Interferon regulatory factor-4 (IRF-4) is a hematopoietic cell–restricted transcription factor important for hematopoietic development and immune response regulation. It was also originally identified as the product of a proto-oncogene involved in chromosomal translocations in multiple myeloma. In contrast to its oncogenic function in late stages of B lymphopoiesis, expression of IRF-4 is down-regulated in certain myeloid and early B-lymphoid malignancies. In this study, we found that the IRF-4 protein levels are increased in lymphoblastic cells transformed by the BCR/ABL oncogene in response to BCR/ABL tyrosine kinase inhibitor imatinib. We further found that IRF-4 deficiency enhances BCR/ABL transformation of B-lymphoid progenitors in vitro and accelerates disease progression of BCR/ABL-induced acute B-lymphoblastic leukemia (B-ALL) in mice, whereas forced expression of IRF-4 potently suppresses BCR/ABL transformation of B-lymphoid progenitors in vitro and BCR/ABL-induced B-ALL in vivo. Further analysis showed that IRF-4 inhibits growth of BCR/ABL+ B lymphoblasts primarily through negative regulation of cell-cycle progression. These results demonstrate that IRF-4 functions as tumor suppressor in early B-cell development and may allow elucidation of new molecular pathways significant to the lymphoid leukemogenesis by BCR/ABL. The context dependent roles of IRF-4 in oncogenesis should be an important consideration in developing cancer therapies targeting IRF-4.
Introduction
Interferon-regulatory factor-4 (IRF-4) is an IRF family transcription factor important for hematopoietic development and immune processes. IRF-4 is expressed in lymphoid cells, dendritic cells, and macrophages, where it is associated with regulation of important cellular processes, including cell differentiation, apoptosis, DNA repair, and cytokine production
The essential role of IRF-4 in various stages of B lymphocyte development is well characterized. IRF-4 was originally identified as a protein recruited by the Ets transcription factor, Pu.1, to the immunoglobulin κ (Igκ) light-chain enhancer.1 The closely related IRF family member IRF-8 also associates with Pu.1 at the Igκ light-chain locus and functions redundantly with IRF-4 in early B-cell development.2 Mice deficient for both IRF-4 and IRF-8 show a block in B-cell development at the pre-B to immature B-cell transition and, consequently, have an accumulation of cycling pre-B cells.2 In addition to its overlapping role with IRF-8, IRF-4 has unique functions essential for later stages in B-cell development. IRF-4-deficient mice have a block in B-cell maturation from the immature to mature follicular B-cell stage.3 Recent studies revealed that IRF-4 is also required for class switch recombination and plasma cell differentiation.4,5
In addition to its normal function in regulating hematopoiesis, IRF-4 also plays a role in the pathogenesis of hematopoietic malignancies. Chromosome translocation that fuses the Ig heavy chain gene to the IRF-4 locus, resulting in overexpression of IRF-4, was found in a fraction of multiple myeloma cases.6 In addition, overexpression of IRF-4 is linked to poor prognosis in chronic lymphocytic leukemia and to the pathogenesis of adult T-cell leukemia and lymphoma.7,8 These studies indicate that IRF-4, when overexpressed, functions as an oncoprotein. On the other hand, in contrast to its role in promoting tumor progression in late stages of B lymphopoiesis, expression of IRF-4 is down-regulated in some myeloid and early B-lymphoid malignancies. Chronic myelogenous leukemia (CML) is a myeloproliferative disease characterized by the underlying t(9;22)(q34;q11) reciprocal translocation that creates a minute chromosome, known as the Philadelphia chromosome (Ph). The translocation leads to creation and expression of the fusion gene product BCR/ABL, a constitutively active tyrosine kinase.9,10 The disease has a relatively mild chronic phase, an accelerated myeloproliferative phase, and finally a transformation to blast crisis, which is characterized by a block of cell differentiation that results in accumulation of myeloid or B-lymphoid blast cells. In addition to CML, BCR/ABL is also found in 20% of adult and 2% to 5% of pediatric patients with de novo acute B-lymphoblastic leukemia (B-ALL), a leukemia that blocks B-cell development at the pro–B-cell stage.11,12 IRF-4 expression was shown to be down-regulated in patients with CML but restored in response to treatment with interferon-α (IFN-α), and higher IRF-4 expression is associated with a good response to IFN-α treatment.13,,–16 In addition, IRF-4 expression is reduced in pro-B cells transformed by BCR/ABL and v-Abl—the Abelson murine leukemia virus's oncogenic element that is created by a recombination event that fuses viral gag sequences to a truncated c-abl gene. Microarray analysis showed that the IRF-4 mRNA levels are also reduced in patients with Ph+ B-ALL.17 The role of down-regulation of IRF-4 in leukemogenesis is not clear, because it might be either part of pathogenesis (down-regulation of a tumor suppressor) or part of host defense mechanism (suppressing an oncoprotein).
Imatinib mesylate, a selective inhibitor of the ABL tyrosine kinase, has shown a remarkable clinical activity in patients with CML.18 Despite their success in treating patients with CML, imatinib and other ABL kinase inhibitors are not effective in treating patients with Ph+ B-ALL. The remission rate associated with imatinib treatment is approximately 20%, but relapse occurs within weeks to months.19,,–22 Identifying molecular targets critical in the pathogenesis of Ph+ B-ALL is important for developing therapeutic strategies to the disease.
Expression of IRF-4 is increased in response to imatinib treatment in patients with Ph+ B-ALL, patient-derived cell lines, and in BCR/ABL or v-Abl–transformed B cells.17 These data suggest that IRF-4 may be important to mediate imatinib therapy. In this study, we determine the role of IRF-4 in B-lymphoid leukemogenesis by BCR/ABL. We found that the IRF-4 protein levels are increased in response to imatinib treatment in murine lymphoblastic cells transformed by BCR/ABL, and that BCR/ABL transformation of B-lymphoid progenitors in vitro and in vivo is facilitated by loss of IRF-4 and suppressed by forced expression of IRF-4. These results demonstrate that, in contrast to its tumor promoting function in late stages of B-cell development, IRF-4 functions as a tumor suppressor in early B-cell development.
Methods
DNA constructs
Production of MSCV-BCR/ABL-IRES-GFP retroviral constructs was described previously.23 The cDNA for murine IRF-4 was amplified by polymerase chain reaction (PCR) with a 3′ primer containing a NotI site and a 5′ primer containing a ClaI site. The amplified DNA fragment was sequenced to confirm no errors had been introduced. The amplified IRF-4 was cloned into the NotI and ClaI sites of the previously described retroviral vector MSCV-BCR/ABL-GFP-IRES-2xmyc tag24 to generate MSCV-BCR/ABL-GFP-IRES2xmyc tagIRF-4, where IRF-4 is in frame with the myc tag. MSCV-GFP-IRES-2xmyc tagIRF-4 was made by swapping the EcoRI-flanked BCR/ABLGFP from MSCV-BCR/ABLGFP-IRES-IRF-4 with EcoRI-flanked GFP sequence. The MSCV-GFP-IRES-IRF-8myc tag construct was made as described previously25 and used to generate MSCV-BCR/ABL-GFP-IRES-IRF-8myc tag by excising the EcoRI-flanked GFP sequence from MSCV-GFP-IRES-IRF-8myc tag and replacing it with the EcoRI-flanked BCR/ABL-GFP sequence. A modified MSCV construct containing a neomycin resistance gene, MSCV-IRES-Neo, was used to produce MSCV-BCR/ABL-GFP-IRES-Neo by inserting the BCR/ABL-GFP sequence into the EcoRI site preceding the IRES in MSCV-IRES-Neo. The control MSCV-GFP-IRES was made by swapping EcoRI-flanked BCR/ABL-GFP from MSCV-BCR/ABLGFP-IRES-2xmyc tag with EcoRI-flanked GFP sequences. MSCV-RFP was made by excising GFP sequences from MSCV-GFP-IRES-IRF-4 and MSCV-GFP-IRES-IRF-8 with EcoRI and XhoI and replacing it with an enhanced red fluorescent protein (RFP, tdimer2)26 sequences.
Mouse strains
The IRF-4+/− mice3 in C57BL/6 background was kindly provided by Dr H. Singh (University of Chicago, Chicago, IL), with kind permission by Dr T. Mak (Princess Margaret Hospital, Toronto, ON). Mice of IRF-4+/+ (wild type [WT]), IRF-4+/− (Het), and IRF-4−/− (knockout [KO]) were generated through breeding of the IRF-4+/− mice. This study received approval for the use of mice from the Institutional Animal Use Committee of Brandeis University.
Cell culture and retrovirus production
Bosc23 cells27 were maintained in Dulbecco modified Eagle medium containing 10% fetal bovine serum (FBS), 100 U/mL penicillin, and 100 μg/mL streptomycin. BCR/ABL+ B-ALL cell cultures were established by isolating bone marrow (BM) from moribund mice that had been reconstituted with BCR/ABL-infected BM from IRF-4 WT, IRF-4 KO, or IRF-4 Het donor mice. Cells were maintained in RPMI 1640 medium (Invitrogen, Carlsbad, CA) containing 10% FBS, 100 U/mL penicillin, 100 μg/mL streptomycin, and 50 μmol/L 2-mercaptoethanol. Media was changed twice weekly. Within 2 to 3 weeks, cultures consisted of 100% GFP+ malignant B lymphoblasts.
An interleukin-7 (IL-7)–dependent pro–B-cell line, E2AGFP, was kindly provided by Dr Y. Zhuang (Duke University School of Medicine, Durham, NC).28 BCR/ABL transformed E2AGFP pro-B cells were generated by transduction of E2AGFP cells with MSCV-BCR/ABL-IRES-GFP by cosedimentation at 1200 relative centrifugal force (rcf) for 90 minutes in medium containing 50% viral supernatant, 5% FBS, 100 U/mL penicillin, 100 mg/mL streptomycin, 10 ng/mL IL-7, 50 μmol/L 2-mercaptoethanol, and 6 μg/mL Polybrene. IL-7 was removed from the culture media 2 days after transduction to select for IL-7–independent, BCR/ABL-transformed cells. BCR/ABL-transformed E2AGFP cells and primary B-ALL cell cultures were treated with 5, 10, or 20 μmol/L imatinib.18 Cell-cycle analysis was performed using standard bromodeoxyuridine (BrdU) incorporation assays according to protocols described for APC BrdU Flow kit (BD Biosciences, San Jose, CA).
Immunoblotting
BCR/ABL+ B lymphoblast cell lysates were prepared from primary cell cultures described above. Live cells were counted by trypan blue exclusion and resuspended in phosphate-buffered saline (PBS) at a concentration of 2 × 108 cells/mL followed by addition of an equal volume of 2× SDS sample buffer. Samples were boiled for 5 minutes followed by centrifugation to pellet debris, then analyzed by sodium dodecyl sulfate–polyacrylamide gel electrophoresis. Proteins were separated on 6% to 18% polyacrylamide gradient gels, then proteins were transferred to nitrocellulose filters. The filters were probed with anti-ABL monoclonal antibody Ab3 (Oncogene Research Products, Cambridge, MA), anti–IRF-4 polyclonal antibody (Santa Cruz Biotechnology, Santa Cruz, CA), anti-IRF-8 polyclonal antibody (Santa Cruz Biotechnology), anti-dynamin monoclonal antibody (BD Biosciences, San Jose, CA), or anti-myc tag 9E10 (from conditional media of 9E10 hybridoma cell line). Bound antibodies were detected by horseradish peroxidase-conjugated anti–mouse IgG and Super Signal West Femto chemiluminescence reagents (Pierce Biotechnology, Rockford, IL).
Bone marrow colony assays
Bone marrow colony assays for transformation of BM-derived B-lymphoid progenitors were performed as described previously30 with modifications. BM cells were infected by cosedimentation with virus in a volume of 3 ml containing 50% viral supernatant, 5% FBS, 100 U/mL penicillin, 100 μg/mL streptomycin, 5% WEHI-conditioned medium, 10 ng/mL IL-7, and 6 μg/mL Polybrene. The cells were centrifuged at 1200 rcf for 90 minutes then incubated at 37°C for an additional 90 minutes. Cells were washed with PBS, then 2 × 106 cells were plated in triplicate in RPMI 1640 media, 20% FBS, 100 U/mL penicillin, 100 μg/mL streptomycin, 50 μmol/L 2-mercaptoethanol, and 0.3% bacto agar. Cultures were incubated at 37°C, and colonies were counted after 10 days. The Student t test was used for statistical analysis.
Bone marrow transduction and transplantation
The mouse bone marrow transduction and transplantation model for BCR/ABL-induced B-ALL was performed as described previously.31 In brief, bone marrow cells isolated from BALB/cByJ (Taconic Farms, Hudson, NY) or IRF-4 WT, Het, and KO donor mice were infected with retrovirus by cosedimentation at 1200 rcf for 90 minutes in medium containing 50% viral supernatant, 5% FBS, 100 U/mL penicillin, 100 μg/mL streptomycin, 5% WEHI-conditioned medium, 10 ng/mL IL-7, and 6 μg/mL Polybrene. Cells were then incubated at 37°C for 4.5 hours then washed with PBS followed by transplantation of 106 cells into lethally irradiated syngenic recipients. Statistical analysis of survival data were performed with StatView 5 (Abacus Concepts, Berkeley, CA) using the Kaplan-Meier survival analysis and Mantel-Cox (log-rank) test functions.
Flow cytometry analysis
Standard protocols for antibody staining of cell surface proteins were followed.32 Cells from peripheral blood, bone marrow, pleural effusion, or lymph node tumors were treated with ACK (150 mM NH4Cl, 1 mM KHCO3, 0.1 mM Na2EDTA [pH 7.3]) to lyse red blood cells then resuspended in staining buffer (PBS, 1% FBS, and 0.1% sodium azide) and blocked with anti–mouse CD16-CD32 (Fc block; BD Pharmingen, San Diego, CA). Cells were stained with the following antibodies from BD Pharmingen: allophycocyanin (APC)–conjugated Mac1(M1/70), phycoerythrin (PE)–conjugated Mac1, PE-conjugated Gr1 (RB6-8C5), fluorescein isothiocyanate (FITC)–conjugated Gr1, PE-conjugated CD19, APC-conjugated B220, PE-conjugated CD43, PE-conjugated IgM, APC-conjugated streptavidin, and PE-conjugated Bp1. After staining, cells were washed with PBS and resuspended in staining buffer containing propidium iodide to label dead cells. Flow cytometry was performed on a FACSCalibur machine (BD Biosciences) to detect green fluorescent protein (GFP), red fluorescent protein (RFP), and/or antibody-stained cells. Data were analyzed with FlowJo software (TreeStar, Ashland, OR).
Real-time RT-PCR analysis
Total RNA was isolated from 3 BCR/ABL transformed primary B-ALL cell lines derived from IRF-4 WT (no. 8), IRF-4 Het (no. 23), and IRF-4 KO (no. 9) donor BM using the RNeasy Mini kit (QIAGEN, Valencia, CA). The cDNAs were synthesized using the Supercript III First Strand Synthesis System (Invitrogen, CA). All real-time PCR reactions were done using the Rotor-Gene 3000 (Corbett Research, Sydney, Australia). Each reaction contained 5 μL SYBR Green Jumpstart Taq Readymix (Sigma-Aldrich, St Louis, MO), 3.5 μL diethyl pyrocarbonate (DEPC) water, 0.25 μL of forward primer, 0.25 μL of reverse primer, and 1 μL cDNA. The cycle program was as follows: 90 seconds at 95°C followed by 50 cycles of 94°C for 10 seconds, 60°C for 30 seconds, and 72°C for 35 seconds. All experiments were performed in triplicate, and each cell line was represented by 3 independent samples. The housekeeping gene β-2-microglobin (B2m) was used as the internal control. The statistic analysis was done using a Student unpaired t test. All primers were synthesized by Integrated DNA Technologies (Coralville, IA) and diluted to 10 μmol/L. The primers for Cdkn2a were 5′-CGT GAA CAT GTT GTT GAG GC-3′ and 5′-CGA ATC TGC ACC GTA GTT GAG C-3′; for Cdkn2b, 5′-AAC GCC CTG AAC CGC TTC-3′ and 5′-GCAGCAGCAGCT CTG CCA-3′; for Cdkn1b, 5′-TTG GGT CTC AGG CAA ACT CT-3′ and 5′-CGA AGA AGA ATC TTC TGC AGC AGG-3′; for Cdkn1c, 5′-TGC GAA CGA CTT CTT CGC CAA-3′ and 5′-TCT GGC CGT TAG CCT CTA AAC-3′; and for B2m, 5′-GGC CTG TAT GCT ATC CAG AA-3′ and 5′-GAA AGA CCA GTC CTT GCT GA-3′.
Results
Imatinib treatment results in increased expression of IRF-4 in BCR/ABL-induced mouse B-ALL
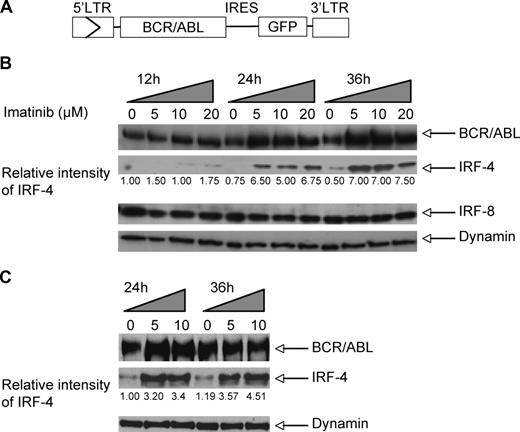
It has been shown that expression of BCR/ABL in B-lymphoid progenitors efficiently induces B-ALL in mice.33,34 To study the role of IRF-4 in BCR/ABL B-ALL using this mouse model, we first examined IRF-4 expression in BCR/ABL-induced mouse B-ALL in response to imatinib treatment. We used MSCV-BCR/ABL-IRES-GFP retrovirus (Figure 1A) to transduce bone marrow cells freshly isolated from donor mice and then transplanted the infected marrow cells into lethally irradiated syngeneic recipients. The recipient mice developed B-ALL–like disease in 5 to 10 weeks after bone marrow transplantation (BMT). Bone marrow cells were isolated from the BCR/ABL BMT mice that succumbed to B-ALL and then cultured in the absence of cytokines to select for BCR/ABL expressing GFP+ malignant B lymphoblasts. After 3 weeks, the cultures consisted of 100% GFP+ B lymphoblasts with pro–B-cell phenotype: B220+, CD19+, CD43+, Bp-1+, and IgM− (data not shown). The B lymphoblast cell cultures were treated with 0, 5, 10 and 20 μmol/L imatinib. Western blot analysis shows that IRF-4 expression levels are dramatically increased within 24 hours in response to imatinib treatment (Figure 1B). Real-time RT-PCR analysis of IRF-4 RNA levels in the same cultured B lymphoblasts show that IRF-4 transcript levels are increased in response to imatinib treatment (Figure S1, available on the Blood website; see the Supplemental Materials link at the top of the online article), indicating that BCR/ABL regulates IRF-4 expression at the transcription level. It is noteworthy that the protein levels of the closely related IRF family member IRF-8 are not increased under the same conditions (Figure 1B). Analysis of the effect of imatinib treatment on cell-cycle progression in the primary cell cultures shows a significant increase in the percentage of cells in G1/G0 and in the percentage of apoptotic cells (Figure S2). There is no significant difference in cell cycle kinetics between cells treated with 5 and 10 μmol/L imatinib, suggesting that saturating conditions were achieved at 5 μmol/L imatinib.
Increase of IRF-4 expression in BCR/ABL+ B lymphoblast cells in response to imatinib treatment. (A) Diagram of MSCV-BCR/ABL-IRES-GFP retroviral construct used to induce B-ALL in mice and transduce E2AGFP pro-B cells. (B) Expression BCR/ABL, IRF-4, and IRF-8 in imatinib-treated or untreated B lymphoblasts derived from BCR/ABL BMT mice suffering from B-ALL, as detected by immunoblotting with an anti-ABL monoclonal antibody (Ab-3; top panel), anti–IRF-4 polyclonal antibody (second panel from top), anti–IRF-8 polyclonal antibody (third panel from top), and anti-dynamin monoclonal antibody (loading control, bottom panel). (C) Expression of BCR/ABL and IRF-4 in imatinib-treated or -untreated BCR/ABL-transformed E2AGFP cells as detected by immunoblotting with antibodies described in panel B.
Increase of IRF-4 expression in BCR/ABL+ B lymphoblast cells in response to imatinib treatment. (A) Diagram of MSCV-BCR/ABL-IRES-GFP retroviral construct used to induce B-ALL in mice and transduce E2AGFP pro-B cells. (B) Expression BCR/ABL, IRF-4, and IRF-8 in imatinib-treated or untreated B lymphoblasts derived from BCR/ABL BMT mice suffering from B-ALL, as detected by immunoblotting with an anti-ABL monoclonal antibody (Ab-3; top panel), anti–IRF-4 polyclonal antibody (second panel from top), anti–IRF-8 polyclonal antibody (third panel from top), and anti-dynamin monoclonal antibody (loading control, bottom panel). (C) Expression of BCR/ABL and IRF-4 in imatinib-treated or -untreated BCR/ABL-transformed E2AGFP cells as detected by immunoblotting with antibodies described in panel B.
We also examined the effect of imatinib on IRF-4 protein levels in a pro-B cell line (E2AGFP) transformed by BCR/ABL. Consistent with our observations in the primary B-ALL cells, imatinib induced up-regulation of IRF-4 in these cells as well (Figure 1C).
IRF-4 deficiency facilitates BCR/ABL transformation of B-lymphoid progenitors
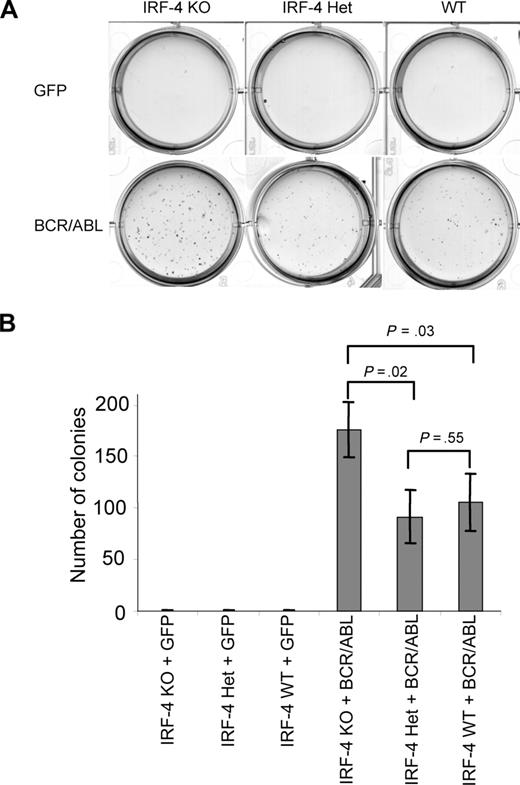
The down-regulation of IRF-4 in BCR/ABL+ B-ALL and increase of IRF-4 expression in response to imatinib suggest that IRF-4 may negatively regulate lymphoid leukemogenesis by BCR/ABL. To assess the role of IRF-4 in the pathogenesis of BCR/ABL+ B-ALL, we examined the effect of both loss of and forced expression of IRF-4 in transformation of lymphoid cells by BCR/ABL. Because BCR/ABL reduces, but does not eliminate, IRF-4 expression, we predicted that if IRF-4 functioned as a tumor suppressor, knockout of the IRF-4 gene would facilitate BCR/ABL leukemogenesis. To test this hypothesis, we first examined the effect of IRF-4 deficiency on B-lymphoid cell transformation by BCR/ABL in vitro using a lymphoid colony formation assay.30 In brief, bone marrow cells isolated from IRF-4+/−, IRF-4−/−, and WT mice were infected with MSCV-IRES-GFP or MSCV-BCR/ABL-IRES-GFP retroviral supernatant by cosedimentation in the presence of IL-7. Cells were then plated in soft agar in the absence of cytokines and incubated at 37°C for 10 days. The GFP vector control did not induce cytokine independent colony formation in cultures for any type of donor as expected. BCR/ABL did induce lymphoid colony formation in cultures derived from WT, IRF-4+/−, and IRF-4−/− BM, but there were significantly more colonies in cultures from BCR/ABL-infected IRF-4−/− bone marrow compared with BCR/ABL-infected WT or IRF-4+/− BM (Figure 2A,B). The number of colonies in cultures from IRF-4+/− and WT donors, on the other hand, was not significantly different. Similar results were obtained in a repeating experiment (data not shown). These data demonstrate that complete loss of IRF-4 facilitates BCR/ABL transformation of B-lymphoid progenitors, indicating that IRF-4 functions in inhibiting B-lymphoid transformation by BCR/ABL.
IRF-4 deficiency facilitates BCR/ABL transformation of B-lymphoid progenitors. Bone marrow from IRF-4 WT, IRF-4 Het, or IRF-4 KO mice was infected with MSCV retrovirus containing sequences for BCR/ABL-IRES-GFP or GFP, then triplicates of 2 × 106 cells were plated in soft agar media in the absence of cytokines. Colonies were counted after 10 days. Representative soft agar plates and quantitation of the corresponding colonies are presented in panel A and panel B, respectively.
IRF-4 deficiency facilitates BCR/ABL transformation of B-lymphoid progenitors. Bone marrow from IRF-4 WT, IRF-4 Het, or IRF-4 KO mice was infected with MSCV retrovirus containing sequences for BCR/ABL-IRES-GFP or GFP, then triplicates of 2 × 106 cells were plated in soft agar media in the absence of cytokines. Colonies were counted after 10 days. Representative soft agar plates and quantitation of the corresponding colonies are presented in panel A and panel B, respectively.
IRF-4 deficiency accelerates disease progression in a BCR/ABL-induced B-ALL mouse model
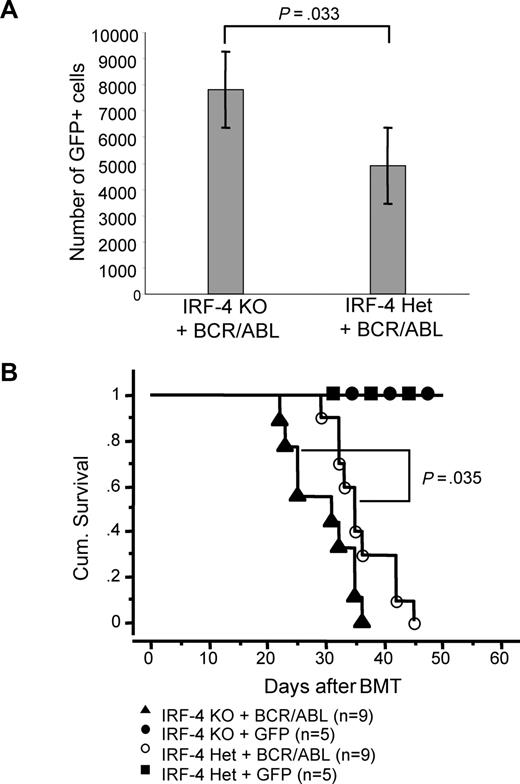
Having shown that loss of IRF-4 enhances the transforming potential of BCR/ABL in B lymphoid cells in vitro, we moved to investigate whether IRF-4 deficiency affects BCR/ABL lymphoid leukemogenesis in vivo. We infected bone marrow cells isolated from IRF-4+/− and IRF-4−/− mice with MSCV-IRES-GFP or MSCV-BCR/ABL-IRES-GFP retrovirus, and then transplanted them into wild-type recipient mice. Because there was no difference in the transformation capacity of BCR/ABL between WT and IRF-4+/− genetic background, we excluded the WT donors from this experiment. Analysis of GFP+ cells in recipient mice 15 days after BMT shows that mice reconstituted with MSCV-BCR/ABL-GFP–infected cells from IRF-4−/− donors have a significantly higher number of GFP+ cells compared with mice reconstituted with BCR/ABL-infected IRF-4+/− BM (Figure 3A). These data suggest that BCR/ABL-infected IRF-4−/− BM cells expanded faster compared with BCR/ABL-infected IRF-4+/− BM cells in vivo. Consistently, mice reconstituted with BCR/ABL-infected IRF-4−/− BM succumbed to a B-ALL disease and die significantly faster compared with mice reconstituted with BCR/ABL-infected IRF-4+/− BM (Figure 3B). These results are consistent with the idea that IRF-4 is a tumor suppressor in early B-lymphoid cell development.
IRF-4 deficiency accelerates disease progression in a BCR/ABL-induced B-ALL mouse model. (A) Number of GFP+ cells in peripheral blood of mice reconstituted with IRF-4 KO BM infected with BCR/ABL-IRES-GFP is significantly higher than the number of GFP+ cells from mice reconstituted with IRF-4 Het BM infected with BCR/ABL-IRES-GFP (P = .03). There were 9 mice in each group, and all mice were used to obtain these results. (B) Survival of mice receiving transplantation of IRF-4 Het or IRF-4 KO bone marrow cells infected with BCR/ABL-GFP– or GFP-containing retroviruses. Survival curves were generated by Kaplan-Meier survival analysis. Statistical analysis of survival data were performed with StatView 5 (Abacus Concepts) using the Kaplan-Meier survival analysis and Mantel-Cox (log-rank) test functions.
IRF-4 deficiency accelerates disease progression in a BCR/ABL-induced B-ALL mouse model. (A) Number of GFP+ cells in peripheral blood of mice reconstituted with IRF-4 KO BM infected with BCR/ABL-IRES-GFP is significantly higher than the number of GFP+ cells from mice reconstituted with IRF-4 Het BM infected with BCR/ABL-IRES-GFP (P = .03). There were 9 mice in each group, and all mice were used to obtain these results. (B) Survival of mice receiving transplantation of IRF-4 Het or IRF-4 KO bone marrow cells infected with BCR/ABL-GFP– or GFP-containing retroviruses. Survival curves were generated by Kaplan-Meier survival analysis. Statistical analysis of survival data were performed with StatView 5 (Abacus Concepts) using the Kaplan-Meier survival analysis and Mantel-Cox (log-rank) test functions.
Forced expression of IRF-4 inhibits BCR/ABL transformation of B-lymphoid progenitors in vitro
To determine whether reconstituted expression of IRF-4 affects BCR/ABL transformation of B-lymphoid cells, we investigated the effects of forced expression of IRF-4 on BCR/ABL-induced colony formation of BM-derived B lymphoid progenitors in vitro. We also included IRF-8 in this experiment for a comparison.
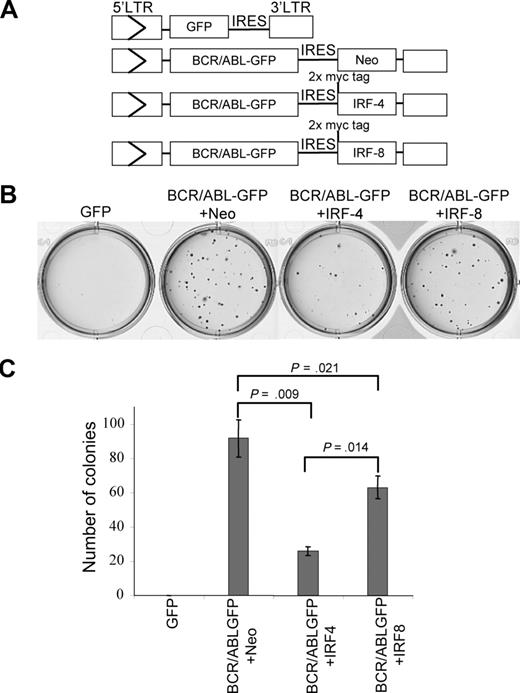
We examined the abilities of retroviral constructs MSCV-BCR/ABL-GFP+Neo, MSCV-BCR/ABL-GFP+IRF-4, MSCV-BCR/ABL-GFP+IRF-8, and MSCV-GFP (Figure 4A) to stimulate growth of BM-derived B-lymphoid cells in soft agar. Tests in NIH3T3 fibroblast and 32D myeloid cell lines showed that BCR/ABL expression and protein tyrosine phosphorylation were not affected by IRF-4 or IRF-8 expression (data not shown). Expression of BCR/ABL, myc-tagged IRF-4, and myc-tagged IRF-8 was detected in transduced NIH3T3, 32D cells, and E2AGFP B-lymphoid cells (data not shown and Figure S3A). Densitometry analysis showed that the relative expression level of ectopically expressed IRF-4 is comparable with that of the endogenous IRF-4 from imatinib-treated BCR/ABL transformed E2AGFP cells (0.88 vs 1.13; Figure S3A). These results suggest that any effects of forced expression of IRF-4 from MSCV-BCR/ABL-IRES-IRF-4 are probably not due to expression levels exceeding the normal capability of IRF-4 expression within the cell.
IRF-4 suppresses BCR/ABL–stimulated B-lymphoid colony formation. Bone marrow cells freshly isolated from mice were infected with titer-matched MSCV constructs containing BCR/ABL-GFP+Neo, BCR/ABL-GFP+IRF-4, BCR/ABL-GFP+IRF-8, or GFP (A), then triplicates of 2 × 106 cells were plated in soft agar media in the absence of cytokines. Colonies were counted after 10 days. Representative soft agar plates and quantitation of the corresponding colonies are presented in panel B and panel C, respectively.
IRF-4 suppresses BCR/ABL–stimulated B-lymphoid colony formation. Bone marrow cells freshly isolated from mice were infected with titer-matched MSCV constructs containing BCR/ABL-GFP+Neo, BCR/ABL-GFP+IRF-4, BCR/ABL-GFP+IRF-8, or GFP (A), then triplicates of 2 × 106 cells were plated in soft agar media in the absence of cytokines. Colonies were counted after 10 days. Representative soft agar plates and quantitation of the corresponding colonies are presented in panel B and panel C, respectively.
As expected, BCR/ABL-GFP, but not the GFP control, stimulated colony formation in soft agar. Cultures infected with BCR/ABL-GFP+IRF-4 and BCR/ABL-GFP+IRF-8, on the other hand, had smaller and significantly fewer colonies after 10 days compared with BCR/ABL-GFP+Neo-infected cultures (Figure 4B,C). In addition, BCR/ABL-GFP+IRF-4–infected cultures formed significantly fewer colonies than BCR/ABL-GFP+IRF-8 cultures (Figure 4B,C). These results show that forced expression of IRF-4 potently inhibits BCR/ABL-mediated B-lymphoid transformation in vitro and that forced expression of IRF-8 also inhibits colony formation but to a lesser degree compared with IRF-4.
Forced expression of IRF-4 suppresses BCR/ABL-induced B-ALL in mice
To test the ability of IRF-4 to inhibit B-lymphoid leukemogenesis directly, we determined whether coexpression of IRF-4 with BCR/ABL affected the pathogenesis of BCR/ABL-induced B-ALL in the mouse model described above. Again, IRF-8 was included for a comparison. Titer-matched BCR/ABL-GFP+Neo, BCR/ABL-GFP+IRF-4, BCR/ABL-GFP+IRF-8, and GFP MSCV retroviruses were used to transduce bone marrow cells freshly isolated from mice, followed by transplantation of the infected marrow cells into lethally irradiated syngeneic recipients.
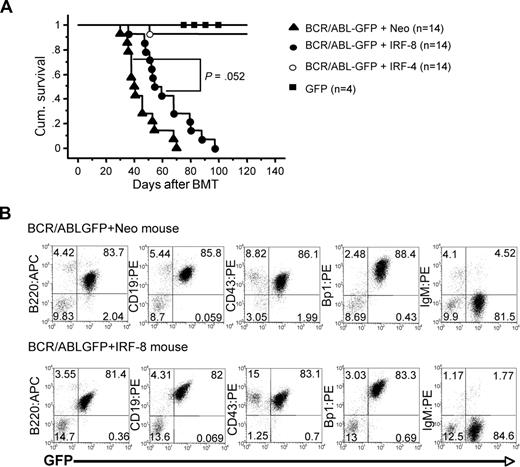
As expected, mice transplanted with bone marrow containing GFP alone showed no signs of disease in 6 months of observation, whereas mice transplanted with BCR/ABL-GFP+Neo-infected bone marrow became moribund within 5 to 10 weeks after BMT and died of a B-ALL–like disease (Figure 5A,B). Analysis of moribund mice showed moderate enlargement of spleen and lymph nodes and a bloody pleural effusion that was probably the cause of death. Some mice also developed lymph node tumors and rear-leg paralysis. Fluorescence-activated cell sorting (FACS) analysis of pleural effusion (Figure 5B) as well as lymph node tumors, bone marrow, and spleen (data not shown) indicates that the malignant GFP+ blasts are B220+, CD19+, CD43+, Bp1+, and IgM−. This phenotype is similar to what is observed at the pro-B stage of B-cell development in mice and reflects what is observed in patients with Ph+ B-ALL.35,36
IRF-4 suppresses B-lymphoid leukemogenesis by BCR/ABL in mice. (A) Survival of mice receiving transplantation of bone marrow cells infected with BCR/ABL-GFP+Neo-, BCR/ABL-GFP+IRF-4-, BCR/ABL-GFP+IRF-8-, or GFP-containing retroviruses. Survival curves were generated by Kaplan-Meier survival analysis. One BCR/ABL-GFP+IRF-4 BMT mouse succumbed to disease and died, whereas 13 of 14 mice remain alive in more than 1 year of observation. (B) Immunophenotype of pleural effusion from moribund BCR/ABL-GFP+Neo (top panel) and BCR/ABL-GFP+IRF-8 (bottom panel) BMT mice.
IRF-4 suppresses B-lymphoid leukemogenesis by BCR/ABL in mice. (A) Survival of mice receiving transplantation of bone marrow cells infected with BCR/ABL-GFP+Neo-, BCR/ABL-GFP+IRF-4-, BCR/ABL-GFP+IRF-8-, or GFP-containing retroviruses. Survival curves were generated by Kaplan-Meier survival analysis. One BCR/ABL-GFP+IRF-4 BMT mouse succumbed to disease and died, whereas 13 of 14 mice remain alive in more than 1 year of observation. (B) Immunophenotype of pleural effusion from moribund BCR/ABL-GFP+Neo (top panel) and BCR/ABL-GFP+IRF-8 (bottom panel) BMT mice.
The BCR/ABL-GFP+IRF-8 BMT mice survived longer than the BCR/ABL-GFP+Neo BMT mice with a borderline significance (P = .052). Analysis of moribund mice showed that 13 of 14 mice developed a B-lymphoid malignancy with the same disease phenotype as observed for diseased BCR/ABL-GFP+Neo BMT mice (Figure 5B). One BCR/ABL-GFP+IRF-8 BMT mouse developed a CML-like disease characterized by expansion of mature granulocytic cells and pulmonary hemorrhage (data not shown).
The BCR/ABL-GFP+IRF-4 BMT mice survived significantly longer than both BCR/ABL-GFP+Neo and BCR/ABL-GFP+IRF-8 BMT mice (Figure 5A). Thirteen of 14 BCR/ABL-GFP+IRF-4 BMT mice remained alive even at the end of the 6-month observation period. This suggests that IRF-4 is much more potent than IRF-8 at suppressing BCR/ABL-induced B-ALL in vivo. The mice that remained alive show no signs of disease or any evidence of GFP+ malignant blasts in the peripheral blood even after 1 year. Postmortem analysis of the only BCR/ABL-GFP+IRF-4 BMT mouse that succumbed to disease revealed that the cause of death was probably due to a bloody pleural effusion characteristic of a B-ALL–like disease. However, tissues from this mouse were decayed and unsuitable for further analysis. These results indicate that forced expression of IRF-4 is a potent tumor suppressor for B-lymphoid leukemogenesis by BCR/ABL.
IRF-4 inhibits proliferation of BCR/ABL+ B lymphoblasts
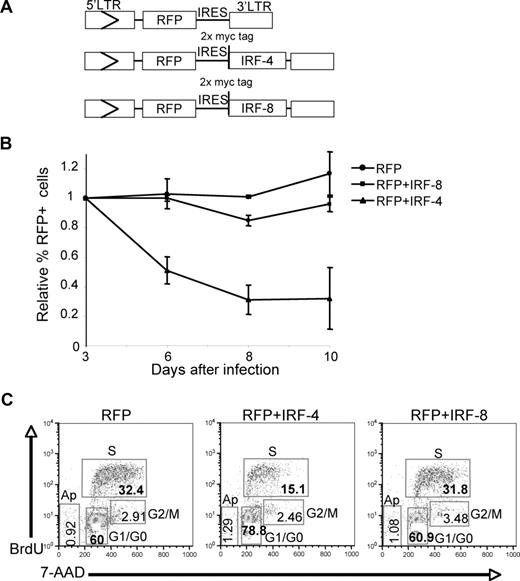
To gain insights into the mechanism by which IRF-4 suppresses lymphoid leukemogenesis, we determined if ectopic expression of IRF-4 affects cell proliferation and/or survival of BCR/ABL+ B-ALL cells. IRF-4 or IRF-8 sequences were cloned into an MSCV retroviral vector containing a red fluorescent protein (RFP) gene as depicted in Figure 6A. The primary GFP+ BCR/ABL+ B lymphoblast cultures described above were transduced by cosedimentation with RFP, RFP+IRF-4, or RFP+IRF-8 retroviruses. Western blot analysis showed that myc-tagged IRF-4 and myc-tagged IRF-8 are expressed in transduced cells (Figure S3B). The lower expression level of IRF-4 comparing IRF-8 may be due to a negative selection of high IRF-4 expressing cells due to its antitumor activity as shown below. Densitometry analysis of IRF-4 expression levels in B-ALL cells transduced with RFP-IRES-IRF-4 showed that the relative intensity of IRF-4 expression (combined endogenous and ectopic) is comparable with that of the endogenous IRF-4 in imatinib-treated nontransduced B-ALL cells (1.10 vs 0.88; Figure S3C).
IRF-4 inhibits proliferation in BCR/ABL+ B-lymphoblasts. (A) Retroviral constructs used to transduce RFP + IRF-4, RFP + IRF-8, and RFP genes. (B) Relative percentage of RFP-expressing cells for BCR/ABL+ B lymphoblast cultures derived from BM of moribund BCR/ABL BMT mice suffering from B-ALL like disease. Triplicates of cultures were infected with retroviruses depicted in (A) and RFP expression was determined by FACS analysis. The percentage of RFP-expressing cells for each time point was normalized to the initial percentage of infected cells determined at 3 days after infection. (C) Cell-cycle analysis of RFP positive cells from BM cultures infected with RFP, RFP + IRF-4, or RFP + IRF-8. Analysis of BrdU incorporation and 7-amino-actinomycin D (7-AAD) levels allowed distinction of cell-cycle phases G1/G0, G2/M, S, and dying/dead cells (Ap). Percentage of cells in each phase is indicated within the gate.
IRF-4 inhibits proliferation in BCR/ABL+ B-lymphoblasts. (A) Retroviral constructs used to transduce RFP + IRF-4, RFP + IRF-8, and RFP genes. (B) Relative percentage of RFP-expressing cells for BCR/ABL+ B lymphoblast cultures derived from BM of moribund BCR/ABL BMT mice suffering from B-ALL like disease. Triplicates of cultures were infected with retroviruses depicted in (A) and RFP expression was determined by FACS analysis. The percentage of RFP-expressing cells for each time point was normalized to the initial percentage of infected cells determined at 3 days after infection. (C) Cell-cycle analysis of RFP positive cells from BM cultures infected with RFP, RFP + IRF-4, or RFP + IRF-8. Analysis of BrdU incorporation and 7-amino-actinomycin D (7-AAD) levels allowed distinction of cell-cycle phases G1/G0, G2/M, S, and dying/dead cells (Ap). Percentage of cells in each phase is indicated within the gate.
The initial percentage of transduced cells for each infected culture was assessed at 3 days after transduction by FACS analysis for the RFP expression. The percentage of cells expressing RFP, RFP+ IRF-4, or RFP+ IRF-8 was monitored for 10 days. The data show that the percentage cells expressing the RFP vector alone remains relatively constant over time (Figure 6B). In contrast, there is a progressive decrease in the percentage of RFP+ IRF-4 cells (Figure 6B). The percentage of RFP+ IRF-8–expressing cells is decreased moderately over time, but the reduction is less dramatic compared IRF-4 infected cultures (Figure 6B). Next, we determined the effect of ectopic expression of IRF-4 on cell-cycle progression at 4 days after infection. Cell-cycle analysis of RFP+ cells shows that cells expressing RFP+ IRF-4 have a significantly reduced number of cells in S phase (P = 2.66 × 10−6) with a corresponding significant increase in the number of cells in G0/G1 (P = .0013) compared with cells expressing RFP alone (Figure 6C). Cells expressing RFP+ IRF-8 had a cell-cycle profile similar to that of cells with RFP alone and showed no significant difference in the number of cells in any particular cell-cycle phase. In addition, we observed no significant difference in the proportions of dying/dead cells for RFP+, RFP+ IRF-4, or RFP+ IRF-8 populations. These results suggest that IRF-4 exerts tumor suppressor function primarily through the negative regulation of cell-cycle progression of B-lymphoblasts. It's possible that the effects on cell- cycle progression observed in imatinib-treated cells may be mediated in part by the activity of IRF-4.
To gain insight into the mechanism by which IRF-4 inhibits cell-cycle progression, we analyzed growth rate and expression of various cell-cycle inhibitors in cultured B-ALL cells derived from MSCV-BCR/ABL-IRES-GFP transduced BM cells from IRF-4 WT (no. 8), IRF-4 Het (no. 23), and IRF-4 KO (no. 9) donor mice. An equal number of cells were seeded in triplicate for growth analysis of the above cell lines. After 3 days, there were significantly more cells in IRF-4 KO B-ALL cells compared with IRF-4 WT and IRF-4 Het B-ALL cells (P = .01 and P = .002, respectively; Figure S4A). Real-time RT-PCR analysis shows no significant difference in mRNA levels for Cdkn1b, Cdkn1c, and Cdkn2a in IRF-4 WT, Het, or KO B-ALL cells (Figure S4B-D). However, we observed a statistically significant decrease in RNA levels for the cell-cycle regulator Cdkn2b in the IRF-4 KO B-ALL cells compared with that in IRF-4 WT or IRF-4 Het B-ALL cells (P < .05; Figure S4E). These results suggest that Cdkn2b is a target gene of IRF-4 transcriptionally and may involve in the negative regulation of proliferation of BCR/ABL B-ALL cells by IRF-4. A Western blot analysis to compare the Cdkn2b protein levels in IRF-4 KO B-ALL cells versus IRF-4 WT or IRF-4 Het B-ALL cells was not conclusive as a result of poor quality of an anti-Cdkn2b antibody. Target genes of IRF-4 that mediate its tumor suppressor function need to be identified systematically in the future.
Discussion
In this study, we demonstrated that the IRF-4 protein levels are increased in BCR/ABL+ murine B-ALL in response to imatinib treatment, IRF-4 deficiency facilitates BCR/ABL-mediated transformation of B-lymphoid progenitors in vitro and accelerates progression of BCR/ABL-induced B-ALL in mice, and that forced expression of IRF-4 effectively suppresses lymphoid leukemogenesis by BCR/ABL. These data indicate that IRF-4 has tumor suppressor activity in early B-cell development and suggest that down-regulation of IRF-4 may play an important role in the pathogenesis of BCR/ABL+ B-ALL.
The molecular mechanism by which IRF-4 suppresses B-lymphoid leukemogenesis is not clear. Our data suggest that IRF-4 may inhibit cell-cycle progression of lymphoblasts (Figure 6). It is possible that down-regulation of IRF-4 provides a proliferative advantage for BCR/ABL transformed B-lymphoid cells and inhibits differentiation of the pro–B-malignant clones by preventing cell-cycle exit. Our data are consistent with the previous observation that IRF-4 deficiency causes a lymphoproliferative disorder in mice over time.3
It has been shown that IRF-4 and IRF-8 have redundant functions in early B-cell development. However, in this study, we found that IRF-4 is a more potent suppressor for BCR/ABL-induced B-lymphoid leukemia compared with IRF-8. Expression of IRF-8 prolongs survival in the B-ALL mouse model, whereas expression of IRF-4 almost completely blocks the disease onset. These results indicate that IRF-4 and IRF-8 share some overlapping activity in suppressing B-lymphoid leukemogenesis but IRF-4 may have unique properties that make it a more potent tumor suppressor. It has been shown that IRF-4 has unique activity important for B-cell maturation.3,4 As mentioned earlier, IRF-4–deficient mice develop severe lymphadenopathy over time.3 IRF-8–deficient mice, on the other hand, show no obvious abnormalities in B-cell development.2,37 At the molecular level, although both IRF-4 and IRF-8 bind to the Ets family transcription factor Pu.1, it has been demonstrated that the IRF-4/Pu.1 complex is a more potent inducer of transcription than IRF-8/Pu.1 in macrophages and B cells.38 It is possible that these differences contribute to the more potent tumor suppressor activity of IRF-4 in early B-lymphoid cells. It is important to note that IRF-4 is much less abundant than IRF-8 in macrophages,39 therefore, although it is a more potent inducer of transcription, the overall activity of IRF-4/Pu.1 complex was shown to be less than that of IRF-8/Pu.1 in macrophages.
Microarray analysis of patient-derived BCR/ABL+ B cells show that expression of the IRF-8 mRNA is reduced compared with B cells isolated from healthy donors.17 However, we did not observe significant increase of the IRF-8 protein levels in imatinib-treated BCR/ABL+ mouse B-ALL cells (Figure 1). This discrepancy may attribute to the detection of transcript versus protein. Alternatively down-regulation of IRF-8 in BCR/ABL+ B-ALL may occur in a kinase-independent manner and, therefore, treatment with imatinib would not have an effect on the expression level of IRF-8. In addition, in several leukemic cell lines where IRF-4 and IRF-8 are down-regulated, the promoter region of IRF-4, but not IRF-8, is hypermethylated thus inhibiting transcription.16 This suggests that BCR/ABL-mediated down-regulation of IRF-4 and IRF-8 may occur by distinct mechanisms. Finally, because IRF-4 is a more potent tumor suppressor in early B-lymphoid cells, down-regulation of IRF-4 may be an earlier and more important event than that of IRF-8 in lymphoid leukemogenesis by BCR/ABL. A more detailed comparison of IRF-8 expression levels in the malignant blasts and the normal pro-B counterpart will help clarify whether or not IRF-8 is down-regulated in BCR/ABL+ B-ALL.
Imatinib and second-generation ABL kinase inhibitors are not effective in treating BCR/ABL+ B-ALL or CML lymphoid blast crisis.36 Continued effort in finding a treatment for these BCR/ABL-related malignancies is needed. The finding that IRF-4 is a potent tumor suppressor may allow elucidation of new molecular pathways significant to the pathogenesis of BCR/ABL+ B-ALL. Elucidating the molecular mechanism by which IRF-4 suppresses transformation may provide insights important to drug development for BCR/ABL-induced B-ALL, CML lymphoid blast crisis, and potentially other subsets of B-ALL. In addition, the context dependent roles of IRF-4 in oncogenesis should be an important consideration in developing cancer therapies targeting IRF-4. Further dissecting the mechanisms by which IRF-4 functions as an oncogene or a tumor suppressor gene is crucial for developing therapeutic strategies for precisely targeting the IRF-4 oncogenic pathway as well as for taking advantage of the IRF-4 tumor suppressor pathway.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Drs Tak Mak and Harinder Hsingh for providing the IRF-4−/− mice, Dr Yuan Zhuang for providing the Il-7–dependent E2AGFP pro-B cell line, and Ms Noreen Francis for helping with cell sorting.
This work was supported by National Cancer Institute grant CA68008 (R.R.).
National Institutes of Health
Authorship
Contribution: R.R. designed research, analyzed data, and drafted the manuscript; J.A. designed the study, performed research, analyzed data, and drafted the manuscript; and X.C. performed part of the research and helped draft the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Ruibao Ren, Brandeis University, Rosenstiel Center, MS029, 415 South Street, Waltham, MA 02454-9110; e-mail: ren@brandeis.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal