Acute graft-versus-host disease (GVHD) occurs less frequently after umbilical cord blood transplantation (UCBT). More recent investigations include the use of 2 partially human leukocyte antigen (HLA)–matched UCB units, or double UCB graft, to meet the minimum cell-dose requirement. The purpose of this analysis was to assess the relative risk of acute GVHD in 265 consecutive patients receiving transplants with UCB graft composed of 1 (n = 80) or 2 (n = 185) units. The incidence of grade III-IV acute GVHD was similar between cohorts. However, the incidence of grade II-IV acute GVHD was higher among double UCBT recipients (58 vs 39%, P < .01). Three risk factors for grade II-IV acute GVHD were identified in multiple regression analysis: use of 2 UCB units, use of nonmyeloablative conditioning, and absence of antithymocyte globulin in the conditioning regimen. Transplantation-related mortality (TRM) at 1 year, however, was significantly lower after double UCBT (24 vs 39%, P = .02) even if recipients had grade II-IV acute GVHD (20 vs 39%, P = .05). These data suggest that, despite a higher incidence of grade II acute GVHD in recipients of 2 partially HLA-matched UCB units, there is no adverse effect on TRM. This study is registered at http://www.clinicaltrials.gov under the identifiers NCT00305682 and NCT00309842.
Introduction
In general, acute graft-versus-host disease (GVHD) is a major cause of morbidity and mortality after allogeneic hematopoietic stem cell transplantation (HSCT).1,,–4 Risk factors for the development of acute GVHD have consistently included recipient age, cytomegalovirus (CMV) serostatus, donor HSC source, and human leukocyte antigen (HLA) disparity.
Increasingly, umbilical cord blood (UCB) is chosen as an alternative to bone marrow or peripheral blood stem cells, especially for those without an HLA-matched donor.5,,,,,,,–13 Despite 1 to 2 antigen disparities between the donor and host, acute GVHD occurs with lower frequency after UCB transplantation (UCBT) relative to that observed after HLA-matched marrow from unrelated donors.14,–16 Risk factors for acute GVHD in patients receiving unrelated donor UCB have yet to be clarified. HLA match,6,7,17 patient age,7 and CMV serostatus6 have been reported as risk factors in some studies and not others.9,10,18 Therefore, we sought to identify potential risk factors in 265 consecutive recipients of UCB treated at a single institution. In this analysis, we also sought to determine the impact on the incidence of GVHD of double UCBT pioneered at the University of Minnesota as a strategy to overcome the limited number of cells available in a single unit, particularly for adults and adolescents.
Methods
Study design
Clinical and laboratory data were systematically and prospectively collected on all patients undergoing HSCT and entered into the University of Minnesota Blood and Marrow Transplant (BMT) Database. All transplant protocols were reviewed and approved by the Cancer Protocol Review Committee and Human Subjects Institutional Review Board (IRB) at the University of Minnesota. All patients and/or guardians signed IRB-approved informed consent in accordance with the Declaration of Helsinki.
Between January 1994 and June 2006, 450 patients underwent UCBT at the University of Minnesota. Because a focus of this analysis was to determine the effect of double UCBT on the risk of GVHD, recipients less than 10 years of age were ineligible (n = 140), as only 7 double UCB transplantations had been performed in younger patients over the time period. Other exclusion criteria were: use of a 3/6 HLA-matched UCB unit (n = 3) or prior allogeneic transplantation (n = 42). Therefore, this analysis included 265 eligible patients aged greater than 10 years. Eighty patients were transplanted with a single UCB unit, and 185 patients were transplanted with 2 UCB units. At the time of analysis, all patients had 12-110 months of follow-up (median 36 months).
Patient characteristics
Patient characteristics, including year of transplantation and recipient age, weight, sex, recipient CMV serostatus, and underlying diagnosis, are shown in Table 1. Double UCBT was initiated in the year 2000; therefore, for these recipients, follow-up was shorter. Patients received a double UCB graft if they lacked a suitable single UCB, as determined by cell-dose criteria.12,13,19 Single UCB recipients were younger with a median age of 23 (range 10-65) versus 45 (range 10-69) years (P < .01) and weighed less with a median of 63 (range 30-107) versus 78 (range 33-149) kilograms (P < .01) compared with recipients of 2 UCB units. Underlying diagnoses were similar with approximately half of the patients in each group having acute leukemia (Table 1). Standard risk was defined as patients with acute leukemia in first or second complete remission, chronic myelogenous leukemia in first chronic phase, myelodysplastic syndrome without excess blasts, or nonmalignant diseases. All other patients were considered high risk.
Patient and transplantation characteristics
| Factors . | Single . | Double . | P . |
|---|---|---|---|
| Total | 80 | 185 | |
| Year at transplantation | < .01 | ||
| 1994-1997 | 7 (8%) | 0 | |
| 1998-2000 | 23 (29%) | 2 (1%) | |
| 2001-2003 | 39 (49%) | 64 (35%) | |
| 2004-2005 | 11 (14%) | 99 (54%) | |
| 2006 (first half) | 0 | 20 (10%) | |
| Recipient age at transplantation | |||
| 10-18 | 35 (44%) | 23 (12%) | < .01 |
| ≥ 18 | 45 (56%) | 162 (88%) | |
| Median (range) | 23 (10-65) | 45 (10-69) | < .01 |
| Recipient weight at transplantation, median (range) | 63 (30-107) | 78 (33-149) | < .01 |
| Recipient sex | .08 | ||
| Male | 40 (50%) | 114 (62%) | |
| Female | 40 (50%) | 71 (38%) | |
| Recipient CMV serostatus | .38 | ||
| Negative | 41 (51%) | 84 (45%) | |
| Positive | 39 (49%) | 101 (55%) | |
| Recipient diagnosis | .28 | ||
| ALL | 16 (20%) | 31 (17%) | |
| AML | 30 (38%) | 67 (36%) | |
| CML | 6 (7%) | 10 (5%) | |
| MDS | 7 (9%) | 13 (7%) | |
| NHL | 7 (9%) | 31 (17%) | |
| Other malignancies | 3 (3%) | 18 (10%) | |
| Aplastic anemia | 4 (5%) | 8 (4%) | |
| Metabolic disorder | 7 (9%) | 7 (4%) | |
| Disease risk | .29 | ||
| Standard | 35 (44%) | 94 (51%) | |
| High | 45 (56%) | 91 (49%) | |
| HLA match (lowest)* | .98 | ||
| 6/6 | 7 (9%) | 15 (8%) | |
| 5/6 | 28 (35%) | 64 (35%) | |
| 4/6 | 45 (56%) | 106 (57%) | |
| HLA match (engrafting unit)† | .87 | ||
| 6/6 | 7 (9%) | 13 (7%) | |
| 5/6 | 28 (35%) | 68 (37%) | |
| 4/6 | 45 (56%) | 104 (56%) | |
| Conditioning | < .01 | ||
| Myeloablative | 61 (76%) | 78 (42%) | |
| Nonmyeloablative | 19 (24%) | 107 (58%) | |
| Use of ATG in the conditioning | < .01 | ||
| Yes | 46 (58%) | 49 (26%) | |
| No | 34 (43%) | 136 (74%) | |
| GVHD prophylaxis | < .01 | ||
| CSA/MP | 46 (58%) | 4 (2%) | |
| CSA/MMF | 33 (41%) | 181 (98%) | |
| CSA/MTX | 1 (1%) | 0 | |
| Total nucleated cell dose, (×107/kg recipient), median (range) | 2.4 (0.9-14.0) | 3.6 (1.1-8.0) | < .01 |
| Total CD34 cell dose (×105/kg recipient), median (range) | 2.8 (0.5-1.9) | 4.7 (0.7-1.7) | < .01 |
| Total CD3 cell dose (×104/kg recipient), median (range) | 0.6 (0.1-56.4) | 1.3 (0.1-3.1) | < .01 |
| Follow-up among survivors, median (range) in years | 4.8 (2.0-9.2) | 2.2 (1.0-5.3) |
| Factors . | Single . | Double . | P . |
|---|---|---|---|
| Total | 80 | 185 | |
| Year at transplantation | < .01 | ||
| 1994-1997 | 7 (8%) | 0 | |
| 1998-2000 | 23 (29%) | 2 (1%) | |
| 2001-2003 | 39 (49%) | 64 (35%) | |
| 2004-2005 | 11 (14%) | 99 (54%) | |
| 2006 (first half) | 0 | 20 (10%) | |
| Recipient age at transplantation | |||
| 10-18 | 35 (44%) | 23 (12%) | < .01 |
| ≥ 18 | 45 (56%) | 162 (88%) | |
| Median (range) | 23 (10-65) | 45 (10-69) | < .01 |
| Recipient weight at transplantation, median (range) | 63 (30-107) | 78 (33-149) | < .01 |
| Recipient sex | .08 | ||
| Male | 40 (50%) | 114 (62%) | |
| Female | 40 (50%) | 71 (38%) | |
| Recipient CMV serostatus | .38 | ||
| Negative | 41 (51%) | 84 (45%) | |
| Positive | 39 (49%) | 101 (55%) | |
| Recipient diagnosis | .28 | ||
| ALL | 16 (20%) | 31 (17%) | |
| AML | 30 (38%) | 67 (36%) | |
| CML | 6 (7%) | 10 (5%) | |
| MDS | 7 (9%) | 13 (7%) | |
| NHL | 7 (9%) | 31 (17%) | |
| Other malignancies | 3 (3%) | 18 (10%) | |
| Aplastic anemia | 4 (5%) | 8 (4%) | |
| Metabolic disorder | 7 (9%) | 7 (4%) | |
| Disease risk | .29 | ||
| Standard | 35 (44%) | 94 (51%) | |
| High | 45 (56%) | 91 (49%) | |
| HLA match (lowest)* | .98 | ||
| 6/6 | 7 (9%) | 15 (8%) | |
| 5/6 | 28 (35%) | 64 (35%) | |
| 4/6 | 45 (56%) | 106 (57%) | |
| HLA match (engrafting unit)† | .87 | ||
| 6/6 | 7 (9%) | 13 (7%) | |
| 5/6 | 28 (35%) | 68 (37%) | |
| 4/6 | 45 (56%) | 104 (56%) | |
| Conditioning | < .01 | ||
| Myeloablative | 61 (76%) | 78 (42%) | |
| Nonmyeloablative | 19 (24%) | 107 (58%) | |
| Use of ATG in the conditioning | < .01 | ||
| Yes | 46 (58%) | 49 (26%) | |
| No | 34 (43%) | 136 (74%) | |
| GVHD prophylaxis | < .01 | ||
| CSA/MP | 46 (58%) | 4 (2%) | |
| CSA/MMF | 33 (41%) | 181 (98%) | |
| CSA/MTX | 1 (1%) | 0 | |
| Total nucleated cell dose, (×107/kg recipient), median (range) | 2.4 (0.9-14.0) | 3.6 (1.1-8.0) | < .01 |
| Total CD34 cell dose (×105/kg recipient), median (range) | 2.8 (0.5-1.9) | 4.7 (0.7-1.7) | < .01 |
| Total CD3 cell dose (×104/kg recipient), median (range) | 0.6 (0.1-56.4) | 1.3 (0.1-3.1) | < .01 |
| Follow-up among survivors, median (range) in years | 4.8 (2.0-9.2) | 2.2 (1.0-5.3) |
ALL indicates acute lymphoblastic leukemia; AML, acute myelogenous leukemia, CML, chronic myelogenous leukemia; MDS, myelodysplastic syndrome; and NHL, non-Hodgkin lymphoma.
High risk refers to acute leukemia in CR1 and CR2, CML in first chronic phase, and MDS without excess blasts or nonmalignant diseases. Standard risk refers to all others.
HLA match equals the poorest matched unit in recipients of two UCB units.
HLA match (engrafting unit) equals the HLA match of units that engrafted long term.
Transplantation characteristics
Transplantation characteristics including HLA match, preparative therapy, GVHD prophylaxis, and UCB graft cell dose are shown in Table 1. Patients and donors were typed for HLA-A and HLA-B at antigen level and for DRB1 at allele level. HLA-C, HLA-DQ, and HLA-DP were not considered in donor unit selection. The HLA match of the single UCB unit and lowest HLA match of the 2 units in recipients of double UCBT were similar. Similarly, the HLA match of the engrafting unit in recipients of 2 UCB units was similar to that in recipients of a single UCB unit.8,11 The majority (76%) of the single UCBT recipients received myeloablative conditioning compared with only 42% of the double UCBT recipients (P < .01). In addition, a greater proportion of the single UCBT recipients received equine antithymocyte globulin (ATG) as part of their conditioning therapy (58% vs 26%, P > .01), and cyclosporine A (CsA) and short-course methylprednisolone as GVHD immunoprophylaxis (98% vs 57%, P < .01). The total nucleated, CD34 and CD3 cell doses infused were significantly lower in recipients of a single unit.
Supportive care
Patients were hospitalized in single rooms with high-efficiency particulate air filtration with positive pressure until neutrophil engraftment (absolute neutrophil count [ANC] ≥ 0.5 × 109/L for 3 consecutive days). Patients all received antibiotic prophylaxis until engraftment. Broad-spectrum intravenous antibacterial and as indicated antifungal antimicrobials were used when patients developed fever. Patients received acyclovir prophylaxis if they were seropositive for herpes simplex virus and/or CMV. Oral trimethoprim-sulfamethoxazole was given for pneumocystis carinii pneumonia prophylaxis after engraftment for 1 year. CMV-seronegative recipients received CMV-safe (seronegative or filtered) blood products. All but 22 patients received granulocyte colony-stimulating factor (G-CSF) 5 μg/kg per day from the day of transplant until ANC was greater than 2.5 × 109/L for 2 consecutive days.
Diagnosis, staging, and grading of acute GVHD
Symptoms of acute GVHD were graded by standard clinical criteria,20,21 modified to include upper gastrointestinal (GI) acute GVHD per the GVHD consensus conference.22,23 Grade of GVHD refers to clinical (not histologic) grade throughout this report. Real-time staging and grading of each organ was determined weekly by the attending physician, supported by laboratory and clinical information and histologic confirmation when possible. The grading scheme was consistent throughout the study period. While all patients GVHD grades were retrospectively reviewed by the Acute GVHD Grading Committee (M.M. and D.W.), the overall grade used in this analysis was determined by a computer algorithm, incorporating all available clinical and pathologic GVHD organ staging data.
Statistical analysis
Data on pretransplant patient characteristics, transplantation complications, and outcomes, including maximum acute GVHD score, were prospectively collected by the Biostatistical Support Group at the University of Minnesota using standardized collection procedures.
Cumulative incidence rates and their 95% confidence intervals (CIs) were estimated for engraftment, grade II-IV and grade III-IV acute GVHD, chronic GVHD, and transplantation-related mortality (TRM). Event times for neutrophil engraftment were measured from the date of transplantation to the date of neutrophil recovery, with censoring for early death (ie, death before day 21 without neutrophil recovery; n = 4).24 Patients who had very delayed engraftment (ie, those who achieved an ANC ≥ 5 × 108/L after day 42) were scored as having primary graft failure.
The multivariate modification of cumulative incidence statistics of Fine and Gray were used to evaluate the univariate and multiple effects of risk factors on outcome.25 The outcome variables were engraftment, GVHD, and TRM.
Results
Acute GVHD
For the entire cohort of patients, the cumulative incidence of grade II-IV and grade III-IV acute GVHD at day 100 after UCBT was 52% (95% CI, 45%-59%) and 19% (95% CI, 14%-24%), respectively. While no factor was independently associated with the risk of grade III-IV acute GVHD, 3 factors were associated with the development of grade II-IV acute GVHD in multiple regression analysis, namely, transplantation of 2 UCB units, use of a nonmyeloablative conditioning regimen, and absence of ATG in the conditioning regimen (Table 2). Recipients of double UCBT had a 2-fold increased risk of developing grade II-IV acute GVHD (relative risk [RR], 2.0; 95% CI, 1.2-3.4; P = .01) compared with single UCB transplantation. While recipients of a nonmyeloablative conditioning regimen had higher risk of grade II-IV acute GVHD (RR, 1.7; 95% CI, 1.1-2.5; P = .01), use of ATG in the conditioning regimen was associated with a lower relative risk (RR, 0.5; 95% CI, 0.3-0.9; P = .02). Recipient age, type of GVHD prophylaxis, cell dose, and degree of donor-recipient HLA disparity had no significant independent effect on the risk of grade II-IV acute GVHD.
Factors associated with grade II-IV acute GVHD: multiple regression analysis
| Factor . | RR of acute GVHD (95% CI) . | P . |
|---|---|---|
| Number of donors | ||
| One* | 1.0 | |
| Two | 2.0 (1.2-3.4) | .01 |
| Conditioning | ||
| Myeloablative* | 1.0 | |
| Nonmyeloablative | 1.7 (1.1-2.5) | .01 |
| ATG in conditioning | ||
| No* | 1.0 | |
| Yes | 0.5 (0.3-0.9) | .02 |
| MMF as GVHD prophylaxis | ||
| No* | 1.0 | |
| Yes | 0.5 (0.2-1.3) | .14 |
| HLA matching (engrafting unit) | ||
| 6/6* | 1.0 | |
| 5/6 | 1.9 (0.9-4.2) | .11 |
| 4/6 | 1.4 (0.7-3.2) | .36 |
| Disease risk | ||
| Standard* | 1.0 | |
| High | 0.7 (0.5-1.1) | .10 |
| Factor . | RR of acute GVHD (95% CI) . | P . |
|---|---|---|
| Number of donors | ||
| One* | 1.0 | |
| Two | 2.0 (1.2-3.4) | .01 |
| Conditioning | ||
| Myeloablative* | 1.0 | |
| Nonmyeloablative | 1.7 (1.1-2.5) | .01 |
| ATG in conditioning | ||
| No* | 1.0 | |
| Yes | 0.5 (0.3-0.9) | .02 |
| MMF as GVHD prophylaxis | ||
| No* | 1.0 | |
| Yes | 0.5 (0.2-1.3) | .14 |
| HLA matching (engrafting unit) | ||
| 6/6* | 1.0 | |
| 5/6 | 1.9 (0.9-4.2) | .11 |
| 4/6 | 1.4 (0.7-3.2) | .36 |
| Disease risk | ||
| Standard* | 1.0 | |
| High | 0.7 (0.5-1.1) | .10 |
Reference group.
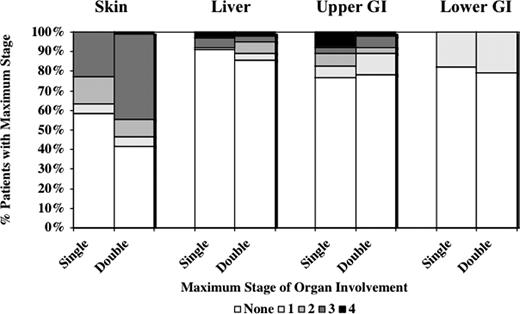
For double UCB graft recipients, the incidence of grade II-IV acute GVHD was 58% (95% CI, 50%-66%) compared with 39% (95% CI, 28%-50%) in single UCB unit recipients (P < .01), as shown in Figure 1. In addition, acute GVHD developed earlier after double UCBT at a median of 28 (range 11-75) days compared with 36 (range, 14-86) days after single UCBT. In contrast, the incidence of more severe grade III-IV acute GVHD was similar between groups (ie, 19% [95% CI, 14%-25%] with a double and 18% [95% CI, 9%-26%] with a single UCB graft). As shown in Figure 2, recipients of 2 UCB units had a higher incidence of grade II disease only, manifest by a higher incidence of stage III skin GVHD only (44 vs 23%, P = .02). Liver and gastrointestinal acute GVHD was similar between recipients of a single and double UCB grafts.
Cumulative incidence of grade II-IV acute GVHD by number of UCB units transplanted.
Cumulative incidence of grade II-IV acute GVHD by number of UCB units transplanted.
Maximum stage of acute GVHD by organ system in recipients of a single and double UCB transplant.
Maximum stage of acute GVHD by organ system in recipients of a single and double UCB transplant.
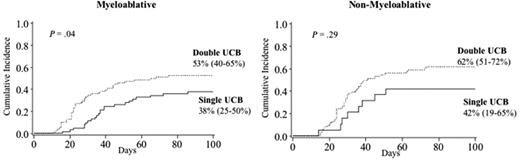
For the 139 patients who received a myeloablative conditioning, the incidence of grade II-IV acute GVHD was higher in recipients of double UCBT (53% [95% CI, 40%-65%]) versus single UCBT (38% [95% CI, 25%-50%], P = .04) and occurred earlier (median of 23 [range 11-75] days vs 37 [range 16-86]) in recipients of a double UCBT, as shown in Figure 3. Similarly, for the 126 patients who received a nonmyeloablative conditioning, 62% (95% CI, 51%-72%) of double UCBT recipients developed grade II-IV acute GVHD compared with 42% (95% CI, 19%-65%) of single UCBT recipients (P = .29).
Cumulative incidence of grade II-IV acute GVHD in recipients by conditioning regimen. (Left panel) Recipients of myeloablative conditioning regimen. (Right panel) Recipients of nonmyeloablative conditioning regimen.
Cumulative incidence of grade II-IV acute GVHD in recipients by conditioning regimen. (Left panel) Recipients of myeloablative conditioning regimen. (Right panel) Recipients of nonmyeloablative conditioning regimen.
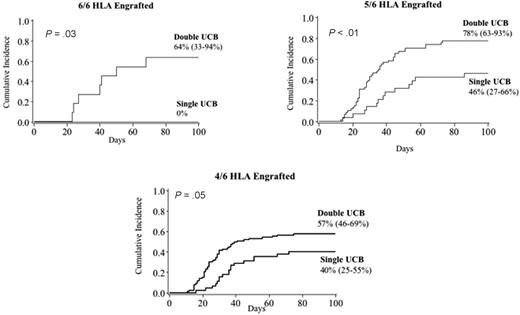
The effects of donor:recipient HLA match and GVHD prophylaxis were also examined. None of the 7 patients who received a 6/6 HLA-matched, single UCB graft developed acute GVHD compared with 60% (95% CI, 31%-89%) of 15 patients who received 2 6/6 HLA-matched UCB units (P = .02). HLA match of the infused single unit had no effect on risk of acute GVHD. Similarly, as shown in Figure 4, HLA match of the engrafting unit had no direct effect on the risk of acute GVHD. However, when evaluated by single versus double UCBT, use of 2 units was consistently associated with a higher risk regardless of the HLA match of the engrafting unit to the recipient. While recipients of CsA and methylprednisone may have had a lower risk of acute GVHD, this association was confounded by the infusion of ATG before transplantation in all patients. In contrast, most recipients of CsA and mycophenolate mofetil (MMF) immunoprophylaxis did not receive ATG. In recipients of CsA and MMF, a higher incidence of grade II-IV acute GVHD was observed in recipients of 2 UCB units (59% [95% CI, 50%-67%]) versus 1 UCB unit (39% [95% CI, 28%-50%], P < .01).
Cumulative incidence of grade II-IV acute GVHD by HLA match of the engrafted UCB unit.
Cumulative incidence of grade II-IV acute GVHD by HLA match of the engrafted UCB unit.
When examined in 3 separate age cohorts (10-18 years, 18-45 years, and > 45 years), there was a trend toward more frequent acute GVHD in recipients of double versus single UCBT in all 3 age groups (59% [95% CI, 36-82] vs 31% [95% CI, 15-47], P = .07; 54% [95% CI, 41%-67%] vs 43% [95% CI, 24%-62%], P = .13; and 61% [95% CI, 49%-72%] vs 47% [95% CI, 22%-72%], P = .44), respectively.
Chronic GVHD
For the entire cohort of patients, the cumulative incidence of chronic GVHD after UCBT was 17% (95% CI, 13%-22%). The incidence was similar in recipients of a single (18% [95% CI, 9%-26%]) and double (17% [95% CI, 12%-23%]) UCBT (P = .47). As reported with other HSC sources, the probability of developing chronic GVHD was higher in patients with prior grade II-IV acute GVHD (26% [95% CI, 18%-34%]) compared with those without (8% [95% CI, 8%-13%], P < .01). Among those with a history of grade II-IV acute GVHD, the rates of chronic GVHD were 29% (95% CI, 5%-46%) and 25% (95% CI, 7%-43%) for recipients of a single versus double UCBT (P = .46).
Transplantation-related mortality
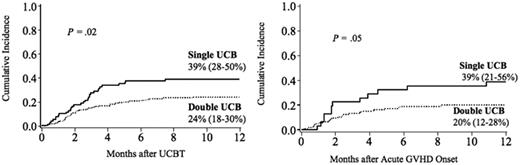
TRM at 1 year was 39% (95% CI, 28%-50%) and 24% (95% CI, 18%-30%) after a single and double UCBT (P = .02), respectively (Figure 5 left panel). Similarly, among patients who developed grade II-IV acute GVHD, TRM at 1 year following the onset of acute GVHD was lower in recipients of double UCBT (20% [95% CI, 12%-28%]) versus single UCBT (39% [95% CI, 21%-56%], P = .05) (Figure 5 right panel). At 3 years after the onset of acute GVHD, there was still a trend toward lower TRM in recipients of double UCBT (29% [95% CI, 13%-45%]) versus single UCBT (36% [95% CI, 26%-46%], P = .11). While risk of grade III-IV acute GVHD was similar in recipients of a single and double UCBT, TRM was 43% (95% CI, 19%-67%) after single compared with 20% (95% CI, 7%-33%) after a double UCB transplantation (P = .06).
Cumulative incidence of TRM at 1 year after transplantation. (Left panel) All patients. (Right panel) Patients with grade II-IV acute GVHD.
Cumulative incidence of TRM at 1 year after transplantation. (Left panel) All patients. (Right panel) Patients with grade II-IV acute GVHD.
In multiple regression analysis, the only factor associated with TRM after grade II-IV acute GVHD was the number of infused UCB units (Table 3). In patients with grade II-IV acute GVHD, the use of 2 UCB units was associated with a lower risk of TRM (RR, 0.4; 0.2-0.9; P = .03). There was no significant effect of recipient age, weight, sex, diagnosis, disease risk, type of conditioning (myeloablative vs nonmyeloablative), use of ATG, use of MMF, cell dose, HLA matching, time from diagnosis to transplantation, or time to onset of acute GVHD on TRM.
Factors associated with TRM in patients with grade II-IV acute GVHD: multiple regression analysis
| Factor . | RR of TRM (95% CI) . | P . |
|---|---|---|
| Number of donors | ||
| One* | 1.0 | |
| Two | 0.4 (0.2-0.9) | .03 |
| Disease | ||
| Other malignant* | 1.0 | |
| Leukemia (CR1,CP1) | 1.7 (0.4-7.0) | .44 |
| Leukemia (other) | 1.9 (0.6-5.9) | .24 |
| Nonmalignant | 3.1 (0.9-10.5) | .07 |
| Disease risk | ||
| Standard* | 1.0 | .52 |
| High | 1.4 (0.5-3.9) | |
| HLA | ||
| 6/6* | 1.0 | |
| 5/6 | 0.4 (0.1-1.5) | .18 |
| 4/6 | 0.4 (0.1-1.4) | .14 |
| Factor . | RR of TRM (95% CI) . | P . |
|---|---|---|
| Number of donors | ||
| One* | 1.0 | |
| Two | 0.4 (0.2-0.9) | .03 |
| Disease | ||
| Other malignant* | 1.0 | |
| Leukemia (CR1,CP1) | 1.7 (0.4-7.0) | .44 |
| Leukemia (other) | 1.9 (0.6-5.9) | .24 |
| Nonmalignant | 3.1 (0.9-10.5) | .07 |
| Disease risk | ||
| Standard* | 1.0 | .52 |
| High | 1.4 (0.5-3.9) | |
| HLA | ||
| 6/6* | 1.0 | |
| 5/6 | 0.4 (0.1-1.5) | .18 |
| 4/6 | 0.4 (0.1-1.4) | .14 |
Reference group.
Discussion
Acute GVHD after allogeneic HSCT is often associated with increased TRM and worse survival. The purpose of this analysis was to investigate potential risk factors for acute GVHD after UCBT. Risk factors for acute GVHD after UCBT have varied between reports. While some have found associations with patient age,7 HLA disparity,6,7 and CMV serostatus,6 others have not.9,10,18 Rates of acute GVHD in single UCBT recipients in the present analysis are similar to those reported by others.6,7,26,–28 However, this analysis benefits from the use of one consistent diagnostic and treatment approach for acute GVHD over time. This is the first analysis evaluating the potential impact of double UCBT on the risk of acute GVHD.
Based on this analysis, 3 findings emerge: (1) acute GVHD occurs more often, and at an earlier time point after transplantation of 2 partially HLA-matched UCB units compared with a single unit; (2) when acute GVHD occurs after a double UCBT, it is more likely grade II in severity with involvement principally of the skin; and (3) TRM is significantly lower in patients with acute GVHD when receiving transplants with 2 UCB units.
A potential mechanism for the observed increase in acute GVHD after double UCBT may be the higher cell dose. While the dose of CD3+ T cells is statistically higher, the absolute difference in total numbers of CD3+ T cells is not appreciable (median 0.6 vs 1.3 × 107/kg). Additionally, some as yet unknown impact of transient double chimerism may play a role in limiting the severity and adverse clinical consequences of GVHD after double UCBT. Alternatively, the increased incidence of acute GVHD after double UCBT may be due to a graft-versus-graft effect, similar to an in vivo mixed lymphocyte reaction (MLR).
More detailed analyses on the speed of engraftment and immune reconstitution after single versus double UCBT will be of interest. Such analyses are part of an ongoing Blood and Marrow Transplant Clinical Trial Network prospective randomized trial testing double versus single UCB grafts in children with leukemia.
The focus of this analysis was on risk of acute GVHD and impact on TRM. While of considerable interest, the impact of acute GVHD on relapse risk was not evaluated with this dataset because of disease heterogeneity. A separate analysis is underway evaluating the impact of single versus double UCBT in specific diseases and by conditioning regimen.
The increased risk of acute GVHD was predominantly limited to the skin. While there was more stage III skin acute GVHD after double UCBT, liver and gastrointestinal tract acute GVHD was similar. Interestingly, the grade II acute GVHD associated with double UCBT in this study was associated with lower TRM. While the mechanism for this observation is unclear, it is possible that lower TRM is principally related to the higher cell dose associated with double UCBT with an effect that is independent of acute GVHD. Alternatively, double UCBT, which generally results in transient dual chimerism with 1 unit eventually predominating,11,13 may somehow modulate the severity of acute GVHD and as such, lessen its clinical consequences.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank the nurses, nurse coordinators, and physicians who cared for these patients and their families. In addition, we gratefully acknowledge the research nurses who collected the GVHD data.
This work was supported in part by National Cancer Institute grant PO1-CA65493, and a grant from the Children's Cancer Research Fund.
National Institutes of Health
Authorship
Contribution: All authors contributed equally to the conception, design, and interpretation of data and the final manuscript; Q.C. and T.E.D. performed the statistical analysis; and M.L.M. had primary responsibility for drafting the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Margaret L. MacMillan, Department of Pediatrics, University of Minnesota, MMC 484, 420 Delaware Street SE, Minneapolis, MN 55455; e-mail: macmi002@umn.edu.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal