The regulation of the interaction between the immune system and antigens, which may lead to the induction of immune tolerance, is critical both under physiologic conditions and in different pathological settings. In the past few years, major strides have been made in our understanding of the molecular and cellular bases of this process. Novel pathways have been identified and several novel therapeutic agents are currently under clinical investigation for those diseases in which the normal balance between activation and suppression of the immune response is altered. The tryptophan catabolic enzyme, indoleamine 2,3-dioxygenase (IDO), is one of the key players involved in the inhibition of cell proliferation, including that of activated T cells. Recent works have demonstrated a crucial role for IDO in the induction of immune tolerance during infection, pregnancy, transplantation, autoimmunity, and neoplasias, including hematologic malignancies. In this review, the role of IDO in the induction of immunologic tolerance is addressed with a specific focus on its recently discovered effect on hematologic malignancies.
Introduction
Indoleamine 2,3-dioxygenase (IDO) is an intracellular heme-containing enzyme that catalyzes the initial rate-limiting step in tryptophan degradation along the kynurenine pathway.1 Tryptophan starvation by IDO consumption inhibits T-cell activation,1,–3 whereas products of tryptophan catabolism, such as kynurenine derivatives and O2 free radicals, regulate T-cell proliferation and survival.1,4 IDO is widely expressed in human tissues and cell subsets and is induced during inflammation by IFN-γ and other inflammatory cytokines.5 Recent works have demonstrated a crucial role for IDO in the induction of immune tolerance during infection, pregnancy, transplantation, autoimmunity, and neoplasias, including hematologic malignancies.1,6,7
In the hematology field, regulatory dendritic cells (DCs) and bone marrow (BM)–derived mesenchymal stem cells (MSCs) expressing IDO have the capacity to suppress T-cell responses to autoantigens and alloantigens.8,9 Moreover, acute myeloid leukemia (AML) cells have been recently demonstrated to express a functionally active form of the IDO protein, which is capable of inhibiting the T-cell response through the expansion of regulatory T cells (Tregs).10
Although several reviews addressing the enzymatic activity of IDO have recently been published, the aim of the present paper is to summarize the most recent data about IDO expression in hematopoietic cells and its role as a potential therapeutic target in hematologic malignancies.
General and biologic aspects
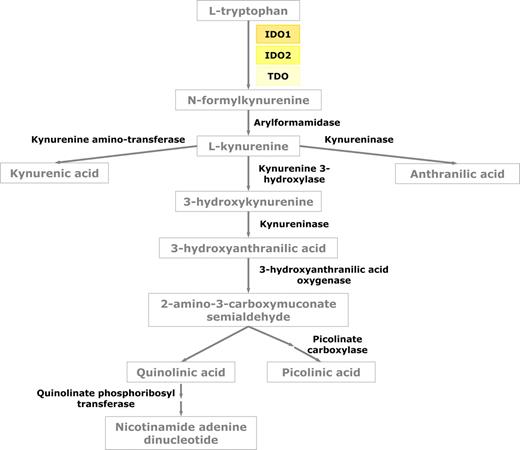
Tryptophan is an amino acid required by all life forms for protein synthesis and other important metabolic functions, such as the synthesis of the essential cellular factor, nicotinamide adenine dinucleotide (NAD+) and the synthesis of serotonin. Dietary tryptophan is delivered to the liver through the hepatic portal system, and the pool of tryptophan that is not used for protein synthesis can either be degraded in the liver or distributed into the bloodstream where it is captured and used by cells throughout the body. Since the 1970s, several studies have demonstrated that 2 different enzymes initiate the catabolism of tryptophan through a series of metabolic steps known collectively as the kynurenine pathway. Tryptophan 2,3-dioxygenase, or TDO, is an enzyme that is primarily expressed in the liver, is induced by tryptophan and metabolic steroids, and is highly specific for tryptophan. In contrast, IDO is found in many tissues, is induced by inflammatory stimuli, and has less substrate specificity. More recently, a novel gene with homology to IDO has been reported and then subsequently demonstrated to encode an enzyme that catabolizes tryptophan.11,12 This enzyme has been referred to as IDO-2 on the basis of its structural similarity to IDO (Figure 1). However, recent reports have postulated that the 2 forms of IDO may have a differential spectrum of expression and they may have different responses to IDO inhibitors.13
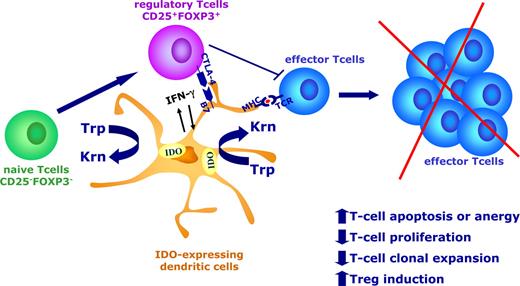
In the last few years, IDO has been extensively investigated since the demonstration of its critical role in regulating immune tolerance to foreign antigens within tissue microenvironments.1 The regulation of the IDO gene, protein, and enzymatic activity is complex and still not completely elucidated. Physiologically, IDO is expressed by a wide variety of cell types in response to several stimuli. Within the immune system, certain subsets of antigen-presenting cells (APCs), including macrophages and DCs, have the capacity to up- and down-regulate IDO expression in response to external stimuli, depending on the microenvironment (Figure 2).14,–16 Proinflammatory signals, such as IFN-γ as well as signals from T cells, are known to induce IDO expression in DCs.17,18 In particular, in murine splenic CD8+ DCs and in human monocyte-derived DCs (Mo-DCs), IDO can be induced by the exposure to IFN-γ through the intermediate modulation of a series of relevant genes, including the Tyropb gene via IRF-8 and the signaling adapter DAP12.16,19 Ligation of CD40 on DCs, after inducing the early production of inflammatory mediators via canonical NF-κB signaling, is also known to up-regulate an anti-inflammatory pathway, including IDO expression, via a noncanonical NF-κB signaling mechanism.20,21 Moreover, both in mouse and human DCs, cytotoxic T lymphocyte–associated antigen 4 (CTLA-4) either in a soluble form (CTLA-4-Ig) or bound to the cell membrane of Tregs activates an IDO-dependent tolerogenic program requiring high levels of IFN-γ.18,22,–24 In contrast to the hypothesis that IDO is strictly inducible by inflammatory stimuli, recent reports have clearly demonstrated that certain subsets of human myeloid DCs might constitutively express IDO to suppress allogeneic T-cell immune responses.25,26 Taken together, these data contribute to a move toward the identification of a tolerogenic signature in DCs based on the expression of the IDO gene and protein.16 This platform can be used to investigate the molecular basis for IDO expression in cell types other than DCs, including stem cells and tumor cells.
IDO-mediated tryptophan degradation by DCs results in multiple effects, including inhibition of T-cell proliferation, increased T-cell apoptosis, and de novo formation of Tregs. Proinflammatory signals, such as IFN-γ, as well as signals from T cells are known to induce IDO expression in DCs. In particular, CTLA-4 bound to the cell membrane of Tregs activates an IDO-dependent tolerogenic program in DCs. IDO expression by DCs results in inhibition of T-cell effector function, including proliferation and clonal expansion, and reduced survival. Moreover, IDO-expressing DCs may favor the emergence of CD4+CD25+Foxp3+ Tregs by the expansion/conversion from naive CD25−Foxp3− T cells.
IDO-mediated tryptophan degradation by DCs results in multiple effects, including inhibition of T-cell proliferation, increased T-cell apoptosis, and de novo formation of Tregs. Proinflammatory signals, such as IFN-γ, as well as signals from T cells are known to induce IDO expression in DCs. In particular, CTLA-4 bound to the cell membrane of Tregs activates an IDO-dependent tolerogenic program in DCs. IDO expression by DCs results in inhibition of T-cell effector function, including proliferation and clonal expansion, and reduced survival. Moreover, IDO-expressing DCs may favor the emergence of CD4+CD25+Foxp3+ Tregs by the expansion/conversion from naive CD25−Foxp3− T cells.
IDO as a mechanism of acquired tolerance
IDO was originally described to contribute to maternal-fetal tolerance, which is considered an example of foreign antigens to which the immune system should remain tolerant.27 Thus, IDO involvement in such a pivotal aspect of human reproduction indicates that an IDO-dependent metabolic pathway is part of a very basic, ancestral, and evolutionarily conserved mechanism of immune tolerance. Recent experiments in IDO−/− KO mice were not able to demonstrate a role for IDO in central and homeostatic immune tolerance.28 In contrast, several in vivo reports clearly demonstrate that IDO significantly contributes to acquired peripheral tolerance.29,,–32
Two main hypotheses have been proposed to explain the IDO effect in immune regulation.33 First, the tryptophan depletion theory, which emerges from the idea that starving pathogens of tryptophan may result in their elimination, is based on the demonstration that IDO-mediated depletion of tryptophan from the tissue microenvironment or culture medium leads to reduced proliferation and increased apoptosis of T cells.3,34 In this view, Munn et al proposed that the lack of tryptophan is the mechanism responsible for preventing allogeneic fetal rejection.27 More recently, it has been demonstrated that some of the metabolites derived from tryptophan degradation along the kynurenine pathway are immunosuppressive by causing T-cell apoptosis (tryptophan utilization theory).4,35,36 Interestingly, the stress-responsive kinase, general control nonderepressible 2 (GCN2), has been identified as a signaling molecule that enables T cells to respond to stress conditions created by IDO.37,38 Although still speculative, local tryptophan depletion as well as increased kynurenine metabolites may act by up-regulating GCN2, which results in impaired T-cell function.
Naturally arising CD4+CD25+ Foxp3+ Tregs are known to suppress most types of immune responses, including antitumor immunity.39 IDO is expressed and is functionally active in the placenta, which, in turn, is infiltrated by CD4+CD25+ Tregs.40 Moreover, Candida albicans infection increases the number of Tregs due to an IDO induction in host APCs.41 In human cancers, tumor-draining lymph nodes contain IDO-expressing DCs, which enhance Treg function.14,42,43 More recently, Puccetti and collaborators demonstrated that in a nontumoral mouse model, tryptophan catabolism favors the emergence of CD25+Foxp3+ Tregs by their conversion from CD25−Foxp3− cells (Fallarino et al38 ). Taken together, these data support the notion that IDO-mediated tryptophan degradation results in multiple effects, including the inhibition of T-cell proliferation, increased T-cell apoptosis, and de novo induction of Tregs, which all lead to an impairment of the cellular immune response. In this context, DCs, which may up-regulate IDO upon environmental stimuli, are believed to play a critical role (Figure 2).
IDO in human pathology
The contribution of IDO to the control of a T cell–mediated immune response is supported by several studies carried out in a variety of pathological conditions, including infections, autoimmunity, transplantation, and cancer.
Infections
Early studies have demonstrated a role for IDO in the inhibition of viruses and intracellular pathogens, such as Toxoplasma gondii and Chlamydia psittaci.44,45 Moreover, the activity of IDO on the induction of a specific inhibitory environment during chronic infection has recently been revisited. In particular, IFN-dependent expression of IDO by different cell subsets, including DCs, is often seen during infections and represents an innate mechanism of pathogen elimination.46 Listeria monocytogenes infection of the mouse placenta, which leads to granulomatous disease, results in the induction of IDO, which, in turn, plays a role in counteracting the bacterial infection while maintaining a barrier to T cells to prevent fetal rejection.47 Similar observations were made with other granulomatous infections caused by Bartonella henselae and Mycobacterium tuberculosis, where IDO-expressing APCs prevent T cell–mediated granuloma destruction and subsequent pathogen dissemination.48 More recently, a critical role for IDO has been found in the generation of immunologic resistance to fungal infections, including candidiasis and aspergillosis. In this setting, DC expression of IDO, tryptophan catabolites, and Tregs all cooperate in regulating the fine balance between inflammation, which is required for protection, especially at the early pathogen encounter phase, and tolerance, which is necessary to down-modulate potentially overwhelming inflammatory responses.41,49
Autoimmune diseases
In animal models of autoimmunity, such as experimental autoimmune encephalomyelitis (EAE), a large body of evidence clearly supports the role of IDO-mediated tryptophan catabolite generation in skewing a Th1 into a Th2 immune response, resulting in reduced inflammation and tissue damage. Interestingly, an orally active tryptophan metabolite is able to reverse paralysis in mice with EAE.50 In IDO−/− KO mice, IDO promotes Th2-mediated allergy airway inflammation via unique effects on lung DCs.51 In patients with rheumatoid arthritis (RA), another T cell–mediated autoimmune disorder, synovial DCs have been shown to express a functional form of IDO that inhibits T-cell proliferation.52 IDO up-regulation is induced by IFN-γ, which is also responsible for activation of T cells. It is then conceivable that, in this setting, IDO immunosuppressive activity represents a negative feedback effect induced by hyperactivation of T-cell immunity. This observation is in accordance with the hypothesis that one of the biologic functions of IDO may be a counter-regulatory mechanism to suppress excessive immune activation. From this viewpoint, inhibiting IDO activity in autoimmune diseases may have a detrimental effect and may worsen the clinical manifestations, which are induced by a Th1-mediated immune response.
Th1 cells have long been associated with the pathogenesis of many autoimmune diseases. However, the observation of disease in mice deficient in molecules involved in Th1-cell differentiation raised the possibility that other effector T cells were responsible for inducing autoimmunity. Recently, a new CD4+ effector T-cell subset that produces IL-17 (Th17) has emerged. These cells are characterized as preferential producers of interleukin-17A (IL-17A), IL-17F, IL-21, and IL-22.53 Th17 cells and their effector cytokines mediate host defensive mechanisms to various infections, and are involved in the pathogenesis of many autoimmune diseases. In the context of pathogenic inflammation to fungi, IL-17 and IDO-dependent production of kynurenins have been recently proven to play opposing roles.49 Given the emerging pathogenetic role of Th17 cells in autoimmunity, IDO modulation and the in vivo administration of synthetic kynurenins may represent a novel therapeutic means of contrasting Th17 pathway in this setting.
Transplantation
Recently, the immunosuppressive role of IDO has been widely investigated for the induction of graft tolerance. In several transplant models, increased IDO activity in transplanted cells has been demonstrated to have antirejection properties both in vitro and in vivo.54,–56 Several authors reported the capacity of immunosuppressive agents, such as CTLA-4Ig and CD40Ig, to favor an acceptance of the transplanted organ by inducing an immune tolerance.55 As recently demonstrated, much of this effect is mediated through the up-regulation of IDO expression in DCs and endothelial cells, which induce a peripheral tolerogenic pathway via the increase of Tregs.22,55 Moreover, in an MHC-mismatched renal allograft model, spontaneous acceptance of the transplanted organ involves several mechanisms leading to Treg expansion via IDO expression in DCs.56
Allogeneic hematopoietic stem cell transplantation (HSCT) is often complicated by side effects that derive from the profound alteration of the immune system. Graft-versus-host disease (GVHD) is the main cause of morbidity and mortality after HSCT. In recent years, our knowledge of the pathophysiology of GVHD has increased, but the immunologic mechanisms by which GVHD is regulated both at local and systemic levels are still incompletely understood. Infections and T-cell responses directed against alloantigens are known to induce inflammation, which needs a series of negative feedbacks to be efficiently counterbalanced. Among these, the induction of IDO, which mediates anti-inflammatory activities and T-cell inhibition via tryptophan catabolism, have been postulated.57
Recently, IDO has been demonstrated to act as a critical regulator of GVHD in a mouse model of HSCT.58 By using an IDO−/− KO mouse model, it has been demonstrated that the absence of IDO results in increased colon GVHD and reduced survival of transplanted tissue in IDO−/− mice compared with wild-type mice. Interestingly, in IDO−/− mice, the major effect is isolated to the site of GVHD, mainly in the gut, with increased infiltration of proliferating CD4+ and CD8+ T cells, whereas there was little to no systemic effect found (ie, activation of T cells in lymphoid organs and/or induction of Tregs). The critical role for IDO expression in orchestrating T-cell function during GVHD at the site of inflammation represents a major advance in our knowledge on the environmental effects influencing GVHD tissue injury and offers a rationale for a novel approach to GVHD management. Interestingly, MSCs, which are currently under clinical investigation for the induction of immunologic tolerance within allogeneic stem cell transplantation,59 have been shown to express a functionally active form of IDO after exposure to IFN-γ.9 This observation provides a rationale for exploiting IDO-mediated tolerance as part of a cell therapy approach to GVHD.
Cancer
During tumor development, cancer cells acquire certain cell-intrinsic characteristics, such as immortalization, growth signal self-sufficiency, and apoptosis resistance, as well as some properties that are defined through the interaction with the host environment (cell extrinsic). Among the latter, the capacity of tumor cells to interact with the immune system is typical and may represent a crucial step in the process of malignant transformation. Indeed, several reports support the hypothesis that the immune system–tumor cell interaction plays a critical and dual role in tumor development both by eliminating tumor cells and by facilitating tumor escape from immune control. Recent evidence has demonstrated that the immune system can readily destroy transformed cells but can also promote the selection of neoplastic clones with reduced immunogenicity, thus providing developing tumors with a mechanism to escape immunologic detection and elimination.60
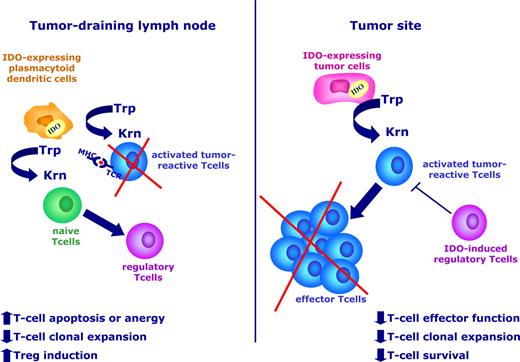
Recently, a wide variety of human solid tumors have been demonstrated to express an active IDO protein, and transfection of IDO into tumor cells prevents their rejection by preimmunized hosts.6,43 The antitumor effect of IDO blockade is completely dependent on the presence of a fully competent immune system, thus suggesting that IDO acts by deregulating the host immune response and plays a pivotal role in the inhibition of tumor-specific immunity. The mechanism(s) by which IDO expression leads to the induction of immune tolerance to tumors has been widely investigated in recent years. These studies suggest that IDO protein is critically involved in this process both at the tumor site, where the depletion of tryptophan and the increase of immunosuppressive metabolites results in reduced effector function, clonal expansion, and survival of antigen-specific T cells, and at the level of the tumor-draining lymph node, where a population of IDO-expressing plasmacytoid DCs have a tolerogenic effect on T cells during their encounter with antigen-loaded APCs (Figure 3). Interestingly, IDO-expressing DCs have been observed in sentinel lymph nodes of patients with melanoma and are associated with poor clinical outcome.14
IDO protein is involved in the induction of tumor tolerance. IDO is up-regulated in both tumor cells (right) and in a subset of regulatory/plasmacytoid DCs within tumor-draining lymph nodes (left). At the tumor site, tryptophan degradation impairs the effector function of antigen-specific T cells and reduces the immune-mediated control of tumor growth. In tumor-draining lymph nodes, regulatory DCs create a tolerogenic microenvironment, where the presentation of relevant tumor antigens to developing T cells may be defective, which leads to reduced numbers of antigen-specific T cells and increased numbers of Tregs.
IDO protein is involved in the induction of tumor tolerance. IDO is up-regulated in both tumor cells (right) and in a subset of regulatory/plasmacytoid DCs within tumor-draining lymph nodes (left). At the tumor site, tryptophan degradation impairs the effector function of antigen-specific T cells and reduces the immune-mediated control of tumor growth. In tumor-draining lymph nodes, regulatory DCs create a tolerogenic microenvironment, where the presentation of relevant tumor antigens to developing T cells may be defective, which leads to reduced numbers of antigen-specific T cells and increased numbers of Tregs.
In humans, IDO overexpression has been correlated with poor prognosis in patients with ovarian carcinoma, endometrial carcinoma, and colon carcinoma.61,–63 In particular, for colorectal cancer, IDO expression by tumor cells is associated with a reduction in tumor-infiltrating lymphocytes.63 These data are in agreement with the hypothesis that increased IDO activity within a tumor site creates an immunosuppressive microenvironment that inhibits effector antigen-specific T cells. The genetic basis of IDO gene regulation in tumor cells as well as its interplay with other aspects of malignant conversion, such as tumor cell proliferation and apoptosis, is not completely elucidated. One recent report demonstrated that IDO is under the genetic control of BIN1, which acts as a cancer suppressive gene and is down-regulated in many human malignancies.64 In particular, deletion of the BIN1 gene from cells results in the overexpression of IDO gene by IFN-γ. These experimental data may suggest that, at least in some cases, IDO is constitutively expressed by tumor cells as part of the genetic changes involved in malignant transformation and provide a platform to investigate the molecular link between tumor cell transformation and the interaction of tumor cells with the host immune system. Alternatively, since IDO is known to be induced in different cell subsets upon stimulation with IFN-γ, its expression by tumor cells may be a response to inflammatory cytokines, which are produced in response to the initial host immune activation against the tumor.
The role of IDO in hematology
The leukemogenic process has been widely investigated over the last 3 decades and major advances have been made in our understanding of the molecular and cellular bases of leukemias.65 Moreover, several reports support the notion that the leukemic clone is influenced by the host immune system, which can counteract the development and growth of leukemic cells. Clinical and biologic results from allogeneic HSCT represent the major evidence that leukemia cells are sensitive to the antitumor activity of the immune system. Since the first observation that allogeneic BM transplantation offered a clinical advantage over autologous transplantation, much more attention has been given to the role of adoptive immunotherapy over a conditioning regimen as a means to eradicate tumor cells. In particular, donor lymphocyte infusions (DLIs) in patients with chronic myeloid leukemia (CML) who relapse after HSCT are able to reinduce hematologic and molecular complete remission.66 Moreover, tumor-specific T lymphocytes have been demonstrated to participate in the elimination of CML cells in patients undergoing HSCT or IFN-α–based treatment.67 More recently, patients with AML have been successfully treated with a leukemia-specific vaccine formulation, which resulted in the eradication of minimal residual disease.68 These results, along with the increasing use of so-called mini-transplants, are the proof of principle for the crucial activity of antitumor immunity in controlling leukemia growth.
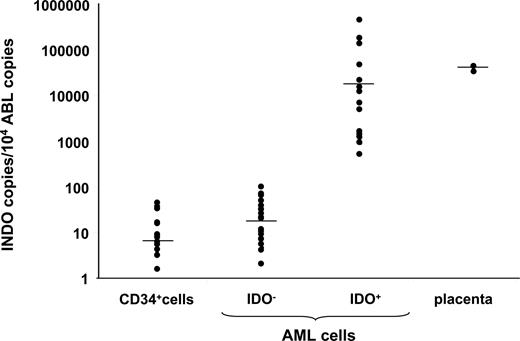
Along with the demonstration of the capacity of the immune system to recognize and destroy leukemia cells, recent reports have also provided evidence that leukemia cells exert several mechanisms to escape tumor-specific immune responses. AML cells prevent T-cell activation and proliferation both through a cell-to-cell contact mechanism and by producing soluble factors inhibiting the Th1 immune response.69,70 Transgene expression of costimulatory molecules into AML cells is not sufficient to restore T-cell function, thus suggesting that the leukemic microenvironment is very important in inducing the T-cell deficit.70 Moreover, CML cells are known to shape an antileukemia immune response by depleting high-avidity T-cell clones.71 We demonstrated that AML cells, but not their normal counterparts (ie, CD34+ hematopoietic stem/progenitor cells [HSCs]), express an active IDO protein that converts tryptophan into kynurenine and inhibits allogeneic T-cell proliferation.8 The differential expression of IDO among normal CD34+ HSCs and leukemic cells supports the hypothesis that, in a subset of AML patients, the constitutive expression of IDO protein is associated with the leukemic transformation and may play a role in leukemia development. IDO gene expression by leukemic blasts has been recently analyzed using a quantitative reverse-transcription–polymerase chain reaction (RT-PCR) method. Fifty-four newly diagnosed AML patients have been studied. According to the IDO transcript level, AML patients could be subdivided into 2 groups (Figure 4; A.C., unpublished data, February 2009). In particular, a group of patients expressed IDO at the same level as normal BM cells (IDO negative, n = 40), whereas 14 patients (26%) showed a significantly increased IDO mRNA expression at a level comparable with that of the positive control (placenta cells). We and others6 have also shown that there is a direct correlation between the level of mRNA and the presence of detectable amounts of protein, which is measurable by enzymatic activity. Indeed, only leukemic samples with a high expression of IDO mRNA have detectable amounts of protein, which was measured by Western blot analysis and immunohistochemistry, and consequently, show significant production of tryptophan metabolites along the kynurenine pathway.7 The clinical relevance of IDO expression by AML cells is under active clinical investigation by several groups. As mentioned above, IDO expression by solid tumors confers a poor clinical outcome. Very recently, IDO overexpression in AML blasts has been confirmed by other groups.72,,–75 IDO activity of AML cells can be measured from patients' sera by high-performance liquid chromatography (HPLC) and is inducible by IFN-γ.72 Moreover, correlation of IDO expression with well-known prognostic factors and survival identified high IDO expression as a strong negative independent predicting variable for overall survival and relapse-free survival.75
IDO expression by AML cells. IDO gene expression by AML blasts has been analyzed using a quantitative RT-PCR method in 54 newly diagnosed patients. IDO mRNA level was compared with that of Abelson (ABL) control gene. According to the IDO transcript level, AML patients could be subdivided into 2 groups. In particular, a group of patients (n = 40) expressed IDO at the same level as normal BM-derived CD34+ cells (IDO−). The remaining samples (n = 14) showed a significantly increased IDO mRNA expression that was at a level comparable with that of the positive control placenta cells (IDO+).
IDO expression by AML cells. IDO gene expression by AML blasts has been analyzed using a quantitative RT-PCR method in 54 newly diagnosed patients. IDO mRNA level was compared with that of Abelson (ABL) control gene. According to the IDO transcript level, AML patients could be subdivided into 2 groups. In particular, a group of patients (n = 40) expressed IDO at the same level as normal BM-derived CD34+ cells (IDO−). The remaining samples (n = 14) showed a significantly increased IDO mRNA expression that was at a level comparable with that of the positive control placenta cells (IDO+).
A significantly increased number of Tregs have been observed in several tumors, such as lung, breast, pancreatic, ovarian carcinomas, and melanoma, and Tregs have been found to suppress the tumor-specific immune response.76,–78 In hematologic malignancies, an increase in circulating CD4+CD25+ Tregs has been demonstrated in Hodgkin lymphoma, non-Hodgkin lymphoma (NHL), chronic lymphocytic leukemia, AML, multiple myeloma, and high-risk myelodysplastic syndromes.79,,,,–84 One recent comprehensive survey of Treg frequency in NHL has been published.85 The authors found that all subtypes of lymphomas had increased Tregs and their number was associated with disease stage and other biomarkers correlated with tumor burden (ie, lactate dehydrogenase). Interestingly, there were abnormally high numbers of Tregs in both the peripheral blood and locally involved tissues, thus suggesting that the microenvironment induced by lymphoma cells may be responsible for Treg induction.
The origin of the increased number of Tregs in cancer patients needs further elucidation. Murine tumors of different histologic types are capable of expanding Treg numbers by converting CD4+CD25− into CD25+ T cells.86 It was postulated that tumor-derived soluble factors, such as transforming growth factor-β1,87 may play a critical role in inducing the Treg conversion. Other possibilities include the recruitment and/or expansion of the circulating Treg population within tumoral tissues. We recently showed that AML cells induce in vitro CD4+CD25+Foxp3+ Tregs from the Foxp3−CD25− T-cell fraction via expression of IDO protein and modulation of tryptophan catabolism.10 Increased levels of circulating Tregs have been associated with a high expression of IDO protein by AML cells. The same results were obtained using a mouse model of leukemia/lymphoma, where the transgenic overexpression of IDO in tumor cells induced an increase in the Treg frequency in peripheral blood and within lymphatic tissues, such as the spleen and lymph nodes. These data point to a direct role for IDO production in the expansion of converting Tregs by AML cells and are in agreement with the results by Puccetti and collaborators demonstrating that, in a nontumoral mouse model, tryptophan catabolism favors the emergence of CD25+Foxp3+ Tregs by conversion from CD25−Foxp3− cells (Fallarino et al38 ). It remains to be elucidated how the modulation of tryptophan catabolism by IDO-expressing AML cells may be implicated in the conversion of CD4+CD25− cells into CD4+CD25+ cells. A tumor microenvironment containing reduced concentrations of tryptophan and high concentrations of kynurenine may play a role in the tumor-mediated induction of Tregs. Interestingly, the accumulation of small molecules, such as l-arginine, nitric oxide, and polyamines, within the tumor microenvironment has recently been demonstrated to affect tumor-infiltrating cell populations, including Tregs.88 More recently, ATP metabolites, which may be generated by Tregs within tumor microenvironment, have been demonstrated to suppress DC maturation and to induce IDO expression through triggering adenosine receptors.89
IDO as a target for cancer therapy
Several preclinical studies have demonstrated that IDO inhibition may result in a therapeutic effect, especially in murine models of cancer. IDO inhibitors include small molecules, such as 1-methyl-tryptophan (1-MT), which abrogates IDO enzymatic activity by competing with the normal tryptophan substrate. Initially, 1-MT was used in vivo to test the effect of IDO in tumor cells, which had been genetically modified to overexpress IDO protein.6 These studies demonstrated that 1-MT can slow down the development and growth of IDO-expressing tumor cells, but it cannot induce their regression. Similarly, in the spontaneous mammary tumors that develop in the MMTV-neu/HER2 transgenic mouse model of breast cancer, 1-MT was effective at reducing tumor growth, but did not affect tumor-free survival.64 However, when combined with different types of cytotoxic chemotherapeutic agents, 1-MT induced regression of MMTV-neu/HER2 tumors. This effect was mediated by a T-cell pathway since it was abolished by the depletion of T cells before the treatment. Collectively, these data indicate that the combination of an immunomodulatory agent inhibiting IDO activity and chemotherapeutics may be more effective than the single agents used alone. Indeed, it is well known that cancer chemotherapy may favor antigen presentation by causing the release of tumor antigens from dying tumor cells.90 Moreover, some cytotoxic agents may act by inactivating tumor-protective Tregs, thus increasing the efficacy of immunotherapeutic interventions.91 However, in most studies, chemotherapy is not sufficient to elicit a protective antitumor immune response against cancer. It would be interesting to determine whether inhibiting IDO after chemotherapy would result in better outcomes since it persistently breaks the tolerance to tumors, thus eliciting an effective immune response.
1-MT exists as both d and l isomers. Although d-1-MT has recently entered a phase 1 clinical trial as immune adjuvant for cancer immunotherapy (http://www.clinicaltrial.gov), it is still a matter of debate which isomer would be more powerful as an immune-modulating agent. Based on its chemical structure, it would be expected that only l-1-MT may exert a significant effect in inhibiting IDO enzymatic activity. The superiority of l-1-MT was clearly demonstrated when both molecules were tested to inhibit IDO-expressing cell lines.92 However, when the 2 compounds were compared in mixed lymphocyte reactions to suppress in vitro IDO-mediated tolerogenic effect on T cells, d-1-MT proved superior to l-1-MT.3 In addition, reports using in vivo murine models of cancer demonstrated that d-1-MT inhibited IDO activity better. Interestingly, recent data show that IDO2 is preferentially inhibited by d-1-MT, in contrast to IDO, which is inhibited by l-1-MT.13 Recent reports have demonstrated a differential expression of IDO and IDO2 by different cell subsets. It is then possible that the contrasting results obtained with 1-MT isoforms under different experimental conditions may be a result of the differential expression and susceptibility to inhibition of IDO targets. Other IDO-inhibitor molecules are currently under investigation.93 In particular, the nontoxic inhibitor, methyl-thiohydantoin tryptophan, and the natural product, brassinin, both display great potency in inhibiting IDO activity in a similar way as 1-MT.93,94 Given the promising preclinical results in inhibiting the tolerance induction to tumors, it is of major interest for clinicians to clearly demonstrate which IDO-inhibitor should be used for clinical purposes.
Conclusions
As discussed in this review, IDO has recently emerged as a key regulator of the immune response. IDO activity has been widely investigated and is currently a well-established inducer of the immune tolerance to tumors. Taken together, these data offer a rationale for the clinical investigation of the capacity of IDO inhibitors to increase the efficacy of anticancer immunotherapy, in addition to conventional chemotherapeutic agents. In particular, active vaccination may significantly benefit from a multipronged approach, which includes the destruction of tumor cells by chemotherapy with a subsequent release of tumor antigens and prevention of the IDO-mediated immunologic tolerance. Ongoing and future clinical studies should both address whether IDO suppression significantly impacts the efficacy of immunotherapeutic interventions against cancer and provide evidence to determine whether IDO expression represents a fundamental pathway in tumor development, including hematologic malignancies. Besides tumors, the demonstration of a critical effect for IDO in reducing Th1-mediated tissue damage has significant implications for a variety of clinical settings, including GVHD. GVHD is the main cause of morbidity and mortality after HSCT and novel approaches of GVHD treatment are necessary. The modulation of IDO in GVHD target organs may represent an interesting strategy to limit GVHD by acting at sites where host and donor cells interact, whereas other interventions, such as Treg infusional therapy, may be used to induce systemic T-cell tolerance within lymphoid organs. Future clinical applications of these recent biologic advances are warranted since the interest in developing therapeutic targets against the IDO pathway is expected to be continuously growing.
Acknowledgments
The authors thank Dr Mario Colombo for his critical review of the paper. The authors apologize to colleagues whose work could not be cited due to space limitations.
This work was supported by Consiglio Nazionale delle Ricerche (CNR, Rome, Italy; N. CU03.00334), Italian Ministry of Health (Progetto Strategico Oncologia, 2006), Regione Emilia-Romagna (Progetti di Ricerca Università-Regione Emilia Romagna, Progetto Medicina Rigenerativa, 2007), Italian Association Against Leukemia, Section of Bologna (BolognAIL), and Ministero dell'Istruzione, dell'Università e della Ricerca (MIUR; PRIN 2006).
Authorship
Contribution: A.C. designed the research, analyzed the data, and wrote the paper; S.T. and V.S. performed research and analyzed the data; M.B. critically reviewed the paper; and R.M.L. designed the research and critically reviewed the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Antonio Curti, Department of Hematology and Oncological Sciences L & A Seràgnoli, Via Massarenti, 9, 40138, Bologna, Italy; e-mail: antonio.curti2@unibo.it.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal