMFI for IFN-γ. One representative experiment of 3. (E) Similar experiment as in panel D. Anti-Ly49D stimulation of poly IC–activated NK cells, numbers indicate the percentage of IFN-γ+ cells and GeoMFI for IFN-γ+. (F) The percentage of IFN-γ+ (top graph) and GeoMFI (bottom graph) in Ly49A+C−I−Ly49G2− IL-15/18–activated NK cells from Db and Dd mice after stimulation with either YAC-1 or its β2m-deficient variant AH-2−. YAC-1 and A.H-2− both display very low levels of MHC class I at the cell surface and, as they gave identical results, all experiments were combined in the same figures. In total, 7 experiments were performed. Data are expressed as mean relative values compared with MHC−/− NK cells from the same experiment. *P < .05; **P < .01; ***P < .001.
These experiments also revealed an increase in the average content of IFN-γ in NK cells from mice expressing multiple MHC class I alleles as measured by the GeoMFI IFN-γ+ (Figure 5C). This was not seen in unstimulated cells or in cells treated with the unspecific stimulant PMA plus ionomycin (Figure S3). To further study the finding that individual cells seemed to produce varying amounts of cytokine depending on the educating allele, we compared IFN-γ production in single Ly49A-expressing NK cells that were educated in the context of a weak (Db) versus a strong (Dd) ligand. To optimize resolution for this type of analysis, we forced cytokine production by cultures in IL-15/18. More single-Ly49A+ NK cells responded with IFN-γ production in Dd single mice than in Db single mice, and these NK cells also responded, on average, more strongly at the single cell level (Figure 5D). The result was even clearer for NK cells from mice pretreated with poly IC (Figure 5E).
To verify that the quantitative responses in NK cells from different MHC backgrounds was not an artifact of directed stimulation of specific activating receptors only, we cocultured NK cells with YAC-1 tumor cells and investigated IFN-γ secretion in single Ly49A+ IL-15/18-activated NK cells from Db and Dd mice. Even in this case, more NK cells produced IFN-γ from Dd single mice than from Db single mice, and they also produced, on average, more IFN-γ per cell (Figure 5F).
We conclude that the education of NK cells by an MHC class I context with high educating impact not only leads to a higher frequency of responding NK cells, but also to responding NK cells that are more likely to exhibit 2 rather than 1 effector function and to elicit a quantitatively stronger response to a given stimulus. These experiments also show that the activation state of the NK cell at the time of receptor stimulation did not affect the differences between NK cells that had been set during education, at least not activation imposed by IL-15/18 and type I IFN inducers. Altogether, our results imply a model where the potency of each individual NK cell can vary along a continuum depending on the extent of inhibitory input it receives during education.
NK cells expressing more than one inhibitory receptor for self–MHC class I are more likely to respond to activation than NK cells with a single receptor
So far, we had varied the inhibitory input by changing, or adding, MHC class I ligands, and analyzed how this affected individual NK-cell subsets defined by one particular single-Ly49 receptor. We next asked whether variations in inhibitory input caused by coexpression of several inhibitory receptors in a constant MHC class I environment would have a similar effect on NK-cell responsiveness. To address this question, we stimulated NK cells expressing only 1, 2, or 3 known self-specific inhibitory receptors with antibodies against NKG2D and analyzed the degranulation capacity in each subset. In a mouse expressing the weakly educating MHC class I allele Db, we found that NK cells expressing Ly49A and C together, but no other Ly49/NKG2 receptors, showed significantly higher responsiveness than NK cells expressing only Ly49A or only Ly49C (Figure 6A). NK cells expressing also a third self-receptor, NKG2A, showed an even higher proportion of responsive cells (Figure 6A). Thus, the coexpression of multiple inhibitory self-receptors can also act to increase the inhibitory input and consequently lead to increased responsiveness in an environment where the ligands are kept constant.
Synergistic effects of certain combinations of inhibitory receptors on NK-cell responsiveness. (A) Relative frequency of CD107a+ NK cells expressing the combinations of Ly49A, Ly49C, and NKG2A in Db single mice. Ly49C and Ly49I were gated away. (B) A similar analysis in Dd single mice, including Ly49A, Ly49G2, and NKG2A, excluding Ly49C and Ly49I. (C) A similar analysis in KbDb mice, including Ly49C, Ly49I, and NKG2A, excluding Ly49A and Ly49G2. (D) Effect on CD107a-expression of adding the 2 excluded receptors in each mouse. Data are expressed as mean percentages of CD107a+ NK cells in a given subset relative to the receptor-negative NK cells from the same mouse strain and experiment. Mean fold change with SEM from at least 8 experiments. *P < .05; **P < .01; ***P < .001.
Synergistic effects of certain combinations of inhibitory receptors on NK-cell responsiveness. (A) Relative frequency of CD107a+ NK cells expressing the combinations of Ly49A, Ly49C, and NKG2A in Db single mice. Ly49C and Ly49I were gated away. (B) A similar analysis in Dd single mice, including Ly49A, Ly49G2, and NKG2A, excluding Ly49C and Ly49I. (C) A similar analysis in KbDb mice, including Ly49C, Ly49I, and NKG2A, excluding Ly49A and Ly49G2. (D) Effect on CD107a-expression of adding the 2 excluded receptors in each mouse. Data are expressed as mean percentages of CD107a+ NK cells in a given subset relative to the receptor-negative NK cells from the same mouse strain and experiment. Mean fold change with SEM from at least 8 experiments. *P < .05; **P < .01; ***P < .001.
To test whether this pattern would emerge also in a mouse expressing a strong educating allele, Dd, we compared the proportion of responding cells among NK cells expressing only Ly49A, G2, and NKG2A, alone or in combinations. Interestingly, all 3 single-expressing subsets showed a rather high level of responsiveness (Figure 6B). Here, expression of these receptors in pairs led only to a small and insignificant increase in responsiveness, while coexpression of all 3 receptors synergized and led to a significantly higher response than that seen with the single-expressing NK cells. Thus, while synergistic effects between different receptors can be observed in both strains, they seem to be more pronounced in the context of a weakly educating MHC class I allele.
We next tested for synergy effects in a situation with more than one educating MHC class I allele. To this end, we chose NK cells expressing combinations of the Kb-specific receptors Ly49C and I and the receptor NKG2A, known to recognize Qa-1b in mice with Db (but not Kb) leader peptides. In a mouse expressing both Kb and Db, a synergistic effect was noted between NK cells expressing NKG2A and either Ly49C or I (Figure 6C). However, no increased responsiveness was seen in the presence of all 3 receptors, as compared with the double receptor expressing subsets. This implies that in this particular case, the system may be saturated upon expression of 2 self-specific receptors, overall supporting a quantitative view on NK-cell education controlled by the number and affinities of individual MHC class I alleles. When the 2 receptors excluded in the previous analysis were included, no further synergies were seen in single Db or Dd mice (Figure 6D). In contrast, a significant increase in responsiveness was seen in KbDb mice in which NK cells expressing Ly49A, G2, C, I, and NKG2A were compared with NK cells expressing Ly49C, I, and NKG2A (Figure 6D). These results further corroborate the notion that NK-cell responsiveness is dynamically determined by an interplay between the number and type of MHC class I alleles and the entire Ly49 repertoire.
Discussion
Multicolor flow cytometry is a powerful tool that allows dissections of how specific phenotypes are linked to effector functions in subsets of lymphocytes. In this study, this technique has allowed us to identify a cellular correlate to the educating impact imposed by MHC class I alleles on NK-cell function, providing an analysis in the mouse at a level of resolution that has not previously been reported. The successive introduction of MHC class I alleles in the host genome in mice, starting with 1 allele and ending with 3, led to a gradually increased efficiency of missing self-rejection. A similar situation was seen when a single MHC class I allele with a strong educating impact was compared with a single allele with a weak educating impact. Our analysis demonstrated that at least 2 quantitative aspects contribute to the increased NK-cell functionality as a result of stronger inhibitory signaling: (1) a gradual increase in the number of NK cells in the responsive NK-cell pool; and (2) a gradually increased functional efficiency of individual NK effector cells within this responsive pool. These results provide evidence for a quantitative tuning of NK-cell responsiveness determined by the binding strength of the MHC class I/Ly49 interaction. This notion was also supported by our finding that NK-cell responsiveness was higher in NK cells expressing multiple inhibitory self-receptors than in NK cells expressing only one self-receptor, in the presence of a given MHC class I repertoire.
Before discussing the model that we propose to account for our observations, it is important to compare our results regarding the binding specificities of Ly49 receptors to previously reported results. In our functional studies of NK-cell subsets, we found evidence for education in several unexpected MHC-Ly49 combinations. For example, we found that NK cells expressing either Ly49I or Ly49G2 alone were both educated by Db in our assay. Earlier binding data using Db tetramers or cells in vitro are, however, mostly negative.20,21 We also noted that Ly49A-expressing NK cells in KbDb mice tended to respond more vigorously compared with mice expressing Db alone. While this difference was small and must be considered uncertain, the reason we think it may be significant is our observation that single Ly49A-expressing NK cells clearly develop functional responsiveness in Kb single mice, implying a role for Kb in the education of Ly49A+ NK cells (P.B., unpublished data, May 2008). These observations are nevertheless at odds with in vitro data failing to demonstrate binding between Ly49A and Kb.20,21 Further corroborating this view of previously unrecognized interactions contributing to NK-cell education was our finding of increased responsiveness in NK cells expressing the 5 receptors Ly49A, G2, C, I, and NKG2A as compared with NK cells expressing C, I, and NKG2A+ in KbDb-expressing mice (Figure 6B). A possible reason for the discrepancies between our data and previous binding assays is that some Ly49/MHC interactions are too weak to allow tetramer binding or stable cell-cell interactions in vitro. In contrast, interactions within a tissue stroma in vivo would potentially promote more stable and long-lasting conjugates, allowing for productive outcomes of low affinity interactions. Additional FACS stainings including NK-cell inhibitory receptors that were not included in our antibody panel (Ly49E, F, and J) will also be of interest, although the low frequency of those receptors on NK cells (2%-8%) makes it unlikely that they would significantly affect our conclusions regarding single Ly49-expressing NK-cell subsets.29,–31
NK-cell education has been interpreted mainly as an “on-off switch,” that is, either an NK cell becomes responsive or not after interactions with MHC class I. Our previous data,23 recent data on human NK cells,32,–34 and data in this study suggest, however, that the end point of NK-cell education varies along a continuum with respect to the potency of each cell. Based on our observations in this direction, we propose a quantitative model for NK-cell education. According to this model, NK cells sense inhibitory signals in a quantitative manner through their Ly49/NKG2 receptors during education, with different allelic specificities and affinities. The “sum” of these signals then adjusts downstream activating pathways, much like a “rheostat” controlling an electrical circuit. Consequently, a higher inhibitory input during education gives more efficient activation pathways, with 2 consequences: (1) more cells within a given Ly49 subset will fall above a threshold of responsiveness; and (2) for NK cells above this threshold, the ones having received the strongest inhibitory input would display a more efficient response per cell, reflected by the increased likelihood of those NK cells to deliver 2 effector functions (degranulation and IFN-γ secretion) as well as to produce larger quantities of IFN-γ. Recent data on T cell responses to immunization show that qualitative differences in effector responses, including single versus multiple effector responses, determined the degree of protection against Leishmania major,35 suggesting that, like NK cells, T cells capable of mediating more than one effector function may be more potent effectors in vivo. Similar to our data, this study also showed that the T cells that produced multiple rather than single cytokines also contained more IFN-γ per cell,36 suggesting that the level of intracellular IFN-γ reflects an increased capacity not only to make this cytokine, but for functional potency in general. Altogether, this notion strengthens our conclusion that MHC class I alleles with a strong educating impact, or certain combinations of inhibitory receptors acting in synergy, most likely by virtue of a stronger inhibitory signal, directly determines the threshold of activation to match this inhibitory input. Thus, education is not a binary process but a continuous quantitative process influencing the number of responsive cells and the efficiency by which each cell responds. The fact that NK-cell subsets expressing 2 self-receptors were more likely to degranulate after receptor stimulation compared with NK cells expressing only one such receptor manifested a critical prediction of our model and adds substantial strength to it. Importantly, synergy between inhibitory receptors on the functional responsiveness of NK cells has recently been found also in humans, suggesting that human NK cells may be subject to a similar quantitative educational input by MHC class I alleles.32
Our data can be viewed as an extension of either of the previously proposed licensing and disarming models.10,19 The direction of adjustment of activating signaling pathways in response to inhibitory input postulated here could act to either “tune-down” the responsiveness of initially active cells (disarming model), or to “tune-up” initially nonresponsive cells (licensing model). Adjustment of the rheostat could potentially occur in both directions, even after the NK-cell maturation process. Reversible tuning is consistent with observations in MHC class I mosaic mice,37,38 in which tolerance to cells lacking the ligand was reversed within 20 hours of in vitro cultures after separating cells of the 2 phenotypes, supporting the idea of reversible education in mature NK cells.
While we favor a model for dynamic education, modulating the efficiency of the individual NK cells, our data do not exclude a strict selection-based education process, in which NK-cell precursors display different preprogrammed levels of responsiveness. In such a model, only those cells for which activating signaling falls within a range that just about matches inhibitory input would become responsive, and heterogeneity in the precursor pool would thus lead to mature NK cells with different functional efficiencies. Regardless of the correct model, we conclude that MHC-dependent education leads to NK cells with different responsiveness along a gradual continuum, where strong inhibition, either because of one strong or multiple additive MHC class I ligands or multiple inhibitory receptors acting in synergy, induce both a higher number of responsive NK cells and a more efficient function in each responsive NK cell. This notion and the different models that can account for it are important to pursue to understand the basics of NK-cell education and its consequences for interpretation of clinical data, as on HLA-KIR combinations in hematopoietic transplantation and disease epidemiology.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank the staff in the animal facility for excellent help with animal experiments and maintenance; Johan Brännström for technical advice; Susanne Lemieux for providing the hybridoma 4LO3311; and members of the P.H. and K.K. groups for support and valuable input.
This work was supported by individual grants to P.H. and K.K. from the Swedish Cancer Society, the Swedish Research Council, and Karolinska Institutet; and grants to K.K. from the Swedish Foundation for Strategic Research. The work was performed within the IRIS Center, supported by a Center of Excellence grant from the Swedish Foundation for Strategic Research. All organizations providing grants are located in Stockholm, Sweden.
National Institutes of Health
Authorship
Contribution: P.B., P.H., and K.K. designed the experiments; P.B. and T.L. performed the experiments; and P.B., P.H., K.K., and S.J. wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Petter Höglund, Department of Microbiology, Tumor and Cell Biology, Box 280, S-171 77 Stockholm, Sweden; e-mail: petter.hoglund@ki.se.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal